Abstract
The bacterial community and genes involved in geobiocycling of arsenic (As) from sediment impacted by long-term gold mining were characterized through culture-based analysis of As-transforming bacteria and metagenomic studies of the arsC, arrA, and aioA genes. Sediment was collected from the historically gold mining impacted Mina stream, located in one of the world’s largest mining regions known as the “Iron Quadrangle”. A total of 123 As-resistant bacteria were recovered from the enrichment cultures, which were phenotypically and genotypically characterized for As-transformation. A diverse As-resistant bacteria community was found through phylogenetic analyses of the 16S rRNA gene. Bacterial isolates were affiliated with Proteobacteria, Firmicutes, and Actinobacteria and were represented by 20 genera. Most were AsV-reducing (72%), whereas AsIII-oxidizing accounted for 20%. Bacteria harboring the arsC gene predominated (85%), followed by aioA (20%) and arrA (7%). Additionally, we identified two novel As-transforming genera, Thermomonas and Pannonibacter. Metagenomic analysis of arsC, aioA, and arrA sequences confirmed the presence of these genes, with arrA sequences being more closely related to uncultured organisms. Evolutionary analyses revealed high genetic similarity between some arsC and aioA sequences obtained from isolates and clone libraries, suggesting that those isolates may represent environmentally important bacteria acting in As speciation. In addition, our findings show that the diversity of arrA genes is wider than earlier described, once none arrA-OTUs were affiliated with known reference strains. Therefore, the molecular diversity of arrA genes is far from being fully explored deserving further attention.
Introduction
Arsenic occurs naturally in the earth’s crust and is widely distributed in the environment [1], [2]. Natural mineralization and microorganisms enhance arsenic mobilization in the environment, but human interventions, such as gold mining, have aggravated the environmental arsenic contamination arousing health concerns. Water pollution by arsenic is one of the major challenges for public health, primarily due to its carcinogenic potential at low doses [3], [4], [5], [6]. According to Nordstrom [7] over 50 million people in the world are at risk from drinking arsenic-contaminated water. Moreover, given that arsenic has a variety of valence states (+V, +III, 0, −III) with different physicochemical properties, the removal of arsenic from contaminated water bodies is yet a challenge.
In nature, microorganisms have developed different response mechanisms to metabolize As, mainly via reduction and oxidation reactions, leading to its speciation [8]. Previous studies have regarded As speciation as a result of microbial activity in the environment, including some derived from gold-mining activities [1], [2]. However, few bacterial genera involved in As transformation have been found at any of the sites studied [9]–[13]. Thus, a more comprehensive knowledge on the structure of the bacterial community involved in As-transformation in gold-mining sites remains warranted.
The arsenate (AsV) reducing pathways known are the detoxification (arsC gene) and the dissimilatory respiration (arrA/B genes). The organization of ars operons varies greatly between taxa, and the core genes include arsR, arsB and arsC, whereas arsD and arsA genes can eventually be found [1]. The arsC gene encodes the enzyme AsV reductase, which is located in the cytoplasm and is responsible for the biotransformation of AsV to AsIII. This enzyme together with a transmembrane efflux pump, encoded by arsA and arsB genes, is the most common As transformation mechanism in the environment [2], [14]–[16]. Moreover, arrA/B genes encode a periplasmic AsV reductase that works during anaerobic respiration using AsV as the final electron acceptor for energy generation [17]. The AsV dissimilatory respiration reduction has already been described for many bacterial phyla, including obligatory and facultative anaerobic bacteria and some archaea [1].
The microbial oxidation of AsIII was first reported in 1918 and can be mediated by two distinct enzymes: AioBA, hardly studied, and ArxAB, recently described by Zargar et al. [18]. Both enzymes have been found in several heterotrophic and chemolithoautotrophic bacterial species [19]–[22]. Aerobic AsIII oxidation is catalyzed by arsenite oxidase, which uses O2 as terminal electron acceptor, and is encoded by aioB/A genes, formerly referred to as aoxA/B, aroB/A and asoB/A genes [20], [23]. ArxAB detected in AsIII oxidizing bacteria in anoxic conditions, in which nitrate or chlorate reduction is coupled to AsIII oxidation in the chemolithotrophs [24], [25]. Interestingly, members of the genus Ectothiorhodospira are able to use AsIII as electron donor for anoxygenic phototrophic growth [26]. According to Zargar et al. [18] the arxA gene is more closely related to arrA than to aioA genes.
In this research, we bioprospected As-resistant bacteria from As-enrichment culture of sediments collected from a stream located at the Brazilian gold mining area known as the Iron Quadrangle (IQ, Minas Gerais state), one of the world’s largest mining regions. Much concern exists about As-contamination of gold-mining sites in this area because it is estimated that at least 390,000 tons of As have been released into this area since the beginning of gold-mining activity in the 17th century [27]. We also investigated the diversity of As-transforming genes using metagenomic strategies. This included the genes for arsenite oxidase (aioA) and arsenate reductases (arsC and arrA).
Materials and Methods
Ethics Statement
For sampling in Mina stream, no specific permit was required for the described field study. The study location is not privately-owned or protected in any way and we confirm that the field study did not involve endangered or protected species.
Study Area and Sampling
Mina stream (19°58′46.80″S–43°49′17.07″W) is a natural body of water located at the Velhas River Basin (IQ, Minas Gerais state, Brazil) and characterized as backwater (Figure S1). This stream was chosen because is located near a historically impacted gold-mining area. Moreover, previous investigations [28] reported As concentrations superior to those permitted by Brazilian law (Conselho Nacional do Meio Ambiente – CONAMA) and by Canadian Environmental Quality Guidelines (Canadian Council of Ministers of the Environment– CCME).
Bulk water and superficial sediment samples (up to 1.0 cm depth) were collected on 13 July 2011, during the dry season. The typical sediment core can be divided into three zones: oxic, suboxic and anoxic [29]. According to literature the thick oxic zone can extend from several mm up to 10 cm [30], [31]. In this work the sampling site was shallow (20 cm) and therefore highly influenced by the nutrients and oxygen concentrations of the water body. The analyzed sediment was taken from the upper part, representing the oxic zone. Samples were collected aseptically at three points at 1m distance from each other, subsequently pooled in a single sample, and stored at 4°C for bacterial analysis or at −20°C for chemical and molecular analyses.
To assess the bulk water conditions physicochemical characteristics such as temperature, pH, and dissolved oxygen (DO) concentration were measured in situ with a multiprobe (Horiba, model U-22) [30]. Concentrations of total nitrogen (TN), total phosphorus (TP), ammonium (NH4 +-N), nitrite (NO2-N), nitrate (NO3-N), and soluble reactive phosphorus (PO4-P) were measured as previously described [32], [33]. Metal and metalloid concentrations of water and sediment samples were determined by using an inductively coupled plasma-optical emission spectrometer (ICP-OES, Optima 7300 DV, PerkinElmer).
Arsenic Enrichment and Isolation
Sediment (10 g) samples were added to Erlenmeyer flasks containing 100 mL of CDM medium (0.012 mM Fe2SO4, 7 mM Na2SO4, 0.0574 mM K2HPO4, 9.5 mM NaHCO3, 18.7 mM NH4Cl, 8.12 mM MgSO4, 0.457 mM CaCl2 and 44.6 mM sodium lactate as organic carbon source, pH 7.2) with either 2 mM sodium arsenite or 10 mM sodium arsenate and incubated at 28°C for seven days. Then, serial 10-fold dilutions of the enrichment cultures were plated onto CDM agar media (1.5% agar) amended with 2 mM sodium arsenite or 10 mM sodium arsenate to selectively enrich and isolate AsIII- and AsV-resistant bacteria. Plates were incubated at 28°C for five days. The resulting colonies were repeatedly streaked on the same medium to accomplish their purification. The bacterial isolates from AsIII- and AsV-resistant bacteria (named MS-AsIII and MS-AsV, respectively) were stored at −20°C in 25% glycerol.
DNA Extraction from the Cultures and Sediment
Genomic DNA was extracted and purified from each MS-AsIII and MS-AsV isolate using a protocol previously described [34]. Additionally, metagenomic DNA was extracted from 10 g (wet weight) of sediment using the PowerSoil DNA Extraction Kit (MO BIO Laboratories, USA) according to the manufacturer’s instructions. Total DNA from the MS-AsIII and MS-AsV isolates and sediment were quantified by absorbance at 260 nm using a NanoDrop Spectrophotometer (NanoDrop Technologies). DNA purity was assessed using the A260/A280 and A260/A230 ratios. DNA was stored at −20°C until further processing.
PCR Amplification and Construction of Clone Libraries
Briefly, touchdown PCR was carried out by amplifying bacterial MS-AsIII and MS-AsV isolates 16S rRNA gene fragments using the conditions previously described by Freitas et al. [35]. The reactions were performed using the bacterial-targeted primer set 8F (5′-AGAGTTTGATYMTGGCTCAG-3′) and 907R (5′-CCGTCAATTCMTTTRAGT-3′) [36]. Taq DNA polymerase and dNTPs were purchased from Fermentas (Canada) and used in all the PCR reactions.
Metagenomic and genomic DNA were used as template for PCR employing the arsC, arrA and aioA genes for construction of clone libraries and genotypic characterization of the bacterial MS-AsIII and MS-AsV isolates. PCR reactions targeting the arsC, arrA and aioA genes were carried out using primers and conditions as previously described by Sun et al. [37], Malasarn et al. [17] and Hamamura et al. [20], respectively. The arsC gene examined was of the glutaredoxin-dependent arsenate reductase enzyme, ArsC, from Escherichia coli R773 plasmid. The primer chosen has been successfully applied in several investigations of a variety of environmental samples [37], [38].
The amplicons of arsC, arrA, and aioA genes were gel-purified using the Silica Bead DNA Gel Extraction Kit (Fermentas, Canada). PCR products were cloned into the vector pJET1.2/blunt (Fermentas, Canada), and propagated with Escherichia coli XL1-Blue electrocompetent cells according to the manufacturer’s instructions.
Sequencing and Phylogenetic Analysis
Partial 16S rRNA, arsC, arrA, and aioA gene sequences were obtained using BigDye Terminator Cycle Sequencing kit (Life Technologies, USA) according to the manufacturer’s instructions. The nucleotide sequences were quality checked and submitted to GenBank with accession numbers from KC577613 to KC577798. The 16S rRNA gene sequences were analyzed through blastn (http://www.ncbi.nlm.nih.gov) and Classifier search tool (http://rdp.cme.msu.edu) to determine their phylogenetic affiliation. The arsC, arrA, and aioA gene sequences were compared with those available at the GenBank databases using blastn and blastx tools (http://www.ncbi.nlm.nih.gov) to retrieve potential homologs. Operational taxonomic unities (OTUs) from As gene clone libraries were defined with DOTUR software [39] using a cut-off threshold of ≥97% identity. Coverage of the clone libraries was calculated using the equation C = 1−(n/N)×100, where n is the number of unique OTUs and N is the number of sequences analyzed in the library [40].
In total, five fasta files were obtained containing arsC, arrA, aioA, MS-AsIII 16S rRNA, and Ms-AsV 16S rRNA gene sequences. Due to the short length of arsC and arrAamino acid sequences obtained in this study, added to the high similarity of some OTUs and isolates, we decided to reconstruct the phylogenetic relationships of As metabolism genes using nucleotide sequences to increase the phylogenetic signal and avoid overparameterization.
Sets of nucleotide sequences were independently aligned using MAFFT 7 with iterative refinement by the G-INS-i strategy [41]. Multiple sequence alignments were manually refined using Jalview [42]. To optimize the datasets for evolutionary analyses we removed redundancy and sequences too distantly related using the Decrease Redundancy tool available as a resource at ExPaSy (www.expasy.org). The Decrease Redundancy parameters were set as 99 for “% max similarity” and 30 for “% min similarity”. Identical sequences were clustered as single OTUs and filtered alignments were further used in phylogenetic analyses. Identifiers of filtered sequences were later included into the phylogenetic tree. To reconstruct phylogenetic trees we used the maximum likelihood method (ML) as implemented in PhyML [43]. For the phylogenetic reconstruction we tested seven different evolutionary models (HKY85, JC69, K80, F81, F84, TN93, and GTR) using the jModelTest 2 software [44]. The evolutionary model best fitting the data was determined by comparing the likelihood of tested models according to the Akaike Information Criterion (AIC). Statistical support value for each node was computed by approximate likelihood ratio test (aLTR). Trees were visualized and edited using the FigTree software (tree.bio.ed.ac.uk/software/figtree).
Susceptibility and Arsenic Transformation Tests
Minimum inhibitory concentrations (MIC) were established, in triplicate, by the agar dilution method in CDM with 1×105 CFU ml−1 as standard inoculums. CDM plates were supplemented with increasing concentrations (from 2 mM to 1024 mM) of AsIII or AsV and incubated at 28°C for seven days. MIC was defined as the lowest AsIII or AsV concentration that completely inhibited bacterial growth.
The ability to oxidize AsIII and reduce AsV was investigated using a qualitative screening according to [45]. To achieve that, bacterial MS-AsIII and MS-AsV isolates were grown in CDM broth with 100 mg l−1 2 mM sodium arsenite or 100 mg l−1 sodium arsenate until an optical density of 0.4 at 595 nm was reached. After that, 20 µl of 0.01 mol l−1 of potassium permanganate solution were added in 1 ml of bacterial culture. The data were interpreted according to the change in medium color, i.e., a pink color indicated a positive oxidation of AsIII and a yellow color indicated a positive reduction of AsV.
Results
Environmental Parameters
The physicochemical characteristics of the water and sediment samples from the Mina stream are presented in Tables 1 and 2. Data displayed on Table 1 revealed that metal concentrations in the Mina stream exceeded the maximum allowable concentrations established by Brazilian and Canadian environmental regulations [46], [47] for sediment and water. Al, Mn, Fe, Cu, As and Zn were the metals present in the highest concentrations in the sediment sample analyzed.
Table 1. Metal concentration from sediment and water of Mina Stream and limits permitted by law.
| Metals | Sediment (mg kg−1) | CONAMA* (mg kg−1) | Water (mg l−1) | CONAMA** (mg l−1) |
| Fe | 492.8 | NE | 0.52 | 15 |
| Ni | 9.0 | 18 | <0.1 | 2 |
| Mn | 1284.5 | NE | 1.45 | 1 |
| Cu | 387.7 | 35.7 | 0.19 | 1 |
| Pb | 8.7 | 35 | NE | 0.5 |
| Cd | <2.5 | 0.6 | <0.1 | 0.2 |
| Zn | 180.9 | 123 | 0.2 | 5 |
| Al | 2343.2 | NE | <0.5 | NE |
| As | 297.1 | 5.9 | <0.1 | 0.5 |
| Cr | 17.3 | 37.3 | <0.1 | 1 |
| Hg | <2.5 | 0.17 | <0.1 | 0.01 |
NE – Not established.
*CONAMA resolution 344/04.
**CONAMA resolution 430/11.
Table 2. Physicochemical parameters from water of Mina Stream.
| Parameters | Water |
| pH | 6.2 |
| Conductivity (µs cm−1) | 2151 |
| Temperature (°C) | 18.0 |
| Dissolved Oxygen (mg l−1) | 9.1 |
| Redox (mV) | 215 |
| NO3 − -N (µg l−1) | 3103.8 |
| NO2 − -N (µg l−1) | 161.3 |
| NH4 + -N (µg l−1) | 829.5 |
| PO4 3− -P (µg l−1) | 2.3 |
| Total P (µg l−1) | 77.6 |
| Total N (µg l−1) | 2916.8 |
The physicochemical analysis revealed that the Mina stream can be characterized as a mesothermal oxidized environment with highly oxygenated and circum-neutral waters (Table 2). Nitrogen and phosphorus ratio was greater than nine (Table 2). According to Salas & Martino [48], this ratio indicates that the phosphorus was the most limiting nutrient and that the stream can be classified as eutrophic.
Phylogenetic Affiliation
In total, 123 bacterial isolates were recovered from the enrichment cultures (68 and 55 from the MS-AsIII and MS-AsV, respectively). Partial 16S rRNA gene sequences used for phylogenetic analysis were approximately 600 bp long and spanned the V2 to V4 variable regions. MS-AsIII and MS-AsV isolates were categorized into three phyla: Proteobacteria (56% and 59%, respectively, includes alpha, beta, and gamma-Proteobacteria), Firmicutes (36% in both enrichment cultures), and Actinobacteria (8% and 5%). Twenty genera represented these phyla in the Mina stream sample analyzed. Differences in the bacterial composition between the MS-AsIII and MS-AsV enrichment cultures were detected (Table 3 and Figure 1). The resulting Venn diagram shows that a higher bacterial diversity was observed in the MS-AsIII than in the MS-AsV enrichment cultures. Eight genera were specifically found in MS-AsIII and seven were shared between the culture systems (Figure 1).
Table 3. Phylogenetic distribution of the bacterial isolates and their As-metabolism phenotype and genotype.
| Enriched culture | Phylum | Genus | N° of isolates* | Phenotype** | Genotype*** |
| MS-AsV | Proteobacteria | Acinetobacter | 1 | reducer | arsC aioA |
| Brevundimonas | 9 | reducer (3) | arsC [9] aioA [1] | ||
| Diaphorobacter | 1 | reducer | arsC | ||
| Pannonibacter | 2 | reducer (2) | arsC [2] | ||
| Pseudomonas | 6 | reducer (2) | arsC [6] aioA [2] | ||
| Pseudoxanthomonas | 10 | reducer (6) | arsC [7] aioA [1] | ||
| Stenotrophomonas | 5 | reducer (5) | arsC [5] aioA [2] | ||
| Thermomonas | 1 | reducer | arsC | ||
| Firmicutes | Bacillus | 14 | reducer (9), oxidizer (8) | arsC [12] aioA [6] arrA [1] | |
| Exiguobacterium | 1 | reducer | arsC aioA | ||
| Actinobacteria | Microbacterium | 4 | reducer (4) oxidizer (1) | arsC [3] aioA [1] | |
| Rhodococcus | 1 | reducer | arsC | ||
| MS-AsIII | Proteobacteria | Achromobacter | 1 | oxidizer | arsC aioA |
| Acidovorax | 2 | oxidizer(2) | arsC [2] aioA [1] | ||
| Acinetobacter | 4 | reducer (3) | arsC [2] aioA [1] | ||
| Comamonas | 5 | reducer (5) | arsC [5] arrA [2] | ||
| Ochrobactrum | 1 | reducer | arsC | ||
| Pseudomonas | 3 | reducer (3) | arsC [1] aioA [1] | ||
| Pseudoxanthomonas | 17 | reducer (9) oxidizer (5) | arsC [14] aioA [2] arrA [1] | ||
| Shewanella | 1 | reducer | arrA | ||
| Stenotrophomonas | 2 | reducer (2) oxidizer (1) | arsC [1] aioA [1] | ||
| Thermomonas | 2 | reducer (1) | arsC [2] arrA [1] | ||
| Firmicutes | Bacillus | 21 | reducer (19) oxidizer (5) | arsC [21] aioA [2] arrA [3] | |
| Paenibacillus | 1 | reducer oxidizer (1) | arsC | ||
| Staphylococcus | 4 | reducer (4) oxidizer (1) | arsC [3] | ||
| Actinobacteria | Micrococcus | 3 | reducer (2) oxidizer (1) | arsC [1] aioA [1] | |
| Microbacterium | 1 | reducer | - |
*The number represents the total of bacterial isolates identified.
**Values in parentheses indicate the number of As-redox isolates.
***Values in bracket indicate the number of isolates harboring As-metabolism genes.
Figure 1. Venn diagram showing the exclusive and shared bacterial genera retrieved from MS-AsIII and MS-AsV enrichment cultures.
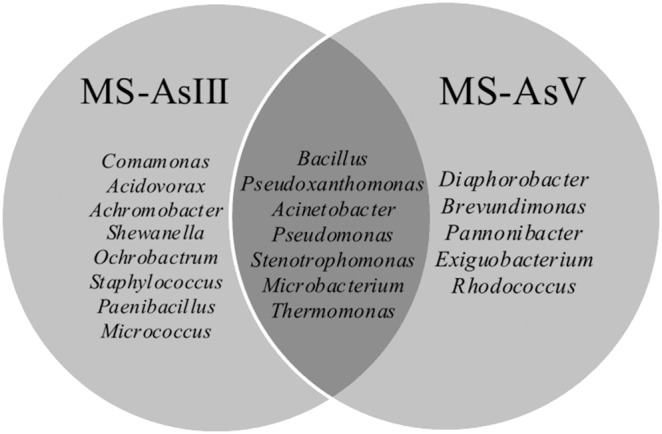
Dominant genera in MS-AsV were Bacillus (26%), Pseudoxanthomonas (18%), and Brevundimonas (16%). The predominant population in MS-AsIII was Bacillus (30%), followed by Pseudoxanthomonas (25%). The other bacteria related to MS-AsIII and MS-AsV are listed in Table 3. Although the Proteobacteria phylum was the most diverse and dominant, the Bacillus (29%) genus was the most abundant and diverse among the genera because it harbored eight identified species.
Characterization of As-reducing and Oxidizing Isolates and Identification of their Genes Involved in As Metabolism
The MICs for the MS-AsIII and MS-AsV isolates were determined. The highest MIC was found for AsV in which 94% of the isolates exhibited values ≥256 mM, whereas 90% of the isolates displayed MICs ranging from 32 mM to 64 mM for the most toxic AsIII.
The As-transformation ability of the isolates was determined with a qualitative test that revealed that 72% of the isolates were AsV-reducing, whereas 20% were AsIII-oxidizing. Of those, 8% presented AsV-reducing as well as AsIII-oxidizing activities. Among the 20 genera identified in both MS-AsIII and MS-AsV enrichment cultures, Acidovorax and Achromobacter presented only AsIII-oxidizing activity. No As-transformation activity was found in 8% of the total of MS-AsIII and MS-AsV isolates (123) (Table 3).
The molecular analysis of the MS-AsIII and MS-AsV isolates unveiled that the arsC gene was the most frequent (85%), followed by aioA (20%) and arrA (7%) (Table 3). Of those, Bacillus was the only genus harboring all three genes, and Shewanella was the only genus which did not harbor the most common gene (arsC) in the isolate analyzed (MS-AsIII-61). Achromobacter and Acidovorax both harbored the aioA gene, confirming the phenotypic data. Thermomonas and Pannonibacter also harbored As resistance genes.
General Features of Clone Libraries
To unveil the molecular diversity of genes involved in As metabolism in the Mina stream sediment, three clone libraries for arsC, arrA, and aioA genes were constructed. One hundred sixty-four sequences were analyzed after quality control and the removal of chimeric sequences. The coverage values of the three libraries (80%, 70% and 63%, respectively for arsC, arrA, and aioA) indicated that most of the diversity of these genes was detected.
Blastx analysis of arsC, aioA, and arrA-OTUs revealed high similarity with sequences from glutaredoxin-glutathione arsenate reductase (from 76 to 100%), molybdopterin-binding arsenite oxidase (from 71 to 96%), and respiratory arsenate reductase (from 64 to 98%) (Tables S1, S2, and S3 in Tables S1). The sequences corresponding to arsC were associated with arsC harboring different bacterial taxa from a variety of environments. The aioA-OTUs were closely related to uncultured and cultured clones from As contaminated environments. Furthermore, all arrA-OTUs were closely related to uncultured clones from rock biofilms of an ancient gold mine and Cache Valley Land Fill sediments, both arsenic-contaminated environments.
Phylogenetic Analyses of 16S rRNA, arsC, aioA, and arrA Genes Sequences
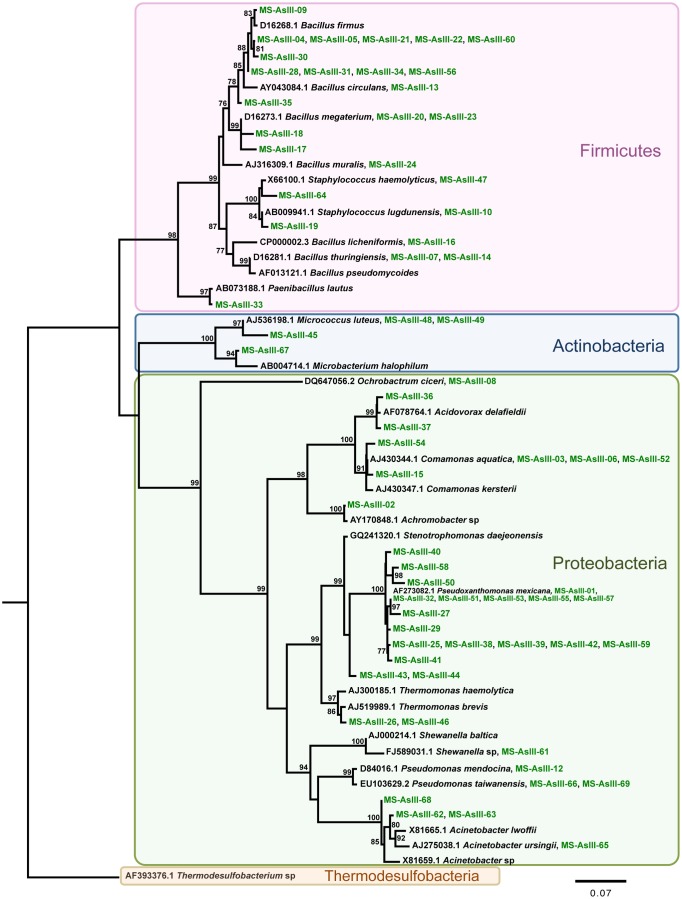
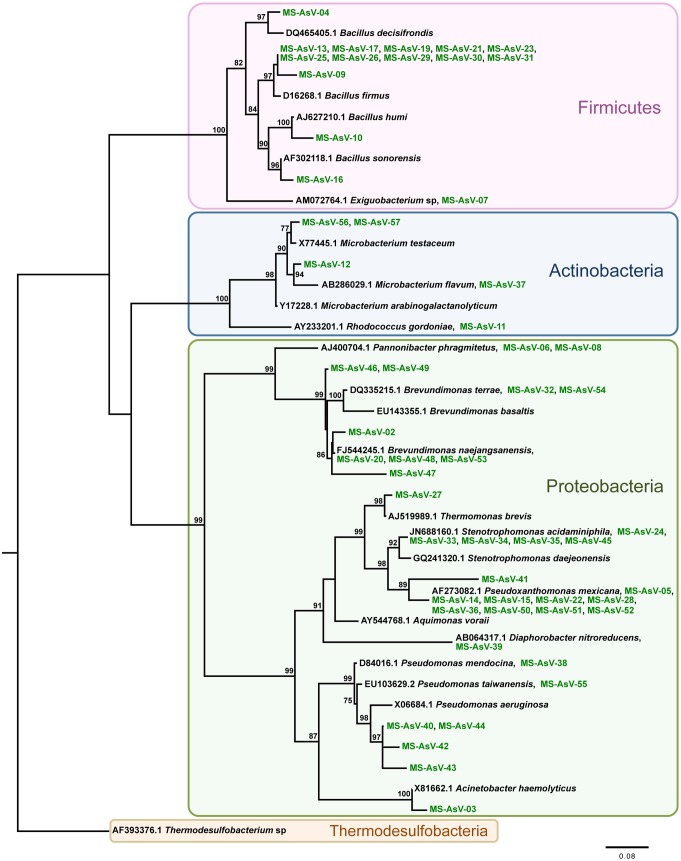
In this study we have amplified, sequenced and reconstructed the evolutionary relationships of 16S rRNA and As metabolism genes encoded by As-resistant bacteria retrieved from a stream located at the Brazilian gold mining area and cultivated on As-enrichment sediment’s culture, as well as As metabolism genes of clone libraries. The phylogeny of the AsIII-resistant bacteria (MS-AsIII) 16S rRNA gene sequences was reconstructed from an alignment containing 57 operational taxonomic units and 719 sites, which represent 99 sequences (Fig. 2). Therefore, 42 sequences were considered redundant by the Decrease Redundancy tool (www.expasy.org). The phylogenetic tree reconstructed by using the maximum likelihood method as implemented in PhyML [43], shows sequence’s clear separation into three strongly supported clades, which have representatives of the Firmicutes, Actinobacteria, and Proteobacteria phyla (Fig. 2). Similar results were obtained for the AsV-resistant bacteria (MS-AsV) 16S rRNA phylogenetic analysis (Fig. 3). The evolutionary history was based on an alignment containing 40 OTUs and 721 sites, representing 79 sequences (Fig. 3). The Decrease Redundancy tool filtered about 50% of the initially selected sequences. The resulting phylogeny also exhibits the presence of three well-supported clades containing Firmicutes, Actinobacteria, and Proteobacteria phyla representatives (Fig. 3).
Figure 2. Evolutionary relationships of AsIII-resistant bacteria (MS-AsIII) 16S rRNA sequences.
A total of 57 nucleotide sequences and 719 sites were analyzed. The phylogeny was reconstructed by maximum likelihoodand TIM3+I+G+F was selected as best fit model. Support values for each node were estimated using the Akaike Likelihood Ratio Test (aLRT). Only support values higher than 70% are shown. Reference sequences retrieved from the non-redundant database of the NCBI are shown in black, bacterial isolates (MS-AsIII and MS-AsV) in green. Different background colors highlight three well-supported clades: Firmicutes, Actinobacteria, and Proteobacteria. Thermodesulfobacteria was used as outgroup.
Figure 3. Evolutionary relationships of AsV-resistant bacteria (MS-AsV) 16S rRNA sequences.
A total of 40 nucleotide sequences and 721 sites were analyzed. The phylogeny was reconstructed by maximum likelihood and TrN+G+F was selected as best fit model. Support values for each node were estimated using the Akaike Likelihood Ratio Test (aLRT). Only support values higher than 70% are shown. Reference sequences retrieved from the non-redundant database of the NCBI are shown in black, bacterial isolates (MS-AsIII and MS-AsV) in green. Different background colors highlight three well-supported clades: Firmicutes, Actinobacteria, and Proteobacteria. Thermodesulfobacteria was used as outgroup.
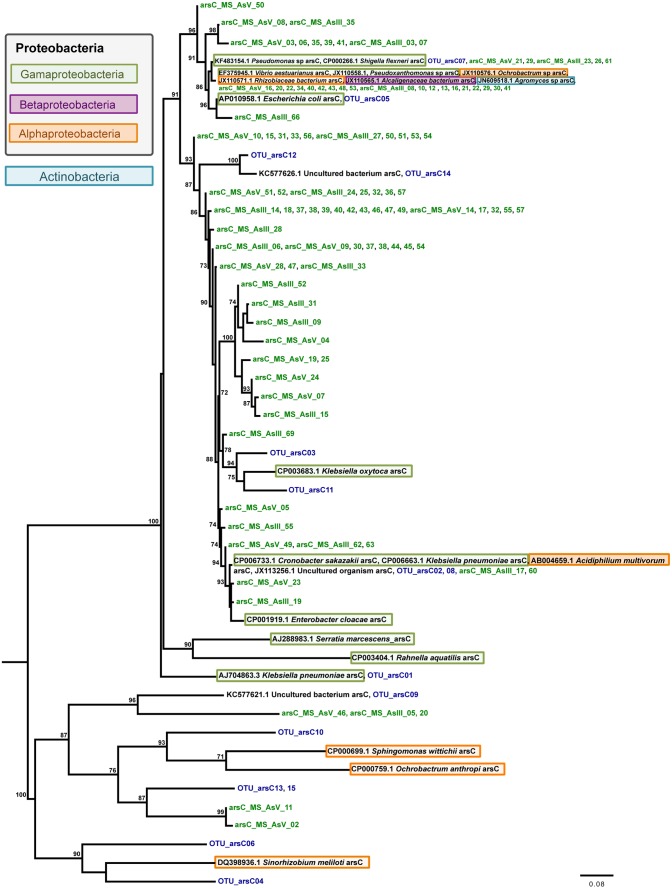
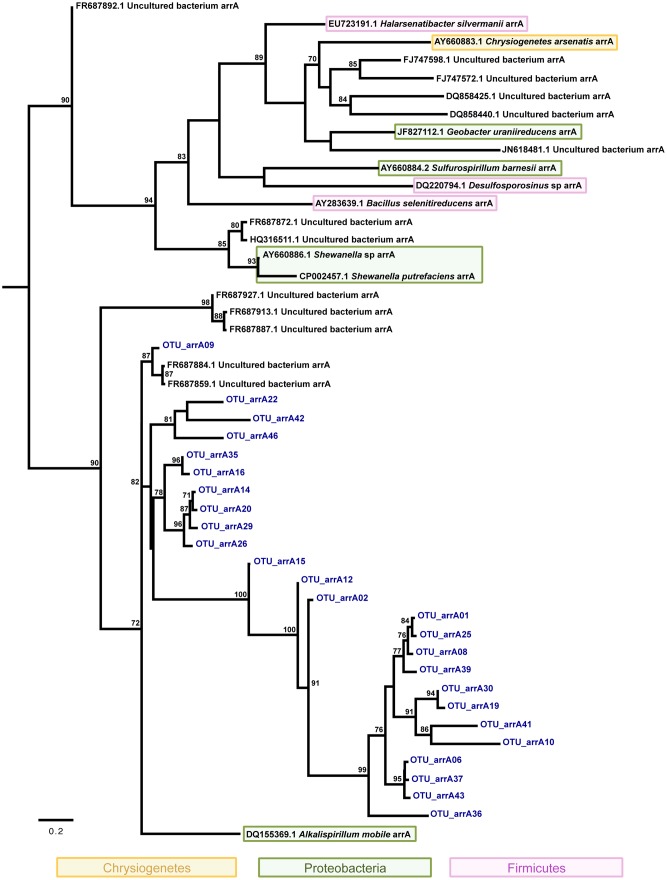
Concerning evolutionary histories of As metabolism genes, the phylogenetic tree of arsC sequences was reconstructed with 48 nucleotide sequences and 352 sites, which represent 142 sequences (Fig. 4). TrN+I+G+F was selected as best fit model. The resulting phylogeny supports the hypothesis that horizontal gene transfer (HGT) seems to have played a role in the widespread distribution of arsC coding gene in Actinobacteria and Proteobacteria. Similar findings were retrieved on the phylogeny reconstructed for arrA sequences based on 47 nucleotide sequences and 242 sites where GTR+I+G+F was selected as best fit model (Fig. 5). On the other hand, the phylogenetic analysis of aioA sequences based on 72 nucleotide sequences and 543 sites shows two clades strongly supported: alpha- and beta-proteobacteria (Fig. 6) without clear evidence of HGT. For this analysis GTR+I+G+F was selected as best fit model. Interestingly, all putative arrA sequences obtained in this study (arrA- OTU) were more closely related to themselves or to sequences from uncultured bacteria, showing that more studies involving arrA sequences will be relevant to better understand the molecular diversity of those genes (Fig. 5).
Figure 4. Evolutionary relationships of arsC sequences.
A total of 48 nucleotide sequences and 352 sites were analyzed. The phylogeny was reconstructed by maximum likelihoodand TrN+I+G+F was selected as best fit model. Support values for each node were estimated using the Akaike Likelihood Ratio Test (aLRT). Only support values higher than 70% are shown. Reference sequences retrieved from the non-redundant database of the NCBI are shown in black, bacterial isolates (MS-AsIII and MS-AsV)in green, and operational taxonomic unities (OTUs) from As gene clone librariesin blue. Different background colors highlight Actinobacteria and three Proteobacteria classes – Gamma-, Beta, and alpha-proteobacteria.
Figure 5. Evolutionary relationships of arrA sequences.
A total of 47 nucleotide sequences and 242 sites were analyzed. The phylogeny was reconstructed by maximum likelihoodand GTR+I+G+F was selected as best fit model. Support values for each node were estimated using the Akaike Likelihood Ratio Test (aLRT). Only support values higher than 70% are shown. Reference sequences retrieved from the non-redundant database of the NCBI are shown in black, bacterial isolates (MS-AsIII and MS-AsV) in green, and operational taxonomic unities (OTUs) from As gene clone libraries in blue. Different background colors highlight three bacterial phyla - Proteobacteria, Firmicutes, and Chrysiogenetes.
Figure 6. Evolutionary relationships of aioA sequences.
A total of 72 nucleotide sequences and 543 sites were analyzed. The phylogeny was reconstructed by maximum likelihood and GTR+I+G+F was selected as best fit model. Support values for each node were estimated using the Akaike Likelihood Ratio Test (aLRT). Only support values higher than 70% are shown. Reference sequences retrieved from the non-redundant database of the NCBI are shown in black, bacterial isolates (MS-AsIII and MS-AsV) in green, and operational taxonomic unities (OTUs) from As gene clone libraries in blue. Different background colors highlight two Proteobacteria classes – beta- and alpha-proteobacteria.
Discussion
The environmental impact of gold mining is presently a major concern because its processes release toxic metals such as As in both soil and groundwater. Considering the relevance of bacteria in the speciation of As in aquatic environments, we bioprospected As-resistant bacteria and As-transforming genes originated from sediments impacted by long-term gold mining. Although some studies have focused in the identification of As-resistant bacterial communities in a long-term As-contaminated environment [9]–[13], [49]–[52], the employment of combination of culture-based physiological and genomic approaches with metagenomic analysis in sediments collected from these areas are scarce [53], [54]. In this study, we reveal a large number of phylogenetically distinct As-resistant bacterial genera retrieved from sediment collected from a stream in a long-term gold-mining area.
We found Bacillus as the dominant genus in both MS-AsIII and MS-AsV enrichment cultures. Members of Bacillus are often found in As-contaminated environments [9], [11], [16], [54] being related to As-reduction and -oxidation, indicating that they are an essential component of As speciation in nature [54], [55]. The observed abundance of Bacillus isolates harboring the arsC and aioA genes confirmed its ubiquity and high As-resistance in As-rich environments, as it is the case of Mina stream sediment. This suggests an important role for Bacillus in As speciation. It also points to a possible use of these natural isolates in future bioremediation projects.
A recent study of our group [56], using culture-independent approach to assess the prokaryotic diversity in Mina stream sediment, revealed the presence of the Thermomonas, Acidovorax, Acinetobacter and Ochobactrum genera also detected in the present study. Moreover, Bandyopadhyay et al. [57] have proposed a novel species of the Pannonibacter genus, Pannonibacterindica, which is able to grow in high concentrations of AsV. However, it should be noted that Thermomonas and Panonnibacter were not previously reported in the literature as As-transforming genera.
The phenotypic and genotypic characterization of the MS-AsIII and MS-AsV bacterial isolates revealed their ability to reduce and oxidize As. Most bacteria (85%) were AsV-resistant bacteria (ARB) harboring the arsC gene, responsible for the reduction of AsV to AsIII, which is the most frequent detoxification reaction among bacteria in the environment [8]. Although the aerobic enrichment culture condition employed in this study could inhibit the growth of dissimilatory arsenate-reducing bacteria (DARB), it is likely that these bacteria were present because the arrA gene was detected. Several reports have evidenced DARB bioremediation potential of As-contaminated samples [2], [54], [58], [59].
AsIII-oxidizing bacterial isolates were minority (20%). This result is in agreement with those reported by Silver & Phung [14], who suggest that most isolates from natural environments lack AsIII-oxidizing ability. In this study, all AsIII-oxidizing isolates were classified as heterotrophic AsIII oxidizers (HAO) spanning 11 genera. However, only isolates belonging to Bacillus, Pseudoxanthomonas, Stenotrophomonas, Micrococcus, Achromobacter, and Acidovorax genera co-presented the oxidizing phenotype and genotype. From an ecological perspective, oxidizing bacteria are important performers in As-contaminated environments because they promote transformation from AsIII into AsV.
In a few isolates (8%), oxidizing and reducing As-transformation activities were not observed in their phenotype and genotype. There are several possible explanations for this. First, the As-transformation gene expression observed in isolates grown in the laboratory is likely to be different from that encountered in these isolates in nature, because of the different conditions of these environments. Second, these differences may reflect mutations in the As-resistance genes studied. Third, alternative resistance genes may be expressed by these isolates [60].
The high diversity and adaptability of the bacterial community disclosed herein could be explained by the presence of multiple copies of As-resistance genes either on bacterial chromosomes or on plasmids as a consequence of pressure created by the long-term contamination that occurs in the Mina stream area. Nevertheless, further studies will be needed to establish this.
Previous studies on As-resistance genes are associated with As-resistant cultivable isolates [10], [16], [59], [61]. Considering that the vast majority of bacteria are uncultivable, this traditional approach has limited our understanding of the extreme functional diversity in natural bacterial communities. Therefore, a metagenomic approach to investigate the functional genes associated with As-transformation in nature is essential to further our current knowledge on this matter. The analysis of arrA sequences revealed that all of them exhibited similarity with those from uncultured organisms. This predominance of uncultured organisms indicates that arrA gene present in Mina stream sediment is expressed by unidentified DARB. The arsC sequences detected in the sediment were similar to those previously reported [37], [62], [63]. The primers used in this study amplified aioA-like sequences [20], [64]. The aioA sequences were similar to several aioA genes of the Proteobacteria phylum. This finding is in agreement with Quéméneur et al. [65], who reported prevalence of AsIII-oxidizing Proteobacteria in mesophilic As-contaminated soils. However, it should be noted that aioA genes have been also detected in non-proteobacterial lineages [53], [69].
Phylogenetic analyses’ findings performed for MS-AsIII 16S rRNA, Ms-AsV 16S rRNA and As metabolism genes were consistent with findings obtained by similarity searches (blastx and blastn, respectively). Overall, the phylogenetic trees reconstructed for MS-AsIII and MS-AsV 16S rRNA sequences show very similar evolutionary histories where the relationships among Firmicutes, Actinobacteria, Proteobacteria, and Thermodesulfobacteria phyla members reflect the current knowledge regarding their evolution [66].
As previously described on the literature, the evolutionary relationships of arsC and arrA homologs (Figs.4 and 5) support the role of horizontal gene transfer (HGT) on the evolution of arsenate oxidases e.g. [67], [68]. The phylogeny reconstructed for arsC homologs (Fig. 4) clearly shows two Ochrobactrum sequences clustered in different well-supported clades suggesting that these two homologs were acquired by HGT from unrelated donors. Although it is known that due to HGT events aioA sequences are not a suitable marker for microbial diversity studies [53], it was not observed on the aioA phylogeny here presented (Fig. 6). Albeit aioA sequences have been detected in non-proteobacterial lineages [53], [69], our findings show two strongly supported clades clustering alpha- and beta-proteobacteria homologs. Such results probably reflect the bias existing on GenBank databases where most aioAsequences available are from proteobacterial lineages.
Overall, evolutionary analyses revealed high genetic similarity between some arsC and aioA sequences obtained from isolates and clone libraries, suggesting that those isolates may represent environmentally important bacteria acting in As speciation. In addition, some arsC, aioA, and arrA sequences were found to be closely related to homologs from uncultured bacteria. Thus, it may be hypothesized that these divergent sequences could represent novel variants of the As-resistance genes or other genes with related function. In addition, our findings show that the diversity of arrA genes is wider than earlier described, once none arrA-OTUs were affiliated with known reference strains. Therefore, the molecular diversity of arrA genes is far from being fully explored deserving further attention.
Altogether, this study is a bioprospection of AsIII-oxidizing and AsV-reducing bacteria and As-transforming genes in sediments impacted by long-term gold mining. Our culture efforts successfully identified a large number of phylogenetically distinct arsenic-resistant bacterial genera and revealed two novel As-transformation genera, Thermomonas and Pannonibacter. Our heterotrophic arsenite oxidizers and DARB isolates open new opportunities for their use in bioremediation of long-term gold-mining impacted areas. Furthermore, metagenomic analysis of As functional genes revealed a predominance of previously unidentified DARB.
Supporting Information
Map showing the sampling site. Crosshatch, red and yellow areas represent mining, urban, and sampling areas, respectively.
(TIF)
This file includes Table S1, S2 and S3. Table S1. Phylogenetic affiliation of aioA OTUs based on blastx protein database. Table S2. Phylogenetic affiliation of arsC OTUs based on blastx protein database. Table S3. Phylogenetic affiliation of arrA OTUs based on blastx protein database.
(DOCX)
Acknowledgments
We appreciate the technical supportf from Laboratório de Análises Químicas/DEMET/UFMG do Instituto Nacional de Ciência e Tecnologia em Recursos Minerais, Água e Biodiversidade – INCT –ACQUA in the chemical analyses. The authors acknowledge the use of the computing resources of the Center for Excellence in Bioinformatics (CEBio//CPqRR/Fiocruz, Brazil).
Funding Statement
Sources of funding: FAPEMIG APQ 00801/12, CNPq n°472411/2012-8, CNPq/INCT no 15206-7. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lièvremont D, Bertin PN, Lett MC (2009) Arsenic in contaminated waters: Biogeochemical cycle, microbial metabolism and biotreatment processes. Biochimie 91: 1229–1237. [DOI] [PubMed] [Google Scholar]
- 2. Páez-Espino D, Tamames J, de Lorenzo V, Cánovas D (2009) Microbial responses toenvironmentalarsenic. Biometals 22: 117–130. [DOI] [PubMed] [Google Scholar]
- 3. Neubauer O (1947) Arsenical cancer - a review. Br J Cancer 1: 192–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhattacharya P, Welch AH, Stollenwerk KG, McLaughlin MJ, Bundschuh J, et al. (2007) Arsenic in the environment: Biology and Chemistry. Sci Total Environ 379: 109–20. [DOI] [PubMed] [Google Scholar]
- 5. McClintock TR, Chen Y, Bundschuh J, Oliver JT, Navoni J, et al. (2012) Arsenic exposure in Latin America: biomarkers, risk assessments and related health effects. Sci Total Environ 429: 76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kruger MC, Bertin PN, Heipieper HJ, Arsène-Ploetze F (2013) Bacterial metabolism of environmental arsenic-mechanisms and biotechnological applications. Appl Microbiol Biotechnol 97: 3827–3841. [DOI] [PubMed] [Google Scholar]
- 7. Nordstrom DK (2002) Public health-worldwide occurrences of arsenic in ground water. Science 296: 2143–2145. [DOI] [PubMed] [Google Scholar]
- 8. Tsai SL, Singh S, Chen W (2009) Arsenic metabolism by microbes in nature and the impact on arsenic remediation. Curr Opin Biotechnol 20: 659–67. [DOI] [PubMed] [Google Scholar]
- 9. Anderson CR, Cook GM (2004) Isolation and characterization of arsenate-reducing bacteria from arsenic-contaminated sites in New Zealand. Curr Microbiol 48: 341–347. [DOI] [PubMed] [Google Scholar]
- 10. Chang JS, Yoon IH, Lee JH, Kim KR, An J, et al. (2008) Arsenic detoxification potential of aox genes in arsenite oxidizing bacteria isolated from natural and constructed wetlands in the Republic of Korea. Environ Geochem Health 32: 95–105. [DOI] [PubMed] [Google Scholar]
- 11. Drewniak L, Styczek A, Majder-Lopatka M, Sklodowska A (2008) Bacteria, hypertolerant to arsenic in the rocks of an ancient gold mine, and their potential role in dissemination of arsenic pollution. Environ Pollut 156: 1069–74. [DOI] [PubMed] [Google Scholar]
- 12. Cai L, Liu G, Rensing C, Wang G (2009) Genes involved in arsenic transformation and resistance associated with different levels of arsenic-contaminated soils. BMC Microbiol 9: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cavalca L, Zanchi R, Corsini A, Colombo M, Romagnoli C, et al. (2010) Arsenic-resistant bacteria associated with roots of the wild Cirsiumarvense (L.) plant from an arsenic polluted soil, and screening of potential plant growth-promoting characteristics. Syst Appl Microbiol 33: 154–64. [DOI] [PubMed] [Google Scholar]
- 14. Silver S, Phung LT (2005) Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl Environ Microbiol 71: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaur S, Kamli MR, Ali A (2009) Diversity of arsenate reductase genes (arsC genes) from arsenic-resistant environmental isolates of E. coli . CurrMicrobiol 59: 288–94. [DOI] [PubMed] [Google Scholar]
- 16. Liao VHC, Chu YJ, Su YC, Hsiao SY, Wei CC, et al. (2011) Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J Contam Hydrol 123: 20–9. [DOI] [PubMed] [Google Scholar]
- 17. Malasarn D, Saltikov CW, Campbell KM, Santini JM, Hering JG, et al. (2004) arrA is a reliable marker for As(V) respiration. Science 36: 455. [DOI] [PubMed] [Google Scholar]
- 18. Zargar K, Conrad A, Bernick DL, Lowe TM, Stolc V, et al. (2012) ArxA, a new clade of arsenite oxidase within the DMSO reductase family of molybdenum oxidoreductases. Environ Microbiol 14: 1635–45. [DOI] [PubMed] [Google Scholar]
- 19. Muller D, Lièvremont D, Simeonova DD, Hubert JC, Lett MC (2003) Arsenite oxidase aox genes from a metal-resistant beta-proteobacterium . J Bacteriol 185: 135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamamura N, Macur RE, Korf S, Ackerman G, Taylor WP, et al. (2009) Linking microbial oxidation of arsenic with detection and phylogenetic analysis of arsenite oxidase genes in diverse geothermal environments. Environ Microbiol 11: 421–31. [DOI] [PubMed] [Google Scholar]
- 21. Stolz JF, Basu P, Oremland RS (2010) Microbial arsenic metabolism: new twists on an old poison. Microbe 5: 53–59. [Google Scholar]
- 22. Slyemi D, Bonnefoy V (2012) How prokaryotes deal with arsenic. Environ Microbiol Reports 4: 571–586. [DOI] [PubMed] [Google Scholar]
- 23. Lett MC, Muller D, Lièvremont D, Silver S, Santini J (2012) Unified nomenclature for genes involved in prokaryotic aerobic arsenite oxidation. J Bacteriol 194: 207–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oremland RS, Saltikov CW, Wolfe-Simon F, Stolz JF (2009) Arsenic in the evolution of earth and extraterrestrial ecosystems. Geomicrobiol J 26: 522–536. [Google Scholar]
- 25. Sun W, Sierra-Alvarez R, Milner L, Field JA (2010) Anaerobic oxidation of arsenite linked to chlorate reduction. Appl Environ Microbiol 76: 6804–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kulp TR, Hoeft SE, Asao M, Madigan MT, Hollibaugh JT, et al. (2008) Arsenic(III) fuels anoxygenic photosynthesis in hot spring biofilms from Mono Lake, California. Science 321: 967–970. [DOI] [PubMed] [Google Scholar]
- 27. Borba RP, Figueiredo BR, Rawlins BG, Matchullat J (2000) Arsenic in water and sediment in the Iron Quadrangle, Minas Gerais state, Brasil. Revista Brasileira de Geociências 30: 554–557. [Google Scholar]
- 28.Instituto Mineiro de Gestão das Águas (IGAM) (2004) Camargos LMM. Plano diretor de recursos hídricos da bacia hidrográfica do rio das Velhas: resumo executivo - Belo Horizonte, MG. Instituto Mineiro de Gestão das Águas, Comitê da Bacia Hidrográfica do Rio das Velhas.
- 29. Salomons W, de Rooij NM, Kerdijk H, Bril J (1987) Sediments as a source for contaminants? Hydrobiologia149: 13–30. [Google Scholar]
- 30. Rasmussen H, Jørgensen BB (1992) Microelectrode studies of seasonal oxygen uptake in a coastal sediment: role of molecular diffusion. Marine Ecology Progress Series 81: 289–303. [Google Scholar]
- 31. Marchand C, Lallier-Verges E, Allenbach M (2011) Redox conditions and heavy metals distribution in mangrove forests receiving shrimp farm effluents (Teremba bay, New Caledonia). J Soils Sediments 11: 529–541. [Google Scholar]
- 32.Mackereth FJH, Heron J, Talling JF (1978) Water analysis: some revised methods for limnologists. Freshwater Biological Association Scientific Publication, Wareham.
- 33.Golterman HL, Clymo RS, Ohnstad MAM (1978) Methods for chemical analysis of fresh waters. Blackwell Scientific Publications, Philadelphia, PA.
- 34. Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, et al. (1995) Entry of Listeriamonocytogenes into hepatocytes requires expression of inIB, a surface protein of the internalin multigene family. Mol Microbiol 16: 251–261. [DOI] [PubMed] [Google Scholar]
- 35. Freitas DB, Lima-Bittencourt CI, Reis MP, Costa PS, Assis PS, et al. (2008) Molecular characterization of early colonizer bacteria from wastes in a steel plant. Lett Appl Microbiol 47: 241–249. [DOI] [PubMed] [Google Scholar]
- 36.Lane DJ (1991) 16S/23S rRNA sequencing. John Wiley and Sons, New York.
- 37. Sun Y, Polishchuk EA, Radoja U, Cullen WR (2004) Identification and quantification of arsC genes in environmental samples by using real-time PCR. J Microbiol Methods 58: 335–49. [DOI] [PubMed] [Google Scholar]
- 38. Sarkar A, Kazy SK, Sar P (2013) Characterization of arsenic resistant bacteria from arsenic rich groundwater of West Bengal, India. Ecotoxicology 2: 363–376. [DOI] [PubMed] [Google Scholar]
- 39. Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71: 1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Good IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40: 237–262. [Google Scholar]
- 41. Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview Version 2–a multiple sequence alignment editor and analysis workbench Bioinformatics. 25: 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol. 59: 307–21. [DOI] [PubMed] [Google Scholar]
- 44. Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salmassi TM, Venkateswaren K, Satomi M, Nealson KH, Newman DK, et al. (2002) Oxidation of arsenite by Agrobacteriumalbertimagni, AOL15, spnov., isolated from Hot Creek, California. Geomicrobiol J 19: 53–66. [Google Scholar]
- 46.Conselho Nacional do Meio Ambiente (CONAMA) (2011) Resolução N° 430, de 13 de maio de 201. URL: http://www.mma.gov.br/port/conama/estr.cfm. Accessed 20 June 2011.
- 47.Canadian Council of Ministers of the Environment (CCME) Canadian Environmental Quality Guidelines. URL: http://www.ccme.ca/. Accessed 02 April 2011.
- 48. Salas HJ, Martino P (1991) A simplified phosphorus trophic state model for warm-water tropical lakes. Water Res 25: 341–350. [Google Scholar]
- 49. Santini JM, Sly LI, Schnagl RD, Macy JM (2000) A new chemolithoautotrophicarsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl Environ Microbiol 66: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santini JM, Sly LI, Wen A, Comrie D, Wulf-Durand P, et al. (2002) New arsenite-oxidizing bacteria isolated from australian gold mining environments- phylogenetic relationships. Geomicrobiol J 19: 67–76. [Google Scholar]
- 51. Santini JM, vanden Hoven RN (2004) Molybdenum-containing arsenite oxidase of the chemolithoautotrophicarsenite oxidizer NT-26. J Bacteriol 186: 1614–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oliveira A, Pampulha ME, Neto MM, Almeida AC (2009) Enumeration and characterization of arsenic-tolerant diazotrophic bacteria in a long-term heavy-metal-contaminated soil.Water Air Soil Pollut. 200: 237–243. [Google Scholar]
- 53. Heinrich-Salmeron A, Cordi A, Brochier-Armanet C, Halter D, Pagnout C, et al. (2011) Unsuspected Diversity of Arsenite-Oxidizing Bacteria as Revealed by Widespread Distribution of the aoxB Gene in Prokaryotes. Appl Environ Microbiol 77: 4685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamamura S, Ike M, Fujita M (2003) Dissimilatory arsenate reduction by a facultative anaerobe, Bacillus sp. strain SF-1. J BiosciBioeng 96: 454–60. [DOI] [PubMed] [Google Scholar]
- 55. Chang JS, Kim IS (2010) Arsenite oxidation by Bacillus sp. strain SeaH-As22w isolated from coastal seawater in Yeosu Bay. Environ Eng Res 15: 15–21. [Google Scholar]
- 56. Reis MP, Barbosa FA, Chartone-Souza E, Nascimento AMA (2013) The prokaryotic community of a historically mining-impacted tropical stream sediment is as diverse as that from a pristine stream sediment. Extremophiles 17: 301–309. [DOI] [PubMed] [Google Scholar]
- 57. Bandyopadhyay S, Schumann P, Das SK (2013) Pannonibacter indica sp. nov., a highly arsenate-tolerant bacterium isolated from a hot spring in India. Arch Microbiol 195: 1–8. [DOI] [PubMed] [Google Scholar]
- 58. Dowdle PR, Laverman AM, Oremland RS (1996) Bacterial dissimilatory reduction of arsenic(V) to arsenic(III) in anoxic sediments. Appl Environ Microbiol 62: 1664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chang YC, Nawata A, Jung K, Kikuchi S (2012) Isolation and characterization of an arsenate-reducing bacterium and its application for arsenic extraction from contaminated soil. J Ind Microbiol Biotechnol 39: 37–44. [DOI] [PubMed] [Google Scholar]
- 60. Achour AR, Bauda P, Billard P (2007) Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res Microbiol 158: 128–37. [DOI] [PubMed] [Google Scholar]
- 61. Sri LSM, Prashant S, Bramha CPV, Nageswara RS, Balaravi P, et al. (2012) Molecular identification of arsenic-resistant estuarine bacteria and characterization of their ars genotype. Ecotoxicology21: 202–12. [DOI] [PubMed] [Google Scholar]
- 62. Rasko DA, Rosovitz MJ, Myers GS, Mongodin EF, Fricke WF, et al. (2008) The pangenome structure of Escherichiacoli: comparative genomic analysis of E. coli commensal and pathogenic isolates.JBacteriol. 190: 6881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gootz TD, Lescoe MK, Dib-Hajj F, Dougherty BA, He W, et al. (2009) Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob Agents Chemother 53: 1998–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Inskeep WP, Macur RE, Hamamura N, Warelow TP, Ward SA, et al. (2007) Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ Microbiol 9: 934–43. [DOI] [PubMed] [Google Scholar]
- 65. Quéméneur M, Heinrich-Salmeron A, Muller D, Lièvremont D, Jauzein M, et al. (2008) Diversity surveys and evolutionary relationships of aoxBgenes in aerobic arsenite-oxidizing bacteria. Appl Environ Microbiol 74: 4567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Olsen GJ, Woese CR, Overbeek R (1994) The winds of (Evolutionary) change: breathing new life into microbiology. J Bacteriol 176: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jackson CR, Dugas SL (2003) Phylogenetic analysis of bacterial and archaeal arsC gene sequences suggests an ancient, common origin for arsenate reductase BMC Evol. Biol.3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Duval S, Ducluzeau AL, Nitschke W, Schoepp-Cothenet B (2008) Enzyme phylogenies as markers for the oxidation state of the environment: the case of respiratory arsenate reductase and related enzymes. BMC Evol Biol 8: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Andres J, Arsène-Ploetze F, Barbe V, Brochier-Armanet C, Cleiss-Arnold J, et al. (2013) Life in an arsenic-containing gold mine: genome and physiology of the autotrophic arsenite-oxidizing bacterium rhizobium sp. NT-26. Genome Biol Evol 5: 934–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Map showing the sampling site. Crosshatch, red and yellow areas represent mining, urban, and sampling areas, respectively.
(TIF)
This file includes Table S1, S2 and S3. Table S1. Phylogenetic affiliation of aioA OTUs based on blastx protein database. Table S2. Phylogenetic affiliation of arsC OTUs based on blastx protein database. Table S3. Phylogenetic affiliation of arrA OTUs based on blastx protein database.
(DOCX)