Abstract
Intercellular communication is a key process in the development and progression of cancer. The dynamic and reciprocal interplays between the tumor and its microenvironment orchestrate events critical to the establishment of primary and metastatic niches and maintenance of a permissive environment at the tumor−stroma interface. Atay and colleagues found that gastrointestinal stromal tumor cells secrete vesicles known as exosomes. These exosomes contain oncogenic KIT and their transfer and uptake by surrounding smooth muscle cells lead to enhanced AKT and MAPK signaling and phenotypic modulation of several cellular processes, including morphological changes, expression of tumor-associated markers, secretion of matrix metalloproteinases, and enhanced tumor cell invasion. This provocative study emphasizes that exosome-mediated signaling within the tumor microenvironment acts as a positive feedback loop that contributes to invasiveness and that interfering with this message delivery system may represent promising therapeutic approaches, not only for GIST, but for other types of cancer.
Keywords: Exosomes, GIST, tumor micro-environment, invasion, feedback loop
Normal and Tumor Cells Secrete Nanosized Vesicles Called Exosomes
In response to physiological and/or pathological cues all cells in the body communicate with each other via secretion of a heterogeneous mixture of vesicles differing in size and composition, including apoptotic bodies, microparticles, shed microvilli, ectosomes and exosomes.1 Exosomes, the focus of our studies, are small membrane vesicles of endocytic origin with a size range of 30 to 150 nm.2 Exosomes are released by a variety of “normal” cells including mast cells (MC),3 dendritic cells4,5 reticulocytes,6 epithelial cells,7 B-cells,8 trophoblastic cells,9,10 and neural cells,11 as well as a variety of tumor cells.12–14 In addition, exosomes are found in various biological fluids including bronchoalveolar lavage,15 blood,16 ascites,17,18 urine,19 pregnancy associated sera,20 breast milk,21 saliva,22 and malignant effusions.17,23 Because of their endosomal origin, exosomes contain several proteins involved in the Endosomal Soritng Complexes Required for Transport (ESCRT) complex (e.g, TSG101, Alix) and in transport and fusion (e.g., Rab11, Rab7, Rab2 and various annexins). Further markers expressed in or on exosomes include tetraspanins (CD81, CD63, CD9), heat shock proteins (HSC70 and HSP90), and cytoskeletal proteins (actin, tubulin and moesin).24–26 In addition, the molecular characterization of various healthy cell type-derived and tumor-derived exosomes revealed enhanced expression of cell-specific and tumor-associated antigens on the exosomal surface. In fact, exosomes isolated from antigen presenting cells harbor MHCII on their surface,8 those from urine possess surface aquaporin-2,19 from reticulocytes contain the transferrin receptor,6 and from T-cells carry the TCR/CD3/zeta complex.27 These cell-specific proteins are thought to represent a means by which exosomes can specifically target various recipient cells by either interaction with cell surface adhesion molecules or through interaction with cell-surface heparan sulfate proteoglycans. Alternatively, exosomes can enter another cell via lipid-dependent endocytosis, in which a high content of sphingomyelin/ganglioside GM3 in the exosomal membranes enhances the fusion efficiency with the plasma membrane of target cells.28 Therefore, exosome internalization by recipient cells appears to be a cell type dependent process, and the extent of exosome internalization likely depends upon the phagocytic abilities of the recipient cell.29 As such, we and others are exploiting this information to isolate specific populations of exosomes from heterogeneous biological fluids for use in early detection and disease monitoring. Proteomic analysis of malignant effusion-derived exosomes from various sources has increased our knowledge of exosome protein composition and likewise, our understanding of the role of exosomes in biological processes.30–33 Proteome analyses have been conducted in a number of cancer-derived exosomes including, mesothelioma,34 melanoma,14 gastric carcinoma,35 breast carcinoma,36 ovarian,18,37 prostate,38 malignant pleural effusions,17,23 brain,24 and colorectal.26,39 These isolated exosomal proteins constitute a “cancer signature” which may help in improving the diagnosis and treatment of cancer patients.
Tumor-Derived Exosomes as Mediators of Intercellular Communication During Tumor Progression
Cellular communication is key to the regulation of physiological and pathological processes.40 During the development and progression of cancer, the cellular composition of the tumor microenvironment is influenced by the activity of the tumor cell41 which recruit and educate host stromal cells into tumor supportive cells that actively participate in tumor progression.42 One way that tumor cells can communicate and alter the microenvironment is by the constitutive release of exosomes.13,43 Recent studies have shown that exosomes produced by tumor cells can interact with target cells by a number of mechanisms, including i) direct stimulation of the target by surface-expressed ligands;44 ii) receptor transfer between the tumor cell and the target;44 iii) horizontal transfer of genetic information to the target,44 and iv) direct stimulation of the target cell by endocytic-expressed surface receptors.45 Growing evidence supports the view that tumors constitutively shed exosomes with pleiotropic immunosuppressive effects46,47 that are protective and supportive of the tumor with effects that range from regulation of tumor growth, to invasion, and to angiogenesis and metastasis.41,46,48 Recently, Al-Nedawi and colleagues demonstrated that exosome mediated transfer of an oncogenic epidermal growth factor variant 3 (EGFRvIII) from human glioma cells to glioma cells lacking the mutant receptor induced expression of EGFRvIII-regulated genes (such as VEGF, Bcl-xL, p27).49 In a subsequent study, oncogenic EGFR from human squamous cell carcinoma taken up by tumor-associated endothelial cells activated MAPK and AKT cell signaling pathways and promoted endothelial VEGF expression.50 Therefore, the regulatory properties attributed to tumor-derived exosomes are essential in shaping the tumor microenvironment and promoting tumor growth.32 Collectively, these studies support a role for exosomes in remodeling the tumor microenvironment into a tumor supportive milieu and thereby contribute to tumor progression via enhanced angiogenesis and metastasis.51,52 In fact, melanoma-associated exosomes have recently been shown to promote metastasis through the preparation of the metastatic niche via crosstalk between the released exosomes and bone marrow progenitor cells.53
GIST Tumor Microenvironment and Tumor-Derived Exosomes
GISTs are the most common mesenchymal tumor of the gastrointestinal tract and are thought to arise from Interstitial Cells of Cajal (ICC),54 named after Santiago Ramón y Cajal, a Spanish pathologist and Nobel laureate. ICCs are found in specific locations within the tunica muscularis of the gastrointestinal tract, and serve as electrical pacemakers and mediators of enteric neurotransmission. Alternatively, GISTs may also arise from interstitial mesenchymal precursor stem cells.55 The majority of GISTs develop in the sub-mucosal layer of the stomach surrounded by smooth muscle cells and interstitial extracellular matrix (ECM) rich in collagen and invade the mucosa in a regulated fashion.56 Nearly 90% of GISTs have a mutation in a tyrosine kinase receptors encoded either by c-KIT or PDGFRA.57 Imatinib mesylate (Gleevec™) is a specific molecular inhibitor of KIT/PDGFRA and is used as the first-line therapy in the treatments of GIST patients.58–62 Although the use of imatinib has drastically changed the outcome of patients with metastatic GIST, additional therapeutic strategies are needed since the vast majority of patients eventually develop resistance to imatinib treatment, leading to disease progression and posing a significant challenge in the clinical management of these tumors.63 Importantly, once a GIST becomes metastatic, the median disease-specific survival of patients is only ~19 months with second- and third-line therapies. Although many studies focused on molecularly defining these tumors,64–66 the importance of the stromal microenvironment during metastasis remains an understudied area of research and clearly needed to be better defined in order to design novel targeted therapeutics.
It is becoming apparent that the tumor microenvironment – non-malignant (stroma) cells, soluble molecules, extracellular matrix components, and exosomes – plays an important role in modulating metastatic properties and sensitivity of tumor cells to therapy. Several studies have shown that ascites-derived exosomes from ovarian cancer patients carry extracellular matrix-remodeling enzymes such as metalloproteinases 2 and 9 (MMP-2, MMP-9),67,68 and urokinase plasminogen activator69,70 leading to an increase in extracellular matrix degradation, which has been shown to increase the invasive phenotype of tumor cells and promote metastasis.71 We believe that by better understanding the myriad of interactions that exist between tumor cells and host cells present in the tumor microenvironment, new insights into the pathogenesis of cancer will be uncovered that will ultimately have profound therapeutic implications.
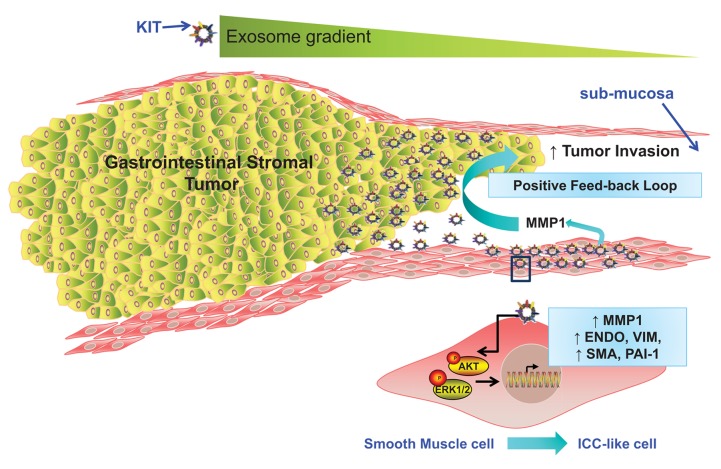
Epithelial cells require mesenchymal transition (EMT) to metastasize; during this process tumor cells dissociate from each other and the ECM and become more motile and able to invade the surrounding stroma.72 In contrast, mesenchymal cells are generally more motile than epithelial cells,73 thus GIST cells have been reported to grow in an endophytic manner parallel to the organ lumen, between the muscularis mucosa and muscularis propria. This finding suggests that a tight regulation exists between the growing tumor and the surrounding stroma.74,75 Matrix metalloproteinases, particularly MMP1, actively shape the stromal microenvironment during sarcoma development.76–78 In fact, a recent study reported that chondrosarcoma cell invasion correlates with MMP1 expression in tumor cells and that a transient downregulation of MMP1 expression decreases invasion in vitro.79,80 In our recent study, we found that GIST cells not only constitutively released low levels of MMP1, but that challenging myometrial smooth muscle cells with GIST patient-derived exosomes (but not exosomes from healthy donors) significantly increased MMP1 production, which in turn enhanced GIST cell invasion.81 We assessed direct/indirect exosome-mediated MMP1 induction using siRNA and inhibitory drug strategies to reduce MMP1 production by myometrial cells and were able to mimic, in vitro, the exosome−MMP1 expression feedback loop (Fig. 1). In particular, in vivo-derived exosomes appeared to be a potent exogenous source of MMP induction in stromal cells, which in turn acted as a pro-invasion factor for GIST cells. It is known that tumor cells acquire some of the required properties for growth and invasion by the specific modification of the tumor microenvironment.79 However, due to the complex nature of these interactions, it is only by altering specific components of this network that it will be possible to identify molecules with pro-tumorigenic and anti-tumorigenic functions. Our study reveals a complex interplay between tumor-derived exosomes and factors produced in response to their internalization by tumor-associated stromal cells. The release of tumor-derived exosomes appears to represent a novel pathway enabling GIST cells to modulate the host microenvironment and thereby promote their ability to invade and spread (Fig. 1). Further studies aiming to elucidate the exact mechanism leading to MMP1 production by smooth muscle cells in response to exosome uptake are ongoing. In fact, a more complete understanding of the mechanisms used by tumor-derived exosomes in the induction of MMPs might permit the development of a successful clinical strategy for novel MMP inhibitors.82,83
Figure 1. Proposed model of tumor-stromal positive feed-back loop mediated by oncogenic KIT-bearing tumor exosomes in the regulation of tumor invasion. GIST cells secrete a gradient of exosomes carrying mutant KIT, which after internalization by surrounding smooth muscle cells activate downstream signaling pathways of KIT (e.g., AKT and MAPK pathways) and induce an enhanced expression of endoglin, vimentin (VIM), smooth muscle actin (SMA), and plasminogen activator inhibitor-1 (PAI-1) in the recipient cells, resembling an ICC-like phenotype. This tumor−stromal interaction creates a positive feed-back loop in which tumor-derived exosome-mediated signaling in stromal cells increases MMP1 secretion. In turn, tumor cells utilized MMP1 to invade the submucosa. This model describes a previously unreported mechanism by which tumor-derived exosomes can modulate their host microenvironment and promote local invasion and potentially distant metastasis.
Another important aspect of tumorigenesis is the epigenetic regulation of gene transcription that mediates cell proliferation, differentiation, and survival which represent additional targets in tumor progression,84 resulting in genomic instability.85 Skog et al. (2008) have demonstrated that glioblastoma-derived microvesicles tRNA to endothelial cells, resulting in the production of pro-angiogenic proteins which promote tumor progression.86 Studies of lung and adenocarcinoma–derived microvesicles (pancreatic and colorectal) indicated a transfer of growth factor encoding mRNA (VEGF, HGF, IL-8, CD44H) to tumor-associated monocytes which enhanced their anti-apoptotic effect and activated the AKT signaling pathway.87 In our recent study, we reported that mutant KIT carrying exosomes modified the transcriptomic, proteomic and secretomic profile of smooth muscle cells via induction of new transcripts within the recipient cells. In addition, we provide the first evidence that large numbers of oncogenic KIT-bearing exosomes are released into the circulation of GIST patients and that these extracellular vesicles represent potent phenotypic modifiers of the tumor microenvironment. Our results indicated that recipient cells that take up these vesicles assume many of the characteristics of ICC cells which in turn secrete significant amounts of interstitial collagenase MMP-1, which is important to enhance invasion of tumor cells in the interstitial stroma. Furthermore, our results confirmed that the resulting cells displayed activation of downstream signaling pathways of KIT, namely AKT and MAPK pathways, and enhanced adhesion to fibronectin and type I collagen. Hence, these data suggest that the release of exosomes may represent a novel pathway enabling tumor cells to modulate the host microenvironment to support tumor invasion.
In summary, although several tumor-stromal communication based on an exosome mediated exchange has been reported,88–91 our recent study provides the first evidence of tumor-stromal communication based on an exosome mediated exchange in GIST. This tumor-stromal feedback loop in which tumor-derived exosome-mediated signaling in host stromal cells increases MMP1 secretion, which in turn enhances tumor cell invasion and further suggests that exosomes released by primary GISTs progressively remodel the host environment, which in turn aids in the tumor's survival (Fig. 1). In addition, our study demonstrated a positive feedback loop that enable the creation of “space” for growing tumors: this is achieved via active release of exosomes, leading to a continual release of exogenous matrix metalloproteinases, such as MMP-1. Although further studies are needed to identify the receptors involved in uptake of tumor-derived exosomes and the molecular mechanisms involved in the production of MMP-1, our study offers novel insights into the pathogenesis of GIST and provide new therapeutic goals aimed to help improve the survival of patients by reducing the development of metastatic disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by a grant from KU Biomedical Research Training Program to S.A. and the National Cancer Institute R01 CA106588 and the National Institute of Health UL1 TR000001-02S1 to A.K.G. The authors would also like to acknowledge support from the University of Kansas Cancer Center, the Kansas Bioscience Authority Eminent Scholar Program, and the Chancellors Distinguished Chair in Biomedical Sciences endowment at KUMC. The funders did not have any involvement in the writing of the article; or the decision to submit the article for publication.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/28231
References
- 1.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006; Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 3.Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–45. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caramori G, Fabbri M, Paioli D, Falcone F, Severino C, Felisatti G, Arar O, Adcock IM, Fan Chung K, Barnes PJ, et al. Asthma is not a common cause of severe chronic respiratory failure in non-smokers: ALOT study. Monaldi Arch Chest Dis. 2005;63:84–7. doi: 10.4081/monaldi.2005.643. [DOI] [PubMed] [Google Scholar]
- 5.Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–20. [PubMed] [Google Scholar]
- 7.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–49. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 8.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am J Reprod Immunol. 2011;65:65–77. doi: 10.1111/j.1600-0897.2010.00880.x. [DOI] [PubMed] [Google Scholar]
- 10.Atay S, Gercel-Taylor C, Taylor DD. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1β production by macrophages. Am J Reprod Immunol. 2011;66:259–69. doi: 10.1111/j.1600-0897.2011.00995.x. [DOI] [PubMed] [Google Scholar]
- 11.Fauré J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–8. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DD, Taylor CG, Jiang CG, Black PH. Characterization of plasma membrane shedding from murine melanoma cells. Int J Cancer. 1988;41:629–35. doi: 10.1002/ijc.2910410425. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DD, Lyons KS, Gerçel-Taylor C. Shed membrane fragment-associated markers for endometrial and ovarian cancers. Gynecol Oncol. 2002;84:443–8. doi: 10.1006/gyno.2001.6551. [DOI] [PubMed] [Google Scholar]
- 14.Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, Patel P, Selby PJ, Banks RE. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–31. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 15.Admyre C, Grunewald J, Thyberg J, Gripenbäck S, Tornling G, Eklund A, Scheynius A, Gabrielsson S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22:578–83. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- 16.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–87. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 17.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 18.Navabi H, Croston D, Hobot J, Clayton A, Zitvogel L, Jasani B, Bailey-Wood R, Wilson K, Tabi Z, Mason MD, et al. Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol Dis. 2005;35:149–52. doi: 10.1016/j.bcmd.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–73. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–42. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 21.Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–78. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa Y, Kanai-Azuma M, Akimoto Y, Kawakami H, Yanoshita R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 2008;31:1059–62. doi: 10.1248/bpb.31.1059. [DOI] [PubMed] [Google Scholar]
- 23.Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol. 2004;31:114–21. doi: 10.1165/rcmb.2003-0238OC. [DOI] [PubMed] [Google Scholar]
- 24.Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541–57. doi: 10.1096/fj.08-122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–99. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 26.Xue H, Lü B, Zhang J, Wu M, Huang Q, Wu Q, Sheng H, Wu D, Hu J, Lai M. Identification of serum biomarkers for colorectal cancer metastasis using a differential secretome approach. J Proteome Res. 2010;9:545–55. doi: 10.1021/pr9008817. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–41. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 28.Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–12. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–87. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 30.Olver C, Vidal M. Proteomic analysis of secreted exosomes. Subcell Biochem. 2007;43:99–131. doi: 10.1007/978-1-4020-5943-8_7. [DOI] [PubMed] [Google Scholar]
- 31.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–83. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 32.Clayton A, Mason MD. Exosomes in tumour immunity. Curr Oncol. 2009;16:46–9. doi: 10.3747/co.v16i3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atay S, Gercel-Taylor C, Kesimer M, Taylor DD. Morphologic and proteomic characterization of exosomes released by cultured extravillous trophoblast cells. Exp Cell Res. 2011;317:1192–202. doi: 10.1016/j.yexcr.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol. 2004;164:1807–15. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baran J, Baj-Krzyworzeka M, Weglarczyk K, Szatanek R, Zembala M, Barbasz J, Czupryna A, Szczepanik A, Zembala M. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010;59:841–50. doi: 10.1007/s00262-009-0808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staubach S, Razawi H, Hanisch FG. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics. 2009;9:2820–35. doi: 10.1002/pmic.200800793. [DOI] [PubMed] [Google Scholar]
- 37.Gercel-Taylor C, Atay S, Tullis RH, Kesimer M, Taylor DD. Nanoparticle analysis of circulating cell-derived vesicles in ovarian cancer patients. Anal Biochem. 2012;428:44–53. doi: 10.1016/j.ab.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, Widmark A. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603–7. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi DS, Lee JM, Park GW, Lim HW, Bang JY, Kim YK, Kwon KH, Kwon HJ, Kim KP, Gho YS. Proteomic analysis of microvesicles derived from human colorectal cancer cells. J Proteome Res. 2007;6:4646–55. doi: 10.1021/pr070192y. [DOI] [PubMed] [Google Scholar]
- 40.Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014–8. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- 41.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–8. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 42.Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008;9:808. doi: 10.1016/S1470-2045(08)70201-8. [DOI] [PubMed] [Google Scholar]
- 43.Taylor DD, Black PH. Shedding of plasma membrane fragments. Neoplastic and developmental importance. Dev Biol (N Y 1985) 1986; 3:33–57. [DOI] [PubMed]
- 44.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 45.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–6. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–5. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 47.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–16. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–22. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 50.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–9. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huber V, Filipazzi P, Iero M, Fais S, Rivoltini L. More insights into the immunosuppressive potential of tumor exosomes. J Transl Med. 2008;6:63. doi: 10.1186/1479-5876-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergmann C, Strauss L, Wieckowski E, Czystowska M, Albers A, Wang Y, Zeidler R, Lang S, Whiteside TL. Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression. Head Neck. 2009;31:371–80. doi: 10.1002/hed.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377–89. doi: 10.1097/00000478-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259–69. [PMC free article] [PubMed] [Google Scholar]
- 56.Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY, ESMO Guidelines Working Group Gastrointestinal stromal tumors: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19:ii35–8. doi: 10.1093/annonc/mdn080. [DOI] [PubMed] [Google Scholar]
- 57.Rink L, Godwin AK. Clinical and molecular characteristics of gastrointestinal stromal tumors in the pediatric and young adult population. Curr Oncol Rep. 2009;11:314–21. doi: 10.1007/s11912-009-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeMatteo RP. The GIST of targeted cancer therapy: a tumor (gastrointestinal stromal tumor), a mutated gene (c-kit), and a molecular inhibitor (STI571) Ann Surg Oncol. 2002;9:831–9. doi: 10.1007/BF02557518. [DOI] [PubMed] [Google Scholar]
- 59.Rink L, Skorobogatko Y, Kossenkov AV, Belinsky MG, Pajak T, Heinrich MC, Blanke CD, von Mehren M, Ochs MF, Eisenberg B, et al. Gene expression signatures and response to imatinib mesylate in gastrointestinal stromal tumor. Mol Cancer Ther. 2009;8:2172–82. doi: 10.1158/1535-7163.MCT-09-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frolov A, Chahwan S, Ochs M, Arnoletti JP, Pan ZZ, Favorova O, Fletcher J, von Mehren M, Eisenberg B, Godwin AK. Response markers and the molecular mechanisms of action of Gleevec in gastrointestinal stromal tumors. Mol Cancer Ther. 2003;2:699–709. [PubMed] [Google Scholar]
- 61.Tarn C, Skorobogatko YV, Taguchi T, Eisenberg B, von Mehren M, Godwin AK. Therapeutic effect of imatinib in gastrointestinal stromal tumors: AKT signaling dependent and independent mechanisms. Cancer Res. 2006;66:5477–86. doi: 10.1158/0008-5472.CAN-05-3906. [DOI] [PubMed] [Google Scholar]
- 62.Belinsky MG, Rink L, Cai KQ, Ochs MF, Eisenberg B, Huang M, von Mehren M, Godwin AK. The insulin-like growth factor system as a potential therapeutic target in gastrointestinal stromal tumors. Cell Cycle. 2008;7:2949–55. doi: 10.4161/cc.7.19.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, Testa JR, Eisenberg B, von Mehren M, Godwin AK. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A. 2008;105:8387–92. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ochs MF, Rink L, Tarn C, Mburu S, Taguchi T, Eisenberg B, Godwin AK. Detection of treatment-induced changes in signaling pathways in gastrointestinal stromal tumors using transcriptomic data. Cancer Res. 2009;69:9125–32. doi: 10.1158/0008-5472.CAN-09-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tarn C, Godwin AK. The molecular pathogenesis of gastrointestinal stromal tumors. Clin Colorectal Cancer. 2006;6:S7–17. doi: 10.3816/CCC.2006.s.002. [DOI] [PubMed] [Google Scholar]
- 66.Belinsky MG, Skorobogatko YV, Rink L, Pei J, Cai KQ, Vanderveer LA, Riddell D, Merkel E, Tarn C, Eisenberg BL, et al. High density DNA array analysis reveals distinct genomic profiles in a subset of gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2009;48:886–96. doi: 10.1002/gcc.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dolo V, D’Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, Vittorelli ML, Canevari S, Pavan A. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis. 1999;17:131–40. doi: 10.1023/A:1006500406240. [DOI] [PubMed] [Google Scholar]
- 68.Dolo V, Ginestra A, Cassará D, Ghersi G, Nagase H, Vittorelli ML. Shed membrane vesicles and selective localization of gelatinases and MMP-9/TIMP-1 complexes. Ann N Y Acad Sci. 1999;878:497–9. doi: 10.1111/j.1749-6632.1999.tb07707.x. [DOI] [PubMed] [Google Scholar]
- 69.Sezonlin M, Dupas S, Le Rü B, Le Gall P, Moyal P, Calatayud PA, Giffard I, Faure N, Silvain JF. Phylogeography and population genetics of the maize stalk borer Busseola fusca (Lepidoptera, Noctuidae) in sub-Saharan Africa. Mol Ecol. 2006;15:407–20. doi: 10.1111/j.1365-294X.2005.02761.x. [DOI] [PubMed] [Google Scholar]
- 70.Graves LE, Ariztia EV, Navari JR, Matzel HJ, Stack MS, Fishman DA. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res. 2004;64:7045–9. doi: 10.1158/0008-5472.CAN-04-1800. [DOI] [PubMed] [Google Scholar]
- 71.McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–23. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 73.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 74.Lin F, Cao J, Gu WL, Fan SF, Li KP, Du H, Chen GQ, Wen MJ, Dai LH, Lai YY. Clinical experience in diagnosis and treatment of malignant gastrointestinal stromal tumors. Kaohsiung J Med Sci. 2012;28:212–5. doi: 10.1016/j.kjms.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 75.von Mehren M, Watson JC. Gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2005;19:547–64, vii. doi: 10.1016/j.hoc.2005.03.010. [vii.] [DOI] [PubMed] [Google Scholar]
- 76.Bendardaf R, Lamlum H, Vihinen P, Ristamäki R, Laine J, Pyrhönen S. Low collagenase-1 (MMP-1) and MT1-MMP expression levels are favourable survival markers in advanced colorectal carcinoma. Oncology. 2003;65:337–46. doi: 10.1159/000074647. [DOI] [PubMed] [Google Scholar]
- 77.Yamashita K, Mori M, Kataoka A, Inoue H, Sugimachi K. The clinical significance of MMP-1 expression in oesophageal carcinoma. Br J Cancer. 2001;84:276–82. doi: 10.1054/bjoc.2000.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sawatsubashi M, Mizokami H, Tokunaga O, Shin T. Expression of MMP-1, TIMP-1, and type I collagen in laryngeal carcinoma. Mod Pathol. 1998;11:878–85. [PubMed] [Google Scholar]
- 79.Dudeja V, Armstrong LH, Gupta P, Ansel H, Askari S, Al-Refaie WB. Emergence of imatinib resistance associated with downregulation of c-kit expression in recurrent gastrointestinal stromal tumor (GIST): optimal timing of resection. J Gastrointest Surg. 2010;14:557–61. doi: 10.1007/s11605-009-1121-2. [DOI] [PubMed] [Google Scholar]
- 80.Yuan J, Dutton CM, Scully SP. RNAi mediated MMP-1 silencing inhibits human chondrosarcoma invasion. J Orthop Res. 2005;23:1467–74. doi: 10.1016/j.orthres.2005.04.004.1100230633. [DOI] [PubMed] [Google Scholar]
- 81.Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A. 2014;111:711–6. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noël A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol. 2008;19:52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 83.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 84.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther. 2009;8:1409–20. doi: 10.1158/1535-7163.MCT-08-0860. [DOI] [PubMed] [Google Scholar]
- 85.Viswanathan M, Sangiliyandi G, Vinod SS, Mohanprasad BK, Shanmugam G. Genomic instability and tumor-specific alterations in oral squamous cell carcinomas assessed by inter-(simple sequence repeat) PCR. Clin Cancer Res. 2003;9:1057–62. [PubMed] [Google Scholar]
- 86.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Brański P, Ratajczak MZ, Zembala M. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–18. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–56. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 89.Cho JA, Park H, Lim EH, Kim KH, Choi JS, Lee JH, Shin JW, Lee KW. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol. 2011;123:379–86. doi: 10.1016/j.ygyno.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–60. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 91.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–30. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]