Abstract
PTEN (phosphatase and tensin homolog on chromosome ten) is a dual protein/lipid phosphatase that dephosphorylates PIP3, thereby inhibiting the AKT/mTOR pathway. This inhibition ultimately decreases protein translation, cell proliferation and cell growth. In the central nervous system, inhibition of PTEN leads to increased stem cell proliferation, somatic, dendritic and axonal growth, accelerated spine maturation, diminished synaptic plasticity, and altered intrinsic excitability. In agreement with these findings, patients carrying single-copy inactivating mutations of PTEN suffer from autism, macrocephaly, mental retardation, and epilepsy.1-9 Understanding the mechanisms through which PTEN modulates the structure, function, and plasticity of cortical networks is a major focus of study. Preventing and reversing the changes induced by loss of Pten in model animals will pave the way for treatments in humans.
Keywords: LTP, PTEN, autism, cortex, development, epilepsy, hippocampus, mTOR, plasticity
Role of Pten in Neuron Morphology and Connectivity
PTEN is essential for both, central nervous system (CNS) development and maintenance of CNS circuit structure and function. The generation of conditional mutant animals has allowed exquisite control over temporal and spatial patterns of Pten expression and delineated specific roles for Pten at each developmental stage. During early embryonic development, Pten deletion in proliferating neural stem/progenitor cells results in increased cell proliferation, and severe defects in cortical, hippocampal and cerebellar lamination.10,11 Pten also inhibits cell proliferation of adult born neural stem cells, controlling self-renewal in the olfactory bulb12 and dentate gyrus.13 After neurons become post-mitotic, Pten deletion or knock-down has dramatic effects on neuronal growth, highlighting the importance of Pten for controlling post-mitotic neuronal development.14,15 Deletion of Pten in differentiated postmitotic cortical and hippocampal neurons leads to progressive somatic and dendritic hypertrophy, and the growth of ectopic dendritic branches.16 Pten deletion also leads to progressive growth of axonal arbors, which is most readily observed in the hippocampal mossy fiber pathway where deletion also leads to enlargement of presynaptic terminals and increased vesicle numbers.16,17 Indeed, Pten is enriched in the axonal compartment and the growth cones during axonal extension, coupling semaphorins (Sema3A) to growth cone collapse.18
The effects of Pten deletion on spine density and morphology are more complex and likely depend on neuronal identity and the differences in spine classification. Recent studies in basolateral amygdala and dentate gyrus granule neurons suggest that Pten deletion does not change overall dendritic protrusion density, but induces a shift in protrusion morphology from thin spines to mushroom spines.19 Rapamycin, the mTORC1 inhibitor prevents somatic, dendritic, and axonal growth induced by Pten deletion, and reverses some but not all these anatomical abnormalities if rapamycin is administered after these changes have already occurred.20
Pten deletion in adult excitatory cortical neurons using α-CaMK2-Cre conditional deletion of floxed-Pten, reveals that Pten is not only necessary for proper cortical development, but also exerts profound control over dendritic growth in adulthood. By using two-photon imaging of the same cortical dendritic arbors over weeks, Chow et al. show that apical dendrites of L2/3 pyramidal neurons that underwent deletion of Pten in adulthood, demonstrate dramatic and progressive growth. Newly elongated dendritic segments form new spines. Importantly, treatment of these mice with the mTORC1 inhibitor, rapamycin, halts dendritic growth, and reduces spine density on the newly grown segments. Adult deletion of Pten in L5 neurons has no effects on apical dendritic arbors, suggesting that Pten exerts distinct effects on different cortical layers in adulthood.21
These structural changes are accompanied by profound alterations in synaptic function. In the dentate gyrus, consistent with the increase in the proportion of mature dendritic spines, Pten knockdown or deletion increases the frequency of excitatory (miniature and spontaneous) postsynaptic currents.19,22 To dissect the presynaptic and postsynaptic contributions to altered functional connectivity in excitatory and inhibitory neurons, Weston and colleagues have carried out studies in autaptic dentate granule cell and striatal cultures. They find that Pten deletion increases evoked synaptic release onto both inhibitory and excitatory neurons, mainly by increasing the number of vesicles available for release.23 These effects are counterbalanced by impaired vesicle fusion induced by hyperactive mTOR signaling, although the prevailing overall effect was an increase in functional connectivity. In the dentate gyrus, these physiological changes have dramatic effects on neuronal synchronization, as loss of Pten in as few as 9% of dentate granule cells lead to development of spontaneous seizures.24 Loss of Pten in auditory cortical neurons enhances the strength of long-range connections inputs from both the contralateral auditory cortex and the thalamus, as well as from local inputs. This hyperconnectivity may constitute a physiological basis for ASD phenotypes associated with problems in processing and integration of complex sensory information.25
Pten and Synaptic Plasticity
In addition to changes in basal synaptic transmission, Pten is also intimately involved in the mechanisms underlying synaptic plasticity. Activity-induced long-term potentiation (LTP) and long-term depression (LTD) of synaptic transmission are two types of enduring changes in neuronal connections that underlie learning and memory functions.26 Conditional alpha-CaMK2-Cre dependent deletion of Pten in mature differentiated CA1 neurons demonstrated that Pten deletion leads to deficits in both major forms of synaptic plasticity (LTP and LTD) before the onset of anatomical abnormalities, suggesting that Pten controls structural synaptic growth and synaptic plasticity through independent mechanisms.27,32 Acute blockade of PI3K impairs LTP in hippocampal CA1 synapses, which reflects the importance of PI3K/Pten balance in synaptic plasticity.28 The precise mechanism through which Pten controls synaptic plasticity is not completely understood. During LTD, NMDA receptor activation triggers the association between Pten and postsynaptic density-95 (PSD-95). This interaction anchors Pten to the postsynaptic membrane, eventually depressing AMPA receptor-mediated synaptic responses.29 Pten may also exert effects on LTP through the hormone leptin, which phosphorylates and inactivates Pten, increasing the membrane expression of GluR1 and thus, the synaptic density of GluR2-lacking AMPA receptors in adult hippocampus.30,31 In dentate granule cells, conditional Pten ablation inhibits metabotropic glutamate receptor (mGluR) and protein synthesis-dependent LTD.32
Changes in the structure of the postsynaptic terminal affect synaptic plasticity regulation. Drebrin is a protein highly enriched in dendrites, and modulates synaptic plasticity by affecting the spine morphology and by regulating neuronal transmission.33 Clustering of Drebrin is regulated by AMPA receptor activity34 and Drebrin regulates the synaptic targeting of NMDA receptors.35 PTEN negatively regulates Drebrin phosphorylation, and neuronal activity induces a dissociation of the PTEN/Drebrin complex. The reduced levels of Drebrin observed in patients with Alzheimer’s disease suggest an important role of PTEN/Drebrin interaction in synaptic plasticity.36
Role of Pten in Intrinsic Excitability
Changes in voltage or calcium-gated conductances which modulate the intrinsic excitability of neurons can sculpt how neuronal networks respond to changes in synaptic weights. These changes can occur at the level of single dendrites,37 within the somatic compartment, or even in the axon initial segment,38 and fine tune the way input is integrated, maintaining the dynamic range of the neuron. There is increasing evidence that Pten and mTOR-related proteins can regulate the expression of intrinsic ion channels, modulating the intrinsic excitability of the cell. The first evidence for this modulation was demonstrated in hippocampal neurons where the mTOR inhibitor rapamycin increased dendritic translation of the potassium channel Kv1.1.39 In the hypothalamus, aging increases mTOR signaling in the pro-opiomelanocortin (POMC), elevating ATP activated potassium (K (ATP)) channel activity, ultimately resulting in obesity.40 In the cerebellum, neuregulin-1 and neuritin increase the density of Kv4.2 channels by engaging the mTOR pathway.41,42
Our own work43 demonstrates that single-copy loss of Pten in the mature visual cortex significantly decreases intrinsic excitability of L2/3 excitatory visual cortical neurons without changing dendritic branching. These neurons demonstrated decreased input resistance and increased amplitude of the spike after hyperpolarization. As calcium activated conductances play a large role in setting the amplitude of the AHP, we tested whether blockade of these conductances could normalize the firing properties and the spike AHP in the Pten mutants. Blockade of SK channels (but not BK channels) rescued the intrinsic firing properties and AHP of Pten deficient neurons. In addition, we found an increased protein expression of the SK2 channel subunit in visual cortex. Finally, we asked whether these changes in the intrinsic firing patterns affected the way about how visual information is processed in vivo. We found that loss of Pten decreased the magnitude of the visually evoked action potential discharges, without altering the orientation selectivity of the neurons. Therefore, single copy loss of Pten, through changes in SK channel expression levels, decreases the responsiveness of excitatory visual cortical neurons. These findings provide additional avenues for research in treating channelopathies induced by PTEN mutations.
PTEN and Human Disease
These physiological changes are especially relevant as de novo or inherited mutations in PTEN are emerging as one of the most validated causes of autism spectrum disorder, intellectual disability and extreme macrocephaly.1-9 These disorders add to the already well-established role of PTEN mutations in causing the PTEN hamartoma tumor syndromes which include Cowden,44 Bannayan-Riley-Ruvalcaba,45 Lhermitte Duclos,44 and Proteus Syndromes.46 In the future, it will be essential to determine which of the complex structural and physiological changes induced by PTEN deficits can be reversed or ameliorated by mTOR inhibition, and which changes will necessitate other interventions to normalize intrinsic excitability, synaptic connectivity, and plasticity.(Fig. 1)
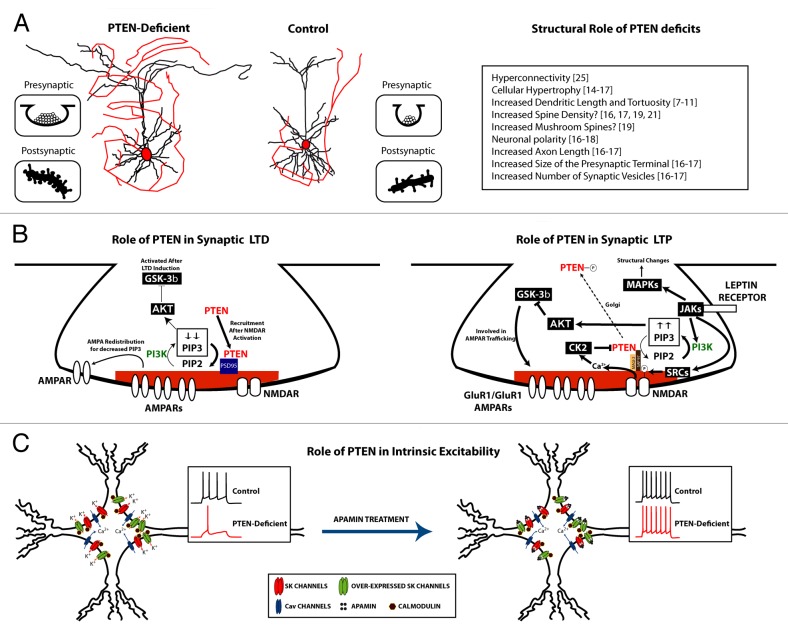
Figure 1. (A) Left: A drawing representing dendritic and axonal growth caused by loss of PTEN. Insets: PTEN loss causes increased synaptic vesicles and dendritic protrusions. Right: List of changes in neuronal anatomy caused by loss of PTEN. The numbers are references supporting each finding. (B) An illustration demonstrating the potential role of PTEN in synaptic long-term depression (LTD) and long term potentiation (LTP). C. A schematic illustrating that loss of PTEN leads to increased expression of calcium-activated potassium channels and decreased intrinsic excitability which is in turn rescued by treatment with the SK channel blocker, apamin.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/28358
References
- 1.Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–21. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman GE, Butter E, Enrile B, Pastore M, Prior TW, Sommer A. Increasing knowledge of PTEN germline mutations: Two additional patients with autism and macrocephaly. Am J Med Genet A. 2007;143A:589–93. doi: 10.1002/ajmg.a.31619. [DOI] [PubMed] [Google Scholar]
- 3.Varga EA, Pastore M, Prior T, Herman GE, McBride KL. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet Med. 2009;11:111–7. doi: 10.1097/GIM.0b013e31818fd762. [DOI] [PubMed] [Google Scholar]
- 4.Orrico A, Galli L, Buoni S, Orsi A, Vonella G, Sorrentino V. Novel PTEN mutations in neurodevelopmental disorders and macrocephaly. Clin Genet. 2009;75:195–8. doi: 10.1111/j.1399-0004.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 5.McBride KL, Varga EA, Pastore MT, Prior TW, Manickam K, Atkin JF, Herman GE. Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res. 2010;3:137–41. doi: 10.1002/aur.132. [DOI] [PubMed] [Google Scholar]
- 6.O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, Carvill G, Kumar A, Lee C, Ankenman K, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–22. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein S, Sharifi-Hannauer P, Martinez-Agosto JA. Macrocephaly as a clinical indicator of genetic subtypes in autism. Autism Res. 2013;6:51–6. doi: 10.1002/aur.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderver A, Tonduti D, Kahn I, Schmidt J, Medne L, Vento J, Chapman KA, Lanpher B, Pearl P, Gropman A, et al. Characteristic brain magnetic resonance imaging pattern in patients with macrocephaly and PTEN mutations. Am J Med Genet A. 2013 doi: 10.1002/ajmg.a.36309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobert JA, Embacher R, Mester JL, Frazier TW, 2nd, Eng C. Biochemical screening and PTEN mutation analysis in individuals with autism spectrum disorders and macrocephaly. Eur J Hum Genet. 2014;22:273–6. doi: 10.1038/ejhg.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–9. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 11.Marino S, Krimpenfort P, Leung C, van der Korput HA, Trapman J, Camenisch I, Berns A, Brandner S. PTEN is essential for cell migration but not for fate determination and tumourigenesis in the cerebellum. Development. 2002;129:3513–22. doi: 10.1242/dev.129.14.3513. [DOI] [PubMed] [Google Scholar]
- 12.Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29:1874–86. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amiri A, Cho W, Zhou J, Birnbaum SG, Sinton CM, McKay RM, Parada LF. Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J Neurosci. 2012;32:5880–90. doi: 10.1523/JNEUROSCI.5462-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, et al. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- 15.Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29:404–11. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- 16.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–88. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser MM, Bayazitov IT, Zakharenko SS, Baker SJ. Phosphatase and tensin homolog, deleted on chromosome 10 deficiency in brain causes defects in synaptic structure, transmission and plasticity, and myelination abnormalities. Neuroscience. 2008;151:476–88. doi: 10.1016/j.neuroscience.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, Eickholt BJ. PTEN couples Sema3A signalling to growth cone collapse. J Cell Sci. 2006;119:951–7. doi: 10.1242/jcs.02801. [DOI] [PubMed] [Google Scholar]
- 19.Haws ME, Jaramillo TC, Espinosa-Becerra F, Widman A, Stuber GD, Sparta DR, Tye KM, Russo SJ, Parada LF, Kaplitt M, et al. PTEN knockdown alters dendritic spine/protrusion morphology, not density. J Comp Neurol. 2014 doi: 10.1002/cne.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–83. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow DK, Groszer M, Pribadi M, Machniki M, Carmichael ST, Liu X, Trachtenberg JT. Laminar and compartmental regulation of dendritic growth in mature cortex. Nat Neurosci. 2009;12:116–8. doi: 10.1038/nn.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luikart BW, Schnell E, Washburn EK, Bensen AL, Tovar KR, Westbrook GL. Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J Neurosci. 2011;31:4345–54. doi: 10.1523/JNEUROSCI.0061-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weston MC, Chen H, Swann JW. Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J Neurosci. 2012;32:11441–52. doi: 10.1523/JNEUROSCI.1283-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, et al. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;75:1022–34. doi: 10.1016/j.neuron.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong Q, Oviedo HV, Trotman LC, Zador AM. PTEN regulation of local and long-range connections in mouse auditory cortex. J Neurosci. 2012;32:1643–52. doi: 10.1523/JNEUROSCI.4480-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Cheng A, Mattson MP. The PTEN phosphatase is essential for long-term depression of hippocampal synapses. Neuromolecular Med. 2006;8:329–36. doi: 10.1385/NMM:8:3:329. [DOI] [PubMed] [Google Scholar]
- 27.Sperow M, Berry RB, Bayazitov IT, Zhu G, Baker SJ, Zakharenko SS. Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration. J Physiol. 2012;590:777–92. doi: 10.1113/jphysiol.2011.220236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–65. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jurado S, Benoist M, Lario A, Knafo S, Petrok CN, Esteban JA. PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J. 2010;29:2827–40. doi: 10.1038/emboj.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moult PR, Cross A, Santos SD, Carvalho AL, Lindsay Y, Connolly CN, Irving AJ, Leslie NR, Harvey J. Leptin regulates AMPA receptor trafficking via PTEN inhibition. J Neurosci. 2010;30:4088–101. doi: 10.1523/JNEUROSCI.3614-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi K, Gertner MJ, Zhou J, Parada LF, Bennett MV, Zukin RS. Dysregulation of synaptic plasticity precedes appearance of morphological defects in a Pten conditional knockout mouse model of autism. Proc Natl Acad Sci U S A. 2013;110:4738–43. doi: 10.1073/pnas.1222803110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanov A, Esclapez M, Pellegrino C, Shirao T, Ferhat L. Drebrin A regulates dendritic spine plasticity and synaptic function in mature cultured hippocampal neurons. J Cell Sci. 2009;122:524–34. doi: 10.1242/jcs.033464. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi H, Yamazaki H, Hanamura K, Sekino Y, Shirao T. Activity of the AMPA receptor regulates drebrin stabilization in dendritic spine morphogenesis. J Cell Sci. 2009;122:1211–9. doi: 10.1242/jcs.043729. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi H, Mizui T, Shirao T. Neurochem. Down-regulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurons. 2006;1:110–5. doi: 10.1111/j.1471-4159.2005.03536.x. [DOI] [PubMed] [Google Scholar]
- 36.Kreis P, Hendricusdottir R, Kay L, Papageorgiou IE, van Diepen M, Mack T, Ryves J, Harwood A, Leslie NR, Kann O, et al. Phosphorylation of the actin binding protein Drebrin at S647 is regulated by neuronal activity and PTEN. PLoS One. 2013;8:e71957. doi: 10.1371/journal.pone.0071957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–41. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 38.Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–4. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raab-Graham KF, Haddick PC, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–8. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 40.Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012;75:425–36. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao JJ, Gao XF, Chow CW, Zhan XQ, Hu CL, Mei YA. Neuritin activates insulin receptor pathway to up-regulate Kv4.2-mediated transient outward K+ current in rat cerebellar granule neurons. J Biol Chem. 2012;287:41534–45. doi: 10.1074/jbc.M112.390260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao JJ, Sun J, Zhao QR, Wang CY, Mei YA. Neuregulin-1/ErbB4 signaling regulates Kv4.2-mediated transient outward K+ current through the Akt/mTOR pathway. Am J Physiol Cell Physiol. 2013;305:C197–206. doi: 10.1152/ajpcell.00041.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Junco-Clemente P, Chow DK, Tring E, Lazaro MT, Trachtenberg JT, Golshani P. Overexpression of calcium-activated potassium channels underlies cortical dysfunction in a model of PTEN-associated autism. Proc Natl Acad Sci U S A. 2013;110:18297–302. doi: 10.1073/pnas.1309207110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–7. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 45.Lynch NE, Lynch SA, McMenamin J, Webb D. Bannayan-Riley-Ruvalcaba syndrome: a cause of extreme macrocephaly and neurodevelopmental delay. Arch Dis Child. 2009;94:553–4. doi: 10.1136/adc.2008.155663. [DOI] [PubMed] [Google Scholar]
- 46.Loffeld A, McLellan NJ, Cole T, Payne SJ, Fricker D, Moss C. Epidermal naevus in Proteus syndrome showing loss of heterozygosity for an inherited PTEN mutation. Br J Dermatol. 2006;154:1194–8. doi: 10.1111/j.1365-2133.2006.07196.x. [DOI] [PubMed] [Google Scholar]