Abstract
Plant growth promoting (PGP) rhizobacteria, a beneficial microbe colonizing plant roots, enhanced crop productivity and offers an attractive way to replace chemical fertilizers, pesticides, and supplements. The keratinous waste which comprises feathers, hairs, nails, skin and wool creates problem of solid waste management due to presence of highly recalcitrant keratin. The multi traits rhizobacteria effective to remove both keratine from the environment by producing keratinase enzyme and to eradicate the chemical fertilizer by providing different PGP activity is novel achievement. In the present study, the effective PM2 strain of PGPR was isolated from rhizospheric soil of mustard (Brassica juncea) field, Pantnagar and they were identified on the basis of different biochemical tests as belonging to Bacillus genera. Different plant growth promoting activity, feather degradation and keratinolytic activity was performed and found very effective toward all the parameters. Furthermore, the efficient strain PM2 was identified on the basis of 16s rRNA sequencing and confirmed as Bacillus cereus. The strain PM2 might be used efficiently for keratinous waste management and PGP activity. Therefore, the present study suggests that Bacillus cereus have multi traits activity which extremely useful for different PGP activity and biotechnological process involving keratin hydrolysis, feather biodegradation or in the leather industry.
Keywords: Feather degradation, keratinase, PM2 strain, phosphate solubilization, rhizobacteria, solid waste management
Introduction
The use of microorganisms with the aim of improving nutrients availability for plants is an important practice and necessary for agriculture.1 During the past couple of decades, the use of plant growth promoting rhizobacteria (PGPR) for sustainable agriculture has increased tremendously in various parts of the world. Significant increases in growth and yield of agronomically important crops in response to inoculation with PGPR have been repeatedly reported.2The beneficial plant-microbes interactions in the rhizospere are determinants of plant health and soil fertility. In the biogeochemical cycles of both inorganic and organic nutrients in the soil and in the maintenance of soil health and quality, soil microorganisms are very important.3 In last few decades a large array of bacteria including species of Pseudomonas, Azospirillum, Azotobacter, Klebsiella, Enterobacter, Alcalisens, Arthobacter, Burkholderia, Bacillus and Serratia have reported to enhance plant growth.4 These mechanisms can be active simultaneously or independently at different stages of plant growth. Among this phosphate solubilization, biological nitrogen fixation, improvement of other plant nutrients uptake, and phytohormone production (like indole-3-acetic acid) are some of the regulators that profoundly influence plant growth.5 A number of IAA biosynthetic pathways have been identified in bacteria and emphasized the requirement of tryptophan as a precursor. This has been confirmed by both conventional6 and molecular genetics methods using mutants.7 In other hands the degradation of feather and keratins by indigenous microbial culture are also very important practice and necessary for environment. Feathers are produced in large amounts as a byproduct at poultry processing plants, reaching millions of tons annually. These feathers produce a big disposable problem in environment. Since feathers are almost are composed of over 90% protein having keratin as main component.8 Keratins, a major class of animal proteins, which are constituents of vertebrate skin, nail, hair, feather, wool etc, are abundant in nature and hard to degrade but have limited uses in practice. Since they are insoluble and resistant to degradation, they create a problem of solid waste management9 and also largely responsible for their high degree of recalcitrance. Little attention is given to the utilization or recycling of these wastes. The accumulation of some of these wastes in nature is considered to be a serious source of pollution and health hazards. A group of proteolytic enzyme which is able to hydrolyze insoluble keratins more efficiently than other proteases is called keratinases produced by some microorganisms. Keratinolytic enzymes may have potential roles in biotechnological processes that involving keratin containing wastes from the poultry and leather industries. Keratinolytic activity has been reported for various bacterial genera, such as Bacillus10,Thermoanaerobacter,11 Chryseobacterium,12 Flavobacterium13 and Vibrio.14 Therefore, keeping in mind that, if keratin-degrading and plant growth promoting characteristic do present in nature in same bacteria, then it will be possible to isolate them removed it) and use them for hydrolysis of feathers and keratins and also plant growth promotion which will help the environment to clean up and increase crop productivity.
Results
Isolation and screening of bacterial strain and in vitro determination of PGPR trait via phosphate solubilisation and IAA Production
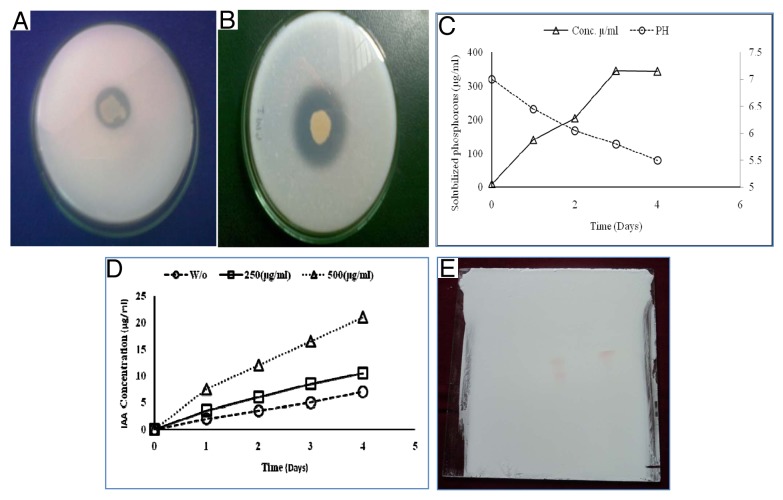
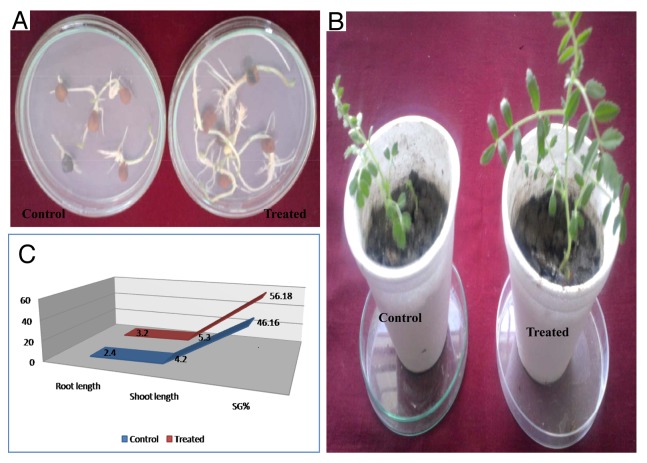
Isolated PM2 strain was successfully isolated from the Rhizospheric soil of mustard field, Pantnagar. The isolated culture was gram positive, rod shaped and raised colonies having smooth surface with elevated margin white or gray in color. Screening was done on the basis of clear halo zone formation on Pikovskaya’s agar plates and Skimmed milk agar plate that portrays the activities of phosphate solubilisation and protease production (Figure 1A and 1B). Isolated strain PM2 was further analyzed for its growth promoting traits such as phosphate solubilization and IAA production (Figure 1C and 1D). Quantitative examine of phosphate solubilization was performed in Pikovskaya’s. The magnitude of soluble phosphate liberated in broth from tri-calcium phosphate solubilization was measured by means of KH2PO4 curve at 600nm to be (135–345 μgml−1) upon 4 d of growth (Figure 1C). The data also displayed a time-dependent boost in the sum of solubilized phosphate, and contrary association with the pH of defined medium. Intermittent examination of pH of the culture filtrate showed a noteworthy decrease from pH 7.0 to 4.40. Thus the results advocate the improved phosphate solubilization with the isolated strain PM2 could be due to result of higher acid production which directly dissolves the rock phosphate (Figure 1C). This investigation discovered the inherent capability of isolated strain PM2 to produce phytohormone. The data shown in Figure 1D, noticeably demonstrate the formation of significant amount of IAA in LB broth medium. The estimation of IAA was performed using the calibration curve of IAA (Figure 1D). The extended incubation of axenic culture for a period of 4 d demonstrated a few degree of decrease in cell viability. Though, adequate bacterial biomass endured in the stationary phase yet its incubation up to 4 d. The filtrate of culture at varying time periods confirmed a straight and time-dependent augment in IAA formation. Improved production of IAA (10.5µgml−1) and (21.oµgml−1) was determined by adding 250µgml−1 and 500 µg ml−1 of tryptophan concentration, correspondingly. Vis-à-vis, 7.04 µg ml−1 IAA in the absence of tryptophan. Furthermore, linear boost in IAA formation till 4 d displayed constancy of the metabolic cells in stationary phase (Figure 1D). Confirmation of IAA was done by Thin layer chromatography (TLC) method, in which the culture filtrate of PM2 strain was use to extract IAA for characterization. The spots of ethyl acetate extract of culture and standard IAA were tested in the solvent mixture of chloroform: ethyl acetate: formic acid (5:3:2). Chromatograms of spots were, sprayed with salkowski reagent that shows almost the same RF value (0.57) (Figure 1E). The growth performance of Brassica juncea plants were evaluated in PM2 strain treated and untreated conditions. In seeds germination test, the percentage seed germination of treated seed were found high (56. 18%) as compare with control (46.16) (Figure 2A). Maximum effect on root length were also observed in germinated seed compare with control seed (Figure 2B), the strain also shows greater length of root and shoot in the harvested plant compare with control (Figure 2C).
Figure 1. Isolation of bacterial strain and in vitro characterization for their PGPR traits. Clear halo zone on Pikovskaya’s agar and Skimmed milk agar plate showing substrate solubilization (A) and Caseins hydrolyzation (B). Phosphate solublizing data using the calibration curve with KH2PO4 at 600nm. The pH variation in the Pikovskaya’s medium during growth of isolates at different time points (C). Productions of indole-3-acetic supplemented with tryptophan 250 µg/mLand 500 µg/mL compared with absence of tryptophan (D). Thin layer chromatography (TLC) of indole-3-acetic acid detected by Salkowiski reagent (E).
Figure 2. Evaluation of plant growth promoting activity of PM2 strain of rhizobacteria. The PM2 strain treated Brassica juncea plants showed higher response of growth parameters such as seed germination (A) Shoot length (B) Growth index (C) as compared with untreated control
Biochemical characterization
Isolated strain PM2 was analyzed for metabolic properties by observing their response to diverse biochemical reactions using specific HiMedia test-kits for carbohydrate and by performing different other test like, Methyl red, Voges prouskaur, Citrate test, Catalase, Oxidase activity, Malonate, ONPG, Arginine, Glucose, Arabinose, Trehalose, Nitrate reduction test, starch hydrolysis, and urease activity test (Table 1).
Table 1. Biochemical tests of isolated strain PM2.
| S.No. | Biochemical test | Strain PM2 |
|---|---|---|
| 1. | Oxidase | -ve |
| 2. | Catalase | + ve |
| 3. | Methyl red | - ve |
| 4. | Vogus-Proskauer | + ve |
| 5. | Starch hydrolysis | + ve |
| 6. | Malonate | + ve |
| 7. | Citrate utilization | - ve |
| 8. | ONPG | + ve |
| 9. | Nitrate | + ve |
| 10. | Arginine | - ve |
| 11. | Sucrose | - ve |
| 12. | Mannitol | + ve |
| 13. | Glucose | + ve |
| 14. | Arabinose | + ve |
| 15. | Trehalose | + ve |
| 16. | Urease test | - ve |
| 17. | Rhamnose | -ve |
| 18. | Cellobiose | - ve |
| 19. | Melizitose | - ve |
| 20. | α-Methyl-D- Mannoside | - ve |
| 21. | Xylitol | - ve |
| 22. | ONPG | + ve |
| 23. | Esculin Hydrolysis | + ve |
In-vitro determination of keratinase production trait: feather degrading activity and production of keratinase and protease
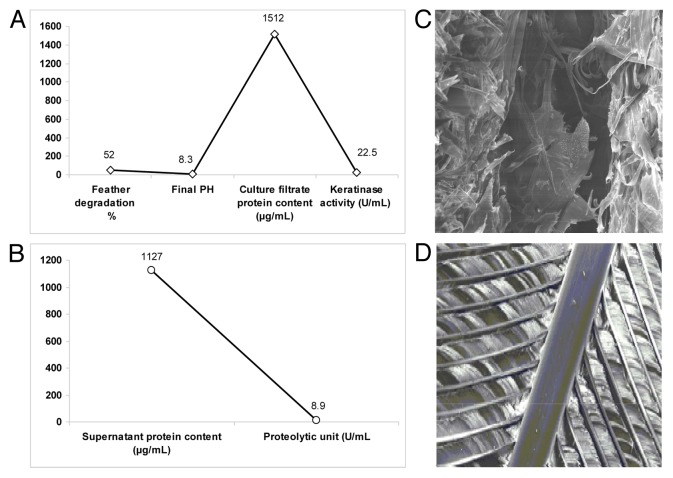
Isolated strain PM2 showed feather degradation of 52% with soluble protein content in culture filtrate i.e., 1512 μg/ml within 48 h of incubation (Figure 3A). The culture supernatant of keratinase production media after centrifugation was used as crude enzyme for keratinase assay. The maximum keratinase activity was found 22.5 (U/ml) at 48 h incubation which gradually decreases when incubated further (Figure 3A). Furthermore, the culture supernatant of protease production media after centrifugation was checked for proteolytic activity. The maximum proteolytic activity was found 8.9 (U/ml) at 48 h (Figure 3B). The PM2 strain was evaluated for its ability to produce keratinase enzyme which effectively degrade feather (Figure 3C) as compared with control (Figure 3D).
Figure 3. In vitro determination of feather degrading activity and production of keratinase and protease. Keratinolytic activity of isolated PM2 strain indicating feather degradation at defined pH and culture filtrate protein (A). Proteolytic activity of isolated PM2 strain was also evaluated (B). SEM micrograph of degraded feather treated with isolated strain PM2 (C) with references to their controls (D).
Crude enzyme characterization
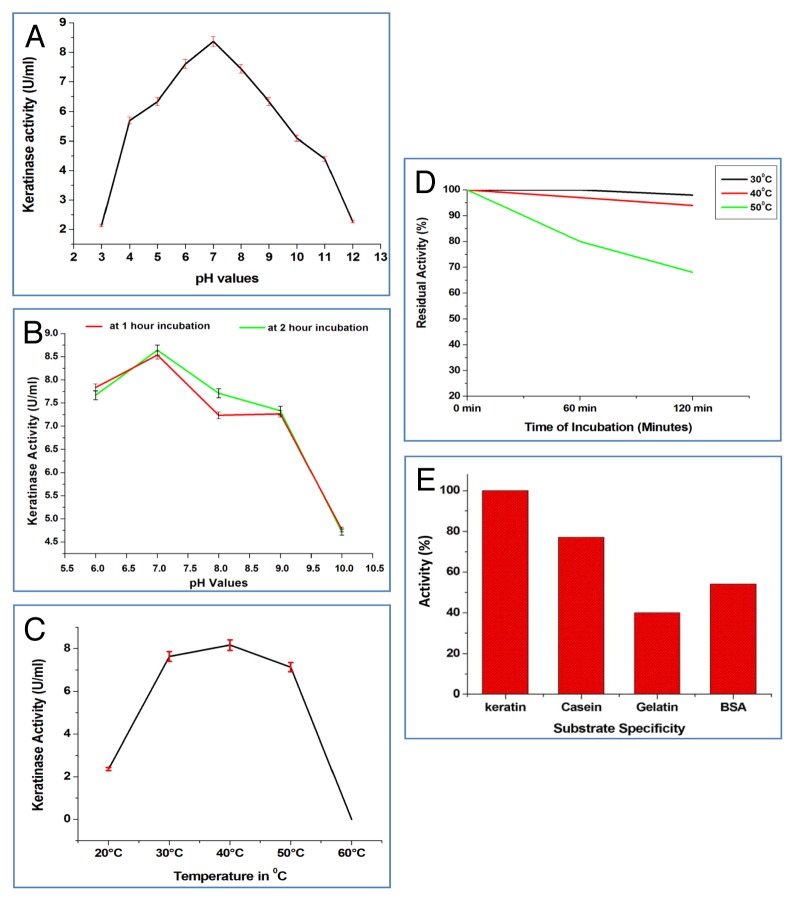
The pH optima of crude keratinase was found to be 7.0 and it was found that keratinase in this study showed more than 80% activity in the range from pH 5.0 to pH 9.0 (Figure 4A). Enzyme showed decline in activity as pH increases or decreases much below pH 7.0 suggesting that it is of neutral keratinolytic protease (Figure 4B). From the data obtained for pH stability studies of keratinase, it was inferred that enzyme was stable in wide pH range from pH 6.0 to 9.0. The temperature optimum for enzyme activity was obtained at 40 °C, which followed by 30° and 50 °C (Figure 4C). The enzyme was stable and showed no fall in activity for 2 h incubation at 40 °C and at 50 °C it shows approx 90% enzyme activity of initial activity after 2 h of incubation (Figure 4D). Among different substrates solution (4mg/ml) tested to evaluate the substrate specificity of keratinase, enzyme showed high affinity toward keratin followed by casein, BSA and gelatin which supports that protease in the present study is a keratinolytic protease (Figure 4E).
Figure 4. Characterize optimum conditions for keratinase activity of PM2 strain of rhizobacteria such as pH optima (A) pH stability (B) Temperature optima (C) Temperature stability (D) Substrate specificity (E).
Molecular characterization and phylogenetic analysis
The isolated strainPM2 was subjected to 16S rRNA sequencing and were analyzed using different bioinformatics tools at Bioserve Biotechnologies, Pvt. Ltd. Hyderabad, Andhra Pradesh, India. The partial sequence data of the isolates was analyzed by BLAST search that showed unambiguous similarity (98%) with Bacillus cereus. Further, the phylogenetic tree was constructed using MEGA4 software by neighbor-joining tree means and the resulted phylogenetic data were examined after sequences alignment of the 16S rRNA of closely related strains (Figure 5). The 16S rRNA gene sequence from strain PM2 was submitted in the GenBank database under Accession number KF673164.
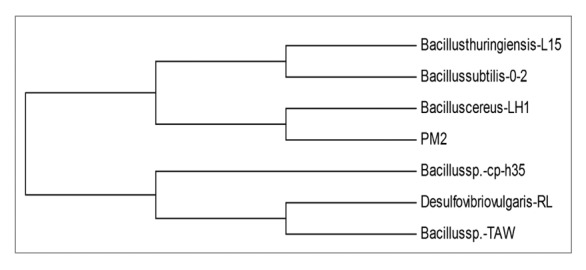
Figure 5. Phylogenetic tree for the 16S rDNA sequences of the bacterial strain PM2 constructed using MEGA4 software by neighbor-joining method.
Discussion
The isolated culture PM2 was gram positive, rod shaped and raised colonies having smooth surface with elevated margin white or gray in color. On screening the clear halo zone formation were found on pikovskaya’s agar plate and skimmed milk agar plate (Figure 1A and 1B), that portray the activities of phosphate solubilisation and protease production. Although, in biochemical test isolated strain was found positive for more substrates, while negative for fewer. This behavior suggests their unique metabolic potential and presumably consider as belong to Basillus sp. based on the morphological and biochemical characteristics. In-vitro determination of PGPR trait, the quantitative estimation of phosphate solubilisation illustrates the time dependent encouragement of phosphate solubilisation with the contrary relationship with pH of the medium (Figure 1C). Thus the results suggest that it could be either result of higher acid production which easily suspends the rock phosphate or chelating calcium ions which liberate P in the solution or incorporation of phosphate released organic compounds via activity of microorganisms by the enzyme “phosphatase” that present in many soil microorganisms. The change of insoluble phosphate to soluble type can be done by a number of microbes found in the soil. A great portion of soil microbes can convert insoluble inorganic phosphates to available form to the plants. The formation of organic acid, phosphatse and its function in phosphate solubilisation is entirely known.15 The relationship of decrease in pH of the culture medium with the amount of phosphate solubilisation is well recognized.16 Although, IAA is one of the most important phytohormone which function as important signal molecule in the regulation of plant development. Enhanced production of IAA were noticed in the presence of tryptophan concentration (Figure 1D) is probably due to tryptophan is the precursor of IAA production, which has been characterized via TLC analysis (Figure 1E). These data suggested that the isolated culture were act as effective bioinoculant. These trends coincided with the previous reports indicating IAA formation in stationary stage of culture.17,18 The conformation of IAA production was performed by TLC, RF value comparing with the RF value of standards IAA (Figure 1E) The same TLC findings are agreements with reported by other scientist.18Although, the evaluation of effect on plant growth parameter of these IAA producing strain PM2 were further studied in seed germination (Figure 2A) and pot experiment (Figure 2B). The data’s obtained from seed germination and pot experiments demonstrated positive effect on root and shoot elongations compare with control (Figure 2C). This indicates that the strain PM2 can improve the plant growth development and thus considered as an effective PGPR. Similar type of results was reported by Bharucha et al.19 and Sivakumar et al.20
In-vitro determination of keratinase and protease production traits of the strain is demonstrated through growing on keratin and casein incorporated media, where degradation of feather by means of using feathers as sole nitrogen and carbon source for their survival in the nutrient limited condition (Figure 3A and 3B). This remarkable activity toward chicken feathers might be ascribed to the actuality that the present enzyme was originally produced in a medium having chicken feathers so it is well adapted to attack it supported by Sharaf and Khalil21 and could offer tremendous potential for the development of biotechnological methods for the hydrolysis of feathers. Similar type of results was bout with the former explanation indicating the keratinase and protease production was found more during initial log phase and become reduce throughout on further incubation.22 The loss of enzyme activity beyond 48 h incubation was probably due to enzyme autolysis or end product inhibition.23 Nagal et al.8 were also reported feather degradation in Bacillus strains previously in the same media. The morphological changes on the surface of feather due to the action of isolated bacterial strain PM2 was noted by comparing with control. The control feather revealed smooth and homogeneous morphology (Figure 3D) while in the case of treated feather, some wrinkled structures were observed, probably due to the microbial action. The exposure of feather with bacterial strain imparted heterogeneous morphology with widened fissure, leading to the formation of cavities with wrinkles and white globular areas (Figure 3C). Thus, the observations suggested the considerable surface deterioration of feather by the virtue of keratinase and protease production from bacterial action. Similar type of results was match with the earlier observations indicating heterogeneous surface morphology in the treated feather compare with control.22 The Keratinolytic activity of bacteria has been characterized via standardizing the optimal conditions.24 Mostly, Keratinases reported from bacteria, actinomycetes and fungi had got their pH optima in neutral to alkaline range.25 In the present study, PM2 a classical PGPR, bears keratinolytic activity which optimal conditions were determine as a function of pH, temperature, time and substrate specificity (Figure 4A-E). Even at 50 °C enzyme shows 90% of its optimum activity at 40 °C but enzyme inactivation takes place abruptly at higher temperatures tested which infers it as mildly thermostable in nature. The optimum temperature of keratinases ranges from 30 to 80 °C. The enzyme from thermophilic F. pennavorans has optimum temperature of 80 °C.25 Thus, the keratinolytic protease from the present isolate can be classified as mild thermostable alkaline keratinase which can be potentially useful for various industrial purposes. The temperature optima and stability of keratinases was found to be very variable, often depending on the source and origin of the isolate.26 Here, temperature optima were 40 °C for keratinase (Figure 4C). Molecular Characterization and phylogenetic analysis disclose the isolated strain unambiguous identity (98%) with all the mention strains of phylogram, while the query cover through Bacillus cereus is 99% and the rest have 98% using bioinformatics tools like BLAST search, Finally the phylogenic tree was constructed using another bioinformatics tools like neighbor-joining method and MEGA4 software
Materials and Methods
Isolation, screening and biochemical characterization of bacterial strain
Bacterial strain was isolated from rhizosphere soil of mustard (Brassica juncea) field, Pantnagar. The rhizospheric soil sample were taken from a depth of 0–15cm, kept in plastic bags and carried to the laboratory. Ten gram of rhizospheric soil was taken from the sample added into a 250 ml of conical flask, and 90 ml of sterile distilled water was added to it. The flask was shaken for 10 min on a rotary shaker, and serial 10-fold dilutions were prepared from the extract and 0.1 ml of each dilution was spread on the plates of nutrient agar medium and the plates were incubated at 37 °C for 2 d. Typical bacterial colonies were observed over the plate. Well isolated single colony was picked up and re-streaked to fresh nutrient agar plate and incubated similarly. The technique was perpetrated thrice and culture was made single colony type. The culture were screened out initially on the basis of their ability to solubilize insoluble inorganic phosphate for PGPR traits, and then, to degrade insoluble casein for protease production, by spotting overnight grown cultures on Pikovskaya’s agar plates and on Skimmed milk agar plate, respectively. Isolated strain, showing clear zone in both the plates around the colony after incubating the plates at 30 °C for 48 to 72 h, named the isolated strain PM2
Further the identification of bacterial strain PM2 was performed.27 Catalase activity was determined by detective bubble formation with 3% H2O2 solution. Oxidase test was determined by using Bacteriological differentiation Oxidase Disc (Hi-Media Laboratories, India). Other important biochemical properties were performed by using biochemical test kits (KB013 HiMEDIA HiBacillusTM identification kit).
Quantitative estimation of phosphate (Pi) released from tricalcium phosphate and indole-3-acetic acid (IAA) production
The isolated PM2 strain was further examined for their ability to release Pi from TCP in broth medium. One ml of overnight culture of the isolate was inoculated to 100 ml of Pikovskaya’s broth.28The inoculated flasks were incubated at 32 °C. The amount of Pi released in the broth was estimated by sampling broth culture at every 24 h. The broth cultures were centrifuged at 10,000 rpm for 10 min to separate the supernatant from the cell growth and insoluble phosphate. The available P in the supernatant was estimated by phosphomolybdic blue color method.29 One ml of the culture supernatant was taken in a 50 ml volumetric flask to which 10 ml of chloromolybdic acid was added and mixed thoroughly. The volume was made up to approximately three fourth with distilled water and 0.25 ml chlorostannous acid was added to it. Immediately, the volume was made to 50 ml with distilled water and mixed thoroughly. After 15 min, the blue color developed was read in a spectrophotometer at 610 nm using a reagent blank. Simultaneously, a standard curve was prepared using various concentrations of P solution. The amount of phosphorus solubilized by the isolate was calculated from the standard curve. Selected isolates PM2 strain was inoculated into three separate 250 ml conical flask, containing, 100 ml of minimal salt medium each, supplemented with tryptophan at a concentration of 500 µg/ ml, 250 µg/ ml and without tryptophan, in the respective flask and incubated at 32 °C under shaking. The amounts of IAA produce in the broth of all flasks were estimated by sampling broth culture at every 24 h. Broth culture were centrifuged at 7500 rpm for 10 min. To one ml of aliquot of the supernatant of the cultures 2 ml of Salkowskis reagent30 was added and incubated at 30 °C for 25 min. Absorption was read at 530 nm and the concentration of IAA in the bacterial strains was determined and quantified by comparison with a standard curve of IAA.31
Extraction of crude IAA and Thin layer Chromatography (TLC)
Single bacterial colony of PM2 was inoculated in 100ml nutrient broth amended with 5mg/ml tryptophan and incubated at 32 °C under shaking. Bacterial culture were centrifuged at 7500 rpm for 10 min to separate the supernatant and subsequently acidified to pH3 with 1N HCL. Extraction was done twice with ethyl acetate at double the volume of supernatant. Extracted ethyl acetate fraction was evaporated to dryness in a rotary evaporator at 40 °C. The extract was dissolve in 0.5ml methanol and kept it at -20 °C. For TLC extracted ethyl acetate fraction (10–30µl) was placed on TLC plate and developed in choloroform: etyl acetate: formic acid (5:3:2). Spot with RF value identical to standard IAA were identified under UV light (254nm) by spraying the plates with salkawaski reagent.
Evaluation of growth performance of Brassica juncea under PM2 treated conditions
To study the effect of IAA producing strain PM2 on growth of plant, seed germination test32 and pot experiment33 were performed. For this, gram (Cicer atrium) seeds were surface sterilized by exposing to 95% ethanol and immersing in 0.2% HgCl2 solution for 3 min. the seeds were then subjected to five times washing with sterile distilled water. 1mL of overnight grown bacterial culture (107-106 cells/mL, 0.5 of OD at 540nm) was applied on each seed for 10 min and treated seeds were dried. For seed germination test the sterile non treated dried seeds as control, soaked with non inoculated media for 10 min and the treated dried seeds were sown on soft agar (0.8%) plates under axenic condition and incubated them at 32 °C for 5 d. The % seed germination and root length were measured as it was consider the main parameter in determined the effect of IAA. For Pot experiment, soil sample were collected, air-dried, sieved and sterilized three times repeatedly by autoclaving before filling the pots. Finally, the sterile non treated dried seeds as control, soaked with non inoculated media and the treated dried seeds were transfer to pots containing sterile soil to a depth of 5mm. Single seed were sown in each pot and the experiment was performed in triplicates. The pots were kept in green house and were observed every day for 15 d. After 15 d the plant were uprooted carefully and the length of root and shoot were measured.
Collection of feathers, keratinase production and enzyme degrading activity via scanning electron microscopy
Chicken feathers were provided by a local poultry farm at Kumaun University, Bhimtal campus. It was washed twice with distilled water and dried at 60 °C overnight and kept at 4 °C, until used. Isolates PM2 strain was cultivated in 100 ml conical flask containing 50 ml cultivation media containing sterile feathers at 32 °C and 150 rpm for 4 d. The crude enzyme was prepared by centrifugation at 4 °C, 10,000 rpm for 20 min. Residual feather in the culture broth of keratinase production media was harvested by filtration through whatmann no. 1 filter paper washed twice with buffer and dried at 65 °C to constant weight. The feather degradation percentage was calculated from the difference in residual feather dry weight between control (Feather without bacterial inoculation) and treated sample. Pattern of detachment of feather fibrils were recorded by periodic observation of the treated feathers using compound light microscope at 10X magnification and scanning electron microscopy.
Protein determination, keratinase and protease assay
The protein content in culture filtrate and crude enzyme (supernatant) was determined by the Method of Lowry et al.34 Keratinase activity was determined by modified method of Letourneau et al.35 using Keratin as substrate. The keratin was suspended in Tris buffer (0.05 M, pH 9) at concentration of 4 mg/ml. The reaction mixture contained 1 ml of enzyme and 1 ml of substrate solution. The sample was incubated at 60 °C for 1 h with regular shaking. After incubation, the reaction was terminated with 2 ml of 10% trichloroacetic acid (TCA) followed by centrifugation at 5000 rpm for 15 min to remove unutilized substrate. The supernatant was measured for release of azo dyes at 595 nm. A control was kept with enzyme and buffer without substrate. One unit of keratinase (1KU) was defined as the amount of enzyme required to increase 0.01absorbance between sample and control at 595 nm in an hour under the specified conditions. Proteolytic activity was assayed by modified Anson method; using 0.5% casein as substrate dissolved in 50 mM Tris buffer pH 8.0. The reaction mixture was incubated at 60 °C for 30 min, and reaction was stopped using 10% TCA. Tyrosine released was estimated using Folin Ciocalteau’s reagent and absorbance was taken at 670 nm. One unit of protease (1 PU) was defined as amount of enzyme required to release 1 μg of tyrosine under the assay condition when reaction was incubated for 1 min.
Crude enzyme characterization
To study the optimum reaction conditions for the PM2 keratinase, the crude enzyme extract was incubated in the appropriate buffer of different pH value from 3.0- 12.0. Buffer used were 0.1M Citrate-phosphate buffer (pH 3.0–6.0), 0.1M TRIS-HCl buffers (pH 7.0–8.0) and 0.1M Glycine-NaOH buffer (pH 9.0–12.0). Effect of pH on enzyme stability was performed by incubating the enzyme solution at different pH ranged from 6–10 for 2 h. The optimum temperature of the crude enzyme was determined by incubating the reaction mixture at different temperature ranging from 20 °C-60 °C. To check the thermostability, the crude enzyme was incubated at temperatures ranging from 70 °C to 90 °C, and after incubation for different time periods viz. One hrs, 2hrs, the reaction mixtures were assayed, and the residual enzymatic activity determined. Substrate specificity of the enzyme was tested for a broad range of substrates (4 mg/ml) such as keratin, casein, BSA, gelatin as soluble substrate. Reaction was carried with normal enzyme assay protocol with keratin replaced by other substrate.
Molecular characterization
Based on screening, potential isolated PM2 strain was characterized on the basis of 16S rRNA sequencing. Polymerase chain reaction (PCR) amplification of the partial 16S rRNA gene region was performed with the bacterial primer set FD1: 5′-ccgaattcgt cgacaacAGA GTTTGATCCT GGCTCAG-3′and RP2: 5′-cccgggatcc aagcttACGG CTACCTTGT TACGACTT-3′, using the Geneamp kit (Applied Biosystems. PCR System 9700, U.S.A) i.e., FD1 and RP2 (FD-forward Distal; RP-reverse proximal).36 About 50–100 ng of the purified DNA were sequenced using BigDye Terminator Cycle. Sequencing Kits (v3.1) (Applied Biosystems, USA), with PCR Conditions: Denaturation – 96 °C- 2min, Final Denaturation- 96 °C -10 s, Annealing - 55 °C -30sec, Extension- 60 °C-4min, No of cycles -35. After 35 cycles, the templates were purified by Ethanol/EDTA precipitation method and sequenced on ABI 3730xls Genetic Analyzer (Applied Biosystems) at Bioserve Biotechnologies, Pvt. Ltd. Hyderabad, Andhra Pradesh, India. The sequence was then analyzed by basic local alignment search tool (BLAST) at National Center for Biotechnology Information, USA (NCBI) database. The isolate was identified based on the similarity scores and the sequence was submitted to NCBI GenBank to get accession numbers. Further, the phylogenetic tree was constructed using the neighbor-joining tree method and the phylogenetic data were obtained by aligning the different sequences of the 16S rRNA of closely related strains.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
MWA is thankful to Department of Science and Technology (DST) for funding under fast track scheme of young scientist. Work on molecular microbiology in VP’s laboratory is partially supported by Department of Biotechnology (DBT), Government of India.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/27683
References
- 1.Freitas ADS, Vieira CL, Santos CERS, Stamford NP, Lyra MCCP. Caracterizacao de rizobios isolados de Jacatupe cultivado em solo salino no Estado de Pernanbuco. Brasil Bragantia. 2007;66:497–504. doi: 10.1590/S0006-87052007000300017. [DOI] [Google Scholar]
- 2.Das AJ, Kumar M, Kumar R. Plant Growth Promoting Rhizobacteria (PGPR): An Alternative of Chemical Fertilizer for Sustainable. Environment Friendly Agriculture. Res J Agric Fores Sci. 2013;1:21–3. [Google Scholar]
- 3.Jeffries S, Gianinazzi S, Perotto S, Turnau K, Barea JM. The contribution of Arbuscular mucorhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils. 2003;37:1–16. [Google Scholar]
- 4.Sakthivel U, Karthikeyan B. Isolation and Characterization of Plant Growth Promoting Rhizobateria (pgpr) from rhizosphere of Cloeus forskohlii grown soil. Int J Rec Sci Res. 2012;2:288–96. [Google Scholar]
- 5.Zaidi A, Khan MS, Ahemad M, Oves M. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol Immunol Hung. 2009;56:263–84. doi: 10.1556/AMicr.56.2009.3.6. [DOI] [PubMed] [Google Scholar]
- 6.Rahman A, Sitepu IR, Tang SY, Hashidoko Y. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci Biotechnol Biochem. 2010;74:2202–8. doi: 10.1271/bbb.100360. [DOI] [PubMed] [Google Scholar]
- 7.Idris EE, Iglesias DJ, Talon M, Borriss R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Interact. 2007;20:619–26. doi: 10.1094/MPMI-20-6-0619. [DOI] [PubMed] [Google Scholar]
- 8.Nagal S, Jain PC. Isolation of keratinolytic bacteria from decomposing feathers. J Microbial world. 2009;11:15–20. [Google Scholar]
- 9.Pillai P, Archana G. Hide depilation and feather disintegration studies with keratinolytic serine protease from a novel Bacillus subtilis isolate. Appl Microbiol Biotechnol. 2008;78:643–50. doi: 10.1007/s00253-008-1355-z. [DOI] [PubMed] [Google Scholar]
- 10.Manczinger L, Rozs M, Lgyi VG, Kevie F. Isolation and characterization of a new keratinolytic Bacillus lichenoformis strain. World J Microbiol Biotechnol. 2003;19:35–9. doi: 10.1023/A:1022576826372. [DOI] [Google Scholar]
- 11.Riessen S, Antranikian G. Isolation of Thermoanaerobacter keratinophilus sp. nov., a novel thermophilic, anaerobic bacterium with keratinolytic activity. Extremophiles. 2001;5:399–408. doi: 10.1007/s007920100209. [DOI] [PubMed] [Google Scholar]
- 12.Riffel A, Brandelli A. Isolation and characterization of a feather-degrading bacterium from the poultry processing industry. J Ind Microbiol Biotechnol. 2002;29:255–8. doi: 10.1038/sj.jim.7000307. [DOI] [PubMed] [Google Scholar]
- 13.Riffel A, Lucas F, Heeb P, Brandelli A. Characterization of a new keratinolytic bacterium that completely degrades native feather keratin. Arch Microbiol. 2003;179:258–65. doi: 10.1007/s00203-003-0525-8. [DOI] [PubMed] [Google Scholar]
- 14.Sangali S, Brandelli A. Feather keratin hydrolysis by a Vibrio sp. strain kr2. J Appl Microbiol. 2000;89:735–43. doi: 10.1046/j.1365-2672.2000.01173.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein AH, Krishnaraj PU. Phosphate solubilizing microorganisms vs. phosphate mobilizing microorganisms: What separates a phenotype from a trait? In: Velazquez E, Rodríguez-Barrueco C, eds. First International Meeting on Microbial Phosphate Solubilization. Springer: 2007; 203-213 [Google Scholar]
- 16.Anwar MS. Isolation and Characterization of phosphate solubilising microorganism. Res J Agric Sci. 2012;3:336–40. [Google Scholar]
- 17.Patil NB, Gajbhiye M, Ahiwale SS, Gunjal AB, Kapadnis BP. Optimization of Indole 3acetic acid (IAA) production by Acetobacter diazotrophicus L1 isolated from Sugarcane. Int J Eng Sci. 2011;2:295–302. [Google Scholar]
- 18.Sahasrabudhe MM. Screening of rhizobia for indole acetic acid production, Scholars Research Library. Ann Biol Res. 2011;2:460–8. [Google Scholar]
- 19.Bharucha U, Patel K, Trivedi UB. Optimization of Indole Acetic Acid Production by Pseudomonas putida UB1 and its Effect as Plant Growth-Promoting Rhizobacteria on Mustard (Brassica nigra) Agric Res. 2013;2:215–21. doi: 10.1007/s40003-013-0065-7. [DOI] [Google Scholar]
- 20.Sivakumar T, Shankar T, Vijayabaskar P, Ramasubramanian V. Plant growth promoting activity of nickel tolerant Bacillus cereus TS1. J Agric Technol. 2012;8:2101–2113. [Google Scholar]
- 21.Sharaf EF, Khalil NM. Keratinolytic activity of purified alkaline keratinase produced by Scopulariopsis brevicaulis (Sacc.) and its amino acids profile. Saudi J Biol Sci. 2011;18:117–21. doi: 10.1016/j.sjbs.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeonga JH, Mileeb O. DongJeona Y, DoKima J, RiLeea N, Leea CY, JooSona H. Production of keratinolytic enzyme by a newly isolated feather-degrading Stenotrophomonas maltophilia that produces plant growth-promoting activity. Process Biochem. 2010;45:1738–45. doi: 10.1016/j.procbio.2010.07.020. [DOI] [Google Scholar]
- 23.Syed DG, Lee JC, Li WJ, Kim CJ, Agasar D. Production, characterization and application of keratinase from Streptomyces gulbargensis. Bioresour Technol. 2009;100:1868–71. doi: 10.1016/j.biortech.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 24.Verma A, Hukum SP, Shalini P, Agarwal S. Exploring the keratin degradation potential of Bacillus Strains isolated from Indian soil sites. JPAM. 2011;5:755–60. [Google Scholar]
- 25.Verma A, Ansari MW, Anwar MS, Agrawal R, Agrawal S. Alkaline protease from Thermoactinomyces sp. RS1 mitigates industrial pollution. Protoplasma. 2013 doi: 10.1007/s00709-013-0559-y. [DOI] [PubMed] [Google Scholar]
- 26.Brandelli A, Daroit DJ, Riffel A. Biochemical features of microbial keratinases and their production and applications. Appl Microbiol Biotechnol. 2010;85:1735–50. doi: 10.1007/s00253-009-2398-5. [DOI] [PubMed] [Google Scholar]
- 27.Holt JG, Krieg NR, Sneath PHA. Bargey’s Mannual of Determinative Bacteriology, 19th edition, Williams and Wilkins, Baltimore 1994. [Google Scholar]
- 28.Pikovskaya RI. Mobilization of phosphorous in soil connection with the vital activity of some microbial species. Microbiologia. 1948;17:362–70. [Google Scholar]
- 29.Jackson ML. Soil Chemical analysis. Prentice Hall of India Pvt, Ltd., New Delhi 1973. [Google Scholar]
- 30.Gordon SA, Weber RP. Colorimetric estimation of indole-acetic acid. Plant Physiol. 1951;26:192–5. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen S, Bose T, Basu K, Acharya R, Samjapati N, Acharya K. In-vitro antagonistic effect of fluorescent Pseudomonas BRL-1 against Sclerotium rolfsii. Indian Phytopath. 2006;59:227–30. [Google Scholar]
- 32.Kravchenko LV, Azarova TS, Makarova NM, Tikhonovich IA. The effect of tryptophane of plant root metabolites on the phytostimulating activity of rhizobacteria. Microibiologica. 2004;3:195. [PubMed] [Google Scholar]
- 33.Strom B, Garhardson B. Differential reaction of wheat and Pea genotypes to root inoculation with growth affecting rhizosphere bacteria, plant. Soil. 1988:109–263. [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 35.Letourneau F, Soussotte V, Bressollier P, Branland P, Verneuil B. Keratinolytic activity of Streptomyces sp. S.K1-02: a new isolated strain. Lett Appl Microbiol. 1998;26:77–80. doi: 10.1046/j.1472-765X.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 36.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]