Abstract
JAK-STAT3 signaling, while regulating many aspects of cancer development and progression, promotes invasion and metastasis through activation of key metastasis promoting genes such as WASF3. STAT3 promotes WASF3 expression and JAK2 independently activates it, which is required for invasion. JAK-STAT3 signaling is dependent on WASF3 function, since its inactivation in cells expressing JAK-STAT3 suppresses invasion. WASF3 overexpression leads to activation of NFκB and ZEB1 which also promote invasion through regulation of target genes involved in metastasis. NFκB frequently cooperates with STAT3 to upregulate metastasis promoting genes such as matrix metalloproteinases and cytokines, as well as to suppress microRNAs which can suppresses invasion. This better understanding of the complex role played by JAK-STAT3 in the regulation of cell movement, invasion, and metastasis provides opportunities to suppress this lethal aspect of cancer progression by not only targeting the JAK and STAT3 proteins directly, but also some of the downstream effectors of JAK-STAT3 signaling.
Keywords: STAT3, invasion, metastasis, motility, cancer, JAK2, WASF3
Introduction
Cancer mortality is largely due to metastatic spread of the primary tumor.1 A wide variety of genes and micro-RNAs (miRNA) appear to be implicated in the metastasis process,2-5 in many cases independently of the dysregulation of genes leading to uncontrolled cell proliferation.6 It is becoming clear, however, that despite the plethora of reports implicating single genes in metastasis, there may be a few master regulators that coordinate the process. Activation of the JAK-STAT3 pathway, in particular, has been repeatedly correlated with increased invasion and metastasis in a wide variety of cancer types7 although, until recently, there have been few mechanistic insights into how this occurs. Identification of key proteins is required for the transduction of the JAK-STAT3 signal specifically to promote invasion, however, brings many disparate observations together and begins to define critical nodes in the overall signaling process that might be exploited to suppress metastasis in a clinical setting. In this article we review the emerging evidence that specifically implicates JAK-STAT3 signaling in cell movement and invasion and investigate some of the complex regulatory relationships between other transcription factors and miRNA in this process. We now describe how activation of JAK-STAT3 signaling leads to upregulation of other key proteins that are primarily involved in orchestrating cell movement and invasion and which may prove to be targets for suppression of invasion and metastasis.
The Invasion and Metastasis Process
Not all epithelial cancer cells are capable of expressing the metastatic phenotype, and those that do must be able to escape the constraints of the primary tumor and enter the circulatory system (blood or lymphatic). This process requires production of enzymes that can break down basement membranes to allow invasion, which is a prerequisite for metastasis. Invasion also depends on reorganization of the actin cytoskeleton to facilitate migration/invasion. The ability of these cancer cells to leave the tumor mass depends on losing cell-cell contact early in the process, which has been associated with a change in cell shape referred to as the epithelial-to-mesenchyme transition (EMT). EMT8 is associated with the loss of cell adhesion proteins such as E-cadherin.9 Once in the circulatory system, the isolated cancer cell must survive before eventually exiting at a distant site to establish as a micro-colony. The mechanics of this process have been reviewed extensively elsewhere.10 Genes that enhance all of these individual capabilities are considered metastasis promoting, and must be activated in concert for metastasis to be achieved.2-5 This process requires responses to external signaling cues from growth factors and cytokines, which are transmitted through extracellular receptors via adaptor protein complexes to regulate gene expression by transcription factors. Within this continuum, the same control pathways also regulate the ability of cells to move (motility) and migrate by regulating actin dynamics, and invade and metastasize by re-orchestrating gene expression. While strictly speaking, analysis of the metastatic phenotype ultimately requires an in vivo model, there are a number of in vitro phenotypes that have been commonly used to predict metastasis with remarkable accuracy and are broken down into motility, migration, and invasion. In this review we accept these phenotypes as part of the overall process in support of the role of JAK-STAT3 signaling in metastasis.
The STAT Family of Genes
Of the seven STAT family members (STAT1, 2, 3, 4, 5A, 5B, and 6),11 STAT2 is largely involved with viral infections12 and STATs 4 and 6 are primarily involved in lymphocyte function,13 particularly associated with helper T cells.11 While there are reports that STAT5A/B can promote invasion and metastasis,14,15 it now appears that STAT5, at least in prostate cancer, may be more involved in promoting growth and preventing apoptosis.16 STAT1 and STAT3 have been implicated in cancer progression but with opposite roles. STAT1 appears to promote anti-proliferative and pro-apoptotic responses in cancer cells17 and, while STAT3 can clearly promote proliferation, in contrast to the other family members, it has been consistently implicated in promoting cell migration and invasion17,18 as well as metastasis16 in many cancer cell types and is, therefore, the focus of this review.
Involvement of JAK-STAT3 Signaling in Cellular Phenotypes Associated with Metastasis
In normal cells, STAT3 signaling is under tight spatial and temporal control as a result of negative feedback mechanisms involving the suppressors of cytokine signaling (SOCS), in particular SOCS3, and tyrosine phosphatases but, in cancer cells, constitutive activation of STAT3 is ubiquitous.7 STAT3 activation requires phosphorylation (typically at tyrosine 705) and this can be achieved directly by growth factor receptors that have inherent tyrosine kinase activity, such as EGFR, ERBB2, VEGFR, and PDGFR, in response to growth factor stimulation.7 These receptors may also recruit non-receptor kinases such as SRC and ABL which can also activate STAT3. Constitutive activation of these receptors by either their overexpression or as a result of mutation, has been associated with increased invasion and metastasis in many cancer cell types.19-22 STAT3 can also be activated by members of the interleukin family of cytokines,7 but since cytokine receptors do not have intrinsic tyrosine kinase activity, they must recruit kinases such as members of JAKs. JAKs phosphorylate tyrosine residues on the STAT clients facilitating STAT3 activation, which is required for its relocation to the nucleus, leading to transcription of the target genes.
The constitutive activation of STATs in cancer cells is usually facilitated through excessive stimulation by cytokines or growth factors, which may be produced by the tumors themselves (autocrine stimulation) or be derived from either stromal or inflammatory cells associated with tumor development (paracrine stimulation), which has led to the notion that the microenvironment in the metastatic niche is important for successful metastasis.23 STAT3 can also stimulate its own activation by regulating genes (e.g., IL-6, IL-10, and EGF) that promote its increased activation or are themselves direct STAT3 activators (e.g., RAS, SRC, and ABL). Thus, paracrine production of IL-6 can promote autocrine STAT3 signaling.24 In experimental systems, JAK-STAT3 signaling can be conveniently activated using IL-6, which has been shown to increase migration and invasion in vitro25-27 and metastasis in vivo.24 Consistently, downregulation of cytokines and their receptors in different cell systems is associated with a less aggressive phenotype.28
Phosphoactivation of STAT3 by JAKs is required for the dimerization that is essential for STAT3 to function as a transcription factor.7 Intuitively, therefore, it might be expected that STAT3 promotes invasion and metastasis as a consequence of regulating genes involved in this process. Indeed, STAT3 is known to regulate expression of essential components of the metastasis continuum, such as the matrix metalloproteinases (MMP) that degrade the basement membranes and extracellular matrix.29 Most aggressive cancer cells overexpress MMPs which facilitate intravasation into the vasculature and extravasation at the metastatic site. Other targets of the STAT3 transcription factor are also intimately involved in the invasion and metastasis process. It has been consistently observed, for example, that HSP70 and HSP90 promote invasion and metastasis30-33 and both are regulated by STAT3.23 STAT3 also regulates HIF1a, which induces many other genes related to invasion and metastasis such as VEGFR.23 Thus, activation of STAT3 has a broad impact on the expression of genes that promote invasion.
The idea that the pro-metastatic effects of STAT3 signaling results from upregulation of metastasis genes, however, may be too simplistic, since there are examples where, even in the presence of constitutive STAT3 signaling and IL-6 responsiveness, inactivation of other genes can still suppress invasion.26 Furthermore, the fact that overexpression of these metastasis promoting genes in cells that normally do not express STAT3 can promote invasion, argues for a STAT3-independent mechanism.26 We have recently demonstrated, for example, that while the expression of the WASF3 pro-metastasis gene is regulated by STAT3, its ability to regulate invasion depends on its direct phosphoactivation by JAK2, independently of STAT3 activation.26
JAK-STAT3 Regulation of the WASF3 Metastasis Promoter Gene
Early evidence implicating JAK-STAT3 signaling in the invasion/metastasis process was largely correlative, with increased STAT3 expression seen in advanced stage tumors,34,35 at the leading edges of invasive cancers24 and associated with survival.36,37 There were, however, few mechanistic insights into the underlying basis of this effect from these studies. For cancer cells to escape the restraints of the parental tumor, they must become able to dissociate from the surrounding cells. Epithelial cells maintain cell contact though adhesion molecules such as the claudins38 and E-cadherin.9 EMT is characterized by loss of E-cadherin expression39 and aggressive subtypes of cancer have low claudin expression.40 The relevance of STAT3 to this observation came with the demonstration that E-cadherin expression was downregulated by transcription factors such as ZEB, Twist, and Snail, which are activated by STAT3.41,42 Losing cell–cell contact by cancer cells, however, is only one component in successfully acquiring the invasive phenotype, since these cells must also be able to reorganize their actin cytoskeleton to facilitate structural changes that are essential for cell migration and invasion.43 Among a variety of genes involved in actin cytoskeleton reorganization, WASF3 in particular, has been shown to be associated with invasion and metastasis44 and is upregulated in high-grade tumors.45
WASF3 is a member of the Wiskott–Aldrich syndrome family of proteins (WASF),46 which are involved in regulating actin cytoskeleton dynamics47 through recruitment of the ARP2/3 complex48,49 which promotes actin polymerization. As a result, increased production of membrane structures such as lamellipodia occurs,44 which is associated with cell motility, migration and invasion.50 Knockdown of WASF3 in a variety of cell types in vitro, leads to loss of invasion50,51 and, where it has been examined, metastasis in vivo.50,52 This loss of invasion and metastasis is independent of the genetic background (e.g., mutant p53, mutant RAS, etc.) of the cancer cells and, whether or not they express STAT3. Overexpression of WASF3 in non-invasive cancer cells enhances invasion.53 Inactivation of WASF3 in cells that constitutively express activated STAT3, however, still leads to suppression of invasion and metastasis, demonstrating that the activation of the STAT3-regulated genes described above is not sufficient to maintain the metastatic phenotype.53 Several studies demonstrate that increasing WASF3 levels leads to increased invasion,53,54 and IL-6 treatment of cancer cells leads to increased expression levels and increased activation levels of WASF3.53 STAT3 was shown to bind to elements in the WASF3 promoter, upregulating its expression in response to IL-6 treatment,53 which led to increased invasion potential. To promote invasion, WASF3 must be phosphoactivated, which can be achieved by different kinases such as PI3K,44 ABL,55 and JAK2.53 When JAK2 is deficient in these cells, however, migration is suppressed demonstrating that JAK2-dependent activation of WASF3 is required for increased migration and by inference, invasion, and metastasis, as we have shown in zebrafish models of human cancer cell metastasis.56 As a consequence of loss of JAK2, WASF3 levels are further reduced because of the concomitant loss of activated STAT3. Thus, activation of WASF3 by JAK2 is the critical event promoting migration in these and other cell types, with the coincident activation of STAT3 leading to increased WASF3 protein levels (Fig. 1). In the study of WASF3 activation, JAK2 was shown to be the critical activating kinase, even though JAK1 was also identified in the WASF3 immunocomplex.53 JAK1 is a major activator of STAT3 in many cell systems7 and, in the highly metastatic U2A fibrosarcoma cells, was required for invasion,57,58 with loss of JAK1 expression suppressing metastasis to a lesser extent.56 It appears, therefore, that JAK1/2 may have the same role in promoting invasion but possibly in a cell context-dependent manner.
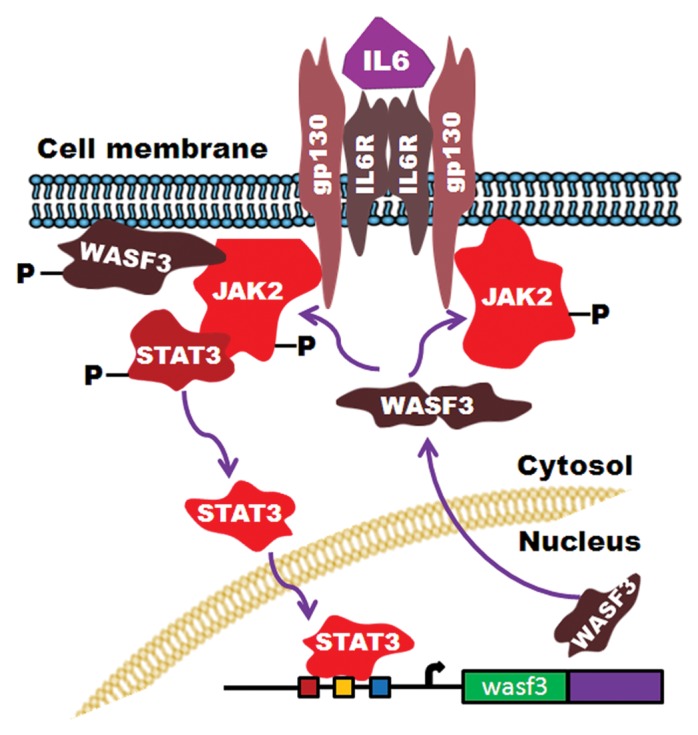
Figure 1. Summary of the IL-6/JAK-STAT interaction with the WASF3 gene. Activation of STAT3 leads to increased expression of WASF3 which is then recruited to the membrane where it is activated by JAK2 to promote invasion.
The ability of IL-6 to promote invasion and metastasis, therefore, is clearly related to its promotion of WASF3 activation, since knockdown of WASF3 in many cell types suppresses invasion despite their constitutive expression of STAT3 and JAK1/2.44,50,51 This JAK2-dependent promotion of invasion by WASF3 appears to be due to its ability to influence expression of the KISS1 metastasis suppressor gene.59 KISS1, through its receptor GPR54, regulates the function of the IκBα repressor of NFκB, which normally sequesters the p65 subunit of NFκB in the cytoplasm.60 High WASF3 levels lead to downregulation of KISS1,59 which in turn leads to phosphorylation-mediated degradation of IκBα, allowing NFκB to move into the nucleus and activate pro-metastasis genes such as MMPs and IL-6.59,60 NFκB also regulates ZEB1 expression directly,61 which promotes EMT in part through downregulation of E-cadherin. ZEB1 also suppresses expression of a number of different microRNAs which have also been associated with metastasis, notably the miRNA200 family62,63 which can regulate WASF3 mRNA stability.64 Increased ZEB1 activity, therefore, as a result of increased NFκB activity following WASF3 overexpression, suppresses expression of miRNA200 family members, thus creating a regulatory loop to increase WASF3 levels further.61 These observations demonstrate the complexity of the JAK2-STAT3 influence on invasion.
STAT3 also has indirect effects on WASF3-promotion of invasion. We recently showed that WASF3 is stabilized by HSP70,54 and suppression of HSP70 function led to reduced invasion in a WASF3-dependent manner. In addition, while WASF3 is not an HSP90 client protein, it is dependent on HSP90 for its activation since ABL kinase is a client of HSP90, and ABL can activate WASF3. This loss of HSP90 prevents WASF3 activation leading to reduced invasion.54 Since both HSP70 and HSP90 are regulated by STAT3,23 these proteins further impact on the ability of WASF3 to control cell invasion.
MicroRNA Deregulation Promotes Cancer Progression and Motility through Regulation of JAK-STAT3
MiRNAs are non-coding RNAs that target mRNAs for decay or interfere with translation, usually through binding to the 3′ untranslated region of their targets.65 Generally, miRNAs are abnormally expressed and hyper-activated in tumors, exerting oncogenic effects on multiple pathways causing increased proliferation and reduced apoptosis. There is, however, a subset of miRNAs that are directly associated with metastasis and invasion which are referred to as the “metastamirs”.66 These miRNAs regulate the function of genes important in metastasis, and individual miRNAs can target many different genes simultaneously. The interaction between STAT3 and various miRNAs involves several regulatory feedback loops associated with invasion67 where either STAT3 drives expression of miRNAs that promote invasion68,69 or where miRNAs suppress the function of STAT3 inhibitors such as SOCS and PIAS.70,71 MiRNAs can also bind directly to the 3′UTR of STAT3 to regulate its activity,72 thereby suppressing invasion and metastasis.73 As expected, downregulation of JAK2 by miRNAs has the same effect, since they lead to decreased levels of activated STAT3 which affects proliferation74 and, presumably, invasion.
STAT3-NFκB Interactions
Extracellular cues transmit signals to regulatory hubs within the cancer cell that influence gene expression related to cell movement and invasion. These signals are typically not linear events and considerable cross talk between regulatory genes is involved in the complex regulation of phenotypes such as invasion. From the WASF3 studies, for example, IL-6 treatment produced a coordinated activation of NFκB through upregulation and activation of WASF3 by JAK2-STAT3. Chromatin binding studies demonstrate a significant overlap between the genes regulated by these two transcription factors, and it has been shown that they co-regulate a sub set of genes associated with invasion.7 Cooperation between NFκB and STAT3 to promote invasion has also been demonstrated at a number of levels.75 For example, STAT3 and NFκB both regulate the expression of proinvasive genes23 including cytokines, and they are also activated by the cytokines they regulate.73 Both are these transcription factors are also centrally involved with inflammation,75 which also promotes tumor progression. At a mechanistic level, STAT3 can bind directly to the NFκB p65 subunit, sequestering it in the nucleus and leading to prolonged NFκB activation.61,76,77 In our studies, we demonstrated that WASF3 can increase NFκB activity by releasing it from KISS1-mediated IκBα repression, creating a feed forward loop promoting motility and invasion through activation of IL-6-STAT3, which further increases WASF3 expression levels.
Targeting the JAK-STAT3 Pathway to Suppress Invasion and Metastasis
The clear role of JAK-STAT3 signaling in promoting invasion and metastasis opens the possibility of targeting these proteins, and possibly associated pathways, to control these phenotypes. Genetic inactivation of STAT3 and JAK2 in model cell systems leads to a reduction in motility and invasion (see above), suggesting that pharmacological targeting of these proteins might have the same effect.78 Increasingly, JAK-STAT3 targeting drugs are being developed, as well as other pharmacological agents that affect genes that impact on JAK-STAT3 activation.18,78 For example, using antibodies that target either IL-6, or the soluble or membrane bound IL-6 receptors, STAT signaling can be downregulated, and these agents are currently in clinical trials. Direct targeting of JAK1 and JAK2 using agents such as AG490 have also been shown to suppress invasion of cancer cells in vitro,78 and knockdown of these kinases suppresses metastasis in animal models.24 Similarly, pharmacologically targeting STAT3 directly leads to suppression of invasion.78 As the signaling pathways become more defined, it is likely that other JAK-STAT3 nodes will be identified that may be equally important targets for a more complete blockade of metastasis.
Conclusion
JAK-STAT3 signaling is clearly a central regulatory hub in the overall process leading to invasion and metastasis. It is becoming clear, however, that its role in this process is made more complex through the diversity of interactions with other signaling pathways. STAT3 also has many other functions beyond metastasis and, in considering targeting JAK-STAT3 as a treatment to suppress invasion and metastasis, a better understanding of the metastasis-specific interactions will be required to provide specificity and avoid unfortunate side effects that might be related to its other functions. In this case, defining proteins that are dependent on JAK-STAT3 function, but which are more specific for the metastasis phenotype, may present better targets. It is likely that teasing out the specific role of STAT3 in the development of metastasis, however, will depend on defining the consequences of interactions with other regulatory molecules that may complicate the analysis if they operate in a cell specific and/or temporal-specific context.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- ABL
Abelson leukemia virus
- ARP
actin related protein
- ERBB
erythroblastic leukemia viral oncogene, family member B
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial to mesenchyme transition
- HIF
hypoxia inducible factor
- HSP
heat shock protein
- IκBα
inhibitor of NFκB
- IL
interleukin
- JAK
Janus kinase
- KISS
kisspeptin
- MMP
matrix metalloproteinase
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PDGFR
platelet derived growth factor receptor
- PIAS
protein inhibitor of activated STAT
- RAS
Rat sarcoma gene
- SOCS
suppressor of cytokine signaling
- SRC
sarcoma kinase
- STAT
signal transducer and activator of transcription
- VEGFR
vascular endothelial growth factor receptor
- WASF
Wiskott Aldrich syndrome family
- ZEB
zinc finger homeodomain enhancer-binding protein
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/28086
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Pencheva N, Tavazoie SF. Control of metastatic progression by microRNA regulatory networks. Nat Cell Biol. 2013;15:546–54. doi: 10.1038/ncb2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perlikos F, Harrington KJ, Syrigos KN. Key molecular mechanisms in lung cancer invasion and metastasis: a comprehensive review. Crit Rev Oncol Hematol. 2013;87:1–11. doi: 10.1016/j.critrevonc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Trapé AP, Gonzalez-Angulo AM. Breast cancer and metastasis: on the way toward individualized therapy. Cancer Genomics Proteomics. 2012;9:297–310. [PubMed] [Google Scholar]
- 5.Roodman GD. Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Rev. 2012;31:569–78. doi: 10.1007/s10555-012-9372-x. [DOI] [PubMed] [Google Scholar]
- 6.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 8.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–5. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury FZ, Farrar JD. STAT2: A shape-shifting anti-viral super STAT. JAKSTAT. 2013;2:e23633. doi: 10.4161/jkst.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene. 2000;19:2577–84. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- 14.Moser C, Ruemmele P, Gehmert S, Schenk H, Kreutz MP, Mycielska ME, Hackl C, Kroemer A, Schnitzbauer AA, Stoeltzing O, et al. STAT5b as molecular target in pancreatic cancer--inhibition of tumor growth, angiogenesis, and metastases. Neoplasia. 2012;14:915–25. doi: 10.1593/neo.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu L, Vogiatzi P, Puhr M, Dagvadorj A, Lutz J, Ryder A, Addya S, Fortina P, Cooper C, Leiby B, et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr Relat Cancer. 2010;17:481–93. doi: 10.1677/ERC-09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Addya S, Fortina P, Dasgupta A, Hyslop T, et al. Transcription factor Stat3 stimulates metastatic behavior of human prostate cancer cells in vivo, whereas Stat5b has a preferential role in the promotion of prostate cancer cell viability and tumor growth. Am J Pathol. 2010;176:1959–72. doi: 10.2353/ajpath.2010.090653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avalle L, Pensa S, Regis G, Novelli F, Poli V. STAT1 and STAT3 in tumorigenesis: A matter of balance. JAKSTAT. 2012;1:65–72. doi: 10.4161/jkst.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devarajan E, Huang S. STAT3 as a central regulator of tumor metastases. Curr Mol Med. 2009;9:626–33. doi: 10.2174/156652409788488720. [DOI] [PubMed] [Google Scholar]
- 19.Chuang TC, Hsu SC, Cheng YT, Shao WS, Wu K, Fang GS, Ou CC, Wang V. Magnolol down-regulates HER2 gene expression, leading to inhibition of HER2-mediated metastatic potential in ovarian cancer cells. Cancer Lett. 2011;311:11–9. doi: 10.1016/j.canlet.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Johnson E, Seachrist DD, DeLeon-Rodriguez CM, Lozada KL, Miedler J, Abdul-Karim FW, Keri RA. HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42. J Biol Chem. 2010;285:29491–501. doi: 10.1074/jbc.M110.136770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jechlinger M, Sommer A, Moriggl R, Seither P, Kraut N, Capodiecci P, Donovan M, Cordon-Cardo C, Beug H, Grünert S. Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest. 2006;116:1561–70. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue P, Zhang X, Paladino D, Sengupta B, Ahmad S, Holloway RW, Ingersoll SB, Turkson J. Hyperactive EGF receptor, Jaks and Stat3 signaling promote enhanced colony-forming ability, motility and migration of cisplatin-resistant ovarian cancer cells. Oncogene. 2012;31:2309–22. doi: 10.1038/onc.2011.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al. The IL-6/JAK-STAT3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–62. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Li G, Li R, Shen J, He Q, Deng L, Zhang C, Zhang J. IL-6 promotion of glioblastoma cell invasion and angiogenesis in U251 and T98G cell lines. J Neurooncol. 2010;100:165–76. doi: 10.1007/s11060-010-0158-0. [DOI] [PubMed] [Google Scholar]
- 26.Teng Y, Ghoshal P, Ngoka L, Mei Y, Cowell JK. Critical role of the WASF3 gene in JAK2/STAT3 regulation of cancer cell motility. Carcinogenesis. 2013;34:1994–9. doi: 10.1093/carcin/bgt167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C, Yang G, Jiang T, Huang K, Cao J, Qiu Z. Effects of IL-6 and AG490 on regulation of Stat3 signaling pathway and invasion of human pancreatic cancer cells in vitro. J Exp Clin Cancer Res. 2010;29:51. doi: 10.1186/1756-9966-29-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK-STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406–17. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 29.Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013;34:2041–51. doi: 10.1007/s13277-013-0842-8. [DOI] [PubMed] [Google Scholar]
- 30.Taiyab A, Rao ChM. HSP90 modulates actin dynamics: inhibition of HSP90 leads to decreased cell motility and impairs invasion. Biochim Biophys Acta. 2011;1813:213–21. doi: 10.1016/j.bbamcr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Garg M, Kanojia D, Saini S, Suri S, Gupta A, Surolia A, Suri A. Germ cell-specific heat shock protein 70-2 is expressed in cervical carcinoma and is involved in the growth, migration, and invasion of cervical cells. Cancer. 2010;116:3785–96. doi: 10.1002/cncr.25218. [DOI] [PubMed] [Google Scholar]
- 32.Garg M, Kanojia D, Seth A, Kumar R, Gupta A, Surolia A, Suri A. Heat shock protein 70-2 (HSP70-2) expression in bladder urothelial carcinoma is associated with tumour progression and promotes migration and invasion. Eur J Cancer. 2010;46:207–15. doi: 10.1016/j.ejca.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Yan Z, Huang L, Guo M, Zhang Z, Guo C. Cell surface heat shock protein 90 modulates prostate cancer cell adhesion and invasion through the integrin-β1/focal adhesion kinase/c-Src signaling pathway. Oncol Rep. 2011;25:1343–51. doi: 10.3892/or.2011.1202. [DOI] [PubMed] [Google Scholar]
- 34.Jiang R, Jin Z, Liu Z, Sun L, Wang L, Li K. Correlation of activated STAT3 expression with clinicopathologic features in lung adenocarcinoma and squamous cell carcinoma. Mol Diagn Ther. 2011;15:347–52. doi: 10.1007/BF03256470. [DOI] [PubMed] [Google Scholar]
- 35.Lassmann S, Schuster I, Walch A, Göbel H, Jütting U, Makowiec F, Hopt U, Werner M. STAT3 mRNA and protein expression in colorectal cancer: effects on STAT3-inducible targets linked to cell survival and proliferation. J Clin Pathol. 2007;60:173–9. doi: 10.1136/jcp.2005.035113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J Gastroenterol. 2010;16:5380–7. doi: 10.3748/wjg.v16.i42.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takemoto S, Ushijima K, Kawano K, Yamaguchi T, Terada A, Fujiyoshi N, Nishio S, Tsuda N, Ijichi M, Kakuma T, et al. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br J Cancer. 2009;101:967–72. doi: 10.1038/sj.bjc.6605212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turksen K, Troy TC. Junctions gone bad: claudins and loss of the barrier in cancer. Biochim Biophys Acta. 2011;1816:73–9. doi: 10.1016/j.bbcan.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong H, Hong J, Du W, Lin YW, Ren LL, Wang YC, Su WY, Wang JL, Cui Y, Wang ZH, et al. Roles of STAT3 and ZEB1 proteins in E-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem. 2012;287:5819–32. doi: 10.1074/jbc.M111.295964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2012;22:90–106. doi: 10.1038/cr.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24:277–83. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sossey-Alaoui K, Li X, Ranalli TA, Cowell JK. WAVE-3 mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:21748–55. doi: 10.1074/jbc.M500503200. [DOI] [PubMed] [Google Scholar]
- 45.Kulkarni S, Augoff K, Rivera L, McCue B, Khoury T, Groman A, Zhang L, Tian L, Sossey-Alaoui K. Increased expression levels of WAVE3 are associated with the progression and metastasis of triple negative breast cancer. PLoS One. 2012;7:e42895. doi: 10.1371/journal.pone.0042895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sossey-Alaoui K, Su G, Malaj E, Roe B, Cowell JK. WAVE3, an actin-polymerization gene, is truncated and inactivated as a result of a constitutional t(1;13)(q21;q12) chromosome translocation in a patient with ganglioneuroblastoma. Oncogene. 2002;21:5967–74. doi: 10.1038/sj.onc.1205734. [DOI] [PubMed] [Google Scholar]
- 47.Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J Cell Sci. 2001;114:1801–9. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- 48.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 49.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 50.Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, Cowell JK. WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res. 2005;308:135–45. doi: 10.1016/j.yexcr.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Teng Y, Ren MQ, Cheney R, Sharma S, Cowell JK. Inactivation of the WASF3 gene in prostate cancer cells leads to suppression of tumorigenicity and metastases. Br J Cancer. 2010;103:1066–75. doi: 10.1038/sj.bjc.6605850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sossey-Alaoui K, Safina A, Li X, Vaughan MM, Hicks DG, Bakin AV, Cowell JK. Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am J Pathol. 2007;170:2112–21. doi: 10.2353/ajpath.2007.060975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teng Y, Ghoshal P, Ngoka L, Mei Y, Cowell JK. Critical role of the WASF3 gene in JAK2/STAT3 regulation of cancer cell motility. Carcinogenesis. 2013;34:1994–9. doi: 10.1093/carcin/bgt167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghoshal P, Teng Y, Lesoon LA, Cowell JK. HIF1A induces expression of the WASF3 metastasis-associated gene under hypoxic conditions. Int J Cancer. 2012;131:E905–15. doi: 10.1002/ijc.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sossey-Alaoui K, Li X, Cowell JK. c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J Biol Chem. 2007;282:26257–65. doi: 10.1074/jbc.M701484200. [DOI] [PubMed] [Google Scholar]
- 56.Teng Y, Xie X, Walker S, White DT, Mumm JS, Cowell JK. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer. 2013;13:453. doi: 10.1186/1471-2407-13-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burfoot MS, Rogers NC, Watling D, Smith JM, Pons S, Paonessaw G, Pellegrini S, White MF, Kerr IM. Janus kinase-dependent activation of insulin receptor substrate 1 in response to interleukin-4, oncostatin M, and the interferons. J Biol Chem. 1997;272:24183–90. doi: 10.1074/jbc.272.39.24183. [DOI] [PubMed] [Google Scholar]
- 58.Kohlhuber F, Rogers NC, Watling D, Feng J, Guschin D, Briscoe J, Witthuhn BA, Kotenko SV, Pestka S, Stark GR, et al. A JAK1/JAK2 chimera can sustain alpha and gamma interferon responses. Mol Cell Biol. 1997;17:695–706. doi: 10.1128/mcb.17.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teng Y, Liu M, Cowell JK. Functional interrelationship between the WASF3 and KISS1 metastasis-associated genes in breast cancer cells. Int J Cancer. 2011;129:2825–35. doi: 10.1002/ijc.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho SG, Li D, Stafford LJ, Luo J, Rodriguez-Villanueva M, Wang Y, Liu M. KiSS1 suppresses TNFalpha-induced breast cancer cell invasion via an inhibition of RhoA-mediated NF-kappaB activation. J Cell Biochem. 2009;107:1139–49. doi: 10.1002/jcb.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teng Y, Mei Y, Hawthorn L, Cowell JK. WASF3 regulates miR-200 inactivation by ZEB1 through suppression of KISS1 leading to increased invasiveness in breast cancer cells. Oncogene. 2014;33:203–11. doi: 10.1038/onc.2012.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–54. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 63.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 64.Sossey-Alaoui K, Bialkowska K, Plow EF. The miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasion. J Biol Chem. 2009;284:33019–29. doi: 10.1074/jbc.M109.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hurst DR, Edmonds MD, Welch DR. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res. 2009;69:7495–8. doi: 10.1158/0008-5472.CAN-09-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cao Q, Li YY, He WF, Zhang ZZ, Zhou Q, Liu X, Shen Y, Huang TT. Interplay between microRNAs and the STAT3 signaling pathway in human cancers. Physiol Genomics. 2013;45:1206–14. doi: 10.1152/physiolgenomics.00122.2013. [DOI] [PubMed] [Google Scholar]
- 68.Lin HY, Chiang CH, Hung WC. STAT3 upregulates miR-92a to inhibit RECK expression and to promote invasiveness of lung cancer cells. Br J Cancer. 2013;109:731–8. doi: 10.1038/bjc.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–23. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collins AS, McCoy CE, Lloyd AT, O’Farrelly C, Stevenson NJ. miR-19a: an effective regulator of SOCS3 and enhancer of JAK-STAT signalling. PLoS One. 2013;8:e69090. doi: 10.1371/journal.pone.0069090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu W, Takanashi M, Borjigin N, Ohno SI, Fujita K, Hoshino S, Osaka Y, Tsuchida A, Kuroda M. MicroRNA-18a modulates STAT3 activity through negative regulation of PIAS3 during gastric adenocarcinogenesis. Br J Cancer. 2013;108:653–61. doi: 10.1038/bjc.2012.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao G, Zhang JG, Shi Y, Qin Q, Liu Y, Wang B, Tian K, Deng SC, Li X, Zhu S, et al. MiR-130b is a prognostic marker and inhibits cell proliferation and invasion in pancreatic cancer through targeting STAT3. PLoS One. 2013;8:e73803. doi: 10.1371/journal.pone.0073803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31:3513–23. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu H, Huang M, Cao P, Wang T, Shu Y, Liu P. MiR-135a targets JAK2 and inhibits gastric cancer cell proliferation. Cancer Biol Ther. 2012;13:281–8. doi: 10.4161/cbt.18943. [DOI] [PubMed] [Google Scholar]
- 75.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–93. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–9. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sansone P, Bromberg J. Targeting the interleukin-6/JAK-STAT pathway in human malignancies. J Clin Oncol. 2012;30:1005–14. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]


