Abstract
Background
Racial disparities in healthcare in the United States are widespread and have been well documented. However, it is unknown whether racial disparities exist in the use of blood transfusion for patients undergoing major surgery.
Methods
We used the University HealthSystem Consortium database (2009-2011) to examine racial disparities in perioperative red blood cells (RBCs) transfusion in patients undergoing coronary artery bypass surgery (CABG), total hip replacement (THR), and colectomy. We estimated multivariable logistic regressions to examine whether black patients are more likely than white patients to receive perioperative RBC transfusion, and to investigate potential sources of racial disparities.
Results
After adjusting for patient-level factors, black patients were more likely to receive RBC transfusions for CABG (AOR = 1.41, 95% CI: [1.13, 1.76], p = 0.002) and THR (AOR = 1.39, 95% CI: [1.20, 1.62], p < 0.001), but not for colectomy (AOR = 1.08, 95% CI: [0.90, 1.30], p = 0.40). Black-white disparities in blood transfusion persisted after controlling for patient insurance and hospital effects (CABG: AOR = 1.42, 95% CI: [1.30, 1.56], p < 0.001; THR: AOR = 1.43, 95% CI: [1.29, 1.58], p < 0.001).
Conclusions
We detected racial disparities in the use of blood transfusion for CABG and THR (black patients tended to receive more transfusions compared with whites), but not for colectomy. Reporting racial disparities in contemporary transfusion practices may help reduce potentially unnecessary blood transfusions in minority patients.
Background
Racial disparities in health and health care have been well documented in the literature. Reducing racial disparities has been consistently highlighted as a national priority. Black Americans have a 50% higher age-adjusted mortality rate than white Americans [1]. Differences in health care between races are a major contributor to this striking difference in mortality outcomes [2,3]. Compared with whites, blacks are less likely to receive invasive cardiac interventions [4,5], high-cost surgical procedures [6,7], effective preventive care [8,9], medically necessary mental health services [10,11], and new medical technologies [12,13]. By contrast, black patients have significantly higher rates of interventions suggestive of less than optimal management of chronic diseases such as bilateral orchiectomies and lower-extremity amputations [14,15]. Numerous studies have documented that blacks are more likely to obtain care from lower-quality physicians and lower-quality hospitals [3,16-23]. Despite national efforts to reduce racial disparities over the last two decades, the AHRQ-funded National Healthcare Disparities Report calls attention to the fact that racial gaps in health care access and quality are not improving [15]. Given that racial and ethnic minorities currently constitute about a third of the U.S. population and are expected to make up more than half the population in 2050 [24], improving overall health outcomes cannot be accomplished without substantially improving outcomes among non-White patients [25,26].
Recent studies have focused attention on the wide variability in transfusion practices in surgery, and the association between blood transfusion and adverse outcomes. In 2007, over 15 million units of allogeneic red blood cells (RBC) were transfused in the United States with the majority administered to patients undergoing surgery [27]. Once considered a relatively safe procedure, blood transfusion is associated with increased risk of mortality and major complications in surgical patients [28-38]. However, it remains unclear whether there are differences in the use of blood transfusion between black and white patients undergoing surgery.
To our knowledge, no study has examined whether minority patients undergoing major surgery are more likely to receive blood transfusion. The primary goal of this study was to determine whether black patients undergoing major surgery were more likely to receive perioperative RBC transfusion compared with white patients. We used national all-payers data from major academic medical centers in patients undergoing three common surgical procedures with significant transfusion rates: coronary artery bypass surgery (CABG), total hip replacement (THR), and colectomy. If black-white differences in blood transfusion were found to exist, our secondary goal was to determine whether the basis for black-white differences in blood transfusion was due [1] to higher rates of blood use in black-serving hospitals, or [2] to higher rates of blood use in black patients compared to white patients treated at the same hospital.
Methods
Data source
The source of data was the University HealthSystem Consortium (UHC) database (January 2009 - September 2011). The time frame was chosen to represent the most contemporary data available and to minimize the effect of transfusion practice change over time. The UHC is a national alliance of academic medical centers and their affiliated hospitals. The UHC database consists of administrative records for all hospitalizations in its member institutions. This represents about 90% of the nation’s hospitalizations at academic medical centers. The UHC database includes patient demographic characteristics, admission status (emergent, urgent, elective, and trauma), ICD-9-CM diagnostic codes, ICD-9-CM procedure codes, present-on-admission (POA) codes, 3 M APR-DRG severity of illness, AHRQ comorbidities [39], in-hospital death, cost, and detailed blood use information. The race information in the UHC database is comprehensive and complete. A unique strength of the UHC database is that it contains full inpatient transfusion information [40]. The UHC dataset also includes encrypted hospital identifiers. No specific patient, physician, or hospital was examined in the analysis, and the study proposal was granted exemption by the University of Rochester Research Subjects Review Board.
Study population
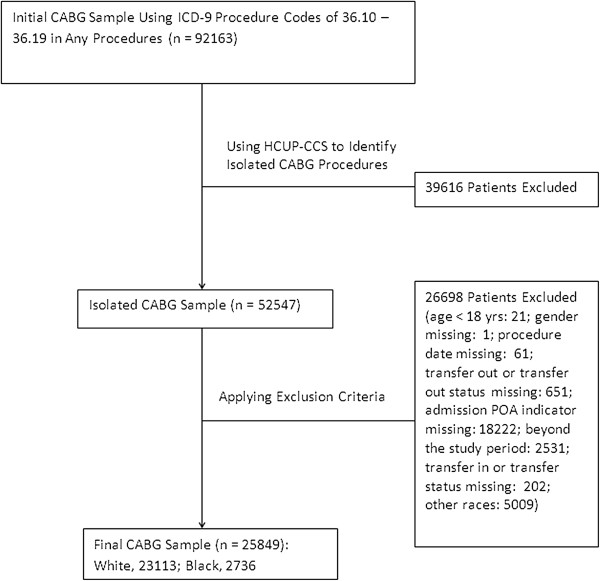
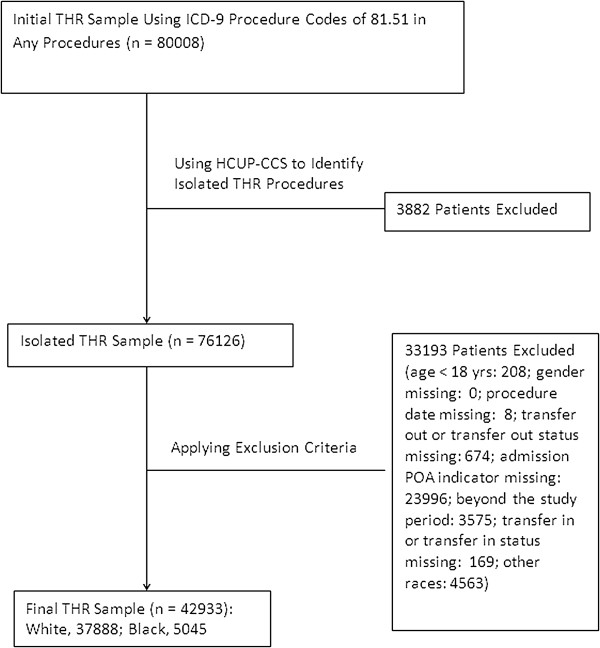
Black or white patients who received one of the following three surgical procedures were included in the analysis: isolated coronary artery bypass surgery (CABG), isolated total hip replacement (THR), or isolated colectomy. We used two steps to identify the patients who underwent isolated surgery. First, we used ICD-9-CM procedure codes to identify CABG (36.10 - 36.19), THR (81.51), and colectomy (45.73-45.76). Second, we applied the Healthcare Cost and Utilization Project (HCUP) clinical classification software (CCS) for ICD-9-CM (available at: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) to identify the patients who underwent primary isolated CABG, THR, or colectomy surgery during the hospitalization. These procedures were selected because they are commonly performed major surgical procedures representing cardiac surgery, orthopedics, and general surgery; and patients undergoing these procedures have relatively high transfusion rates. We excluded patients whose age was less than 18 years old and patients whose important demographic characteristics (e.g., gender) or important healthcare information (e.g., procedure date, transfer out status, transfer in status, admission POA indicator, and admission status) were missing. The final study sample comprised 25,849 isolated CABG patients, 42,933 isolated THR patients, and 8,255 isolated colectomy patients in 87 hospitals (see Figures 1, 2, 3).
Figure 1.
Flow chart for CABG sampling.
Figure 2.
Flow chart for THR sampling.
Figure 3.
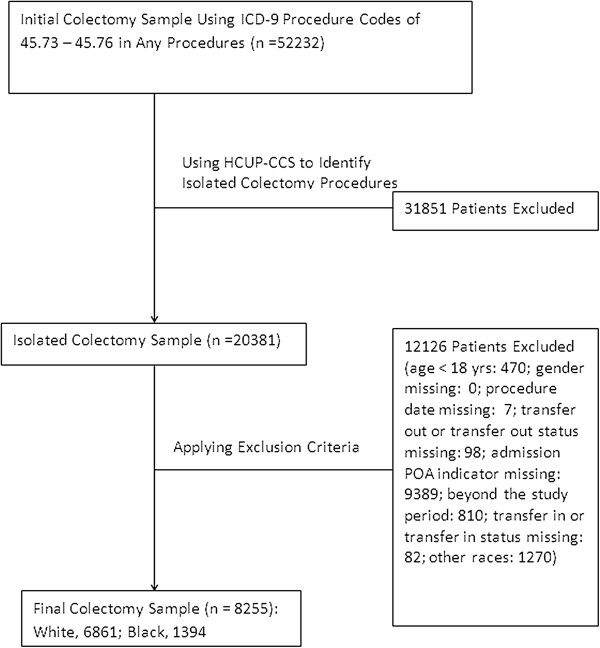
Flow chart for colectomy sampling.
Variable definition
RBC Transfusion
The UHC dataset contains usage information of perioperative (intraoperative and postoperative) allogeneic packed RBC transfusions.
Hospital concentration of black patients undergoing surgery
The hospital’s concentration of black patients undergoing surgery was grouped into quartiles: quartile 1 (≤ 8%), quartile 2 (8-13%), quartile 3 (13-27%), and quartile 4 (> 27%).
Statistical analyses
Baseline characteristics were summarized as frequency, percentage, median (inter- quartile range) as appropriate for each racial group for each surgical procedure. Comparison between racial groups (white vs. black) were made using Chi square test or Fisher exact test for categorical variables, and t test for continuous variables (see Table 1).
Table 1.
Demographic characteristics of patients undergoing isolated major surgery
| Characteristics |
CABG |
Total hip replacement |
Colectomy |
|||
|---|---|---|---|---|---|---|
|
White |
Black |
White |
Black |
White |
Black |
|
| (n = 23,113) | (n = 2,736) | (n = 37,888) | (n = 5,045) | (n = 6,861) | (n = 1,394) | |
| RBC transfused (%) |
10,284 (44.5) |
1,599 (58.4) |
10,680 (28.2) |
1,701 (33.7) |
1,341 (19.6) |
314 (22.5) |
| Age, median (IQR) |
65 (58-73) |
61 (55-69) |
63 (55-72) |
57 (49-65) |
62 (50-73) |
59 (48-68) |
| Female |
5,124 (22.2) |
1,135 (41.5) |
20,552 (54.2) |
2,738 (54.3)# |
3,403 (49.6) |
720 (51.7)# |
| Primary payer |
|
|
|
|
|
|
| Private |
8,802 (38.1) |
761 (27.8) |
17,849 (47.1) |
1,772 (35.1) |
3,082 (44.9) |
389 (27.9) |
| Medicare |
11,441 (49.5) |
1,291 (47.2) |
17,067 (45.1) |
2,035 (40.3) |
2,984 (43.5) |
540 (38.7) |
| Medicaid |
1,032 (4.5) |
330 (12.1) |
1,446 (3.8) |
798 (15.8) |
338 (4.9) |
236 (16.9) |
| Self-pay |
697 (3.0) |
164 (6.0) |
324 (0.9) |
108 (2.1) |
209 (3.1) |
105 (7.5) |
| Other |
1,120 (4.9) |
181 (6.6) |
1,163 (3.1) |
324 (6.4) |
241 (3.5) |
122 (8.8) |
| Admission status |
|
|
|
|
|
|
| Emergent |
4,749 (20.6) |
817 (29.9) |
1,099 (2.9) |
141 (2.8)# |
1,508 (22.0) |
464 (33.3) |
| Urgent |
6,715 (29.1) |
763 (27.9) |
895 (2.4) |
170 (3.4) |
702 (10.2) |
141 (10.1)# |
| Elective |
11,615 (50.3) |
1,152 (42.1) |
35,818 (94.5) |
4,725 (93.7) |
4,602 (67.1) |
763 (54.7) |
| Trauma |
6 (0.03) |
1 (0.04) |
67 (0.2) |
7 (0.2)# |
43 (0.6) |
22 (1.6) |
| Comorbidity |
|
|
|
|
|
|
| Congestive heart failure |
59 (0.3) |
14 (0.5) |
877 (2.3) |
169 (3.4) |
364 (5.3) |
101 (7.3) |
| Valve disease |
29 (0.1) |
5 (0.2)# |
1,475 (3.9) |
96 (1.9) |
326 (4.8) |
45 (3.2) |
| Pulmonary circ disease |
19 (0.1) |
3 (0.1)# |
300 (0.8) |
76 (1.5) |
104 (1.5) |
31 (2.2)# |
| Periphery vascular disease |
3,557 (15.4) |
490 (17.9) |
756 (2.0) |
96 (1.9)# |
333 (4.9) |
57 (4.1)# |
| Hypertension |
16,100 (69.7) |
1,706 (62.4) |
19,924 (52.6) |
3,250 (64.4) |
3,129 (45.6) |
818 (58.7) |
| Paralysis |
111 (0.5) |
22 (0.8) |
108 (0.3) |
19 (0.4)# |
61 (0.9) |
18 (1.3)# |
| Other neurology disorder |
715 (3.1) |
84 (3.1)# |
1,306 (3.5) |
147 (2.9) |
295 (4.3) |
49 (3.5)# |
| Chronic pulmonary disease |
4,557 (19.7) |
520 (19.0)# |
4,732 (12.5) |
806 (16.0) |
987 (14.4) |
176 (12.6)# |
| Diabetes w/o complications |
7,148 (30.9) |
1,071 (39.1) |
3,816 (10.1) |
827 (16.4) |
889 (13.0) |
285 (20.4) |
| Diabetes with complications |
1,757 (7.6) |
319 (11.7) |
417 (1.1) |
83 (1.65) |
136 (2.0) |
35 (2.5)# |
| Hypothyroid |
2,316 (10.0) |
160 (5.9) |
5,082 (13.4) |
288 (5.7) |
703 (10.3) |
64 (4.6) |
| Renal failure |
3,129 (13.5) |
711 (26.0) |
1,321 (3.5) |
357 (7.1) |
395 (5.8) |
123 (8.8) |
| Liver disease |
411 (1.8) |
58 (2.1)# |
535 (1.4) |
127 (2.5) |
170 (2.5) |
26 (1.9)# |
| Peptic ulcer |
9 (0.04) |
0 (0.0)# |
7 (0.02) |
0 (0.0)# |
3 (0.04) |
1 (0.07)# |
| AIDS |
27 (0.12) |
15 (0.6) |
88 (0.2) |
69 (1.4) |
8 (0.1) |
7 (0.5) |
| Lymphoma |
122 (0.5) |
11 (0.4)# |
240 (0.6) |
34 (0.7)# |
47 (0.7) |
7 (0.5)# |
| Metastatic cancer |
50 (0.2) |
3 (0.1)# |
225 (0.6) |
21 (0.4)# |
962 (14.0) |
208 (14.9)# |
| Tumor w/o metastasis |
328 (1.4) |
44 (1.6)# |
263 (0.7) |
23 (0.5) |
205 (3.0) |
43 (3.1)# |
| Rheumatoid arthritis |
478 (2.1) |
66 (2.4)# |
1,460 (3.9) |
335 (6.6) |
179 (2.6) |
31 (2.2)# |
| Coagulopathy* |
121 (0.5) |
12 (0.4)# |
183 (0.5) |
14 (0.3) |
32 (0.5) |
4 (0.3)# |
| Obese |
4,564 (19.8) |
566 (20.7)# |
4,955 (13.1) |
864 (17.1) |
696 (10.1) |
178 (12.8) |
| Weight loss |
204 (0.9) |
34 (1.2)# |
110 (0.3) |
26 (0.5) |
317 (4.6) |
91 (6.5) |
| Fluid electrolyte disorder |
1,112 (4.8) |
195 (7.1) |
718 (1.9) |
108 (2.1)# |
467 (6.8) |
149 (10.7) |
| Blood loss anemia |
48 (0.2) |
14 (0.5) |
62 (0.2) |
9 (0.2)# |
93 (1.4) |
47 (3.4) |
| Deficiency anemia |
1,880 (8.1) |
476 (17.4) |
1,913 (5.1) |
423 (8.4) |
934 (13.6) |
262 (18.8) |
| Alcohol abuse |
804 (3.5) |
135 (4.9) |
693 (1.8) |
137 (2.7) |
167 (2.4) |
66 (4.7) |
| Drug abuse |
296 (1.3) |
161 (5.9) |
334 (0.9) |
170 (3.4) |
85 (1.2) |
58 (4.2) |
| Psychoses |
374 (1.6) |
49 (1.8)# |
657 (1.7) |
119 (2.4) |
154 (2.2) |
49 (3.5) |
| Depression | 1,906 (8.3) | 149 (5.5) | 4,660 (12.3) | 394 (7.8) | 628 (9.2) | 68 (4.9) |
Unless otherwise indicated, results are presented as number of patients and percentages.
Abbreviations: IQR inter-quartile range, AIDS acquired immune deficiency syndrome. All p values < 0.05 except marked with #.
*This refers to the newly defined coagulopathy used in the analysis, which recoded ICD-9 codes of 286.6, 286.7, 286.9, 287.4, and 287.5 as 0 for this comorbidity variable. The reason for the recoding is that the coagulopathy codes could be complications related to blood transfusion.
The outcome variable was whether a patient received any perioperative RBC transfusion. A patient-level binary variable was coded as 1 if the patient received RBC transfusion perioperatively and as 0 otherwise. The primary independent variable of interest was race (white vs. black). The unit of analysis in the study was the patient.
To examine racial disparities in blood transfusion, a series of multivariable logistic regression models for each surgical procedure were fit to the patient-level data in a sequential manner. The baseline model (model 1) examined the association between race and transfusion, controlling for patient demographics (age and gender), admission status, APR-DRG severity of illness, POA status, and AHRQ comorbidities (including anemia). To determine whether black-white differences in transfusion persisted within payer groups, we interacted race and payer status (model 2).
In order to examine whether the source of black-white differences in blood transfusion was due to between-hospital or within-hospital effects, we estimated two additional models. In the first model, we added dummy variables representing the hospital quartile of black patient concentration to the baseline model (model 3). In the second model, we added hospital fixed effects to the baseline model (model 4). Robust variance estimators were used because patient outcomes within the same hospital may be correlated [41]. Data management, analyses, and regression diagnostics were performed using Stata MP version 11.0 (StataCorp, College Station, TX). All statistical tests were 2-sided and P < 0.05 was considered significant.
Results
In comparison with whites, black patients were younger, less well-insured, had more co-morbidities (Table 1). Black patients were more likely to receive perioperative RBC transfusions for two of the three procedures examined. For isolated CABG, 58.4% of black patients received one or more units of red blood cells, compared to 44.5% for white patients (CABG: OR = 1.75, 95% CI: [1.42, 2.17], p < 0.001). For isolated THR, 33.7% of black patients received RBC transfusion, compared to 28.2% for white patients (THR: OR = 1.30, 95% CI: [1.11, 1.51], p = 0.001). For isolated colectomy, 22.5% of black patients received one or more units of RBCs, compared to 19.6% for white patients (Colectomy: OR = 1.20, 95% CI: [0.99, 1.44], p = 0.053) (Table 2).
Table 2.
Risk-adjusted RBC transfusion in major surgery as a function of race
|
Major surgery |
Unadjusted |
Baseline
a
|
Within Hospitals
b
|
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | AOR (95% CI) | P value | AOR (95% CI) | P value | |
| CABG |
|
|
|
|
|
|
| Whites |
(Reference) |
|
(Reference) |
|
(Reference) |
|
| Blacks |
1.75 (1.42-2.17) |
<0.001 |
1.41 (1.13-1.76) |
0.002 |
1.42 (1.30-1.56) |
<0.001 |
| THR |
|
|
|
|
|
|
| Whites |
(Reference) |
|
(Reference) |
|
(Reference) |
|
| Blacks |
1.30 (1.11-1.51) |
0.001 |
1.39 (1.20-1.62) |
<0.001 |
1.43 (1.29-1.58) |
<0.001 |
| Colectomy |
|
|
|
|
|
|
| Whites |
(Reference) |
|
(Reference) |
|
(Reference) |
|
| Blacks | 1.20 (0.997-1.44) | 0.053 | 1.08 (0.90-1.30) | 0.40 | 1.01 (0.85-1.19) | 0.92 |
OR: odds ratio, AOR: adjusted odds ratio, CI: confidence interval, CABG: coronary artery bypass surgery, THR: total hip replacement.
aIndicates patient level risk adjustment model (age, gender, admission status, APR-DRG severity of illness, and AHRQ comorbidities).
bIndicates within-hospital risk adjustment model using hospital fixed effects model (all patient risk factors above plus hospital fixed effects).
After adjusting for patient level covariates, black patients undergoing CABG surgery had a 41% higher odds of receiving perioperative RBC transfusion (AOR = 1.41, 95% CI: [1.13, 1.76], p = 0.002), and blacks undergoing THR had a 39% higher odds of transfusion (AOR = 1.39, 95% CI: [1.20, 1.62], p < 0.001) compared to white patients. We did not detect significant racial difference in transfusion for isolated colectomy (AOR = 1.08, 95% CI: [0.90, 1.30], p = 0.40) (Table 2).
After also adjusting for insurance status, black-white differences in transfusion still existed for patients with identical insurance coverage. Compared to white patients with private insurance, black patients with private insurance had 62% and 27% increased odds of being transfused for isolated CABG (AOR = 1.62, 95% CI: [1.33, 1.97], p < 0.001) and isolated THR (AOR = 1.27, 95% CI: [1.06, 1.52], p = 0.01), respectively. Compared to white patients with Medicare, black patients with Medicare had 37% and 39% higher odds of receiving transfusion for isolated CABG (AOR = 1.37, 95% CI: [1.04, 1.79], p = 0.02) and isolated THR (AOR = 1.39, 95% CI: [1.18, 1.62], p < 0.001), respectively (Figure 4). Black-white differences in blood use were very similar before and after controlling for insurance status (results not shown).
Figure 4.
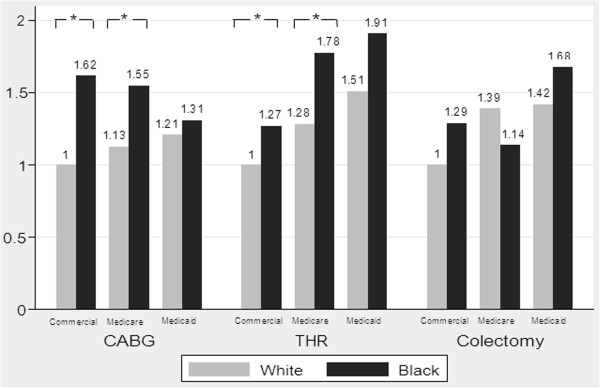
Impact of patient insurance on racial disparities in the use of blood transfusion in major surgery. AOR: Adjusted odds ratio; *p<0.05 for pairwise comparison with identical insurance coverage. This figure is based on the risk adjusted models controlling for patient-level factors (age, gender, admission status, APR-DRG severity of illness, and AHRQ comorbidities) and full interaction terms between race (black vs. white) and patient insurance.
We did not find evidence of an association between blood use and the hospital concentration of black patients for patients undergoing THR or CABG surgeries (Table 3). Finally, black-white differences in the use of perioperative RBC transfusion remained almost unchanged after we included hospitals fixed effects (Table 2). These results suggest that black-white differences in blood use were more likely to be caused by “within hospital variation” rather than “across hospital variation” [2,42]. Each of the multivariable regression models exhibited good discrimination: the Cstatistics were all greater than 0.72. The Hosmer-Lemeshow statistic ranged between 4.16 and 44.8, consistent with acceptable model calibration given the well-known sensitivity of the Hosmer-Lemeshow statistic to sample size [43].
Table 3.
Association between RBC transfusion and hospital concentration of black patients
| Hospital concentration of black patients |
CABG |
THR |
Colectomy |
|||
|---|---|---|---|---|---|---|
| AOR (95% CI) | P value | AOR (95% CI) | P value | AOR (95% CI) | P value | |
| Q1 (Low: ≤8%) |
(Reference) |
|
(Reference) |
|
(Reference) |
|
| Q2 (Medium: 8-13%) |
0.85 (0.56-1.30) |
0.46 |
1.07 (0.66-1.74) |
0.79 |
0.89 (0.61-1.29) |
0.54 |
| Q3 (Medium high: 13-27%) |
1.06 (0.68-1.66) |
0.79 |
1.06 (0.63-1.80) |
0.82 |
1.27 (0.91-1.76) |
0.15 |
| Q4 (High: >27%) | 1.29 (0.73-2.28) | 0.38 | 1.24 (0.74-2.10) | 0.41 | 1.48 (1.07-2.04) | 0.02 |
AOR: adjusted odds ratio, CI: confidence interval, CABG: coronary artery bypass surgery, THR: total hip replacement.
Discussion
Using a contemporary nationwide database and controlling for patient-level factors, we demonstrated that black patients undergoing CABG and total hip replacement in academic medical centers were 40% more likely to receive a blood transfusion compared to white patients. This black-white gap in transfusion practice persisted after adjusting for patient insurance status. Our analyses show that racial disparities in blood transfusion in major surgery exist and that such disparities reflect differences in transfusion practices for black and white patients within the same hospital. Greater use of blood transfusions in black patients may reflect lower surgical technical quality leading to greater blood loss in black compared to white patients [44].
Almost a decade ago, the Institute of Medicine (IOM) report unequal treatment
Confronting Racial and Ethnic Disparities in Healthcare summarized the striking finding that blacks tended to receive lower quality healthcare across a wide range of clinical services. For example, blacks were more likely to receive lower-extremity amputations [45]. This influential report also concluded that the racial differences may stem from patient characteristics (e.g., minority patients may be more likely to delay seeking healthcare), clinical encounters (e.g., medical uncertainty can “open the door” for physicians’ stereotypes and biases to affect their clinical judgment of minority patients), and health systems (e.g., fragmented healthcare delivery systems present cultural and linguistic barriers for minority patients). In response, numerous high-profile initiatives at both national and local levels were designed and implemented to mitigate or eliminate racial and ethnic disparities in healthcare. However, the 2010 National Healthcare Disparities Report demonstrated that racial disparities in healthcare have persisted despite these efforts [15].
The stubborn persistence of racial disparities over time may, in part, reflect a lack of understanding of the underlying causes of racial disparities in healthcare. More recently, a new explanation for racial disparities in health care has drawn great attention because it leads to different policy implications to overcome disparities. Supported by empirical studies, this explanation proposes that racial and ethnic disparities are determined by both discrimination (i.e., “within provider variation”, “who you are”) and segregation (i.e., “across provider variation”, “where you live”) [2,7,12,42,46]. The former highlights the importance of race in the clinical encounter, whereas the latter relates more to geographic variations in the quality of care patterns for all patients. Using this framework, new studies have found that some types of racial disparities in healthcare were largely caused by discrimination [47,48] while others were mainly attributable to segregation [21,49]. To reduce the former type of racial disparities, efforts to improve patient-physician communication and to enhance “patient-centered” care during the clinical encounter are recommended. These efforts include physician cultural competency training, expansions in the numbers of minority physicians in the hospital, hospital’s adoption of patient-centered information technology, and hospital’s efforts to improve effective communication and to promote “communities of care” [45,50-53]. To reduce latter type of racial disparities, interventions to improve performance of particular hospitals which served disproportionately high concentrations of minority patients but provided suboptimal quality of care are needed. These interventions could involve system-level quality initiatives such as pay for performance to incentivize quality improvement in low-quality black-serving hospitals [49,54].
Our findings of racial disparities in transfusion in isolated CABG and THR surgeries not only fill the knowledge gap of contemporary transfusion practices but also suggest the main cause of such disparities - discrimination (“within hospital variation”) during the clinical encounter. This may reflect, in part, complexity of the medical decision making process underlying transfusion decisions. The decision to transfuse a patient can be complex (whether, what, and how much to transfuse) and can involve more than one physician (surgeon, anesthesiologist, critical care medicine physician). Some of the variation in transfusion practices may be due to variation in surgical blood loss, and may account for some of the racial variation in the use of blood transfusion. Surgical procedure time, surgeon technical skill, case complexity, and anesthetic management can contribute to the variation in operative blood loss in complex ways. For example, Silber et al reported that black race was associated with increased anesthesia time and significantly longer procedure time in noncardiac surgery [55]. Our results also indicate that patient insurance coverage and racial segregation (“across hospital variation”) were not major contributors to the detected racial disparities in blood transfusion. Therefore, improving insurance coverage and focusing on black-serving hospitals only might have very limited power in reducing racial disparities in blood transfusion.
Our findings have important policy implications. First, as blood transfusion is gaining recognition as an important domain of quality of care, our study suggests that efforts to monitor and reduce racial disparities in transfusion practices are necessary and important. Since blood transfusion is widely employed in surgery, improving quality of care in transfusion therapy with a particular focus on minorities may lead to improved perioperative outcomes. Second, given that these racial gaps are largely due to “within hospital variation”, interventions with the goal of improving patient-physician communication and enhancing patient-centered health care are more likely to be effective and successful in narrowing these disparities. Fueled by the Patient Protection and Affordable Care Act’s emphasis on patient preferences and medical decision making, there is growing enthusiasm in promoting patient-centered care and addressing racial and ethnic disparities. Third, our findings provide further empirical evidence of black-white disparities in health care. Historically, minorities tend to receive fewer interventions associated with improved health outcomes (e.g. PCI for acute myocardial infarction) [4,5]. However, in some cases, black patients are more likely to undergo interventions suggestive of less than optimal management of chronic diseases (e.g. it was also reported that black patients had significantly higher rates of lower-extremity amputations) [14,15]. Since blood transfusion is frequently associated with higher risk of mortality and complications in surgical patients, our findings that black patients undergoing surgery are more likely to receive blood transfusion suggests there is a need to examine the clinical appropriateness of transfusion therapy in minority patients [56,57].
Our study has significant limitations. Most importantly, preoperative hematocrit values were not available in the UHC database. We were able to control only for the presence but not for the severity of anemia. Thus it is likely that some of the black-white differences in blood transfusions may be due to unmeasured variation in patient preoperative hematocrits. Second, it is possible that a small portion of blacks could be misclassified as whites and vice versa [58]. Thus, our results could be biased towards the null and may underestimate the actual racial disparities in blood transfusion. Third, the present study is based on an administrative database. Therefore, it shares drawbacks of using administrative datasets in evaluating quality such as inability to specify clinical definitions for risk factors (e.g., forced reliance on ICD-9 codes), and limited ability to distinguish between complications of care and pre-existing conditions. Fourth, we cannot rule out the possibility of residual confounding because of other unmeasured risk factors which could account for some of the black-white differences in blood transfusion. Fifth, the blood usage information in the UHC database has not been audited. Finally, because only academic hospitals are included in the UHC database, our findings are not necessarily generalizable to non-academic centers.
Conclusions
In summary, black patients are more likely than white patients to receive blood transfusion for CABG and THR surgery, even after controlling for patient factors and insurance status. Furthermore, these racial disparities are most likely due to differences in transfusion practices between black and white patients within the same hospitals, rather than due to black patients undergoing surgery at predominantly black-serving hospitals. Since a lack of awareness of racial disparities in healthcare remains a significant barrier to eliminating unequal treatment [45,59], reporting racial disparities in contemporary transfusion practices may help reduce potentially unnecessary blood transfusions in minority patients.
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
FQ, ME, SL, and LG conceived the research idea, FQ, ME, SL, SH, CD, RP, RW, and LG participated in its design, SH and LG acquired the data, FQ and LG performed the statistical analysis and drafted the manuscript, ME, SL, SH, CD, RP, RW gave critical comments and helped revising the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Feng Qian, Email: fqian@albany.edu.
Michael P Eaton, Email: Michael_Eaton@urmc.rochester.edu.
Stewart J Lustik, Email: Stewart_Lustik@urmc.rochester.edu.
Samuel F Hohmann, Email: hohmann@uhc.edu.
Carol B Diachun, Email: Carol_Diachun@urmc.rochester.edu.
Robert Pasternak, Email: Robert_Pasternak@urmc.rochester.edu.
Richard N Wissler, Email: Dick_Wissler@urmc.rochester.edu.
Laurent G Glance, Email: Laurent_Glance@urmc.rochester.edu.
Acknowledgements
This project was supported by a grant from the Agency for Healthcare and Quality Research (RO1 HS16737) to LG and funding from the University Of Rochester Department of Anesthesiology to LG and FQ.
References
- Dentzer S. A nation at risk for wider health disparities. Health Aff (Millwood) 2011;14:1818. doi: 10.1377/hlthaff.2011.1091. [DOI] [PubMed] [Google Scholar]
- Chandra A. Who you are and where you live: race and the geography of healthcare. Med Care. 2009;14:135–137. doi: 10.1097/MLR.0b013e31819a4c5e. [DOI] [PubMed] [Google Scholar]
- Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;14:2634–2641. doi: 10.1161/CIRCULATIONAHA.105.543231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson ED, Shaw LK, DeLong ER, Pryor DB, Califf RM, Mark DB. Racial variation in the use of coronary-revascularization procedures. Are the differences real? Do they matter? N Engl J Med. 1997;14:480–486. doi: 10.1056/NEJM199702133360706. [DOI] [PubMed] [Google Scholar]
- Chen J, Rathore SS, Radford MJ, Wang Y, Krumholz HM. Racial differences in the use of cardiac catheterization after acute myocardial infarction. N Engl J Med. 2001;14:1443–1449. doi: 10.1056/NEJM200105103441906. [DOI] [PubMed] [Google Scholar]
- Jha AK, Fisher ES, Li Z, Orav JE, Epstein AM. Racial trends in the use of major procedures among the elderly. N Engl J Med. 2005;14:683–691. doi: 10.1056/NEJMsa050672. [DOI] [PubMed] [Google Scholar]
- Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. New Engl J Med. 2003;14:1350–1359. doi: 10.1056/NEJMsa021569. [DOI] [PubMed] [Google Scholar]
- Schneider EC, Cleary PD, Zaslavsky AM, Epstein AM. Racial disparity in influenza vaccination: does managed care narrow the gap between African Americans and whites? JAMA. 2001;14:1455–1460. doi: 10.1001/jama.286.12.1455. [DOI] [PubMed] [Google Scholar]
- Burns RB, McCarthy EP, Freund KM, Marwill SL, Shwartz M, Ash A, Moskowitz MA. Black women receive less mammography even with similar use of primary care. Ann Intern Med. 1996;14:173–182. doi: 10.7326/0003-4819-125-3-199608010-00002. [DOI] [PubMed] [Google Scholar]
- Zuvekas SH, Fleishman JA. Self-rated mental health and racial/ethnic disparities in mental health service use. Med Care. 2008;14:915–923. doi: 10.1097/MLR.0b013e31817919e5. [DOI] [PubMed] [Google Scholar]
- Harris KM, Edlund MJ, Larson S. Racial and ethnic differences in the mental health problems and use of mental health care. Med Care. 2005;14:775–784. doi: 10.1097/01.mlr.0000170405.66264.23. [DOI] [PubMed] [Google Scholar]
- Groeneveld PW, Laufer SB, Garber AM. Technology diffusion, hospital variation, and racial disparities among elderly Medicare beneficiaries 1989-2000. Med Care. 2005;14:320–329. doi: 10.1097/01.mlr.0000156849.15166.ec. [DOI] [PubMed] [Google Scholar]
- Sonel AF, Good CB, Mulgund J, Roe MT, Gibler WB, Smith SC Jr, Cohen MG, Pollack CV Jr, Ohman EM, Peterson ED. CRUSADE Investigators. Racial variations in treatment and outcomes of black and white patients with high-risk non-ST-elevation acute coronary syndromes: insights from CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines?) Circulation. 2005;14:1225–1232. doi: 10.1161/01.CIR.0000157732.03358.64. [DOI] [PubMed] [Google Scholar]
- Gornick ME, Eggers PW, Reilly TW, Mentnech RM, Fitterman LK, Kucken LE, Vladeck BC. Effects of race and income on mortality and use of services among Medicare beneficiaries. New Engl J Med. 1996;14:791–799. doi: 10.1056/NEJM199609123351106. [DOI] [PubMed] [Google Scholar]
- 2010 National Healthcare Disparities Report. Agency for Healthcare Research and Quality US Department of Health and Human Services. Rockville, Maryland: 2010 National Healthcare Disparities Report; 2011. [Google Scholar]
- Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. New Engl J Med. 2004;14:575–584. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- Mukamel DB, Murthy AS, Weimer DL. Racial differences in access to high-quality cardiac surgeons. Am J Public Health. 2000;14:1774–1777. doi: 10.2105/ajph.90.11.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konety SH, Sarrazin MSV, Rosenthal GE. Patient and hospital differences underlying racial variation in outcomes after coronary artery bypass graft surgery. Circulation. 2005;14:1210–1216. doi: 10.1161/01.CIR.0000157728.49918.9F. [DOI] [PubMed] [Google Scholar]
- Barnato AE, Lucas FL, Staiger S, Wennberg DE, Chandra A. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Med Care. 2005;14:308–319. doi: 10.1097/01.mlr.0000156848.62086.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly DP, Lopez L, Isaac T, Jha AK. How do black-serving hospitals perform on patient safety indicators? Implications for national public reporting and pay-for-performance. Med Care. 2010;14:1133–1137. doi: 10.1097/MLR.0b013e3181f81c7e. [DOI] [PubMed] [Google Scholar]
- Hasnain-Wynia R, Baker DW, Nerenz D, Feinglass J, Beal AC, Landrum MB, Behal R, Weissman JS. Disparities in health care are driven by where minority patients seek care. Arch Intern Med. 2007;14:1233–1239. doi: 10.1001/archinte.167.12.1233. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Spencer CS, Richard P, Anderson GF, Powe NR, Laveist TA. Do hospitals provide lower-quality quality care to minorities than to whites? Health Affair. 2008;14:518–527. doi: 10.1377/hlthaff.27.2.518. [DOI] [PubMed] [Google Scholar]
- Jha AK, Orav EJ, Li Z, Epstein AM. Concentration and quality of hospitals that care for elderly black patients. Arch Intern Med. 2007;14:1177–1182. doi: 10.1001/archinte.167.11.1177. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. An older and more diverse nation by midcentury. 2008. Available at: http://www.census.gov/newsroom/releases/archives/population/cb08 -123.html. Accessed October 20, 2011.
- Healthy People 2020. Department of Health and Human Services. Washington, DC: Healthy People 2020; 2010. [Google Scholar]
- Department of Health and Human Services. HHS action plan to reduce racial and ethnic health disparities. Washington, DC: Department of Health and Human Services; 2011. [Google Scholar]
- US Department of Health and Human Services. The 2007 Nationwide Blood Collection and Utilization Survey Report. Washington, D.C: US Department of Health and Human Services; 2007. [Google Scholar]
- Glance LG, Dick AW, Mukamel DB, Fleming FJ, Zollo RA, Wissler R, Salloum R, Meredith UW, Osler TM. Association between Intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;14:283–292. doi: 10.1097/ALN.0b013e3182054d06. [DOI] [PubMed] [Google Scholar]
- Surgenor SD, Kramer RS, Olmstead EM, Ross CS, Sellke FW, Likosky DS, Marrin CA, Helm RE Jr, Leavitt BJ, Morton JR, Charlesworth DC, Clough RA, Hernandez F, Frumiento C, Benak A, DioData C, O’Connor G. Northern New England Cardiovascular Disease Study Group. The association of perioperative red blood cell transfusions and decreased long-term survival after cardiac surgery. Anesth Analg. 2009;14:1741–1746. doi: 10.1213/ane.0b013e3181a2a696. [DOI] [PubMed] [Google Scholar]
- Surgenor SD, DeFoe GR, Fillinger MP, Likosky DS, Groom RC, Clark C, Helm RE, Kramer RS, Leavitt BJ, Klemperer JD, Krumholz CF, Westbrook BM, Galatis DJ, Frumiento C, Ross CS, Olmstead EM, O’Connor GT. Intraoperative red blood cell transfusion during coronary artery bypass graft surgery increases the risk of postoperative low-output heart failure. Circulation. 2006;14:I43–I48. doi: 10.1161/CIRCULATIONAHA.105.001271. [DOI] [PubMed] [Google Scholar]
- Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, Starr NJ, Blackstone EH. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;14:1608–1616. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- Koch CG, Li L, Duncan AI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg. 2006;14:1650–1657. doi: 10.1016/j.athoracsur.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;14:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;14:2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long - term survival after cardiac operation. Ann Thorac Surg. 2002;14:1180–1186. doi: 10.1016/S0003-4975(02)03766-9. [DOI] [PubMed] [Google Scholar]
- Kuduvalli M, Oo AY, Newall N, Grayson AD, Jackson M, Desmond MJ, Fabri BM, Rashid A. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2005;14:592–598. doi: 10.1016/j.ejcts.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Scott BH, Seifert FC, Grimson R. Blood transfusion is associated with increased resource utilisation, morbidity and mortality in cardiac surgery. Ann Card Anaesth. 2008;14:15–19. doi: 10.4103/0971-9784.38444. [DOI] [PubMed] [Google Scholar]
- Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM, Uttley G, Mor V, Sharma SC, Vezeridis M, Khuri SF, Friedmann PD. Operative blood loss, blood transfusion, and 30-day mortality in older patients after major noncardiac surgery. Ann Surg. 2010;14:11–17. doi: 10.1097/SLA.0b013e3181e3e43f. [DOI] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;14:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- University HealthSystem Consortium. Clinical Data Base/Resource Manager. 2011. Available at: http://www.uhc.edu/11536.htm. Accessed November 1, 2011.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley- Interscience Publication; 2000. [Google Scholar]
- Baicker K, Chandra A, Skinner JS, Wennberg JE. Who you are and where you live: how race and geography affect the treatment of medicare beneficiaries. Health Aff (Millwood) 2004;14:44. doi: 10.1377/hlthaff.var.33. Suppl Variation. [DOI] [PubMed] [Google Scholar]
- Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited. Crit Care Med. 2007;14:2052–2056. doi: 10.1097/01.CCM.0000275267.64078.B0. [DOI] [PubMed] [Google Scholar]
- Glance LG, Mukamel DB, Blumberg N, Fleming FJ, Hohmann SF, Dick AW. Association between surgical resident involvement and blood use in noncardiac surgery. Transfusion. 2013. doi:10.1111/trf.12350. [DOI] [PubMed]
- Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academy Press; 2002. [Google Scholar]
- Williams DR, Jackson PB. Social sources of racial disparities in health - Policies in societal domains, far removed from traditional health policy, can have decisive consequences for health. Health Affair. 2005;14:325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. Racial disparities in diabetes care processes, outcomes, and treatment intensity. Med Care. 2003;14:1221–1232. doi: 10.1097/01.MLR.0000093421.64618.9C. [DOI] [PubMed] [Google Scholar]
- Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in medicare patients. J Vasc Surg. 2011;14:420–426. doi: 10.1016/j.jvs.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yin J, Cai XY, Temkin-Greener J, Mukamel DB. Association of race and sites of care with pressure ulcers in high-risk nursing home residents. Jama-J Am Med Assoc. 2011;14:179–186. doi: 10.1001/jama.2011.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas Bustamante A, Chen J. Physicians cite hurdles ranging from lack of coverage to poor communication in providing high-quality care to latinos. Health Aff (Millwood) 2011;14:1921–1929. doi: 10.1377/hlthaff.2011.0344. [DOI] [PubMed] [Google Scholar]
- Jacobs EA, Leos GS, Rathouz PJ, Fu P Jr. Shared networks of interpreter services, at relatively low cost, can help providers serve patients with limited english skills. Health Aff (Millwood) 2011;14:1930–1938. doi: 10.1377/hlthaff.2011.0667. [DOI] [PubMed] [Google Scholar]
- Karliner LS, Jacobs EA, Chen AH, Mutha S. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature. Health Serv Res. 2007;14:727–754. doi: 10.1111/j.1475-6773.2006.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RM, Fiscella K, Lesser CS, Stange KC. Why the nation needs a policy push on patient-centered health care. Health Aff (Millwood) 2010;14:1489–1495. doi: 10.1377/hlthaff.2009.0888. [DOI] [PubMed] [Google Scholar]
- Jha AK, Orav EJ, Epstein AM. Low-quality, high-cost hospitals, mainly in South, care for sharply higher shares of elderly black, Hispanic, and medicaid patients. Health Aff (Millwood) 2011;14:1904–1911. doi: 10.1377/hlthaff.2011.0027. [DOI] [PubMed] [Google Scholar]
- Silber JH, Rosenbaum PR, Zhang XM, Even_Shoshan O. Influence of patient and hospital characteristics on anesthesia time in medicare patients undergoing general and ortbopedic surgery. Anesthesiology. 2007;14:356–364. doi: 10.1097/00000542-200702000-00025. [DOI] [PubMed] [Google Scholar]
- Barocas DA, Gray DT, Fowke JH, Mercaldo ND, Blume JD, Chang SS, Cookson MS, Smith JA Jr, Penson DF. Racial variation in the quality of surgical care for prostate cancer. J Urol. 2012;14(4):1279–1285. doi: 10.1016/j.juro.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley CJ, Raldow AC, Cramer LD, Soulos PR, Long JB, Yu JB, Makarov DV, Gross CP. A new approach to understanding racial disparities in prostate cancer treatment. J Clin Geriatr Oncol. 2013;14:1–8. doi: 10.1016/j.jgo.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressin NR, Chang BH, Hendricks A, Kazis LE. Agreement between administrative data and patients’ self-reports of race/ethnicity. Am J Public Health. 2003;14:1734–1739. doi: 10.2105/AJPH.93.10.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Epstein AM. Governance around quality of care at hospitals that disproportionately care for black patients. J Gen Intern Med. 2012;14(3):297–303. doi: 10.1007/s11606-011-1880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]