Abstract
We report several classes of human interspersed repeats that resemble fossils of DNA transposons, elements that move by excision and reintegration in the genome, whereas previously characterized mammalian repeats all appear to have accumulated by retrotransposition, which involves an RNA intermediate. The human genome contains at least 14 families and > 100,000 degenerate copies of short (180-1200 bp) elements that have 14- to 25-bp terminal inverted repeats and are flanked by either 8 bp or TA target site duplications. We describe two ancient 2.5-kb elements with coding capacity, Tigger1 and -2, that closely resemble pogo, a DNA transposon in Drosophila, and probably were responsible for the distribution of some of the short elements. The deduced pogo and Tigger proteins are related to products of five DNA transposons found in fungi and nematodes, and more distantly, to the Tc1 and mariner transposases. They also are very similar to the major mammalian centromere protein CENP-B, suggesting that this may have a transposase origin. We further identified relatively low-copy-number mariner elements in both human and sheep DNA. These belong to two subfamilies previously identified in insect genomes, suggesting lateral transfer between diverse species.
Full text
PDF
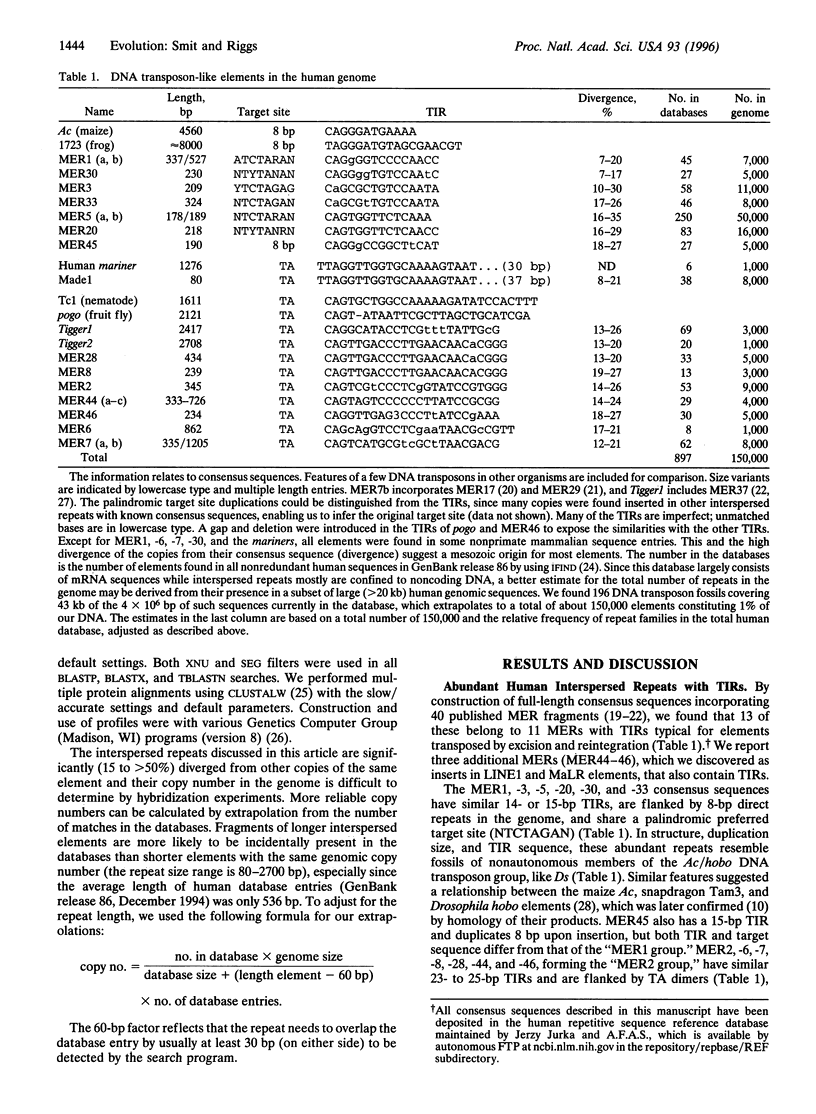


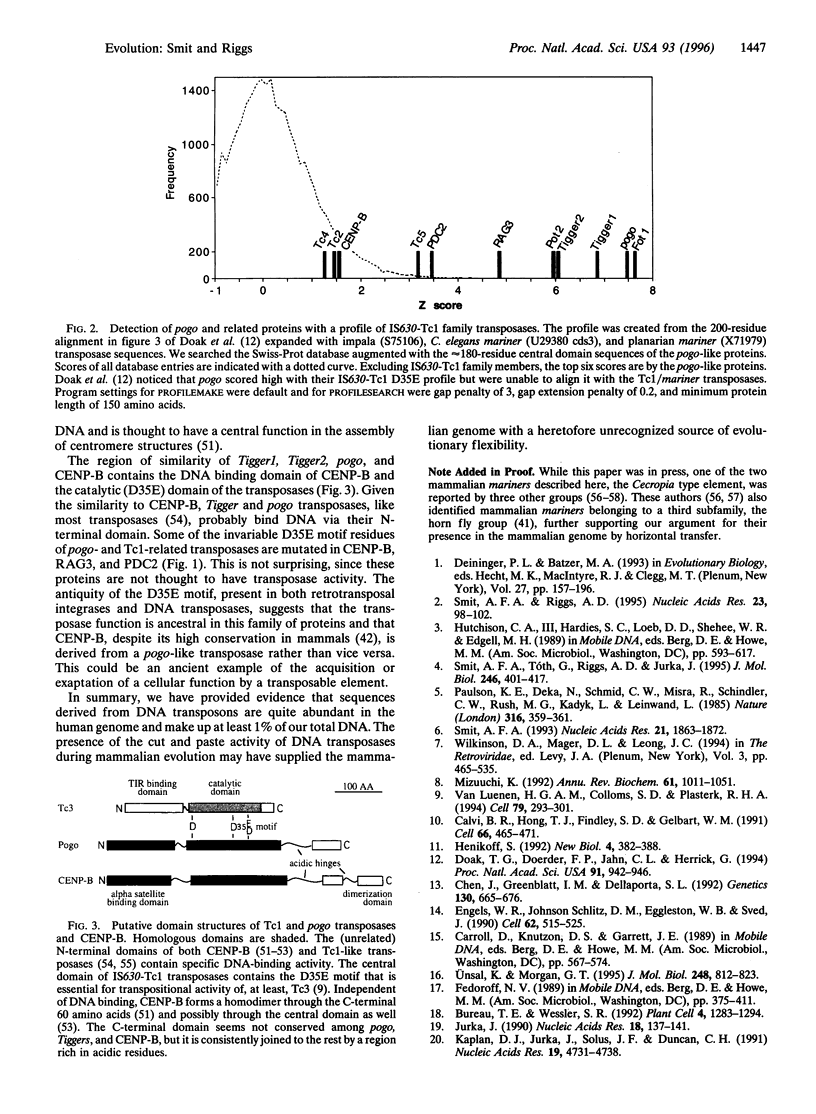

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Auge-Gouillou C., Bigot Y., Pollet N., Hamelin M. H., Meunier-Rotival M., Periquet G. Human and other mammalian genomes contain transposons of the mariner family. FEBS Lett. 1995 Jul 24;368(3):541–546. doi: 10.1016/0014-5793(95)00735-r. [DOI] [PubMed] [Google Scholar]
- Bureau T. E., Wessler S. R. Stowaway: a new family of inverted repeat elements associated with the genes of both monocotyledonous and dicotyledonous plants. Plant Cell. 1994 Jun;6(6):907–916. doi: 10.1105/tpc.6.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau T. E., Wessler S. R. Tourist: a large family of small inverted repeat elements frequently associated with maize genes. Plant Cell. 1992 Oct;4(10):1283–1294. doi: 10.1105/tpc.4.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi B. R., Hong T. J., Findley S. D., Gelbart W. M. Evidence for a common evolutionary origin of inverted repeat transposons in Drosophila and plants: hobo, Activator, and Tam3. Cell. 1991 Aug 9;66(3):465–471. doi: 10.1016/0092-8674(81)90010-6. [DOI] [PubMed] [Google Scholar]
- Chen J., Greenblatt I. M., Dellaporta S. L. Molecular analysis of Ac transposition and DNA replication. Genetics. 1992 Mar;130(3):665–676. doi: 10.1093/genetics/130.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. J., Anderson P. The Tc5 family of transposable elements in Caenorhabditis elegans. Genetics. 1994 Jul;137(3):771–781. doi: 10.1093/genetics/137.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloms S. D., van Luenen H. G., Plasterk R. H. DNA binding activities of the Caenorhabditis elegans Tc3 transposase. Nucleic Acids Res. 1994 Dec 25;22(25):5548–5554. doi: 10.1093/nar/22.25.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daboussi M. J., Langin T., Brygoo Y. Fot1, a new family of fungal transposable elements. Mol Gen Genet. 1992 Mar;232(1):12–16. doi: 10.1007/BF00299131. [DOI] [PubMed] [Google Scholar]
- Deen P. M., Terwel D., Bussemakers M. J., Roubos E. W., Martens G. J. Structural analysis of the entire proopiomelanocortin gene of Xenopus laevis. Eur J Biochem. 1991 Oct 1;201(1):129–137. doi: 10.1111/j.1432-1033.1991.tb16265.x. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Batzer M. A., Hutchison C. A., 3rd, Edgell M. H. Master genes in mammalian repetitive DNA amplification. Trends Genet. 1992 Sep;8(9):307–311. doi: 10.1016/0168-9525(92)90262-3. [DOI] [PubMed] [Google Scholar]
- Doak T. G., Doerder F. P., Jahn C. L., Herrick G. A proposed superfamily of transposase genes: transposon-like elements in ciliated protozoa and a common "D35E" motif. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):942–946. doi: 10.1073/pnas.91.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R., Johnson-Schlitz D. M., Eggleston W. B., Sved J. High-frequency P element loss in Drosophila is homolog dependent. Cell. 1990 Aug 10;62(3):515–525. doi: 10.1016/0092-8674(90)90016-8. [DOI] [PubMed] [Google Scholar]
- Gribskov M., McLachlan A. D., Eisenberg D. Profile analysis: detection of distantly related proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4355–4358. doi: 10.1073/pnas.84.13.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Detection of Caenorhabditis transposon homologs in diverse organisms. New Biol. 1992 Apr;4(4):382–388. [PubMed] [Google Scholar]
- Hohmann S. Characterisation of PDC2, a gene necessary for high level expression of pyruvate decarboxylase structural genes in Saccharomyces cerevisiae. Mol Gen Genet. 1993 Dec;241(5-6):657–666. doi: 10.1007/BF00279908. [DOI] [PubMed] [Google Scholar]
- Iris F. J., Bougueleret L., Prieur S., Caterina D., Primas G., Perrot V., Jurka J., Rodriguez-Tome P., Claverie J. M., Dausset J. Dense Alu clustering and a potential new member of the NF kappa B family within a 90 kilobase HLA class III segment. Nat Genet. 1993 Feb;3(2):137–145. doi: 10.1038/ng0293-137. [DOI] [PubMed] [Google Scholar]
- Jurka J., Kaplan D. J., Duncan C. H., Walichiewicz J., Milosavljevic A., Murali G., Solus J. F. Identification and characterization of new human medium reiteration frequency repeats. Nucleic Acids Res. 1993 Mar 11;21(5):1273–1279. doi: 10.1093/nar/21.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J. Novel families of interspersed repetitive elements from the human genome. Nucleic Acids Res. 1990 Jan 11;18(1):137–141. doi: 10.1093/nar/18.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo P., Leong S. A., Chattoo B. B. Pot2, an inverted repeat transposon from the rice blast fungus Magnaporthe grisea. Mol Gen Genet. 1994 Nov 1;245(3):339–348. doi: 10.1007/BF00290114. [DOI] [PubMed] [Google Scholar]
- Kaplan D. J., Jurka J., Solus J. F., Duncan C. H. Medium reiteration frequency repetitive sequences in the human genome. Nucleic Acids Res. 1991 Sep 11;19(17):4731–4738. doi: 10.1093/nar/19.17.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K., Masumoto H., Ikeda M., Okazaki T. Analysis of protein-DNA and protein-protein interactions of centromere protein B (CENP-B) and properties of the DNA-CENP-B complex in the cell cycle. Mol Cell Biol. 1995 Mar;15(3):1602–1612. doi: 10.1128/mcb.15.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T., Inohara N., Sato I., Tamada T., Kagawa Y., Ohta S. Transcription of the MRP RNA gene in frog stage I oocytes requires a novel cis-element. Biochem Biophys Res Commun. 1994 Jul 15;202(1):225–233. doi: 10.1006/bbrc.1994.1916. [DOI] [PubMed] [Google Scholar]
- Li W., Shaw J. E. A variant Tc4 transposable element in the nematode C. elegans could encode a novel protein. Nucleic Acids Res. 1993 Jan 11;21(1):59–67. doi: 10.1093/nar/21.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfalla G., McInnis M. G., Antonarakis S. E., Uzé G. Structure of the human CRFB4 gene: comparison with its IFNAR neighbor. J Mol Evol. 1995 Sep;41(3):338–344. doi: 10.1007/BF00186545. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K. Transpositional recombination: mechanistic insights from studies of mu and other elements. Annu Rev Biochem. 1992;61:1011–1051. doi: 10.1146/annurev.bi.61.070192.005051. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Oosumi T., Belknap W. R., Garlick B. Mariner transposons in humans. Nature. 1995 Dec 14;378(6558):672–672. doi: 10.1038/378672a0. [DOI] [PubMed] [Google Scholar]
- Paulson K. E., Deka N., Schmid C. W., Misra R., Schindler C. W., Rush M. G., Kadyk L., Leinwand L. A transposon-like element in human DNA. Nature. 1985 Jul 25;316(6026):359–361. doi: 10.1038/316359a0. [DOI] [PubMed] [Google Scholar]
- Pluta A. F., Saitoh N., Goldberg I., Earnshaw W. C. Identification of a subdomain of CENP-B that is necessary and sufficient for localization to the human centromere. J Cell Biol. 1992 Mar;116(5):1081–1093. doi: 10.1083/jcb.116.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polard P., Chandler M. Bacterial transposases and retroviral integrases. Mol Microbiol. 1995 Jan;15(1):13–23. doi: 10.1111/j.1365-2958.1995.tb02217.x. [DOI] [PubMed] [Google Scholar]
- Radice A. D., Bugaj B., Fitch D. H., Emmons S. W. Widespread occurrence of the Tc1 transposon family: Tc1-like transposons from teleost fish. Mol Gen Genet. 1994 Sep 28;244(6):606–612. doi: 10.1007/BF00282750. [DOI] [PubMed] [Google Scholar]
- Robertson H. M. The mariner transposable element is widespread in insects. Nature. 1993 Mar 18;362(6417):241–245. doi: 10.1038/362241a0. [DOI] [PubMed] [Google Scholar]
- Ruvolo V., Hill J. E., Levitt A. The Tc2 transposon of Caenorhabditis elegans has the structure of a self-regulated element. DNA Cell Biol. 1992 Mar;11(2):111–122. doi: 10.1089/dna.1992.11.111. [DOI] [PubMed] [Google Scholar]
- Smit A. F. Identification of a new, abundant superfamily of mammalian LTR-transposons. Nucleic Acids Res. 1993 Apr 25;21(8):1863–1872. doi: 10.1093/nar/21.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A. F., Riggs A. D. MIRs are classic, tRNA-derived SINEs that amplified before the mammalian radiation. Nucleic Acids Res. 1995 Jan 11;23(1):98–102. doi: 10.1093/nar/23.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A. F., Tóth G., Riggs A. D., Jurka J. Ancestral, mammalian-wide subfamilies of LINE-1 repetitive sequences. J Mol Biol. 1995 Feb 24;246(3):401–417. doi: 10.1006/jmbi.1994.0095. [DOI] [PubMed] [Google Scholar]
- Streck R. D., Macgaffey J. E., Beckendorf S. K. The structure of hobo transposable elements and their insertion sites. EMBO J. 1986 Dec 20;5(13):3615–3623. doi: 10.1002/j.1460-2075.1986.tb04690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Hagishita Y., Himeno M. Functional domain structure of human centromere protein B. Implication of the internal and C-terminal self-association domains in centromeric heterochromatin condensation. J Biol Chem. 1994 Sep 30;269(39):24271–24276. [PubMed] [Google Scholar]
- Sullivan K. F., Glass C. A. CENP-B is a highly conserved mammalian centromere protein with homology to the helix-loop-helix family of proteins. Chromosoma. 1991 Jul;100(6):360–370. doi: 10.1007/BF00337514. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor M., Lobocka M., Goodell M., Pettitt J., O'Hare K. The pogo transposable element family of Drosophila melanogaster. Mol Gen Genet. 1992 Mar;232(1):126–134. doi: 10.1007/BF00299145. [DOI] [PubMed] [Google Scholar]
- Unsal K., Morgan G. T. A novel group of families of short interspersed repetitive elements (SINEs) in Xenopus: evidence of a specific target site for DNA-mediated transposition of inverted-repeat SINEs. J Mol Biol. 1995 May 12;248(4):812–823. doi: 10.1006/jmbi.1995.0262. [DOI] [PubMed] [Google Scholar]
- Vos J. C., Plasterk R. H. Tc1 transposase of Caenorhabditis elegans is an endonuclease with a bipartite DNA binding domain. EMBO J. 1994 Dec 15;13(24):6125–6132. doi: 10.1002/j.1460-2075.1994.tb06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren W. D., Atkinson P. W., O'Brochta D. A. The Hermes transposable element from the house fly, Musca domestica, is a short inverted repeat-type element of the hobo, Ac, and Tam3 (hAT) element family. Genet Res. 1994 Oct;64(2):87–97. doi: 10.1017/s0016672300032699. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Ainscough R., Anderson K., Baynes C., Berks M., Bonfield J., Burton J., Connell M., Copsey T., Cooper J. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994 Mar 3;368(6466):32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- Yuan J. Y., Finney M., Tsung N., Horvitz H. R. Tc4, a Caenorhabditis elegans transposable element with an unusual fold-back structure. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3334–3338. doi: 10.1073/pnas.88.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Luenen H. G., Colloms S. D., Plasterk R. H. The mechanism of transposition of Tc3 in C. elegans. Cell. 1994 Oct 21;79(2):293–301. doi: 10.1016/0092-8674(94)90198-8. [DOI] [PubMed] [Google Scholar]