Abstract
Background and Purpose
We showed previously robust neuroprotection with the thrombin inhibitor argatroban, and now sought additional support for its neuroprotective potential.
Methods
We used behavioral and histological endpoints; rigorously blinded the study groups; extended the treatment window to 3 hours following ischemia onset; and used 2 separate models. First, 2-h filament MCAo in 64 male Sprague-Dawley rats was followed by learning and memory testing and quantitative histomporphometry. Randomly assigned treatment was 0.45mg argatroban, saline, or 0.4U thrombin. Second, we used the quantal bioassay (n=272) after 2-hour MCAo to detect the longest time delay after which therapy failed.
Results
Argatroban powerfully and significantly reversed learning and memory deficits due to focal ischemia compared to saline or thrombin (p<0.03, ANOVA). Argatroban was significantly (p<0.05, t-test with Bonferroni) protective when given immediately or after 1, 2, 3 but not 4 hours delay.
Conclusions
We obtained supportive evidence for argatroban protection of the neurovascular unit using behavioral and histological measurements at realistic therapeutic time windows.
Introduction
Thrombin causes cell death via the protease activated receptors (PARs), which are found in endothelial cells, astrocytes and neurons1. The direct thrombin inhibitor, argatroban, inhibited the effect of thrombin in a dose dependent manner using histological markers of cell injury1, 2. Many candidate treatments that protect rodent brain in pre-clinical stroke models, however, failed in clinical trials3. Recently a number of authorities have suggested additional testing requirements for putative neuroprotectants4,5. We sought additional supportive evidence for argatroban protection of the neurovascular unit using testing standards derived from these published guidelines.
Methods
The protocol was approved by the IACUC at Cedars-Sinai Medical Center, following all national guidelines for the care of experimental animals. Male Sprague Dawley rats (n=37, 290g to 310g) were assigned randomly: saline or 1.4units/kg thrombin (Sigma) in 0.2ml administered intra-arterially (IA) over 120min, or 0.45mg argatroban (Axxora) in 0.2ml administered intravenously (IV) for 2 hours2. We used our published methods for middle cerebral artery occlusion (MCAo), behavior, and histology6,1,7.
To determine the optimum therapeutic time window for treatment with argatroban, we randomly assigned 272 male Sprague-Dawley rats, as above, to receive IV saline or “low dose” (10mg/kg) or “high dose” (18mg/kg) argatroban over 24 hours by Alzet mini-pump (model 2001D, Durect Corp). Treatment effects were assessed using the standard quantal bioassay procedure, and TTC staining8. Treatment groups were compared using a t-test of the respective ED50s using a Bonferroni correction for multiple comparisons.
Results
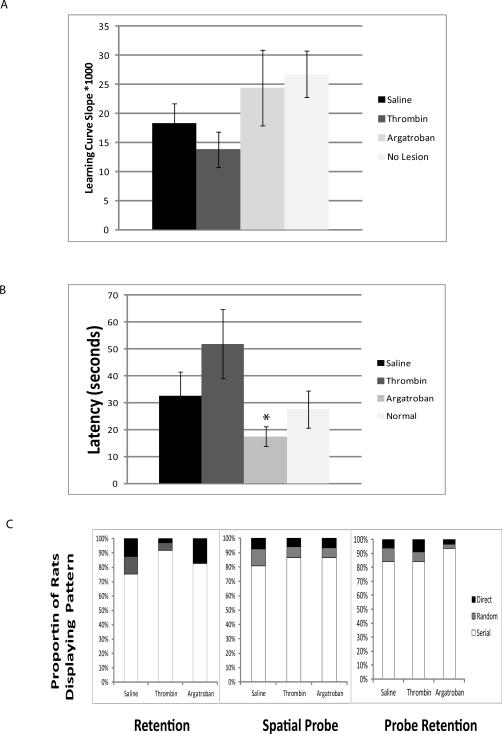
Ischemia significantly reduced the learning curve slope (Fig. 1a, p<0.05, ANOVA), an effect worsened by thrombin and ameliorated by argatroban (Fig. 1a, ANOVA, p<0.05). During the 48 hour retention test, the thrombin treated subjects spent less time in the correct quadrant(NS). A spatial probe test (Fig. 1b) revealed a significant deficit among the thrombin-treated subjects, an effect ameliorated by argatroban1 (p<0.05, ANOVA). Interestingly, upon testing retention of a new location, the thrombin treated animals remembered as well as other treatment groups, suggesting intact reference memory, consistent with previous data that focal ischemia causes impaired learning with preserved memory7. Search strategy analysis (Fig. 1c) revealed a significant association between treatment and search strategy for the retention test (Chi Square, p=0.03) during which argatroban treated subjects used significantly fewer random searches.
Figure 1.
A. Learning Curves. Over 5 days in the Barnes Maze, each animal learned the location of the escape hole. The linear learning curve—the inverse of the mean latency-to-escape on each day—was highest in the ‘no lesion’ controls and slowest in the thrombin group (p=0.03, ANOVA, Tukey's). B. Spatial Probe Escape Latency. The spatial probe assesses the time to locate a new escape location. Saline and thrombin prolonged the time to locate the new escape location1. Argatroban ameliorated this effect (* p<0.05 compared to thrombin treatment). C. Search Strategy. The proportion of searches characterized as direct, serial or random are shown during the retention, spatial probe, and probe retention tests, without error bars for clarity. Argatroban treated subjects used fewer random searches in the retention task (p<0.03, Chi Square).
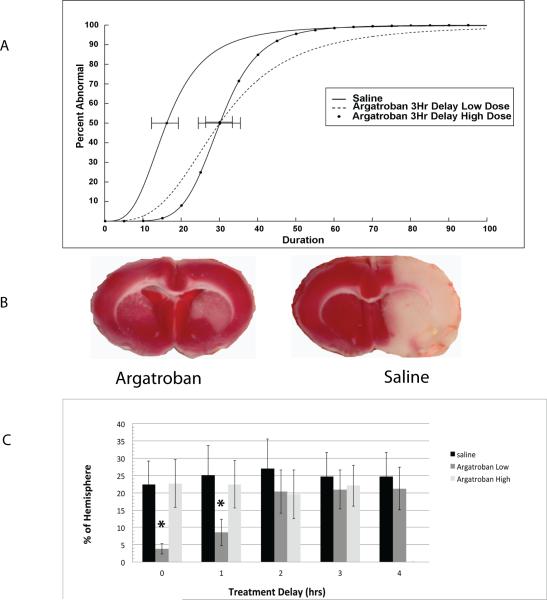
After 1, 2, or 3 hours delay following ischemia onset, both high and low dose argatroban treatment produced a significant protective effect, illustrated for 3 hours delay in Fig. 2a (p<0.05 after t-test and Bonferroni correction). After 4 hours delay there was no protective effect (Table). Using TTC exclusion, the drug showed a significant protective effect (Fig. 2b). These differences were statistically significantly different using a univariate ANOVA that included a delay time vs. treatment interaction term, (p=0.01), but only the low-dose group was protective, and only at 0 or 1 hours delay after stroke onset (Fig. 2c).
Figure 2.
A. Quantal Bioassays. Each bioassay describes the effect of low dose or high dose argatroban given at various delay time points, compared to saline8. The group tolerance to ischemia is characterized by the ED50, minutes of MCAo that renders 50% of the subjects abnormal. The ED50 of each treated group is compared to the saline group after a Bonferroni correction for multiple comparisons, illustrated with curves for those subjects treated after a 3-hour delay. Both low and high dose argatroban were significantly more protective than saline (p<0.05, t-test after Bonferroni). B. Sections after 48 hours with TTC staining. Representative sections from saline and argatroban treated subjects illustrating the pale, non-metabolizing area of lesioned cerebrum reduced by argatroban. Subjects received treatment 3 hours after onset of 2-hour MCAo, and were sacrificed 48 hours later. C. Lesion volume. Mean±SD total lesion volume is shown for each group at the relevant time delays. The volume of metabolic impairment is significantly reduced (improved) by low dose argatroban given immediately or after a 1-hour delay. ** p<0.01, ANOVA with Tukey's.
Table. Summary of Quantal Bioassay Results.
High or Low dose argatroban was compared to saline treatment at various delay times. Each ED50 is shown with a standard error; the p value shown is after correction for multiple comparisons. Argatroban was very effective if given immediately or after a 1, 2 or 3 hour delay. After a 4 hours delay, the beneficial effect was weak, and not statistically significant.
| Group | N | Dose | Delay | P50±SE | t-test compared to Saline |
|---|---|---|---|---|---|
| Saline | 20 | None | 19.4±3.7 | ||
| argatroban | 20 | Low | None | 56.4±11.5 | P<0.01 |
| argatroban | 20 | High | None | 32.74±4.5 | P<0.05 |
| Saline | 19 | 1 hour | 24.0±3.8 | ||
| argatroban | 19 | Low | 1 hour | 46.3±7.8 | P<0.02 |
| argatroban | 19 | High | 1 hour | 40.5±4.9 | P<0.02 |
| Saline | 20 | 2 hours | 14.7±3.2 | ||
| argatroban | 20 | Low | 2 hours | 37.0±4.4 | P<0.01 |
| argatroban | 20 | High | 2 hours | 37.7±3.8 | P<0.02 |
| Saline | 20 | 3 hours | 16.2±3.9 | ||
| argatroban | 20 | Low | 3 hours | 30.1±5.6 | P<0.05 |
| argatroban | 20 | High | 3 hours | 30.1±3.7 | P<0.05 |
| Saline | 20 | 4 hours | 15.5±3.3 | ||
| 20 | Low | 4 hours | 21.9±4.8 | NS |
Discussion
The direct thrombin inhibitor argatroban significantly ameliorates stroke-related behavioral and histological effects in two different models. Thrombin treated subjects performed worse than saline and argatroban treated subjects in learning/memory tasks (Fig. 1). Argatroban treated subjects showed a steeper learning curve during 4 days of training performing like unlesioned controls (Fig. 1a)1. In the Morris Water maze task, as well as in the Barnes Maze task, acquisition of the escape location during training is considered evidence of working memory, and in some reports correlates with hippocampus damage or lesions of the basal nucleus of Meynert9-11. The spatial probe task emphasizes reference memory and the subjects’ must recognize that the escape platform has moved and find the new escape route (Fig. 1b). We have previously shown that the spatial probe task is exquisitely sensitive to large lesions following MCAo12. The argatroban treated subjects clearly used a more efficient search strategy during the retention test (Fig. 1c). During the probe task, although the argatroban animals solved the problem more quickly (Fig. 1b), they used less efficient search strategies (Fig. 1c), suggesting improved ability to recognize and solve the new escape problem because they retained an imprint of the task paradigm.
The quantal bioassay uses a different behavioral outcome, is simpler, and is well suited to studies of the therapeutic time window13,8. We confirmed (Fig. 2 and Table) that argatroban is highly neuroprotective up to 3 hours, a clinically relevant delay4, 14. Correspondingly, argatroban given up to 1 hour after ischemia showed significant benefit on 48-hour lesion volumes (Fig. 2). STAIR and other guidelines have emphasized behavioral outcomes as the more clinically relevant4 and our data supports that conclusion that argatroban therapy is effective as measured with histological or behavioral outcomes.
There are limitations to the studies presented here. Of necessity, the quantal bioassays and TTC lesion volumes were measured after 48 hours, while in separate groups behavior testing and histomorphometry were performed after several weeks. Thus, the results cannot be directly compared. Neither approach considers physiological effects, such as cortical spreading depression, that could mediate behavioral impairments. Further studies using serial functional or blood flow studies could shed light on these phenomena. Traditional limitations of animal studies—lack of blinding and randomization—were avoided in these studies.
We confirmed that the direct thrombin inhibitor argatroban benefits several measures of outcome after MCAo, likely as a result of thrombin inhibition since intra-arterial thrombin worsened outcomes. Future studies will be needed to determine if this effect generalizes to include other thrombin inhibitors.
Acknowledgments
Sources of Funding: this work was supported by the NINDS, R01 NS075930 (Lyden).
Footnotes
Disclosures:
Dr. Lyden discloses a consulting relationship with ZZ Biotech, LLC, as well as a research grant from the National Institutes of Health that supported this research. All other authors state that they have no financial disclosures.
References
- 1.Chen B, Friedman B, Whitney MA, Winkle JA, Lei IF, Olson ES, et al. Thrombin activity associated with neuronal damage during acute focal ischemia. J Neurosci. 2012;32:7622–7631. doi: 10.1523/JNEUROSCI.0369-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris DC, Zhang L, Zhang ZG, Lu M, Berens KL, Brown PM, et al. Extension of the therapeutic window for recombinant tissue plasminogen activator with argatroban in a rat model of embolic stroke. Stroke. 2001;32:2635–2640. doi: 10.1161/hs1101.097390. [DOI] [PubMed] [Google Scholar]
- 3.O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 4.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR Guidelines: Escalating STAIR and STEPS for Effective Translational Research. Transl Stroke Res. 2013;4:279–285. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Winkle JA, Chen B, Lei IF, Pereira B, Rajput PS, Lyden PD. Concurrent middle cerebral artery occlusion and intra-arterial drug infusion via ipsilateral common carotid artery catheter in the rat. J Neurosci Methods. 2012;213:63–69. doi: 10.1016/j.jneumeth.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyden P, Lonzo L, Nunez S, Dockstader T, Mathieu-Costello O, Zivin JA. Effect of ischemic cerebral volume changes on behavior. Behav Brain Res. 1997;87:59. doi: 10.1016/s0166-4328(96)02269-3. [DOI] [PubMed] [Google Scholar]
- 8.Lyden PD, Lonzo L, Nunez S. Combination chemotherapy extends the therapeutic window to 60 minutes after stroke. Journal of Neurotrauma. 1995;12:223. doi: 10.1089/neu.1995.12.223. [DOI] [PubMed] [Google Scholar]
- 9.Morris RGM, Garrud P, Rawlins JNP, O'Keffe JO. Place navigation impaired in rats with hippocampal lesions. Nature (Lond) 1982;297:681. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 10.Rapp JH, Pan XM, Neumann M, Hong M, Hollenbeck K, Liu J. Microemboli composed of cholesterol crystals disrupt the blood-brain barrier and reduce cognition. Stroke. 2008;39:2354–2361. doi: 10.1161/STROKEAHA.107.496737. [DOI] [PubMed] [Google Scholar]
- 11.Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem. 2006;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Shin C, Jackson-Friedman C, Lyden PD. Quantitative effects of nefiracetam on spatial learning of rats after cerebral embolism. J Stroke Cerebrovasc Dis. 2001;10:99–105. doi: 10.1053/jscd.2001.25454. [DOI] [PubMed] [Google Scholar]
- 13.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 14.Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath PM, et al. Good laboratory practice: preventing introduction of bias at the bench. Stroke. 2009;40:e50–52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]