Abstract
Chromogranin A (ChgA) is a beta cell secretory granule protein and a peptide of ChgA, WE14, was recently identified as a ligand for diabetogenic CD4 T cell clones derived from the NOD mouse. In this study we compared responses of human CD4 T cells from recent onset type 1 diabetic (T1D) and control subjects to WE14 and to an enzymatically modified version of this peptide. T cell responders to antigens were detected in PBMCs from study subjects by an indirect CD4 ELISPOT assay for IFN-γ. T1D patients (n=27) were recent onset patients within one year of diagnosis, typed for HLA-DQ8. Controls (n=31) were either 1st degree relatives with no antibodies or from the HLA-matched general population cohort of DAISY/TEDDY. A second cohort of patients (n=11) and control subjects (n=11) was tested at lower peptide concentrations. We found that WE14 is recognized by T cells from diabetic subjects vs. controls in a dose dependent manner. Treatment of WE14 with transglutaminase increased reactivity to the peptide in some patients. This work suggests that ChgA is an important target antigen in human T1D subjects and that post-translational modification may play a role in its reactivity and relationship to disease.
Keywords: Type 1 Diabetes, Chromogranin A, WE14, Autoreactive CD4 T cells, Human, Transglutaminase, Post-translational Modification, Autoantigen
1. Introduction
Autoreactive T cells directed toward β-cell antigens play a central role in the destruction of β-cells in human type 1 diabetes (T1D) and in the NOD mouse model of T1D. The presence of autoreactive T cells in individuals may indicate an ongoing attack on β-cells and can serve as an important biomarker for pre-diabetic status. This time-window in disease development, before overt hyperglycemia, is of key interest for therapeutic intervention strategies, such as antigen-specific tolerance induction, to prevent disease onset [1, 2].
Using a proteomic strategy, we identified Chromogranin A (ChgA) as the target antigen for diabetogenic CD4 T cell clones, including the widely used BDC-2.5 T cell, derived from NOD mice [3]. The peptide WE14, a naturally occurring cleavage product of ChgA, is a weak antigen for these T cell clones in vitro, but the antigenic activity of the peptide is dramatically increased upon treatment with the enzyme tissue transglutaminase (TGase), which is known to covalently crosslink proteins through the formation of isopeptide bonds as well as catalyze glutamine deamidation reactions, such as those that occur in the posttranslational modification of gliadin in celiac disease. In the case of ChgA-reactive T cell clones from non-obese diabetic (NOD) mice, crosslinking rather than deamidation is involved in rendering WE14 more antigenic upon TGase treatment [4]. Since the amino acid sequences of human and mouse WE14 are identical, with the exception of one amino acid [5], we tested T cells from newly diagnosed T1D patients and controls for responses to human WE14 or a TGase-converted form of this peptide. We employed an indirect ELISPOT assay to compare responses to WE14 and TGase-modified WE14 in CD4 T cells from diabetic and control subjects.
2. Materials and Methods
2.1 Study participants
Participants of both genders, aged 9–44 years, and typed for HLA DQ8, were recruited from patients, relatives, and volunteers attending the Barbara Davis Center for Diabetes, in accordance with protocols approved by the Colorado Multiple Institutional Review Board. Subjects with clinical disease of over one year duration at the time of blood draw were excluded from the study, as were control subjects who tested positive for one or more diabetes autoantibodies (insulin autoantibody [IAA], autoantibody to GAD65 [GADA], ICA512, or ZnT8A). An initial cohort (Cohort 1, see Table 1) was used to look at the effect of 20 and 40 μM doses of WE14 and TGase-treated WE14. A second cohort (Cohort 2, see Table 1) was recruited to examine a range of lower peptide concentrations.
Table 1.
Demographics of Subjects
| Cohort 1 | Cohort 2 | |||
|---|---|---|---|---|
| Controls* | T1D** | Controls* | T1D** | |
| Number of subjects | 31 | 27 | 11 | 11 |
| Age at blood draw (median, range) | 25, 8–44 | 18, 9–39 | 26, 16–33 | 15, 10–23 |
| Gender M/F | 10/21 | 16/11 | 4/7 | 6/5 |
| Antibody Positive (IAA, GAD65, IA- 2, ZNT8) | 1***/28 | 24/27 | 0/11 | 11/11 |
| HLA DQ8 | 26/30 | 24/27 | 2/11 | 4/11 |
| HLA DR3/DR4 | 3/30 | 10/27 | 1/11 | 1/11 |
Controls are either 1st degree relatives with no antibodies or HLA matched general population cohort of DAISY/TEDDY
T1D are recent onset patients within 6 months of diagnosis who were typed for HLA-DQ8
Antibody-positive control was not removed from the dataset; p-values did not differ significantly when data were analyzed with and without this value.
2.2 Peptides
Peptides P1 (Proinsulin C19-A3; GSLQPLALEGSLQKRGIV), Insulin B9-23 (SHLVEALYLVCGERG), DR3-restricted GAD3 (GAD65 335–352; TAGTTVYGAFDPLLAVAD), DR4-restricted GAD4 (GAD65 554–575; VNFFRMVISNPAATHQDIDFLI), and R2 (IA-2 853–872; SFYLKNVQTQETRTLTQFHF) were synthesized at > 95% purity (U. of Colorado Cancer Center Proteomics Core). A DQ8-restricted epitope of HA (Influenza Hemagglutinin MP 185–205; EEVDMTPADALDDFD), the kind gift of W. Kwok, Benaroya Inst., was synthesized at > 95% purity (GenScript USA Inc. 860 Centennial Ave., Piscataway, NJ 08854,USA). WE14 (ChgA342-355; WSKMDQLAKELTAE) was synthesized at > 95% purity (Chi Scientific). All peptides were added to assays from stocks of 10 mM in DMSO. Pediacil® and Tetanus Toxoid were used as control antigens.
2.3 Transglutaminase treatment
Cohort 1: A solution of 250 μl of WE14 in PBS (5 mg/ml) was added to 4.75 ml of a reaction mixture containing 1.1 mM EDTA, 2.1 mM DTT, 52.6 mM NaCl, 52.6 mM HEPES (pH 8.0), 42.1 mM CaCl2 and 105 mU/ml TGase (Sigma). This mixture was incubated for 4 h at 37° C and then centrifuged at 1000 g for 10 min; the pellet was resuspended in 375 μl PBS and 10 μl aliquots were frozen at −80° C. Cohort 2: A solution of 100 μl of WE14 in H2O (5 mg/ml) was added to 400 μl of a reaction mixture containing 6.25 mM DTT, 62.5 mM NaCl, 62.5 mM Tris (pH 8.2), 12.5 mM CaCl2 and 250 mU/ml TGase (Sigma). The solution was incubated for 7 h at 37° C followed by freezing of 22 μl aliquots at −80° C. Control samples (WE14 only, TGase only) were prepared in the same manner.
2.4 ELISPOT analysis
Indirect ELISPOT analyses were conducted as described previously [6], using the human IFN-γ ELISPOT kit (U-CyTech Biosciences, Utrecht, The Netherlands). Briefly, PBMCs from subjects were incubated with peptide or antigens for 48 hours and subsequently transferred to plates coated with the anti–IFN-γ capture antibody followed by overnight incubation. Cells were removed and wells were washed. Spots were then formed by sequential incubations with the biotinylated second site anti–IFN-γ, gold-labeled goat anti-biotin, and a precipitating silver substrate; spots were enumerated with a Bioreader 4000 Pro X (BIOSYS, Karben, Germany). For each antigen tested, the total spots from three 96 well plates are added, reflecting spot forming cells per 9 x 105 total cells. In this study, a stimulation index (SI) of > 2.0 was considered a positive response (SI = total spots for analyte/total spots for DMSO). All subjects were tested with ChgA peptide (WE14) and control peptides (tetanus, Pediacel). Due to cell number limitations, some subjects were not tested with all previously identified diabetes-related peptides although for each analyte the percentage tested was > 66%.
2.5 Autoantibody measurements
IAA, GADA, and ICA512 were determined by the UCD DERC clinical core using established assays [7, 8]. ZnT8A were either determined using the “standard” RIA [9], or a modified procedure using a trimeric probe containing sequentially the R, Q, and W variants of the ZnT8 C-terminal domain [10].
2.6 HLA genotyping
HLA genotyping was performed by the UCD DERC clinical core. Individual DRB1 and DQB1 alleles were identified by reverse hybridization of PCR amplicons (18) to either sequence specific oligonucleotide bead arrays (DRB1; Luminex xMAP; One Λ, Canoga Park, CA), or linear arrays (DQB1; Roche Molecular Systems, Alameda, CA), respectively.
2.7 Statistics
The stimulation Index (SI) for each peptide/antigen was computed and then an unpaired two-tailed Student’s T test was performed for each peptide/antigen within their respective cohort. Significance was defined as < 0.05. All data was analyzed using Prism software (GraphPad Software, San Diego, CA).
3. Results
3.1 Population characteristics
Recent onset patients averaged 15.5 years of age with a range of 9–39 vs. control subjects who averaged 25.5 years (14–44). The majority of both subject populations were HLA-DQ8, the proposed restriction element for reactivity to WE14. Most of the T1D patients were positive for at least one autoantibody (IAA, GADA, ICA512, and ZnT8).
3.2 T cell responses of first cohort to WE14
Patient and control PBMC were analyzed for T cell responses to WE14 and other antigens by ELISPOT. Results for the first cohort are illustrated in Table 2 and show that both controls and T1D patients responded strongly to the positive control, tetanus toxoid, with no statistical difference between the two groups. In contrast, as reported previously [11] a higher number of IFN-γ producing cells to the insulin peptide B9-23 could be detected in T1D patients. In addition, DR4-restricted peptides from GAD65 (GAD 4) and proinsulin (P 1), previously reported to elicit a strong response in PBMCs from T1D patients [12], trended toward but did not reach significance in this cohort. We also observed no significant differences between T1D patients and controls to a peptide from I-A2 (R 2). Analysis of WE14 responses in 27 T1D subjects vs. 31 control subjects demonstrated that T1D subjects reacted more strongly to WE14 compared to control subjects at both concentrations tested, a result that was statistically significant for the highest concentration of untreated WE14 at 40 μM (T1D: SI 2.6 vs. Con: SI 1.5, p = 0.042) and almost significant at 20 μM (T1D: SI 2.5 vs. Con: SI 1.5, p = 0.086). T1D subjects also reacted more strongly to TGase-treated WE14, and as shown in Table 2, this result was statistically significant at concentrations of WE14 at 40 μM (T1D: SI 3.5 vs. Con: SI 1.8, p = 0.040) and 20 μM (T1D: SI 2.8 vs. Con: SI 1.3, p = 0.005).
Table 2.
Cohort 1 - T cell Responses to defined autoantigens and WE14 TGase treated and untreated
| ANTIGEN | T1D Mean SI | +/− | Control Mean SI | +/− | P-Value* |
|---|---|---|---|---|---|
| Tetanus Toxoid | 58.1 | 73.9 | 61.9 | 81.1 | 0.895 |
| Pediacel® | 123.4 | 95.8 | 145.2 | 132.1 | 0.452 |
| P 1 | 2.5 | 3.1 | 0.9 | 0.8 | 0.079 |
| B 9-23 | 2.4 | 2.7 | 1.1 | 1.5 | 0.047 |
| GAD 3 | 2.5 | 3.7 | 1.7 | 1.7 | 0.441 |
| GAD 4 | 3.1 | 3.9 | 1.8 | 2.1 | 0.148 |
| R 2 (IA-2) | 2.0 | 2.4 | 2.2 | 2.7 | 0.873 |
| WE14, 20 μM | 2.5 | 2.3 | 1.5 | 1.5 | 0.086 |
| WE14, 40 μM | 2.6 | 2.7 | 1.5 | 1.4 | 0.042 |
| WE14 + TGase, 20 μM** | 2.8 | 3 | 1.3 | 1.3 | 0.005 |
| WE14 + TGase, 40 μM** | 3.5 | 4.2 | 1.8 | 1.4 | 0.040 |
| TGase only | 1.3 | 1.4 | 1.4 | 1.7 | 0.680 |
SI was computed as total spot numbers per analyte/total spot numbers for no antigen. Results were compared using Student’s T test. Values which were significant are in bold type. Spot numbers for no antigen for type 1 patients was 2.5 +/− 2.0 and for control subjects 2.7 +/− 3.4.
Due to the TGase-treatment protocol used for cohort 1, the concentrations shown represent theoretical maximal concentrations. Actual concentrations may be significantly lower.
3.3 T cell responses to lower concentrations of WE14
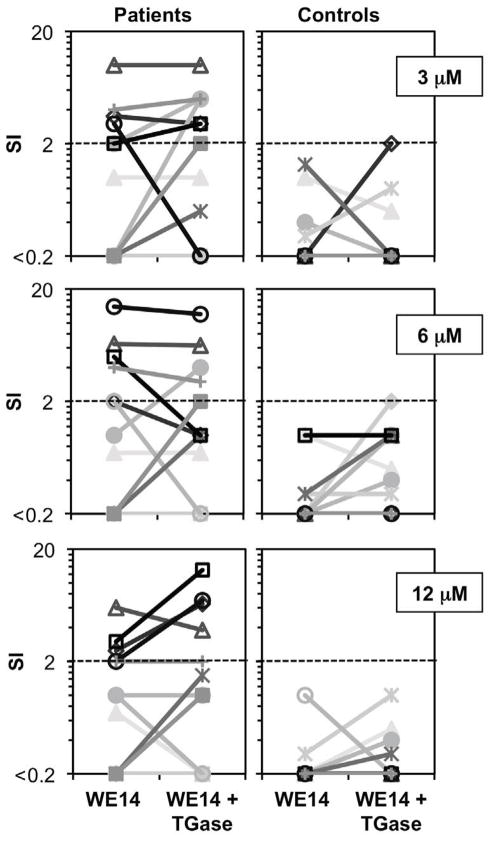
Based on observations from cohort 1, we tested whether lower concentrations of untreated as well as TGase-treated WE14 could elicit T cell responses using a second cohort of diabetic and control subjects (see Table 1). With these samples, we used an optimized TGase-treatment protocol that led to an improved gain in antigenicity for murine WE14 when tested with the BDC-2.5 T cell clone (data not shown). Compared to the control subjects, we detected statistically significant differences in responses from T1D patients to WE14 ranging from 3 to 12 μM, with or without TGase (Table 3, Fig. 1). As shown in Fig. 1, T cell responses of control subjects to treated or untreated WE14 did not exceed a stimulation index (SI) of 2.0 at any of the peptide concentrations tested, in clear contrast to the responses detected in patients. At a peptide concentration of 3 μM the patient group contained 3/11 subjects that exceeded the SI of 2 for the untreated peptide and 5/11 subjects exceeded the SI of 2 for the TGase-treated peptide. At a peptide concentration of 6 μM the patient group contained 4/11 subjects that exceeded the SI of 2 for both the treated and untreated peptide. At a peptide concentration of 12 μM this group contained 3/11 subjects that exceeded the SI of 2 for the untreated peptide and 4/11 for the treated peptide. Overall, the gain of antigenicity for the TGase-treated peptides was most noticeable at a peptide concentration of 12 μM. Interestingly in this cohort, 4 out of 7 WE14 responders bear HLA alleles other than DQ8, suggesting that other alleles can present this peptide.
Table 3.
Cohort 2 - T cell Responses to defined autoantigens and WE14 TGase-treated and untreated
| ANTIGEN | T1D Mean SI | +/− | Control Mean SI | +/− | P-Value* |
|---|---|---|---|---|---|
| Pediacel® | 256.4 | 143.0 | 245.8 | 95.7 | 0.841 |
| WE14, 3 μM** | 1.5 | 1.6 | 0.3 | 0.5 | 0.028 |
| WE14, 6 μM | 2.6 | 4.3 | 0.3 | 0.4 | 0.068 |
| WE14, 12 μM | 1.4 | 1.8 | 0.1 | 0.3 | 0.033 |
| WE14 + TGase, 3 μM** | 2.2 | 2.2 | 0.3 | 0.6 | 0.014 |
| WE14 + TGase, 6 μM | 2.8 | 3.6 | 0.7 | 0.6 | 0.063 |
| WE14 + TGase, 12 μM | 2.1 | 2.6 | 0.2 | 0.3 | 0.027 |
| TGase only** | 1.0 | 1.3 | 0.5 | 0.5 | 0.223 |
SI was computed as total spot numbers per analyte/total spot numbers for no antigen. Results were compared using Student’s T test. Values which were significant are in bold type. Spot numbers for no antigen for type 1 patients was 1.5 +/− 2.8 and for control subjects 1.1 +/− 1.6.
One patient was not tested at this condition.
FIG 1.
Dose titration responses of cohort 2 subjects to TGase-treated and untreated WE14. PBMCs from T1D patients and controls were incubated with various concentrations of WE14 and TGase-treated WE14. IFN-γ producing cells were detected by indirect ELISPOT analysis and SI values were calculated as described in the Methods section.
4. Discussion
Results of this study of cellular autoimmunity indicate that the ChgA-derived peptide WE14 is a target for autoreactive cells in new onset T1D patients. Additionally, modification of WE14 by the enzyme TGase improves the antigenicity of this peptide in some patients. ChgA is an attractive target of the immune response since it is localized in the beta cell granule and so joins insulin, IA-2 and ZnT8 in that respect [10]. Post-translational modification of proteins may strengthen weak immune responses or lead to novel responses to these neo-antigens and so can accelerate the autoimmune process targeting the beta cell [13]. Our data suggests that WE14 is recognized by new onset T1D subjects compared to control subjects. The 48 hour ELISPOT assay favors CD4 vs. CD8 T cell responses [6], but in these studies we cannot exclude that the reactivity we observed was also due to CD8 T cells. A limitation of this preliminary study is the small sample size and its focus on particular HLA alleles. In future studies, we will not only examine more subjects, but also extend these observations to prediabetic subjects. We hope to examine the temporal relationship of reactivity to untreated WE14 and TGase-treated WE14 as subjects progress towards diabetes and also examine when reactivity occurs in comparison to other autoantigenic peptides that have been identified. By design, most of our patients were DQ8, which is the human equivalent of the restriction element used by NOD mice (I-Ag7), and therefore we assume that the T cell reactivity we observed to WE14 is due to presentation by DQ8. Based on the experiments performed to date, we have seen reactivity in 5 patients and 4 control subjects who do not bear HLA DQ8 and therefore other alleles including disease-related DQ2 cannot be excluded from presenting this peptide.
Enzymatic modification of the WE14 peptide led to marked increases in antigenicity in experiments performed with murine T cell clones, suggesting that post-translational modification could contribute to the generation of an autoantigenic peptide from ChgA. TGase catalyzes a variety of protein modifications, chief of which are deamidation and crosslinking [14] [15]. Deamidation was ruled out as the mechanism for enhanced antigenic activity of WE14 in the NOD mouse system as a deamidated version of this peptide was no more antigenic than unmodified WE14 [4]. On the other hand, data obtained from biochemical and mass spectrometric analysis indicate that high molecular weight aggregates between WE14 and TGase are formed and that crosslinking reactions may lead to the increased antigenicity of the peptide after exposure to TGase (Delong et al., unpublished data). Since beta granule proteins should not under normal circumstances elicit an immune response, aberrant post-translational modification of peptides provides an attractive hypothesis for how beta cell self-antigens are generated. In conclusion, we have demonstrated that WE14 appears to be targeted by human CD4 T cells in T1D subjects. Validation and subsequent comparison to other diabetes-related antigens should improve our ability to define T cell reactivity in type 1 diabetes.
Supplementary Material
Highlights.
New onset type 1 diabetes patients have a significantly elevated T cell response to the peptide WE14.
Some patients show an improved T cell response to the transglutaminase-treated peptide WE14.
Posttranslationally modified antigens may play a critical role in the development of type 1 diabetes.
Acknowledgments
We thank the DAISY and TEDDY studies for providing control subjects for these studies.
This work was supported by JDRF Innovative grant 5-2009-724 (KH), JDRF postdoctoral fellowship 3-2010-453 (TD), NIH K01 1K01DK094941-01 (TD), NIH 5T32AI007405-20 (RB), NIH P30DK057516 (DERC core) and JDRF 4-2007-1056 (JDRF center grant).
Footnotes
Peter Gottlieb and Kathryn Haskins are the guarantors of this work, had full access to all the data, and take full responsibility for the integrity of data and the accuracy of data analysis.
Conflicts of interest disclosure
The authors declare no conflict of interest related to this study.
Author contributions: P.G., T.D., R.B., L.F., R.W., and K.H. designed and/or performed experimental work; G.C., M.R., and A.M. provided patients and reagents utilized in these studies. T.D., P.G., R.B., and K.H. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Peter A Gottlieb, Email: Peter.Gottlieb@UCDenver.edu.
Thomas Delong, Email: Thomas.Delong@UCDenver.edu.
Rocky L. Baker, Email: Rocky.Baker@UCDenver.edu.
Lisa Fitzgerald-Miller, Email: Lisa.Fitzgerald-Miller@UCDenver.edu.
Rebecca Wagner, Email: Rebecca.Wagner@UCDenver.edu.
Gabrielle Cook, Email: Gabrielle.Cook@UCDenver.edu.
Marian R. Rewers, Email: Marian.Rewers@UCDenver.edu.
Aaron Michels, Email: Aaron.Michels@UCDenver.edu.
Kathryn Haskins, Email: Katie.Haskins@UCDenver.edu.
References
- 1.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. The New England journal of medicine. 1986;314:1360–8. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Steinman L. Immune therapy for autoimmune diseases. Science. 2004;305:212–6. doi: 10.1126/science.1099896. [DOI] [PubMed] [Google Scholar]
- 3.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nature immunology. 2010;11:225–31. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delong T, Baker RL, He J, Barbour G, Bradley B, Haskins K. Diabetogenic T-cell clones recognize an altered peptide of chromogranin A. Diabetes. 2012;61:3239–46. doi: 10.2337/db12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curry WJ, Shaw C, Johnston CF, Thim L, Buchanan KD. Isolation and primary structure of a novel chromogranin A-derived peptide, WE-14, from a human midgut carcinoid tumour. FEBS letters. 1992;301:319–21. doi: 10.1016/0014-5793(92)80266-j. [DOI] [PubMed] [Google Scholar]
- 6.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. The Journal of clinical investigation. 2004;113:451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianani R, Rabin DU, Verge CF, Yu L, Babu SR, Pietropaolo M, et al. ICA512 autoantibody radioassay. Diabetes. 1995;44:1340–4. doi: 10.2337/diab.44.11.1340. [DOI] [PubMed] [Google Scholar]
- 8.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1701–6. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17040–5. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzlau JM, Frisch LM, Hutton JC, Davidson HW. Mapping of conformational autoantibody epitopes in ZNT8. Diabetes/metabolism research and reviews. 2011;27:883–6. doi: 10.1002/dmrr.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. The Journal of clinical investigation. 2001;107:173–80. doi: 10.1172/JCI8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagata M, Kotani R, Moriyama H, Yokono K, Roep BO, Peakman M. Detection of autoreactive T cells in type 1 diabetes using coded autoantigens and an immunoglobulin-free cytokine ELISPOT assay: report from the fourth immunology of diabetes society T cell workshop. Annals of the New York Academy of Sciences. 2004;1037:10–5. doi: 10.1196/annals.1337.002. [DOI] [PubMed] [Google Scholar]
- 13.Delong T, Baker RL, He J, Haskins K. Novel autoantigens for diabetogenic CD4 T cells in autoimmune diabetes. Immunologic research. 2013;55:167–72. doi: 10.1007/s12026-012-8375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folk JE, Finlayson JS. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Advances in protein chemistry. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- 15.Folk JE. Transglutaminases. Annual review of biochemistry. 1980;49:517–31. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.