Abstract
Obesity and eating disorders are prevailing health concerns worldwide. It is important to understand the regulation of food intake and energy metabolism. Thiamine (vitamin B1) is an essential nutrient. Thiamine deficiency (TD) can cause a number of disorders in humans, such as Beriberi and Wernicke-Korsakoff syndrome. We demonstrated here that TD caused anorexia in C57BL/6 mice. After feeding a TD diet for 16 days, the mice displayed a significant decrease in food intake and an increase in resting energy expenditure (REE), which resulted in a severe weight loss. At the 22nd day, the food intake was reduced by 69% and 74% for male and female mice, respectively in TD group. The REE increased by 9 folds in TD group. The loss of body weight (17–24%) was similar between male and female animals and mainly resulted from the reduction of fat mass (49% decrease). Re-supplementation of thiamine (benfotiamine) restored animal's appetite, leading to a total recovery of body weight. The hypothalamic AMPK is a critical regulator of food intake. TD inhibited the phosphorylation of AMPK in the arcuate nucleus (ARN) and paraventricular nucleus (PVN) of the hypothalamus without affecting its expression. TD-induced inhibition of AMPK phosphorylation was reversed once thiamine was re-supplemented. In contrast, TD increased AMPK phosphorylation in the skeletal muscle and upregulated the uncoupling protein (UCP)-1 in brown adipose tissues which was consistent with increased basal energy expenditure. Re-administration of thiamine stabilized AMPK phosphorylation in the skeletal muscle as well as energy expenditure. Taken together, TD may induce anorexia by inhibiting hypothalamic AMPK activity. With a simultaneous increase in energy expenditure, TD caused an overall body weight loss. The results suggest that the status of thiamine levels in the body may affect food intake and body weight.
Keywords: AMP-activated protein kinase, nutrition, neurological disorders, obesity, vitamin B1
INTRODUCTION
Thiamine, also known as vitamin B1, is an essential nutrient and plays an important role in metabolic and cellular function in all living organisms. Thiamine is found in high concentrations in skeletal muscle, heart, liver, kidneys and brain. The thiamine-dependent enzyme α-ketoglutarate dehydrogenase complex (KGDHC) is a key and perhaps rate-controlling enzyme of the citric acid cycle. In the brain, thiamine is crucial to several biochemical pathways, such as intermediate carbohydrate and lipid metabolism, as well as the production glucose-derived neurotransmitters acetylcholine and gamma-aminobutyric acid (GABA) (Butterworth et al., 1986). The central nervous system (CNS) is particularly sensitive to the disturbance of thiamine due to its heavy dependence on oxidative metabolism. Thiamine deficiency (TD) causes neurological deficits and a number of disorders in humans, such as Beriberi, Wernicke's encephalopathy and Wernicke-Korsakoff syndrome (Vetreno et al., 2012). The most common cause of TD is chronic alcohol consumption, inadequate uptake or deficient absorption of thiamine (Jhala and Hazell, 2011). TD-induced neurological manifestations include ataxia, nystagmus, ophthalmoplegia, change in consciousness/mental status and memory deficits (Sechi and Serra, 2007). TD in rodents causes similar neurological symptoms, providing good experimental models to investigate anatomical and physiological underpinnings of human neurological disorders and underlying mechanisms (Vetreno et al., 2012). Studies using TD rodent models have revealed numerous neuroanatomical, neurochemical, and behavioral changes. For example, TD induced an increase of extracellular glutamate level in the thalamus (Langlais and Zhang, 1993), a decrease of GABA activity in the media thalamus (Heroux and Butterworth, 1988) and alterations in dopamine metabolism (Mousseau et al., 1996). The abnormal behaviors associated with TD include memory and learning impairment on tasks (Nakagawasai et al., 2000, Nakagawasai et al., 2001), increase of pain threshold to noxious heat stimulation (Tadano et al., 1995), and a loss of righting reflex and body weight (Zhang et al., 1995, Nakagawasai et al., 2004). TD The loss of body weight is observed in TD rodent model and patients with Wernicke-Korsakoff syndrome (Nakagawasai et al., 2004, Saad et al., 2010). However, it is unclear how TD causes body weight loss.
Herein, we show that a TD diet significantly decreased food intake which was accompanied by an increase in energy expenditure in C57BL/6 mice, resulting in a body weight loss. Thiamine re-supplementation restored food intake, and reversed TD-induced body weight loss. Adenosine monophosphate-activated protein kinase (AMPK) in the hypothalamus has emerged as a key kinase regulating neuroendocrine feedback control of food intake and energy metabolism (Stark et al., 2012). Our data indicate that TD inhibits hypothalamic AMPK phosphorylation and therefore TD-mediated anorexia may be mediated by alterations in AMPK activity in the brain.
MATERIALS AND METHODS
Animals and diets
Ten–week–old C57BL/6 mice were obtained from Harlan Laboratories (Indianapolis, IN), and housed under a 12-h light–dark cycle with free access to food and water in the Division of Lab Animal Resources at the University of Kentucky. Experimental protocols involving the animals were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
After a one week habituation period, mice were randomly selected and assigned to one of three dietary groups: 1) the control group was provided daily with a normal liquid diet. The normal liquid diet contained 1.5 mg thiamine HCl/liter, and was made from powder (product No. F1259SP, Bio-Serv, Frenchtown, NJ) following manufacturer's instructions; 2) the TD group was provided daily with the same liquid diet as the control group but the thiamine was removed from the diet (product No. F6275SP, Bio-Serv); and 3) the TD+T group at first was provided daily with TD liquid diet, then was switched to a normal liquid diet plus an additional synthetic S-acyl derivative of thiamine, benfotiamine (Sigma-Aldrich, St. Louis, MO), at a dose of 70 mg/kg body weight/day (Volvert et al., 2008). For both control and TD diet, the breakdown of calories is 150 kcal /liter in protein, 360 kcal /liter in fat, and 490 kcal /liter in carbohydrates. During the treatment, the consumed amount of liquid diet for each animal was recorded daily, the body weight for each mouse was weighed every other day, and the gross appearance and general behaviors (e.g., attentiveness, movement, righting reflex and motor control/coordination) were monitored daily. At the end of experiment, for histological staining, animals were deeply anesthetized with ketamine/xylazine (100 mg/kg/10 mg/kg, Butler Schein Animal Health, Dublin, OH) and subjected to transcardial perfusion with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde, then the brain, brown adipose tissues (between the scapulae), and skeletal muscles (in the hindlimb) from each animal were removed, equilibrated in 30% sucrose and later cryosectioned. For immunoblotting analysis, animals were deeply anesthetized and the hypothalamus, brown adipose tissues and skeletal muscles were promptly dissected, frozen in liquid nitrogen and stored at −80°C.
Indirect calorimetry
In rodent models, energy expenditure can be directly assessed by measuring oxygen consumption or heat production (Flier, 2004). However, the measurement of respiratory exchanges has become a means to measure energy expenditure, bypassing the complex, delicate, and costly technique of direct measurement of heat production (Even and Nadkarni, 2012). In our study, energy expenditure was measured by indirect calorimetry which is calculated using oxygen consumption (VO2), carbon dioxide production (VCO2) and physiological constants. Briefly, the mice were grouped as threes in cages and fed liquid diet for 7 days in the regular animal facility. Then they were moved to the TSE LabMaster chamber (one mouse per chamber) for an acclimation period of 7 days and calorimetry of 19 days. The TSE LabMaster Indirect Calorimetry System was used to monitor energy expenditure and activity of mice in individual chambers. Each chamber (235 × 135 × 130 mm) was instrumented to measure oxygen consumption (VO2) and carbon dioxide production (VCO2) from a single animal. Physical activity of the animal on the long axis of the chamber was measured by an array of infrared beams and sensors. Room air was pumped into the chamber and cage air was sampled every 30 minutes through the outlet valve to an in-line oxygen analyzer. In general, the entire system is computer-driven with continuous monitoring of air and activity, and activity was recorded continuously and reported at thirty minute intervals. In addition, food was provided through a dispenser with a liquid diet feeding tube (capacity 50 ml, Bio-Serv), and the consumption of food was recorded daily.
To calculate the resting energy expenditure (REE), the mean value of energy expenditure (EE) was adopted when the parameters were recorded at the animal resting activity status. Data points were collected between 10:00 AM and 6:00 PM (the time during the light phase when human activity in the room is minimized and the mice are resting), and filtered for intervals during which the activity was less than 150 counts. REE (watts/60min) is the equation of [(3.941 × VO2 + 1.106 × VCO2) × 0.001/1.163] and normalized to net kcal feed. The liquid diets are 1 kcal /ml (Bio-Serv).
Body composition analysis
EchoMRI-5000 Whole Body Composition (Echo Medical System, Houston, TX) was used to measure the lean tissue mass and fat mass (g) in mice at the beginning of the experiment and at the end of calorimetry measurement.
Nissl staining
The brain tissue was cryosectioned at 30 μm using a sliding microtome (Leica Microsystems, Wetzlar, Germany) for Nissl staining. Nissl staining was performed in a series composed of every twenty forth section of the brain (12 sections per brain), which was from rostral (Bregma 1.42 mm) to caudal (Bregma – 6.96 mm) based on the reference atlas of The Mouse Brain in Stereotaxic Coordinates (Paxino and Franklin, 2001). The brain sections were stained in 0.1% cresyl violet solution, and differentiated in 95% ethyl alcohol.
Immunohistochemistry
For immunostaining, the brain tissue was sectioned in coronal planes at 30 μm thickness using a sliding microtome. For immunostaining on adipose tissues and skeletal muscles, the tissue was sectioned at 10 μm on a cryostat (Thermo scientific, Kalamazoo, MI). As previously described (Liu et al., 2010), sections were incubated overnight at 4°C with a primary antibody, pAMPKα (polyclonal, 1:250, Cell Signaling Technology, Beverly, MA) or UCP1 (polyclonal, 1:200, Santa Cruz Biotech, Santa Cruz, CA). After washes and incubation with an appropriate secondary antibody (Vector Laboratories, Burlingame, CA), immunoreactive cells were visualized by the avidin-biotin immunoperoxidase method (ABC kits, Vector Laboratories) with chromogen 3, 3'-diaminobenzidine tetrahydrochloride (Sigma-Aldrich). Sections immunostained with UCP1 in adipose tissues and pAMPKα in skeletal muscles were counterstained with Harris hematoxylin to reveal the location of positive signals in the cell body. For immunostaining of pAMPKα, phosphatase inhibitor NaF (1 mM) was added to PBS.
Immunofluorescence
Double immunofluorescent staining was performed on the brain sections as described (Liu et al., 2006). In brief, brain sections were incubated with primary antibody against pAMPKα (polyclonal, 1:100, Cell Signaling Technology) and NPY (monoclonal, 1:500, Abcam, Cambridge, MA) overnight at 4°C. After rinsing in PBS containing 1 mM NaF, sections were treated with Alexa488-conjugated goat anti-rabbit IgG (1:500, Invitrogen, Carlsbad, CA) and Alexa594-conjugated goat anti-mouse IgG (1:500, Invitrogen). The images were recorded using an Olympus microscope (BX61) equipped with a DP70 digital camera (Olympus, Center Valley, PA).
Immunoblotting
The procedure has been previously described with some minor modifications (Liu et al., 2008). Frozen tissue samples were sonicated in 1X RIPA buffer (Cell Signaling Technology) and 1mM PMSF with the sonic dismembrator (Qsonica, Newtown, CT). Protein concentration was determined for each sample by the Lowry assay with the DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Protein samples (30 μg per lane) were separated on an SDS-polyacrylamide gel by electrophoresis, and electrotransferred to a nitrocellulose membrane. The resulting blot was blocked in Tris-buffered saline containing 5% bovine serum albumin overnight at 4°C, then incubated with a primary antibody against target proteins for 2 hr. The primary antibodies used are pAMPKα (polyclonal, 1:1,000, Cell Signaling Technology), UCP1 (polyclonal, 1:500, Santa Cruz Biotech) and α-tubulin (monoclonal, 1:6,000, Cell Signaling Technology). After washing, the blot was incubated with appropriate horseradish peroxidase–conjugated secondary antibody (1:4,000, Sigma-Aldrich), developed by ECL-Plus chemiluminescent reagent (Amersham, Piscataway, NJ) and subsequently exposed using a Gel Logic 2200 Pro Imaging System (Rochester, NY). Signal specificity was ensured by omitting each primary antibody, and bands were normalized according to α-tubulin immunoreactive bands in the same blot. Density measurements for each band were performed with Scion Image software (Scion, Frederick, MD).
Statistical analysis
All data were analyzed using the computer statistical package SigmaPlot (San Jose, CA). The values are expressed as the mean ± SEM, and differences among means were analyzed by using one-way analysis of variance (ANOVA) with treatment as the independent factor. Fisher's Least-Significant-Difference post-hoc analysis was employed when differences were observed in ANOVA testing (p<0.05).
RESULTS
Thiamine deficiency (TD) diet induces anorexia and body weight loss
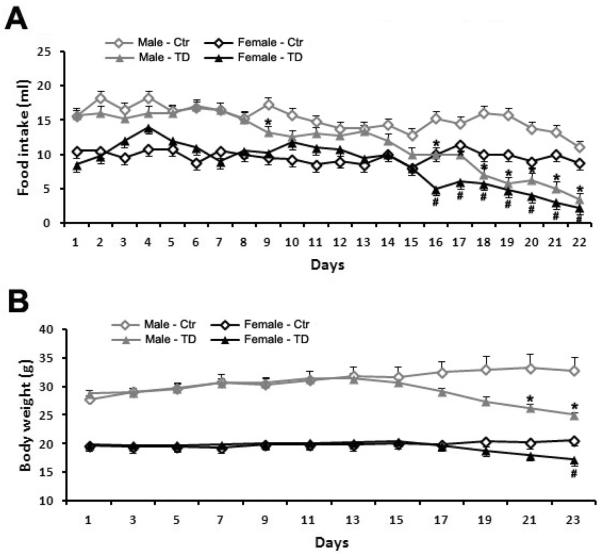
We examined the effects of TD diet on food intake and body weight in both male and female mice. With liquid diet, the food intake can be precisely recorded daily. The body weight was measured every other day. In comparison with their gender-matched controls, the food intake of both male and female mice in TD group significantly decreased on the 16th day and the decrease continued through the 22nd day, the end of experiment (Fig 1A. * p<0.05 vs. control male mice, # p<0.05 vs. control female mice, n = 4 each group). At the end time point, the food intake was reduced by 69.1% for male mice, and 74.3% for female mice in the TD group. Subsequently, a significant loss of body weight was observed since the 21st day in TD feeding male mice, and on the 23rd day in TD feeding female mice when compared to their gender-matched controls (Fig 1B. * p<0.05 vs. control male mice, # p<0.05 vs. control female mice, n = 4 each group). The body weight was decreased by 23.7% in male mice, and 16.7% in female mice in the TD group at the end of the experiment. The body weight loss is slightly more in male mice than in female mice. Thus, in the current mouse model, TD induced anorexia and body weight loss in both male and female animals. In general, there was no significant difference in gross appearance and behaviors, such as alertness, attentiveness and mobility, between control and TD mice. However, the male mice in TD group seemed to become more aggressive and began to fight and bite each other. Therefore, female mice were used for all subsequent studies.
Figure 1.
Effect of TD diet on food intake and body weight in male and female C57BL6 mice. A. Both male and female C57BL6 mice were fed with either a control or TD diet as described under the Experimental Procedures and the food intake was measured daily. A significant decrease in the food intake was observed on the 16th day in the TD group and the decrease continued to the end of the experiment in comparison with that of their gender-matched mice in the control group (*p<0.05 vs. control male mice, #p<0.05 vs. control female mice, n = 4 per group). B. Body weight was recorded for mice in control and TD groups. A significant decrease in the body weight was observed from the 21st day for male and the 23rd day for female mice in the TD group, respectively, in comparison with that of their gender-matched mice in the control group (*p<0.05 vs. control male mice, #p<0.05 vs. control female mice, n = 4 per group).
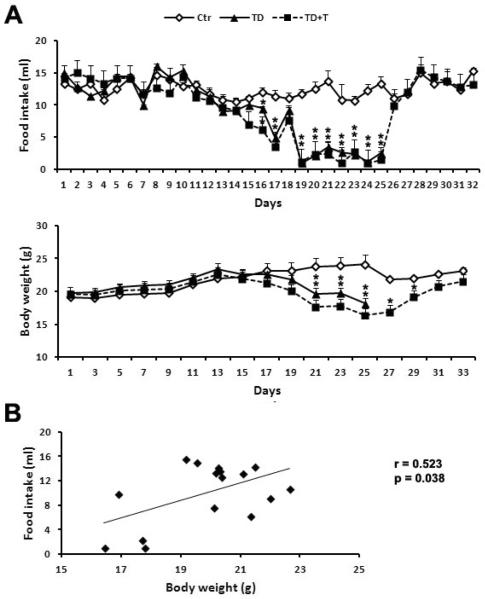
Next, we tested whether thiamine re-supplementation could reverse TD-induced anorexia and body weight loss. In this experiment, adult C57BL/6 female mice were randomly divided into three dietary groups: control, TD, and thiamine re-supplementation (TD+T) groups. In TD+T group, the mice were first fed with TD diet for 25 days; after that, mice were fed with normal diet plus the thiamine supplement benfotiamine. As shown in Fig. 2A, TD caused a significant decrease in food intake and loss of body weight since the 16th and the 21st day, respectively. The thiamine re-supplementation promptly restored the mice's appetite and increased the food intake which reached the levels of control mice; correspondingly, the mice in TD+T group gradually regained the body weight and returned to normal body weight by the 31st day, lasting until the 33rd day. Pearson's correlation coefficient revealed a positive correlation between food intake and body weight in the mice in TD+T group (Fig. 2B, r=0.523, p<0.05).
Figure 2.
Effect of thiamine supplementation on TD-induced decrease in food intake and body weight. A. Female C57BL6 mice were fed with either control or TD diet. The mice in TD+T group were changed to a normal liquid diet supplemented with additional benfotiamine since the 26th day. The food intake and body weight were recorded as described under the Experimental Procedures. *p<0.05 vs. control, n = 6 per group. B. Pearson's correlation coefficient is performed to determine the correlation between changes in food intake and body weight. A positive correlation was demonstrated (r=0.523, p<0.05).
TD enhances energy expenditure and reduces the body mass
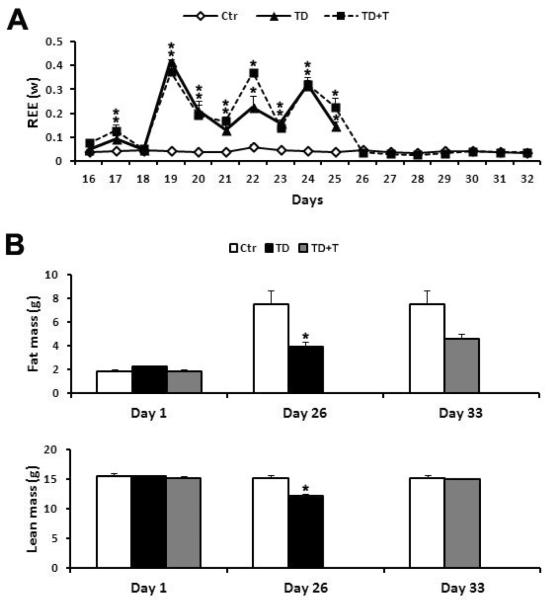
We measured the resting energy expenditure (REE) by indirect calorimetry. After the acclimation period, the data were collected every 30 minutes from the 16th day to the 32nd day. REE for each mouse was normalized with caloric intake and calculated in standardized conditions (fasted status, at rest and thermoneutrality). As shown in Fig. 3A, REE in TD feeding mice was much higher than that in the control subjects from the 19th to 25th day. On the 19th day, the REE in the TD group was approximately 10-fold higher than controls. The thiamine re-supplementation quickly restored REE to control levels since the 26th day and lasted until the end of the experiment.
Figure 3.
Effect of TD on resting energy expenditure (REE) and body composition. A. Female mice were fed with either control or TD diet. The mice in the TD+T group were changed to a normal diet supplemented with benfotiamine on the 26th day. The BEE was measured using indirect calorimetry as described in Experimental Procedures. *p<0.05 vs. control, n = 6 per group. B. At the end of TD feeding (26th day), the body composition including the fat mass and lean mass was measured using EchoMRI technology as described in Experimental Procedures. The body composition of the mice in the TD+T group was measured on the 33rd day at the end of experiment. *p<0.05 vs. control, n = 6 per group.
We determined the body composition which includes both fat mass and lean mass with EchoMRI on the 26th and the 33rd day. In comparison with the control subjects, TD significantly reduced both the fat mass and lean mass on the 26th day, the end time point for TD feeding (Fig. 3B). Interestingly, TD induced a 48.5% fat mass loss and 19.7% lean mass loss on the 26th day; the fat mass was much more affected by TD feeding. However, the thiamine re-supplementation restored the lean mass quickly, and the fat mass slowly on the 33rd day (Fig. 3B).
TD inhibits hypothalamic AMPK phosphorylation
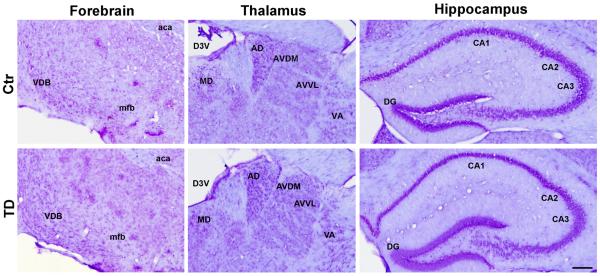
TD in rodents may induce neuronal damage in the central nervous system including the thalamic region (Mitchell and Dalrymple-Alford, 2005), basal forebrain (Pitkin and Savage, 2004), and hippocampus (Nakagawasai et al., 2004). To determine whether TD induced neurodegeneration in our model, we performed a Nissl staining in a series of brain sections composed of every twenty forth section of the brain (12 sections per brain), which contains cortex, striatum, basal forebrain, thalamus, hypothalamus, hippocampus and cerebellum. There was no obvious neuronal loss or damage in the vulnerable brain regions such as forebrain, thalamus, and hippocampus in our TD feeding mice in comparison with controls (Fig 4). We also performed Fluoro-Jade B (Millipore, Bedford, MA) staining on adjacent sections (12 sections per brain), and did not find positive signals on all sections from TD and control subjects (data not shown). Thus, it appeared that our TD feeding model may not induce neurodegeneration in the brain of C57BL6 mice.
Figure 4.
Nissl staining of brain sections from control and TD mice which were sacrificed on the 26th day. General structure and neuronal morphology were examined by conventional Nissl staining as described under the Experimental Procedures. VDB, nucleus of the vertical limb of the diagonal band; mfb, medial forebrain bundle; aca, anterior commissure anterior; MD, mediodorsal thalamic nucleus; AD, anterodorsal thalamic nucleus; AVDM, anterovent thalamic nucleus of dorsomedial part; AVVL, anterovent thalamic nucleus of ventrolateral part; VA, ventral anterior thalamic nucleus; D3V, dorsal 3rd ventricle; CA1, field CA1 hippocampus; CA2, field CA2 hippocampus; CA3, field CA3 hippocampus; DG, dentate gyrus.
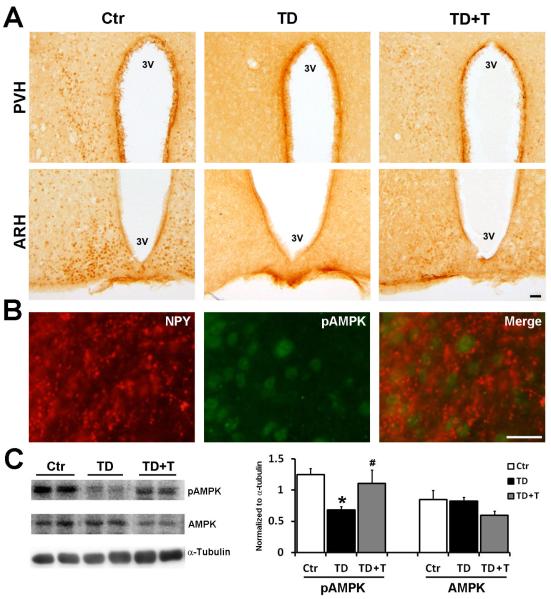
Hypothalamus regulates the interaction between the CNS and the endocrine system, and control food intake, body temperature, sleep and circadian cycles. The heterotrimeric serine/threonine protein kinase AMP-activated protein kinase (AMPK) in the hypothalamus functions as a fuel sensor and has been demonstrated to regulate food intake (Andersson et al., 2004, Minokoshi et al., 2004). AMPK consists of a catalytic α subunit and noncatalytic β and γ subunits. The phosphorylation of AMPKα at Thr172 activates AMPK and has been used as an indirect measurement of AMPK activity (Kim et al., 2004, Shaw et al., 2004). We performed immunohistochemistry (IHC) to determine the expression of activated AMPK [pAMPKα (Thr172)] in the hypothalamus of the mice in the control, TD and TD+T groups. As shown in Fig. 5A, TD decreased the expression of phosphorylated AMPKα in the arcuate and paraventricular hypothalamus (ARH and PVH). The IHC results were confirmed by immunoblotting data which showed that TD reduced the expression of p-AMPKα (Fig. 5C). Interestingly, re-administration of thiamine reversed TD-induced inhibition of AMPKα phosphorylation (Figs. 5A and 5C). In general, there are two critical neuronal populations in the arcuate hypothalamus regulating energy metabolism and they have projections to the PVH; one is the melanocortin system which consists of the subset of neurons that express neuropeptide-Y (NPY), another is a neighboring neuronal population that produces proopiomelanocortin (POMC)-derived peptides (Minokoshi et al., 2004). With double immunofluorescent staining, we showed a partial co-localization of p-AMPKα and NPY in the arcuate nucleus (Fig 5B). However, with currently available antibodies, we could not successfully perform p-AMPKα and POMC double labeling, and therefore were unable to demonstrate the co-localization of p-AMPKα and POMC.
Figure 5.
Effect of TD on the expression and phosphorylation of hypothalamic AMPK. Female C57BL6 mice were fed with either control or TD diet. From the 26th day on, mice in the TD+T group were changed to normal liquid diet supplemented with additional benfotiamine. The mice were sacrificed and perfused on the 26th day in the TD group and the 33rd day in the TD+T and control groups, respectively. A. The phosphorylation of AMPKα in the hypothalamic nuclei of control, TD and TD+T groups was examined by immunohistochemistry (IHC). Scale bar = 200 μm. PVH, paraventricular hypothalamus; ARH, arcuate hypothalamus, 3V, 3rd ventricle. B. Double immunofluorescent staining of phosphorylated AMPK (pAMPKα) and NPY was performed to examine the localization of pAMPKα in the arcuate nucleus. To identify which neuronal population is expressed AMPK in our mouse model, double immunofluorescent staining to detect activated AMPK (green FITC fluorescence) and NPY (red Cy3 fluorescence) expression was conducted and showed a partial co-localization between pAMPKα and NPY in the arcuate nucleus of control subject. Scale bar = 200 μm. C. The expression of pAMPKα, AMPK and α-tubulin in the hypothalamus of the Ctr, TD and TD+T mice was examined by immunoblotting. The change in the expression was quantified by densitometric analysis. *p<0.05 vs. control, # p<0.05 vs. TD, n = 6 per group.
TD activates regulators of energy expenditure in peripheral tissues
Under physiological conditions, body weight is controlled by both food intake and energy expenditure. In rodents, uncoupling protein (UCP)-1 in brown adipose tissue is the main regulator of basal energy expenditure (Lee et al., 2005). UCP1 is located in the mitochondrial inner membrane and dissipates the proton electrochemical energy as heat. We performed immunohistochemistry to detect UCP1 expression in the brown adipose tissue (Fig. 6A). TD increased expression of mitochondrial UCP1 in the brown adipocytes in comparison with control mice (Fig 6A). The finding was supported by immunoblotting results showing an increase in UCP1 expression in the brown adipose tissue of TD mice when compared to controls (Fig. 6B). Re-administration of thiamine restored the expression of UCP1 to control levels.
Figure 6.
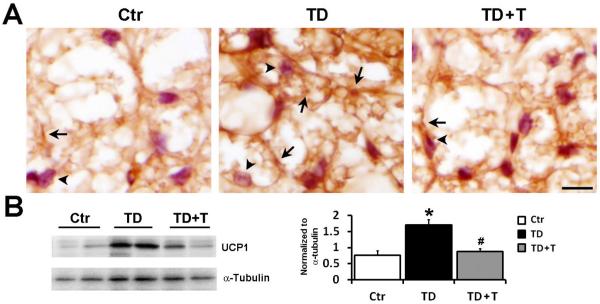
Effect of TD on UCP1 expression in the brown adipose tissue. Female C57BL6 mice were fed with either control or TD diet. From the 26th day on, mice in TD+T group were changed to a normal liquid diet supplemented with additional benfotiamine. The mice were sacrificed and perfused on the 26th day in the TD group and the 33rd day in the TD+T and control groups, respectively. A. The expression of mitochondrial UCP1 (arrows) in the brown adipose tissue of mice in control, TD and TD+T group was demonstrated by IHC. The cells were counterstained with hematoxylin (arrowheads). Scale bar = 200 μm. B. The expression of UCP1 in the brown adipose tissue of control, TD, and TD+T mice was examined by immunoblotting. The change in the expression was quantified by densitometric analysis. *p<0.05 vs. control, # p<0.05 vs. TD, n = 6 per group.
AMPK in the peripheral skeletal tissue is another regulator of energy expenditure (Lee et al., 2005). We examined the expression of p-AMPKα in the skeletal muscle of hindlimb. TD increased the expression of p-AMPKα in the multinucleated myocytes (arrows) in comparison with controls (Fig 7A). Consistently, data from immunoblotting analysis confirmed that TD up-regulated p-AMPKα expression in the skeletal muscle (Fig. 7B). Re-supplementation of thiamine reversed TD-induced up-regulation of p-AMPKα. Taken together, these data suggested that TD stimulated energy expenditure in peripheral tissues.
Figure 7.
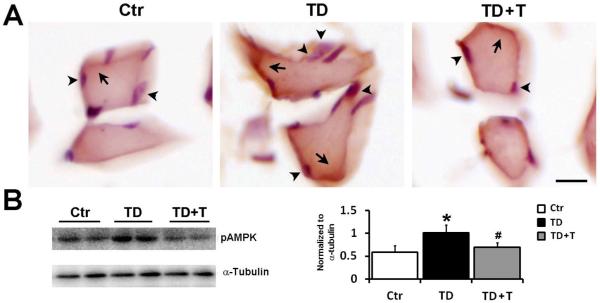
Effect of TD on AMPK phosphorylation in the peripheral skeletal muscle. Female C57BL6 mice were fed with either control or TD diet. From the 26th day on, mice in TD+T group were changed to normal liquid diet supplemented with additional benfotiamine. The mice were sacrificed and perfused on the 26th day in the TD group and the 33rd day in the TD+T and control groups, respectively. A. The expression of phosphorylated AMPKα (p-AMPKα, arrows) in skeletal muscle was demonstrated by IHC. The cells were counterstained with hematoxylin (arrowheads). Scale bar = 200 μm. B. The expression of p-AMPKα and tubulin in the skeletal muscle of the control, TD, and TD+T mice was examined by immunoblotting. The change in the expression was quantified by densitometric analysis. *p<0.05 vs. control, # p<0.05 vs. TD, n = 6 per group.
DISCUSSION
The loss of body weight has been associated with thiamine deficiency (TD) in rodents and patients with Wernicke-Kosakoff syndrome which is mainly caused by TD (Nakagawasai et al., 2004, Saad et al., 2010). However, the underlying mechanisms are unclear. The loss of body weight usually results from either a reduced food intake or enhanced energy expenditure or both. We show here that TD causes a significant decrease in food intake and an increase in basal energy expenditure. The effect of TD on food intake and body weight was gender-independent and reversible. We further demonstrate that TD inhibits hypothalamic AMPK phosphorylation and TD-induced anorexia may be mediated by the modulation of hypothalamic AMPK activity.
In the CNS, the hypothalamus functions as a central regulator of food intake and energy homeostasis. The hypothalamus contains several nuclei, including the arcuate nucleus (ARC), the paraventricular nucleus (PVN), the ventromedial nucleus (VMH), the dorsomedial nucleus (DMH) and the lateral hypothalamic area (LHA). The ARC lies on either side of the third ventricle immediately adjacent to the fenestrated capillaries in the median eminence. This position permits the ARC to sense hormonal and nutrient fluctuations in the plasma (Stark et al., 2012). The ARC is considered the master hypothalamic center for food intake control (Blanco Martinez de Morentin et al., 2011). The ARC neurons produce the orexigenic neuropeptide known as neuropeptide Y (NPY) and agouti-related peptide (AgRP); they also express anorexigenic neuropeptides known as prooppiomelanocortin (POMC), the precursor of α-melanocyte-stimulating hormone (α-MSH) and cocaine- and amphetamine-related transcript (CART) (Pimentel et al., 2013). The ARC neurons expressing orexigenic neuropeptides (NPY/AgRP) mostly project to PVN, while ARC neurons producing anorexigenic products (POMC/α-MSH) project their axons more broadly to PVN, DMH, LHA and the perifornical area (PFA) (Blanco Martinez de Morentin et al., 2011).
AMPK in the hypothalamus has emerged as a key kinase regulating neuroendocrine feedback control of food intake and energy metabolism (Stark et al., 2012). AMPK is an evolutionarily conserved serine/threonine kinase. It is a heterotrimeric protein consisting of α-catalytic subunit and β- and γ-regulatory subunits (Xue and Kahn, 2006). AMPK is a fuel-sensing enzyme activated by physiological and pathological stresses that deplete cellular ATP, including hypoxia, ischemia, glucose deprivation and nutrient deficiency. Phosphorylation of threonine 172 in the α-catalytic subunit increases AMPK activity, which then functions as an intracellular energy sensor that switches off ATP-consuming pathways and switches on ATP-producing pathways such as glucose uptake and fatty acid oxidation (Steinberg and Kemp, 2009). AMPK is highly expressed in several key hypothalamus nuclei, such as ARC, PVN, VMH and LHA (Blanco Martinez de Morentin et al., 2011).
Fasting resulted in increased AMPK activity in multiple hypothalamic regions, whereas re-feeding inhibited it (Minokoshi et al., 2004). Activation of AMPK in the hypothalamus by experimental manipulations is sufficient to increase food intake and body weight, and suppression of hypothalamic AMPK activity is sufficient to decrease parameters (Minokoshi et al., 2004, Xue and Kahn, 2006). It is suggested that the effect of AMPK on food intake is mediated by its regulation of orexigenic/anorexigenic neuropeptides in the hypothalamus (Xue and Kahn, 2006, Stark et al., 2012). For example, inhibition of hypothalamic AMPK activity suppresses the expression of orexigenic NPY and AgRP in arcuate hypothalamus (ARH), whereas activation of AMPK enhances the expression of NPY and AgRP in ARH and melanin-concentrating hormone in the lateral hypothalamus area (LHA) (Minokoshi et al., 2004).
Hypothalamic AMPK mediates neuroendocrine feedback control of food intake and energy metabolisms. A key component of this negative feedback action is the synthesis and release of peripheral metabolic hormones, such as leptin from adipose tissues and ghrelin from the stomach. Leptin and ghrelin represent positive and negative signals of energy balance, respectively. The hypothalamus integrates these signals and regulates food intake and energy expenditure (Stark et al., 2012). Leptin inhibits hypothalamic AMPK activity in the ARC and PVN, but not the VMH and DMH; however, it stimulates AMPK in peripheral tissues, causing a decrease in food intake and an increase in energy expenditure (Minokoshi et al., 2004, Lim et al., 2010). In contrast, ghrelin stimulates hypothalamic AMPK activity and increases food intake (Stark et al., 2012).
We show that TD inhibits the phosphorylation of AMPK in the arcuate and paraventricular hypothalamus (ARH and PVH). AMPK is partially co-localized with NYP positive neurons in the ARH (Fig. 5). Due to unavailability of an appropriate antibody, we could not demonstrate the co-localization of POMC and AMPK. Previous studies have shown that POMC- and AgRP-positive neurons in the ARH express AMPK (Claret et al., 2007). In the current TD feeding model, we did not observe significant neuronal damage, such as neurodegeneration in the brain, which is different from the findings using other TD feeding paradigms. The TD models using TD diet plus injection of thiamine antagonist, such as pyrithiamine, show more severe neurological consequences and produce neurodegeneration and other damages in selected brain regions (Yang et al., 2011).
The mechanisms underlying TD-induced inhibition of AMPK phosphorylation is unclear. There are a number of possibilities. First, TD may affect upstream kinases of AMPK in the ARH. AMPK is regulated by several upstream kinases which include LKB1, TAK1 and calmodulin-dependent protein kinase (CaMKK) (Stark et al., 2012). CaMMK is regulated by intracellular Ca2+ fluctuation. We have previously demonstrated that TD alters intracellular Ca2+ dynamics in the CNS neurons (Lee et al., 2010). Thus, TD may change CaMMK activity by altering intracellular Ca2+ concentration and subsequently alter AMPK activity. Second, TD is known to disrupt glucose metabolism (Jhala and Hazell, 2011, Gibson et al., 2013); the disturbance in glucose metabolism is known to alter AMPK activity. Third, TD may modulate leptin signaling, such as increasing the expression of leptin and/or its receptor or stimulating leptin-mediated intracellular signaling in the hypothalamus. Although it is generally believed that mTOR is a downstream effector of AMPK, a recent study demonstrated that leptin-mediated suppression of AMPK activity is mediated by mTOR (Watterson et al., 2013). We have recently shown TD can modulate mTOR activity in the CNS neurons (Meng et al., 2013). Thus, it is likely that TD may inhibit hypothalamic AMPK activity through altering leptin and mTOR signaling. Fourth, TD may alter the balance of anorexigenic hormones and orexigenic hormones, and higher levels of anorexigenic hormones may inhibit hypothalamic AMPK and reduce food intake. The anorexigenic hormones that have been shown to inhibit hypothalamic AMPK activity include Angptl4/Fiaf, estradiol, insulin, leptin, GLP-1 and resistin; the orexigenic hormones include adiponectin, endocannabinoids, ghrelin and glucocorticoids (Minokoshi et al., 2008, Blanco Martinez de Morentin et al., 2011). It is important to determine whether TD alters the levels of these hormones in future studies.
TD-induced leanness may result from an increase in energy expenditure. Inhibition of hypothalamic AMPK is sufficient to increase energy expenditure. For example, deletion of neuronal protein tyrosine phosphatase 1B (PTP1B) results in decreased hypothalamic AMPK activity, isoform-specific AMPK activation in peripheral tissues, increased energy expenditure and leanness (Xue et al., 2009). Similarly, we show that TD inhibits hypothalamic AMPK activity, but stimulates AMPK activation in skeletal muscles and increases energy expenditure. UCP1 is a key molecule for brown adipose tissue (BAT) thermogenesis (Lowell et al., 1993, Enerback et al., 1997). UCP1 was reported to contribute to the stimulatory effect of leptin on energy expenditure (Okamatsu-Ogura et al., 2011) and may be regulated by hypothalamic neuropeptides (Shi et al., 2013). We show that TD increases UCP1 in the BAT which is consistent with the increased energy expenditure. There is considerable interaction between AMPK and UCP1 (Fritah et al., 2012, Klaus et al., 2012). It is currently unknown, however, whether TD-induced UCP1 expression in the BAT is mediated by AMPK.
In future studies, it is also important to determine whether partial TD has an anorexigenic effect and whether it is a concentration-dependent regulation. It is also interesting to determine whether excessive thiamine uptake can increase food intake and induce obesity. Obesity and eating disorders are a widely prevailing health concern worldwide. Understanding the relationship between the thiamine status and food intake/energy metabolism may provide novel insight into these disorders and develop potential therapeutic approaches by controlling thiamine in the diet.
Thiamine deficiency decreases food intake and increases basal energy expenditure.
Thiamine deficiency induces a severe weight loss.
Thiamine deficiency inhibits hypothalamic AMPK activity.
Thiamine levels may affect food intake and energy metabolism.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institutes of Health (NIH) (AA015407-09). This material is also based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development). It also supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH (8P20 GM103527-06).
Abbreviations
- AMPK
adenosine monophosphate-activated protein kinase
- ARN
arcuate nucleus
- CaMKK
calmodulin-dependent protein kinase
- GABA
gamma-aminobutyric acid
- α-KGDHC
α-ketoglutarate dehydrogenase complex
- α-MSH
α-melanocyte-stimulating hormone
- NPY
neuropeptide-Y
- POMC
prooppiomelanocortin
- PVN
paraventricular nucleus
- REE
resting energy expenditure
- TD
thiamine deficiency
- UCP1
uncoupling protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Blanco Martinez de Morentin P, Gonzalez CR, Saha AK, Martins L, Dieguez C, Vidal-Puig A, Tena-Sempere M, Lopez M. Hypothalamic AMP-activated protein kinase as a mediator of whole body energy balance. Rev Endocr Metab Disord. 2011;12:127–140. doi: 10.1007/s11154-011-9165-5. [DOI] [PubMed] [Google Scholar]
- Butterworth RF, Giguere JF, Besnard AM. Activities of thiamine-dependent enzymes in two experimental models of thiamine-deficiency encephalopathy. 2. alpha-Ketoglutarate dehydrogenase. Neurochem Res. 1986;11:567–577. doi: 10.1007/BF00965326. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Even PC, Nadkarni NA. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. Am J Physiol Regul Integr Comp Physiol. 2012;303:R459–476. doi: 10.1152/ajpregu.00137.2012. [DOI] [PubMed] [Google Scholar]
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- Fritah A, Steel JH, Parker N, Nikolopoulou E, Christian M, Carling D, Parker MG. Absence of RIP140 reveals a pathway regulating glut4-dependent glucose uptake in oxidative skeletal muscle through UCP1-mediated activation of AMPK. PLoS One. 2012;7:e32520. doi: 10.1371/journal.pone.0032520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Hirsch JA, Cirio RT, Jordan BD, Fonzetti P, Elder J. Abnormal thiamine-dependent processes in Alzheimer's Disease. Lessons from diabetes. Mol Cell Neurosci. 2013;55:17–25. doi: 10.1016/j.mcn.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heroux M, Butterworth RF. Reversible alterations of cerebral gamma-aminobutyric acid in pyrithiamine-treated rats: implications for the pathogenesis of Wernicke's encephalopathy. J Neurochem. 1988;51:1221–1226. doi: 10.1111/j.1471-4159.1988.tb03090.x. [DOI] [PubMed] [Google Scholar]
- Jhala SS, Hazell AS. Modeling neurodegenerative disease pathophysiology in thiamine deficiency: consequences of impaired oxidative metabolism. Neurochem Int. 2011;58:248–260. doi: 10.1016/j.neuint.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- Klaus S, Keipert S, Rossmeisl M, Kopecky J. Augmenting energy expenditure by mitochondrial uncoupling: a role of AMP-activated protein kinase. Genes Nutr. 2012;7:369–386. doi: 10.1007/s12263-011-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlais PJ, Zhang SX. Extracellular glutamate is increased in thalamus during thiamine deficiency-induced lesions and is blocked by MK-801. J Neurochem. 1993;61:2175–2182. doi: 10.1111/j.1471-4159.1993.tb07457.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Yang G, Yong Y, Liu Y, Zhao L, Xu J, Zhang X, Wan Y, Feng C, Fan Z, Luo J, Ke ZJ. ADAR2-dependent RNA editing of GluR2 is involved in thiamine deficiency-induced alteration of calcium dynamics. Mol Neurodegener. 2010;5:54. doi: 10.1186/1750-1326-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Koh EH, Won JC, Kim MS, Park JY, Lee KU. Obesity: the role of hypothalamic AMP-activated protein kinase in body weight regulation. Int J Biochem Cell Biol. 2005;37:2254–2259. doi: 10.1016/j.biocel.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Lim CT, Kola B, Korbonits M. AMPK as a mediator of hormonal signalling. J Mol Endocrinol. 2010;44:87–97. doi: 10.1677/JME-09-0063. [DOI] [PubMed] [Google Scholar]
- Liu M, Choi DY, Hunter RL, Pandya JD, Cass WA, Sullivan PG, Kim HC, Gash DM, Bing G. Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J Neurochem. 2010;112:773–783. doi: 10.1111/j.1471-4159.2009.06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Hunter R, Nguyen XV, Kim HC, Bing G. Microsomal epoxide hydrolase deletion enhances tyrosine hydroxylase phosphorylation in mice after MPTP treatment. J Neurosci Res. 2008;86:2792–2801. doi: 10.1002/jnr.21725. [DOI] [PubMed] [Google Scholar]
- Liu M, Sun A, Shin EJ, Liu X, Kim SG, Runyons CR, Markesbery W, Kim HC, Bing G. Expression of microsomal epoxide hydrolase is elevated in Alzheimer's hippocampus and induced by exogenous beta-amyloid and trimethyl-tin. Eur J Neurosci. 2006;23:2027–2034. doi: 10.1111/j.1460-9568.2006.04724.x. [DOI] [PubMed] [Google Scholar]
- Lowell BB, V SS, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- Meng Y, Yong Y, Yang G, Ding H, Fan Z, Tang Y, Luo J, Ke ZJ. Autophagy alleviates neurodegeneration caused by mild impairment of oxidative metabolism. J Neurochem. 2013;126:805–818. doi: 10.1111/jnc.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Shiuchi T, Lee S, Suzuki A, Okamoto S. Role of hypothalamic AMP-kinase in food intake regulation. Nutrition. 2008;24:786–790. doi: 10.1016/j.nut.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Mitchell AS, Dalrymple-Alford JC. Dissociable memory effects after medial thalamus lesions in the rat. Eur J Neurosci. 2005;22:973–985. doi: 10.1111/j.1460-9568.2005.04199.x. [DOI] [PubMed] [Google Scholar]
- Mousseau DD, Rao VL, Butterworth RF. Vesicular dysfunction during experimental thiamine deficiency is indicated by alterations in dopamine metabolism. Eur J Pharmacol. 1996;317:263–267. doi: 10.1016/s0014-2999(96)00842-4. [DOI] [PubMed] [Google Scholar]
- Nakagawasai O, Tadano T, Hozumi S, Tan-No K, Niijima F, Kisara K. Immunohistochemical estimation of brain choline acetyltransferase and somatostatin related to the impairment of avoidance learning induced by thiamine deficiency. Brain Res Bull. 2000;52:189–196. doi: 10.1016/s0361-9230(00)00248-3. [DOI] [PubMed] [Google Scholar]
- Nakagawasai O, Tadano T, Hozumi S, Taniguchi R, Tan-No K, Esashi A, Niijima F, Kisara K. Characteristics of depressive behavior induced by feeding thiamine-deficient diet in mice. Life Sci. 2001;69:1181–1191. doi: 10.1016/s0024-3205(01)01206-1. [DOI] [PubMed] [Google Scholar]
- Nakagawasai O, Yamadera F, Iwasaki K, Arai H, Taniguchi R, Tan-No K, Sasaki H, Tadano T. Effect of kami-untan-to on the impairment of learning and memory induced by thiamine-deficient feeding in mice. Neuroscience. 2004;125:233–241. doi: 10.1016/j.neuroscience.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Okamatsu-Ogura Y, Nio-Kobayashi J, Iwanaga T, Terao A, Kimura K, Saito M. Possible involvement of uncoupling protein 1 in appetite control by leptin. Exp Biol Med (Maywood) 2011;236:1274–1281. doi: 10.1258/ebm.2011.011143. [DOI] [PubMed] [Google Scholar]
- Paxino G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2001 [Google Scholar]
- Pitkin SR, Savage LM. Age-related vulnerability to diencephalic amnesia produced by thiamine deficiency: the role of time of insult. Behav Brain Res. 2004;148:93–105. doi: 10.1016/s0166-4328(03)00208-0. [DOI] [PubMed] [Google Scholar]
- Saad L, Silva LF, Banzato CE, Dantas CR, Garcia C., Jr Anorexia nervosa and Wernicke-Korsakoff syndrome: a case report. J Med Case Rep. 2010;4:217. doi: 10.1186/1752-1947-4-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi G, Serra A. Wernicke's encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6:442–455. doi: 10.1016/S1474-4422(07)70104-7. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, Heilbronn R, Mietzsch M, Weger S, Huang XF, Enriquez RF, Baldock PA, Zhang L, Sainsbury A, Herzog H, Lin S. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013;17:236–248. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Stark R, Ashley SE, Andrews ZB. AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Tadano T, Asao T, Aizawa T, Sakurada S, Abe Y, Yonezawa A, Ando R, Arai Y, Kinemuchi H, Kisara K. Immunohistochemical determination of rat spinal cord substance P, and antinociceptive effect during development of thiamine deficiency. Brain Res. 1995;696:21–29. doi: 10.1016/0006-8993(95)00718-6. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Ramos RL, Anzalone S, Savage LM. Brain and behavioral pathology in an animal model of Wernicke's encephalopathy and Wernicke-Korsakoff Syndrome. Brain Res. 2012;1436:178–192. doi: 10.1016/j.brainres.2011.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volvert ML, Seyen S, Piette M, Evrard B, Gangolf M, Plumier JC, Bettendorff L. Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives. BMC Pharmacol. 2008;8:10. doi: 10.1186/1471-2210-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson KR, Bestow D, Gallagher J, Hamilton DL, Ashford FB, Meakin PJ, Ashford ML. Anorexigenic and orexigenic hormone modulation of Mammalian target of rapamycin complex 1 activity and the regulation of hypothalamic agouti-related protein mRNA expression. Neurosignals. 2013;21:28–41. doi: 10.1159/000334144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Pulinilkunnil T, Murano I, Bence KK, He H, Minokoshi Y, Asakura K, Lee A, Haj F, Furukawa N, Catalano KJ, Delibegovic M, Balschi JA, Cinti S, Neel BG, Kahn BB. Neuronal protein tyrosine phosphatase 1B deficiency results in inhibition of hypothalamic AMPK and isoform-specific activation of AMPK in peripheral tissues. Mol Cell Biol. 2009;29:4563–4573. doi: 10.1128/MCB.01914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Meng Y, Li W, Yong Y, Fan Z, Ding H, Wei Y, Luo J, Ke ZJ. Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain Pathol. 2011;21:279–297. doi: 10.1111/j.1750-3639.2010.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Weilersbacher GS, Henderson SW, Corso T, Olney JW, Langlais PJ. Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. J Neuropathol Exp Neurol. 1995;54:255–267. doi: 10.1097/00005072-199503000-00012. [DOI] [PubMed] [Google Scholar]