Summary
Lampreys are a group of jawless fishes that serve as an important point of comparison for studies of vertebrate evolution. Lampreys and hagfishes are agnathan fishes, the cyclostomes, which sit at a crucial phylogenetic position as the only living sister group of the jawed vertebrates. Comparisons between cyclostomes and jawed vertebrates can help identify shared derived (i.e. synapomorphic) traits that might have been inherited from ancestral early vertebrates, if unlikely to have arisen convergently by chance. One example of a uniquely vertebrate trait is the neural crest, an embryonic tissue that produces many cell types crucial to vertebrate features, such as the craniofacial skeleton, pigmentation of the skin, and much of the peripheral nervous system (Gans and Northcutt, 1983). Invertebrate chordates arguably lack unambiguous neural crest homologs, yet have cells with some similarities, making comparisons with lampreys and jawed vertebrates essential for inferring characteristics of development in early vertebrates, and how they may have evolved from nonvertebrate chordates. Here we review recent research on cyclostome neural crest development, including research on lamprey gene regulatory networks and differentiated neural crest fates.
Keywords: Neural crest, lamprey, neural crest derivatives, vertebrate evolution
Introduction1
Lampreys are jawless fishes (or agnathans), closely related to other living vertebrates, the jawed vertebrates (or gnathostomes). They, along with hagfish, are the only known surviving lineage of once diverse groups of jawless fishes. Living cyclostomes are modern yet they have some anatomic elements that appear to be retained from primitive members of their own groups, and possibly of primitive ancestral vertebrates. Lampreys are readily obtainable, and comparisons between lampreys and vertebrates are useful for the identification of developmental traits that are putatively derived from ancestral vertebrates. Of the two cyclostome groups, lampreys are the more experimentally tractable developmental models, and work has been done on a variety of species. As has been discussed recently (Gans and Northcutt, 1983; Shimeld and Donoghue, 2012), lamprey embryos are amenable to in situ hybridization, antibody staining, microinjection, morpholino oligonucleotide injection, and chemical inhibitor treatment (for protocols see: Nikitina et al., 2009a; Nikitina et al., 2009b; Nikitina et al., 2009c; Sauka-Spengler, 2009). Additionally, the lamprey Petromyzon marinus has been the focus of a recent genome project (Smith et al., 2013). The genome of this species (and lampreys in general; see Kuraku and Kuratani, 2006) is difficult to fully assemble in part because of high percentage GC content, in contrast to the more typical GC content of the lamprey mitochondrial genome (Lee and Kocher, 1995), but this resource will be an essential aspect of future lamprey studies. There are additional germline bacterial artificial chromosome resources for P. marinus and Lethenteron japonicum (Smith et al., 2010; Mehta et al. 2013), which is important because lampreys undergo genomic reduction during their maturation (Mallatt, 1981; Rovainen and Schieber, 1975; Smith et al., 2009).
Lampreys are an increasingly useful model organism and have been used successfully by a growing field of researchers (McCauley and Kuratani, 2008; Shimeld and Donoghue, 2012). Lamprey embryology and developmental genetics have already been crucial in addressing a number of key aspects of early vertebrate evolution, including the evolution of the jaw and evolution of the adaptive immune system (Kuratani et al., 2002; Langille and Hall, 1988a; Shigetani et al., 2002; Shimeld and Donoghue, 2012). Other recent reviews have focused on the developmental biology of lampreys, and their use in a broad variety of experimental contexts (Martin et al., 2009; Shimeld and Donoghue, 2012). In this review, we particularly focus on the ways in which lamprey data have facilitated understanding of the evolution of neural crest.
I. Lamprey phylogeny, anatomy, and fossil record
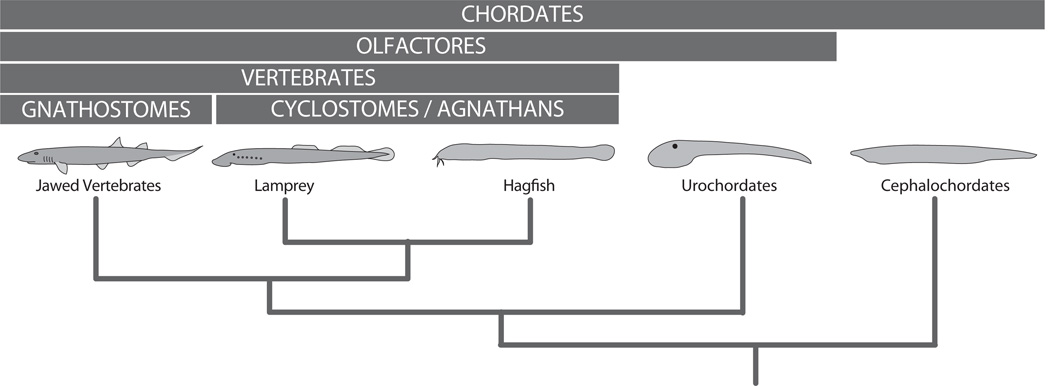
Lampreys and hagfish are jawless fishes with anatomic characters that unambiguously suggest a relatively close relationship with jawless vertebrates. The precise phylogenetic position of hagfish and lampreys relative to the jawed vertebrates has been a difficult issue to resolve, with many early phylogenetic analyses of morphological datasets suggesting that lampreys and jawed vertebrates are sister groups (to the exclusion of hagfish), but molecular phylogenetic data have supported a close relationship between hagfish and lampreys, suggesting they comprise a monophyletic group (Kuraku et al., 1999; Langille and Hall, 1988a). This has remained a contentious issue, but analyses of microRNA sequences, reexamination of morphological datasets, and recent morphological analyses of hagfish (De Beer, 1937; Heimberg et al., 2010; Janvier, 2010; Oisi et al., 2013) suggest that hagfish and lampreys are sister groups, comprising a group called the cyclostomes (See Fig. 1). Of the two cyclostome groups, hagfish are more difficult to acquire, but have recently begun receiving significant attention (Gess et al., 2006; Janvier, 2007; Ota and Kuratani, 2007). Lampreys are by far more accessible, and have been the subject of more developmental analyses. The phylogenetic position of lampreys makes them particularly useful for comparisons with jawed vertebrates, as traits held in common between these groups are possibly homologous by descent.
Figure 1.
Schematic cladogram of interrelationships between select chordate taxa. The labels at top indicate the names of the monophyletic groupings shown beneath.
Lampreys are divided into three major taxa, with a single monophyletic group in the northern hemisphere (including well-known genera such as Petromyzon, Ichthyomyzon, Lethenteron, and Lampetra), and two groups (the Geotria and Mordacia) in the southern hemisphere (Gess et al., 2006; Gill et al., 2003). Size of adult lampreys varies considerably, and partially depends on life history. Some lampreys feed only as larvae, while others parasitize on blood or flesh as adults; parasitic lampreys achieve a larger body size.
Lampreys have quite distinct anatomies at larval (ammocoete) and adult stages. Larval lampreys are diminutive fish that burrow in silty river bottoms, filtering microscopic food particles from the passing current. Their skin is smooth, they lack fully developed eyes, and their mouth opens anteriorly into a space covered by an enclosure projected from the snout, the oral hood. Ammocoetes have a specialized muscular structure, the velum, which forms from the mandibular arch (Gess et al., 2006; Hardisty and Rovainen, 1982). Rhythmic velar contractions ensure there is sufficient water flow through the pharynx to support both feeding and respiration (Bardack and Zangerl, 1968; Mallatt, 1981; Rovainen and Schieber, 1975).
Ammocoetes possess a cartilaginous skeleton that can be described as being divided into viscerocranial and neurocranial regions (See Fig. 2F). Cartilaginous elements arising from the pharyngeal arches fuse to form the branchial basket, which largely comprises the lamprey viscerocranium (Langille and Hall, 1988a; Lund and Janvier, 1986). The branchial basket appears to provide elastic recoil to counteract the movements of pharyngeal muscles (Bardack and Richardson, 1977; Martin et al., 2009). A second major skeletal structure, the mucocartilage, is a single fused connective tissue that reinforces the oral hood and oral apparatus. The mucocartilage has a unique composition, and is only found in ammocoetes. The ammocoete neurocranium includes two parachordal elements and an anterior parachordal, together referred to as trabeculae (Janvier, 2007; Langille and Hall, 1988a). Additional skeletal elements include a pericardial capsule, and otic and nasal capsules (De Beer, 1937; Janvier, 1996), which have received little attention from developmental geneticists.
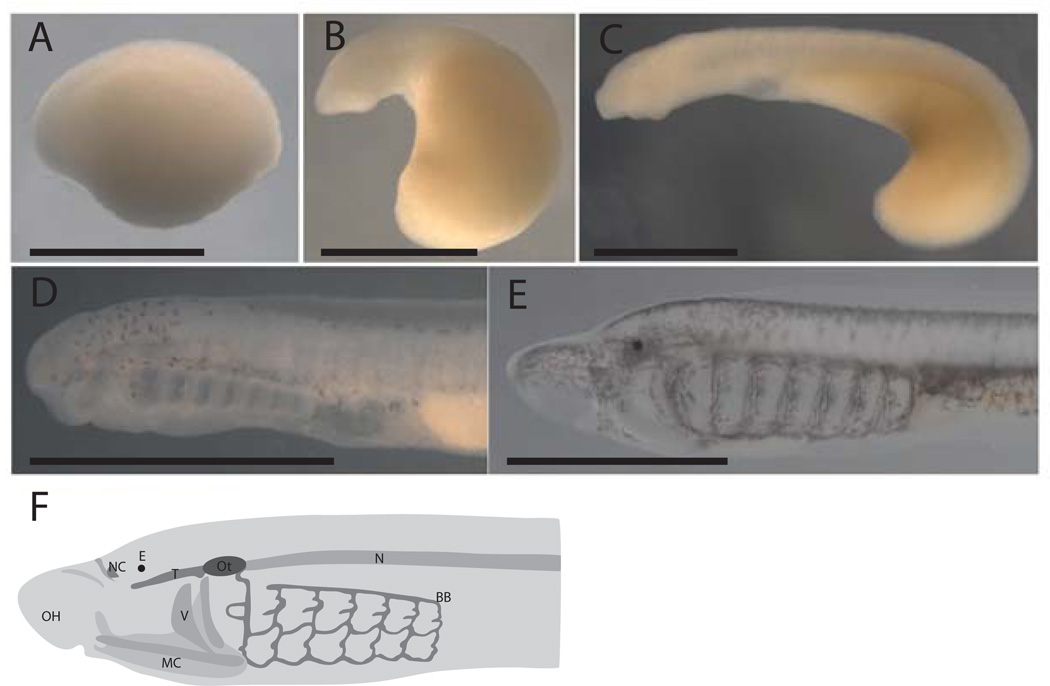
Figure 2.
External morphology during early development of the lamprey P. marinus. A. Embryo after neural rod formation, approximately Tahara Stage 20. B. Embryo at Tahara Stage 22. C. Embryo at Tahara 24.5. D. Embryo at T28 embryo. E. Proammocoete. F. Schematic of a young ammocoete, redrawn after De Beer (1937), and Langille and Hall (1988a). BB: branchial basket, E: eye, MC: mucocartilage, N: notochord, NC: nasal cartilage, OH: oral hood, Ot: Otic capsule, T: trabeculae, V: velum. Bar indicates 1 mm.
Larval lampreys retain the ammocoete body plan for at least several years and, after reaching a suitable size, they go through a significant metamorphosis during which many tissues are reformed and rearranged into adult body structures (De Beer, 1937). These transformations include final development of eye structures, the degradation of mucocartilaginous skeletal elements and their replacement with cartilaginous elements of the adult, and the alteration of the velum from a flow-generating structure to one that acts as a valve to separate feeding (i.e. esophageal) and respiratory (i.e. pharyngeal) channels (Hardisty, 1979). Cartilaginous elements that replace the mucocartilage include an annular cartilage that reinforces the rostral oral opening, paired styliform cartilages, dorsal and lateral elements, cartilaginous elements on their rasping tongue, and a piston cartilage, a key skeletal component that generates the force necessary to feed (De Beer, 1937; Johnels, 1948).
As adults, lampreys are elongated, eel-like fish. As in the ammocoete, the adult integument is smooth, without scales or ossified structures (Hardisty and Potter, 1971). The lamprey mouth, instead of having an opposable jaw, opens as a round sucker with keratinized ‘teeth’. Their fully developed eyes lack intrinsic musculature, and they have a pineal eye that sits on the cranial dorsal midline (Hardisty and Potter, 1971). Their pharynx is perforated by seven round gill slits, which open into muscular pharyngeal pouches. They lack paired fins, but have dorsal, caudal, and anal fins, and some species are quite physically powerful. Many lamprey species are parasitic as adults, and they attach to fish, rasp through the fish integument, and feed on blood or flesh (Hardisty and Potter, 1971). While adults are attached via the sucker, they alternately contract left or right pharyngeal pouches to draw oxygenated water into their pharynx for respiration (Hardisty, 1979). Additional pore musculature controls the aperture of the gill slits.
Lampreys have a lengthy fossil record, and are sometimes regarded as ‘living fossils’ because of their strong resemblance to early fossil material (Gess et al., 2006; Hardisty, 1979; Janvier, 2007). Careful examination of these fossils might provide clues as to which morphological traits have been most stable in lampreys. The earliest fossil lamprey is Priscomyzon, which dates from 360 million years ago (Mya) (Chang et al., 2006; Gess et al., 2006). Priscomyzon shows multiple anatomic traits found in modern lamprey, including a round mouth, a piston cartilage, and seven pharyngeal pouches (Chang et al., 2006; Gess et al., 2006). These traits, and in particular the piston cartilage, are consistent with the animal having had a predatory adult stage, despite its very small size of 4 cm (Gess et al., 2006; Janvier, 2006). Other fossil lampreys include Mayomyzon (Bardack and Zangerl, 1968; Janvier, 2007), 1968), Hardistiella (Janvier, 2007; Lund and Janvier, 1986), and Pipiscius (Bardack and Richardson, 1977; Janvier, 1996), all from the late carboniferous (305 Mya; Janvier, 2007; Janvier et al., 2006). These fossils are also of very small size, and the juxtaposition of small size and morphologies found in adult lampreys might suggest that Mayomyzon lacked a lengthy morphologically distinct larval stage (Gans and Northcutt, 1983; Glenn Northcutt, 2005; Janvier, 1996; Northcutt and Gans, 1983). However, the rostral tip of Mayomyzon shows a smaller oral hood than modern lampreys, leading to the suggestion that Mayomyzon might have been nonparasitic (Hardisty, 1979). Another lamprey from 125 Mya, Mesomyzon mengae (Chang et al., 2006; Piavis, 1971; Richardson and Wright, 2003) exhibits a closer resemblance to living lampreys, with a slightly larger body size and a lengthened snout. All lamprey fossils have skin without a dermal skeleton, a trait likely to have arisen in stem gnathostomes (Gess et al., 2006; Janvier, 2006; Tahara, 1988). These lamprey fossils date to 360 Mya, while the minimum age of hagfish is about 300 Mya (Janvier, 2007; Yamazaki et al., 2003). Assuming monophyletic cyclostomes, based on the ages of stem gnathostome fossils, it is likely that cyclostomes and gnathostomes split no less than 475–500 Mya (Janvier, 2007; Tahara, 1988).
These fossils confirm that some aspects of lamprey development date from early in their evolution and that some traits, like the presence of a piston cartilage, show apparent stability over time. Other traits appear to be quite different in early lampreys. Notably all the early lamprey fossils are small in size compared to current lampreys, with no fossil yet discovered being longer than 10 cm, and each of these fossils has traits, like a piston cartilage, that is associated with the adult morphology of living lampreys (Janvier, 2007). This suggests that early adult lampreys might have been quite small, consistent with a possibly abbreviated or absent larval stage (Janvier, 1996; Koltzoff, 1901). This raises the possibility that traits associated with larval lampreys (including mucocartilage, delayed ocular development, and distinct velar morphology) might have been secondary modifications of the lamprey form. Fossils of juvenile lampreys will be necessary to be certain, but anterior-most larval structures might be particularly derived in lampreys as a result of the evolution of larval feeding strategies.
Another interesting use of fossils for developmental geneticists is that they indicate some aspects of development that are particularly likely to be representative of primitive conditions. One such example is the muscularized pharyngeal pouch of lampreys. Among living animals, these structures are unique to lampreys, and were once considered an oddity of lamprey anatomy. The discovery of a stem-gnathostome fossil (i.e. an ancient jawless fish more closely related to jawed vertebrates than to cyclostomes) of Endeiolepis (370 Mya; Janvier et al., 2006) with very similar pharyngeal pouches suggests that pharyngeal pouches were a general trait of primitive vertebrates that have been secondarily lost in living gnathostomes. Thus, it is possible that lamprey pharyngeal pouch muscle plates represent an example of a primitive condition once common among early vertebrates. If so, then study of neural crest and muscle development in pharyngeal pouch structures offers potentially unique insights into the evolution of a muscularized pharynx, thought to be a key element of the transition from invertebrate filterfeeding to vertebrate predation (Gans and Northcutt, 1983; Glenn Northcutt, 2005; Horigome et al., 1999; Northcutt and Gans, 1983).
II. Lamprey neural crest embryology and morphology
Early embryology of lampreys has been described in several species, including the European brook lamprey Lampetra fluviatilis (Damas, 1944; Horigome et al., 1999), the Atlantic sea lamprey P. marinus (Horigome et al., 1999; Piavis, 1971; Richardson and Wright, 2003), Lampetra reissneri (Horigome et al., 1999; McCauley and Bronner-Fraser, 2003; Tahara, 1988), and the Pacific lamprey Entosphenus tridentatus (McCauley and Bronner-Fraser, 2003; Yamazaki et al., 2003). Generally, embryology of these lamprey species is quite similar, varying only in developmental rate. Rather than relying on staging by embryonic day, which varies between species, lamprey research commonly follows the staging table of (McCauley and Bronner-Fraser, 2003; Tahara, 1988). Lamprey embryos are yolky and quite large (See Fig. 2). Early cleavage is radial and holoblastic, with the mesolecithal yolk distribution leading to significant differences in size of animal and vegetal blastomeres. Following gastrulation, lateral edges of the neural plate rise and fuse at the midline, producing a neural rod that includes precursors of neural crest. The neural lumen is produced secondarily by cavitation.
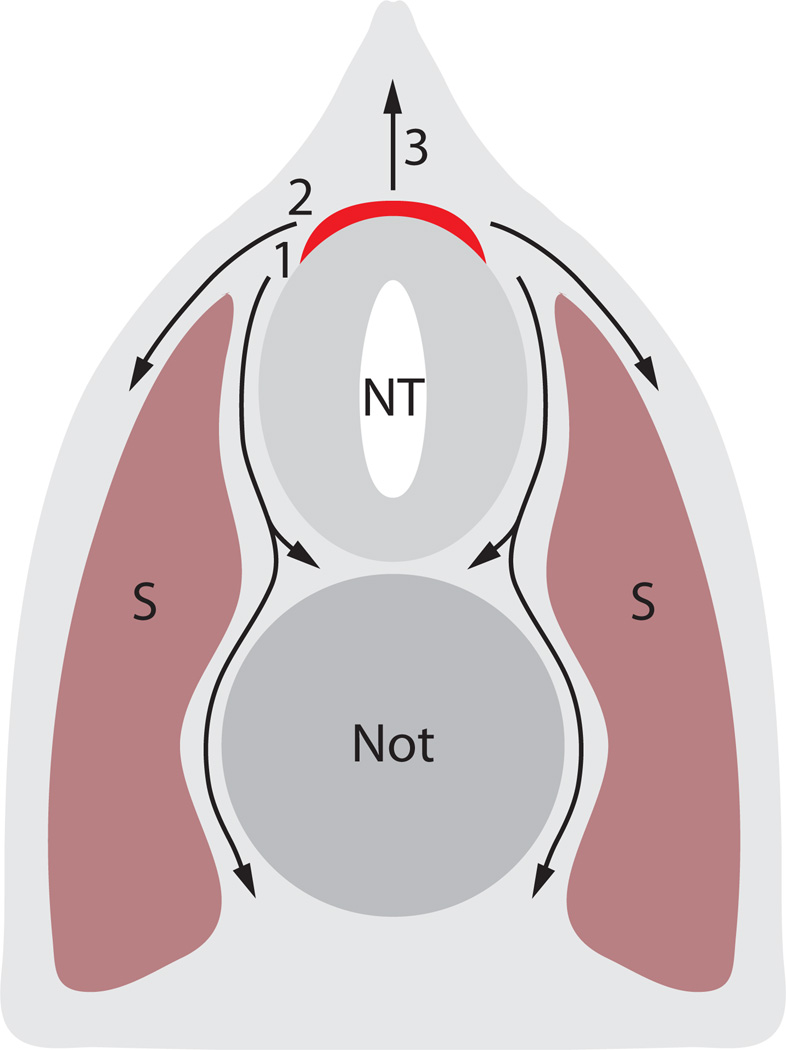
In vertebrates, including lamprey, the neural crest is a transient multipotential and stem cell-like population that produces a wide variety of cell types important to the vertebrate body plans, including skeletal, glial, and pigment cell types. In jawed vertebrates, neural crest cells originate from cells between the neural and non-neural ectoderm, in a region called the neural plate border. Cells from this region elevate during neural tube formation, and generally come to lie in the dorsal neural tube. Neural crest cells delaminate from the adjoining neurepithelium and ectoderm, go through an epithelial to mesenchymal transition, and migrate away from the neural tube. Neural crest cells migrate via routes that vary by axial level and also by species. Generally, they migrate either ventromedially, through or around the somites, or they travel through the dorsolateral pathway, remaining subjacent to epidermis, while migrating to other locations (See Fig. 3). Cells may also remain dorsally and give rise to structures of median and dorsal fins.
Figure 3.
Schematic diagram of neural crest migration pathways through the dorsal half of a vertebrate cross-section. Red color indicates site of origin of premigratory neural crest. 1: ventromedial migration pathway, 2: dorsolateral migration pathway, 3: dorsal migration pathway. S: somite. Not: notochord. NT: neural tube. After Krotoski and Bronner-Fraser (1986).
Lamprey neural crest cells were first identified by Koltzoff (Koltzoff, 1901; McCauley and Bronner-Fraser, 2003). For a discussion of early morphological studies and an important discussion of L. fluviatilis embryonic cranial morphology, please refer to Damas (1944). More recently, Horigome et al (1999) and Kuratani (1997) examined the morphology of L. japonica neural crest cells at premigratory, migratory, and postmigratory stages by a combination of electron microscopy and DiI labeling. L. japonica embryology is very similar to that of P. marinus, and these data offer an excellent model of lamprey neural crest development. Following formation of the lamprey neural rod, presumably, neural crest precursors sit at the dorsal neural tube. In L. japonica, cranial neural crest cells are visible as bulges from the dorsal neural tube at about Tahara St. 20 (Horigome et al., 1999; Meulemans and Bronner-Fraser, 2004). Shortly thereafter, at about Tahara state 20.5, cells begin to delaminate from the neural tube, and they migrate in three streams, termed the trigeminal, hyoid, and branchial streams (Horigome et al., 1999; Meulemans and Bronner-Fraser, 2004). Trigeminal crest originates from midbrain levels to the second rhombomere, and cells migrate via the dorsolateral pathway over the forebrain and mandibular mesoderm. Hyoid stream neural crest cells migrate ventrally from a position adjacent to rhombomere 4, beneath the otic primordium, and into a superficial position within the hyoid arch, bounded by first and second pharyngeal pouches. Branchial crest cells initiate migration from rhombomere 6 and more posterior positions. The precise posterior border of branchial (posteriormost cranial) neural crest cells is indistinct, and migration of trunk neural crest has received little attention in lampreys. Little is known about presumed migration into cardiac and enteric positions. Lamprey neural crest has been shown to migrate along dorsolateral migration pathways in L. japonica and P. marinus (Horigome et al., 1999; McCauley and Bronner-Fraser, 2003; Meulemans and Bronner-Fraser, 2004), but fate-mapping data show that cranial neural crest cells also migrate along a ventromedial pathway in P. marinus (Light et al., 2005; McCauley and Bronner-Fraser, 2003; Sauka-Spengler and Bronner-Fraser, 2006). Comparable fate-mapping analyses have not been completed in L. japonica, but we presume that lamprey cranial crest cells are likely to use both pathways. In gnathostomes, cells traveling the dorsolateral pathway predominantly differentiate into pigment cells, but it is not clear whether particular neural crest fates are associated with, or restricted to, a particular pathway in lampreys.
McCauley and Bronner-Fraser (2003) showed that late-emigrating labeled neural crest cells are capable of migrating to both dorsal and ventral positions within pharyngeal arches. A difference between gnathostome and lamprey neural crest migration is that lamprey cranial neural crest cells migrating into the pharyngeal region posterior to the hyoid arch appear relatively unconstrained, and continue migrating anteriorly and posteriorly until the formation of the posterior pharyngeal (i.e. branchial) arches (McCauley and Bronner-Fraser, 2003).
As in Xenopus (Krotoski and Bronner-Fraser, 1986), some lamprey neural crest migrates adjacent to the notochord: lamprey melanophores are visible surrounding the notochord (Kuratani et al., 1997). It is likely that these cells migrate from the ventral pathway.
III. Gene regulatory networks active in early neural crest cell induction, specification, and maintenance
Extensive examination of neural crest formation in a variety of model species led to the contention that neural crest cell development arises through the activity of a gene regulatory network that is largely conserved throughout vertebrates (Meulemans and Bronner-Fraser, 2004). Interactions between neural crest regulatory network genes result in successive refinement of the distinct fate and behavior of neural crest, establishment of cellular conditions for the maintenance of neural crest fate, establishment of receptive ability to environmental cues governing further differentiation, and control of the epithelial to mesenchymal transformation that crest undergoes in order to migrate away from the neural tube. This network is induced when information from BMP, Wnt, and FGF signaling pathways progressively subdivides ectoderm into three regions: the neural plate, the non-neural ectoderm, and the intervening neural plate border region. Proteins acting as ‘neural plate border specifiers’ include Zic, Msx, Dlx3/5, and Pax3/7 (Meulemans and Bronner-Fraser, 2004). Border specifiers, along with other inductive signals, are responsible for activating ‘neural crest specifiers’, a set of genes whose combined, overlapping expression pattern is indicative of presumptive neural crest cells. The neural crest specification genes include Sox9, Sox10, Msx1/2, AP2, c-Myc, Snail, and Slug. Additional genes generally expressed in early neural crest include Id and Twist homologs (Meulemans and Bronner-Fraser, 2004). Xenopus Id3 is necessary for neural crest stem cell specification and maintenance (Kee, 2005; Light et al., 2005; Sauka-Spengler and Bronner-Fraser, 2006). Neural crest specifier genes in turn activate additional effector genes responsible for activating individual functions of neural crest subtypes (Bronner-Fraser and Sauka-Spengler, 2010). Early neural crest specifier activity leads to the activation of other key effectors of neural crest fate, including altered Cadherin and RhoGTPase activity. For comprehensive reviews of early neural crest cell development and the epithelial to mesenchymal transition, please see (Bronner-Fraser and Sauka-Spengler, 2010; Kerosuo and Bronner-Fraser, 2012; Prasad et al., 2012).
Lamprey neural crest gene regulatory network
Lampreys have a crucial phylogenetic position for making inferences about the state of the neural crest gene regulatory network in early vertebrates. Careful examination of the expression patterns of fifty P. marinus candidate genes with roles in neural crest of gnathostomes has suggested that the lamprey P. marinus uses a neural crest gene regulatory network that is broadly similar to those of jawed vertebrates (Sauka-Spengler et al., 2007). The lamprey P. marinus embryo shows expression of Pax3/7, MsxA, and Zic (though not DlxB), during neural crest border specification, as well as expression of neural crest specification genes at neural crest border specification and neural crest specification stages. Subsequent analyses (Nikitina et al., 2008) have refined this by showing that AP2 and MsxA initially act upstream of other genes (ZicA, Pax3/7, Id, and n-Myc) active in the neural plate border region. These data suggest that formation of the neural crest in lampreys is broadly similar to that of other vertebrates, though there may be some differences in the timing of deployment between cyclostomes and gnathostomes. Although Twist and Ets1 function as neural crest specifier genes in gnathostomes, in situ hybridization failed to detect expression of their homologs in premigratory or early migratory neural crest of lamprey. Rather, these genes were activated in late migrating crest cells within the branchial arches. Such differences may in part explain the changes in neural crest formation in jawed versus jawless vertebrates.
These results show that the majority of the gene regulatory network leading to neural crest formation is conserved between jawless and jawed vertebrates and was already present in the ancestors of all craniate animals. This implies that the earliest origins of neural crest took place in non-vertebrate chordates. It can be difficult to unambiguously identify a crest homolog in the latter, because their body plans are quite different, but knowledge of gene regulatory network structure can provide a supplemental means to test hypotheses about neural crest homologs.
Neural crest origins and putative neural crest homologs in the invertebrate chordates
Neural crest cell derivative fates can be broadly grouped into ectomesenchymal and neuroglial fates (Donoghue et al., 2008). The earliest origins of neural crest are unclear, but it is likely that ectomesenchymal fates of neural crest emerged in early vertebrates (Baker, 2008; Ivashkin and Adameyko, 2013). This is corroborated by the appearance of skeletal elements in fossil lampreys and in the fossils of other early vertebrates. It is possible that the earliest neural crest was a neuroepithelial lineage, and that such a lineage might be present in invertebrate chordates. Several hypotheses have suggested that neural crest originated from a single lineage of fairly differentiated cells arising from the neural border region, for instance cells similar to Rohon-Beard cells (Fritzsch and Northcutt, 1993), or from ascidian pigment cells. Another possibility is that neural crest arose from a multipotent pigment precursor cell (Abitua et al., 2012; Ivashkin and Adameyko, 2013).
Urochordates
Because all vertebrates possess neural crest cells, many have looked to the relatively closely related invertebrate chordates for clues as to the earliest origins of the neural crest. The closest relatives to vertebrates are the urochordates, including ascidians; urochordates and vertebrates are sister groups comprising the group Olfactores (Abitua et al., 2012; Delsuc et al., 2006). There have been several distinct claims about homologous tissues in ascidians. One (Jeffery et al., 2004) suggested that the A7.6 lineage, which produces migratory pigment cells, might be homologous to the neural crest. These cells are mesodermal or mesendodermal (Abitua et al., 2012; Baker, 2008; Jeffery et al., 2004), and expression analyses of their derivatives suggest the cells do not arise from the neural plate border (Jeffery et al., 2008).
Abitua et al (2012) have instead suggested that the a9.49 cell lineage is homologous to neural crest. Crucially, this lineage arises from a neural plate border region that expresses multiple neural plate border and neural crest specification genes, including homologs of Msx, Pax3/7, Zic, AP-2, ID, and Snail (Abitua et al., 2012). The cells normally undergo a short but complex migration (Baker, 2008) to become pigment cells, and the lineage also expresses FoxD, a crucial regulator of neural crest; in Ciona the gene is necessary for MITF expression and pigment formation (Abitua et al., 2012). If the a9.49 cell represents a homolog of neural crest, then it implies the presence of at least a homologous neuroglial lineage early, in the early stem Olfactores that were the common ancestors of vertebrates and ascidians. Overexpression of Twist in a9.49 cells triggers cell migration, leading to the contention that perhaps acquisition of Twist expression, or expression of a similar gene, in a relatively simple neural crest homolog in stem vertebrates might have capacitated gene networks, allowing neural crest elaboration into vertebrate skeletal structures (Abitua et al., 2012).
Cephalochordates
Cephalochordates have no obvious homolog of neural crest cells, but they do have a neural border region that features very similar expression of neural plate border specification genes, including Zic, Msx, and Pax3/7 homologs. However, AP2, FoxD3, and Id expression hasn’t been detected in the border region, though Snail has been detected in this site (Sauka-Spengler and Bronner-Fraser, 2006; Yu, 2010; Yu et al., 2008). The absence of neural crest in amphioxus, as well as apparent absence of any homologous tissue within other deuterostome phyla, such as hemichordates and echinoderms, has typically been interpreted as a primitive character, implying that the earliest neural crest arose in stem vertebrates or stem Olfactores, perhaps in association with novel regulatory elements associated with newly duplicated vertebrate genes (Ota and Kuratani, 2007; Yu et al., 2008).
Another hypothesis suggests that neural crest arose from a multipotent neuroepithelial precursor cell responsible for pigmentation and light reception, which suggests the amphioxus ocellus as a possible neural crest paralog (Ivashkin and Adameyko, 2013).
Patterns of gene subfunctionalization among vertebrate duplicates suggest that core mechanisms of neural crest formation arose prior to vertebrate genome duplications (Medeiros, 2013). Neural crest-like abilities are phylogenetically widespread throughout invertebrates, and a broader understanding of the developmental underpinnings of the sensory cells found in other animals will strengthen assessments of neural crest evolution (Medeiros, 2013).
IV. Neural crest derivatives in lampreys
It is clear from many studies that migrating lamprey neural crest cells give rise to many cell types typical of the neural crest of jawed vertebrates, including melanophores, chondrocytes, and presumably other connective tissues. Ablation or removal of lamprey neural crest cells reduces pigmentation (McCauley and Bronner-Fraser, 2003; Newth, 1951; Langille and Hall, 1988b, Sauka-Spengler and Bronner-Fraser, 2006), and transplanted dorsal neural tissue introduces melanophores into ectopic sites (Newth, 1956). In lampreys, neural crest cell migration into branchial arches is consistent with a role in formation of branchial arch skeletal structures (Horigome et al., 1999; McCauley and Bronner-Fraser, 2003). Langille and Hall (1988b) showed that surgical removal of neural crest and dorsal neural tube at premigratory stages leads to reductions in branchial skeletal elements and trabeculae.
There also are important differences in neural crest derivatives within the vertebrate lineage. For example, the structure of the autonomic system, traditionally divided into sympathetic, parasympathetic, and enteric subdivisions for mammals, is very different in other vertebrates (Nilsson, 2011). The sympathetic system of many jawed vertebrates uses a paravertebral series of sympathetic chain ganglia (Nilsson, 2011). However, interconnections between sympathetic chain ganglia are absent in elasmobranch sharks (Häming et al., 2011; Nilsson, 2011; Young, 1933), and in cyclostomes there are no sympathetic chain ganglia (Fange et al., 1963; Häming et al., 2011; Nicol, 1952). Consistent with these morphological observations, attempts to examine autonomic cell markers have shown that markers homologous to those expressed in chain ganglia of vertebrates – Phox2, Ash/Ascl, and Hand – are not coexpressed at early embryonic stages (Häming et al., 2011).
Certain derivatives that are typical for jawed vertebrates do not appear to be present in lampreys. Notably, lampreys do not possess an easily identifiable structure homologous to sympathetic chain ganglia (Häming et al., 2011; Johnels, 1956). At cranial levels, lampreys have autonomic pathways through the vagus nerve, and possibly through the facial and glossopharyngeal nerves (Nicol, 1952; Tretjakoff, 1927). Fibers innervating the gut (and other visceral organs) throughout much of the trunk derive from spinal neurons, similar to the condition of amphioxus (Fritzsch and Northcutt, 1993).
While there are no obvious homologs of the paravertebral structures, lampreys might have cells homologous to autonomic ganglia. Lampreys have putative autonomic nerve cells adjacent to the cloaca and peripheral nerve plexuses on kidneys and gonads (Johnels, 1956). Nakao and Ishizawa (1982) examined the ultrastructure of cloacal ganglion cells, confirming that their morphology is consistent with autonomic function. This suggests that while lamprey might lack an organized placement of autonomic ganglia in a position adjacent to the spine, homologous cell types might exist. The physiological function of these neurons isn’t clear, but it is likely that they promote gut motility. Understanding the origins of these cells will be important in determining whether these might represent homologs of sympathetic ganglia.
Adrenal chromaffin cells, which derive from neural crest in jawed vertebrates, are an additional neural crest-derived cell type. Lampreys have both cardiac chromaffin cells and extracardiac chromaffin cells (Paiement and McMillan, 1975). Extracardiac chromaffin cells might be homologous to cells of the gnathostome adrenal medulla (Gaskell, 1912; Paiement and McMillan, 1975). The origin of lamprey cardiac chromaffin cells is unknown.
The lamprey heart has two chambers, with components that include neural crest-derived elements in jawed vertebrates. Embryonic lamprey hearts have been reported to have multiple valves, including a sinoatrial valve, an atrioventricular valve, and outflow valves (Farrell, 2007; Lee et al., 2013; Richardson et al., 2010; Shipley). However, the outflow valves are not necessarily homologous to the semilunar valves of amniotes (Bullock et al., 1984; Peters, 1960; Richardson et al., 2010; Schultz et al., 1956), which include neural crest-derived cells (Jain et al., 2011; Nakamura, 2006; Smith et al., 2013).
In gnathostomes, neural crest cells differentiate into pericytes and smooth muscle cells of anterior cranial vasculature (Etchevers et al., 2001). In lampreys, defects in neural crest can lead to dilation of anterior arteries (Newth, 1956), suggesting that neural crest cells are likely to contribute to cranial vasculature. A more precise study of crest interactions with vasculature has not been completed, and it is possible that these defects arise from interactions between neural crest cells and mesoderm, or a general physiological defect (Newth, 1956). Overall the lamprey hematopoietic system is reported to be somewhat similar to that of jawed vertebrates, and there are lymphocytes thought to be homologous to B cells and T cells (Guo et al., 2009; Kasamatsu et al., 2010; Rogozin et al., 2007), though their immune system uses a completely different system of receptors, called VLR receptors, to mediate interactions with foreign molecules. There is a report that lampreys might have a rudimentary thymus (Bajoghli et al., 2011), which in vertebrates includes neural-crest derived elements (Bockman and Kirby, 1984; Foster et al., 2008; Lee et al., 2013; Müller et al., 2008). However, it is unknown whether the lamprey thymoid includes neural crest-derived cells.
Other anatomic neural crest derivatives might be absent in lampreys. Notably, lamprey peripheral neurons are not myelinated, but they are covered with presumptive Schwann cells (Bullock et al., 1984; Peters, 1960; Schultz et al., 1956). These cells may be crest derivatives, but they have not been characterized by molecular genetic methods and their origins are unclear. Interestingly, the lamprey genome has multiple genes associated with myelin production, but use and expression pattern of these genes is unclear (Smith et al., 2013). As mentioned above, lamprey eyes lack intrinsic musculature, which in jawed vertebrates is present and derived from neural crest.
There are still unstudied aspects of neural crest that could be very important in early evolution of crest. Notably, there are numerous interactions between mesoderm and neural crest during the formation of vertebrate cranial muscles. Modification of sites of muscle formation may have been a primary role of early crest. These are not even very well studied in vertebrates, so these analyses must begin in well-established systems, such as avians, zebrafish, and amphibians, that are well suited to addressing these questions.
Conclusion
Species of lampreys living today are by definition modern, competitive, and ecologically successful, yet they are morphologically similar to ancestral groups some 360 millions years distant. Lampreys are at a crucial phylogenetic position, and studies of lamprey anatomy, development, and gene regulation have provided crucial insights into the evolution of neural crest within vertebrates. There are still many open avenues of research, including the evolution of cell communication, and coordination and integration of patterning events in different tissues. Together with comparisons with other non-vertebrate chordates, studies of these gene regulatory networks in lampreys might provide an understanding of the earliest origins of a germ layer. Regardless of precisely when and how neural crest emerged, cyclostomes, and lampreys in particular, will remain crucial for making inferences about the evolutionary elaboration of later neural crest derivatives.
Acknowledgments
We would like to thank C. V. Baker and members of the Bronner Laboratory for helpful discussions. This work was supported by the NIH (R01 NS086907).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations. Mya: million years ago. NC: neural crest.
References
- Abitua PB, Wagner E, Navarrete IA, Levine M. Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature. 2012;492:104–107. doi: 10.1038/nature11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajoghli B, Guo P, Aghaallaei N, Hirano M, Strohmeier C, McCurley N, Bockman DE, Schorpp M, Cooper MD, Boehm T. A thymus candidate in lampreys. Nature. 2011;470:90–94. doi: 10.1038/nature09655. [DOI] [PubMed] [Google Scholar]
- Baker CV. The evolution and elaboration of vertebrate neural crest cells. Curr. Opin. Genet. Dev. 2008;18:536–543. doi: 10.1016/j.gde.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Bardack D, Richardson ES. New agnathous fishes from the Pennsylvanian of Illinois. Fieldiana: Geol. 1977;33:489–510. [Google Scholar]
- Bardack D, Zangerl R. First fossil lamprey: a record from the Pennsylvanian of Illinois. Science. 1968;162:1265–1267. doi: 10.1126/science.162.3859.1265. [DOI] [PubMed] [Google Scholar]
- Bockman D, Kirby M. Dependence of thymus development on derivatives of the neural crest. Science. 1984;223:498–500. doi: 10.1126/science.6606851. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M, Sauka-Spengler T. Assembling neural crest regulatory circuits into a gene regulatory network. Ann. Rev. Cell Devel. Biol. 2010;26:581–603. doi: 10.1146/annurev.cellbio.042308.113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48:145–148. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- Chang M-M, Zhang J, Miao D. A lamprey from the Cretaceous Jehol biota of China. Nature. 2006;441:972–974. doi: 10.1038/nature04730. [DOI] [PubMed] [Google Scholar]
- Damas H. In: Research on the Development of the Lamprey (Lampetra Fluviatilis L.) Saidi Margaret Duggen., translator. Vol. 55. Tunis: Agence Tunisienne de Public-Relations; 1944. p. 349. 1975. [Google Scholar]
- De Beer SG. The development of the vertebrate skull. Oxford: The Clarendon Press; 1937. [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Graham A, Kelsh RN. The origin and evolution of the neural crest. BioEssays. 2008;30:530–541. doi: 10.1002/bies.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development (Cambridge, England) 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Fange R, Johnels AG, Enger PS. The autonomic nervous system. In: Brodal A, Fange R, editors. The biology of Myxine. Oslo: Univ Forlaget; 1963. pp. 124–136. [Google Scholar]
- Farrell AP. Cardiovascular Systems in Primitive Fishes. In: McKenzie DJ, Farrell AP, Brauner CJ, editors. Fish Physiology. London: Elsevier; 2007. pp. 53–120. [Google Scholar]
- Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M. Contribution of neural crest-derived cells in the embryonic and adult thymus. J. Immunol. 2008;180:3183–3189. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Northcutt RG. Cranial and spinal nerve organization in amphioxus and lampreys: evidence for an ancestral craniate pattern. Acta Anat (Basel) 1993;148:96–109. doi: 10.1159/000147529. [DOI] [PubMed] [Google Scholar]
- Gans C, Northcutt RG. Neural crest and the origin of vertebrates: a new head. Science. 1983;220:268–273. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- Gaskell JF. The distribution and physiological action of the suprarenal medullary tissue in petromyzon fluviatilis. J. Physiol. (Lond.) 1912;44:59–67. doi: 10.1113/jphysiol.1912.sp001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gess RW, Coates MI, Rubidge BS. A lamprey from the Devonian period of South Africa. Nature. 2006;443:981–984. doi: 10.1038/nature05150. [DOI] [PubMed] [Google Scholar]
- Gill HS, Renaud CB, Chapleau F, Mayden RL, Potter IC. Phylogeny of Living Parasitic Lampreys (Petromyzontiformes) Based on Morphological Data. Copeia. 2003;2003:687–703. [Google Scholar]
- Glenn Northcutt R. The new head hypothesis revisited. J. Exp. Zool. 2005;304B:274–297. doi: 10.1002/jez.b.21063. [DOI] [PubMed] [Google Scholar]
- Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, Cooper MD. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardisty MW. Biology of the Cyclostomes. London: Chapman & Hall; 1979. [Google Scholar]
- Hardisty MW, Potter IC. The general biology of adult lampreys. In: Hardisty MW, Potter IC, editors. The Biology of Lampreys. Academic Press; 1971. pp. 127–206. [Google Scholar]
- Hardisty MW, Rovainen CM. Morphological and functional aspects of the muscular system. In: Hardisty MW, Potter IC, editors. The Biology of Lampreys. Academic Press; 1982. [Google Scholar]
- Häming D, Simoes-Costa M, Uy B, Valencia J, Sauka-Spengler T, Bronner-Fraser M. Expression of sympathetic nervous system genes in Lamprey suggests their recruitment for specification of a new vertebrate feature. PLoS ONE. 2011;6:e26543. doi: 10.1371/journal.pone.0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg AM, Cowper-Sal-lari R, Sémon M, Donoghue PCJ, Peterson KJ. microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19379–19383. doi: 10.1073/pnas.1010350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horigome N, Myojin M, Ueki T, Hirano S, Aizawa S, Kuratani S. Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Developmental Biology. 1999;207:287–308. doi: 10.1006/dbio.1998.9175. [DOI] [PubMed] [Google Scholar]
- Ivashkin E, Adameyko I. Progenitors of the protochordate ocellus as an evolutionary origin of the neural crest. Evodevo. 2013;4:12. doi: 10.1186/2041-9139-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Engleka KA, Rentschler SL, Manderfield LJ, Li L, Yuan L, Epstein JA. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J. Clin. Invest. 2011;121:422–430. doi: 10.1172/JCI44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier P. Early vertebrates. Oxford: Oxford University Press; 1996. [Google Scholar]
- Janvier P. Palaeontology: modern look for ancient lamprey. Nature. 2006;443:921–924. doi: 10.1038/443921a. [DOI] [PubMed] [Google Scholar]
- Janvier P. Living Primitive Fishes and Fishes From Deep Time. In: McKenzie DJ, Farrell AP, Brauner CJ, editors. Fish Physiology. London: Elsevier; 2007. pp. 1–51. [Google Scholar]
- Janvier P. microRNAs revive old views about jawless vertebrate divergence and evolution. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19137–19138. doi: 10.1073/pnas.1014583107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier P, Desbiens S, Willett JA, Arsenault M. Lamprey-like gills in a gnathostome-related Devonian jawless vertebrate. Nature. 2006;440:1183–1185. doi: 10.1038/nature04471. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Chiba T, Krajka FR, Deyts C, Satoh N, Joly J-S. Trunk lateral cells are neural crest-like cells in the ascidian Ciona intestinalis: Insights into the ancestry and evolution of the neural crest. Developmental Biology. 2008;324:152–160. doi: 10.1016/j.ydbio.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Strickler AG, Yamamoto Y. Migratory neural crest-like cells form body pigmentation in a urochordate embryo. Nature. 2004;431:696–699. doi: 10.1038/nature02975. [DOI] [PubMed] [Google Scholar]
- Johnels AG. On the development and morphology of the skeleton of the head of Petromyzon. Acta Zool. 1948;29:139–279. [Google Scholar]
- Johnels AG. On the peripheral autonomic nervous system of the trunk region of Lampetra planeri. Acta Zool. 1956;37:251–286. [Google Scholar]
- Kasamatsu J, Sutoh Y, Fugo K, Otsuka N, Iwabuchi K, Kasahara M. Identification of a third variable lymphocyte receptor in the lamprey. Proc. Natl. Acad. Sci. U.S.A. 2010;107:14304–14308. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y. To proliferate or to die: role of Id3 in cell cycle progression and survival of neural crest progenitors. Genes & Development. 2005;19:744–755. doi: 10.1101/gad.1257405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerosuo L, Bronner-Fraser M. What is bad in cancer is good in the embryo: importance of EMT in neural crest development. Semin. Cell Dev. Biol. 2012;23:320–332. doi: 10.1016/j.semcdb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzoff NK. Entwicklungsgeschichte des Kopfes von Petromyzon planeri. Bull Soc Nat Moscou. 1901;15:259–289. [Google Scholar]
- Krotoski DM, Bronner-Fraser M. Mapping of neural crest pathways in Xenopus laevis. Prog. Clin. Biol. Res. 1986;217B:229–233. [PubMed] [Google Scholar]
- Kuraku S, Kuratani S. Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences. Zool. Sci. 2006;23:1053–1064. doi: 10.2108/zsj.23.1053. [DOI] [PubMed] [Google Scholar]
- Kuraku S, Hoshiyama D, Katoh K, Suga H. Monophyly of Lampreys and Hagfishes Supported by Nuclear DNA–Coded Genes. J. Mol. Evol. 1999;49:729–735. doi: 10.1007/pl00006595. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Kuraku S, Murakami Y. Lamprey as an evo-devo model: Lessons from comparative embryology and molecular phylogenetics. genesis. 2002;34:175–183. doi: 10.1002/gene.10142. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Ueki T, Aizawa S, Hirano S. Peripheral development of cranial nerves in a cyclostome, Lampetra japonica: morphological distribution of nerve branches and the vertebrate body plan. J. Comp. Neurol. 1997;384:483–500. [PubMed] [Google Scholar]
- Langille RM, Hall BK. Role of the neural crest in development of the trabeculae and branchial arches in embryonic sea lamprey, Petromyzon marinus (L) Development (Cambridge, England) 1988a;102:301–310. [Google Scholar]
- Langille RM, Hall BK. Artificial fertilization, rearing, and timing of stages of embryonic development of the anadromous sea lamprey, Petromyzon marinus L. J. Zool. 1988b;66:549–554. [Google Scholar]
- Lee WJ, Kocher TD. Complete sequence of a sea lamprey (Petromyzon marinus) mitochondrial genome: early establishment of the vertebrate genome organization. Genetics. 1995;139:873–887. doi: 10.1093/genetics/139.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Williams A, Hong CS, You Y, Senoo M, Saint-Jeannet JP. Early development of the thymus in Xenopus laevis. Dev. Dyn. 2013;242:164–178. doi: 10.1002/dvdy.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light W, Vernon AE, Lasorella A, Iavarone A, LaBonne C. Xenopus Id3 is required downstream of Myc for the formation of multipotent neural crest progenitor cells. Development (Cambridge, England) 2005;132:1831–1841. doi: 10.1242/dev.01734. [DOI] [PubMed] [Google Scholar]
- Lund R, Janvier P. A second lamprey from the Lower Carboniferous (Namurian) of Bear Gulch, Montana (USA) Geobios. 1986;19:647–652. [Google Scholar]
- Mallatt J. The suspension feeding mechanism of the larval lamprey Petromyzon marinus. Journal of Zoology. 1981;194:103–142. [Google Scholar]
- Martin WM, Bumm LA, McCauley DW. Development of the viscerocranial skeleton during embryogenesis of the sea lamprey, Petromyzon Marinus. Dev. Dyn. 2009;238:3126–3138. doi: 10.1002/dvdy.22164. [DOI] [PubMed] [Google Scholar]
- McCauley DW, Bronner-Fraser M. Neural crest contributions to the lamprey head. Development (Cambridge, England) 2003;130:2317–2327. doi: 10.1242/dev.00451. [DOI] [PubMed] [Google Scholar]
- McCauley DW, Kuratani S. Cyclostome studies in the context of vertebrate evolution. Zool. Sci. 2008;25:953–954. doi: 10.2108/zsj.25.953. [DOI] [PubMed] [Google Scholar]
- Medeiros DM. The evolution of the neural crest: new perspectives from lamprey and invertebrate neural crest-like cells. WIRES Dev. Biol. 2013;2:1–15. doi: 10.1002/wdev.85. [DOI] [PubMed] [Google Scholar]
- Mehta TK, Ravi V, Yamasaki S, Lee AP, Lian MM, Tay BH, Tohari S, Yanai S, Tay A, Brenner S, Venkatesh B. Evidence for at least six Hox clusters in the Japanese lamprey (Lethenteron japonicum) P. N. A S. 2013 doi: 10.1073/pnas.1315760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Devel Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Müller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M, Rodewald H-R. Neural crest origin of perivascular mesenchyme in the adult thymus. J. Immunol. 2008;180:5344–5351. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Neural Crest Cells Retain Multipotential Characteristics in the Developing Valves and Label the Cardiac Conduction System. Circulation Research. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Nakao T, Ishizawa A. An electron microscopic study of autonomic nerve cells in the cloacal region of the lamprey, Lampetra japonica. J. Neurocytol. 1982;11:517–532. doi: 10.1007/BF01262422. [DOI] [PubMed] [Google Scholar]
- Newth DR. Experiments on the neural crest of the lamprey embryo. Journal of Experimental Biology. 1951;28:247–260. [Google Scholar]
- Newth DR. On the neural crest of the lamprey embryo. J. Embryol. Exp. Morph. 1956;4:358–375. [Google Scholar]
- Nicol JAC. Autonomic nervous systems in lower chordates. Biological Reviews. 1952;27:1–48. [Google Scholar]
- Nikitina N, Bronner-Fraser M, Sauka-Spengler T. Culturing lamprey embryos. Cold Spring Harbor Protocols. 2009a doi: 10.1101/pdb.prot5122. [DOI] [PubMed] [Google Scholar]
- Nikitina N, Bronner-Fraser M, Sauka-Spengler T. Immunostaining of whole-mount and sectioned lamprey embryos. Cold Spring Harbor Protocols. 2009b doi: 10.1101/pdb.prot5126. [DOI] [PubMed] [Google Scholar]
- Nikitina N, Bronner-Fraser M, Sauka-Spengler T. DiI cell labeling in lamprey embryos. Cold Spring Harb Protoc. 2009c doi: 10.1101/pdb.prot5124. [DOI] [PubMed] [Google Scholar]
- Nikitina N, Sauka-Spengler T, Bronner-Fraser M. Dissecting early regulatory relationships in the lamprey neural crest gene network. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20083–20088. doi: 10.1073/pnas.0806009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S. Comparative anatomy of the autonomic nervous system. Autonomic Neuroscience. 2011;165:3–9. doi: 10.1016/j.autneu.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev Biol. 1983;58:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- Oisi Y, Ota KG, Kuraku S, Fujimoto S, Kuratani S. Craniofacial development of hagfishes and the evolution of vertebrates. Nature. 2013;493:175–180. doi: 10.1038/nature11794. [DOI] [PubMed] [Google Scholar]
- Ota KG, Kuratani S. Cyclostome embryology and early evolutionary history of vertebrates. Integrative and Comparative Biology. 2007;47:329–337. doi: 10.1093/icb/icm022. [DOI] [PubMed] [Google Scholar]
- Paiement JM, McMillan DB. The extracardiac chromaffin cells of larval lampreys. Gen. Comp. Endocrinol. 1975;27:495–508. doi: 10.1016/0016-6480(75)90070-2. [DOI] [PubMed] [Google Scholar]
- Peters A. The structure of the peripheral nerves of the lamprey (Lampetra fluviatilis) Journal of Ultrastructure Research. 1960;4:349–359. doi: 10.1016/s0022-5320(60)80027-5. [DOI] [PubMed] [Google Scholar]
- Piavis GW. Embryology. In: Hardisty MW, Potter IC, editors. The Biology of Lampreys. New York, NY: Academic Press; 1971. pp. 361–400. [Google Scholar]
- Prasad MS, Sauka-Spengler T, LaBonne C. Induction of the neural crest state: Control of stem cell attributes by gene regulatory, post-transcription and epigenetic interactions. Dev Biol. 2012;366:10–21. doi: 10.1016/j.ydbio.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MK, Wright GM. Developmental transformations in a normal series of embryos of the sea lamprey Petromyzon marinus (Linnaeus) Journal of Morphology. 2003;257:348–363. doi: 10.1002/jmor.10119. [DOI] [PubMed] [Google Scholar]
- Richardson MK, Admiraal J, Wright GM. Developmental anatomy of lampreys. Biological Reviews. 2010;85:1–33. doi: 10.1111/j.1469-185X.2009.00092.x. [DOI] [PubMed] [Google Scholar]
- Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI, Aravind L, Pancer Z. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nature Immunology. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- Rovainen CM, Schieber MH. Ventilation of larval lampreys. J. Comp. Physiol. 1975;104:185–203. [Google Scholar]
- Sauka-Spengler T. Whole-mount in situ hybridization on lamprey embryos. Cold Spring Harbor Protocols. 2009 doi: 10.1101/pdb.prot5125. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Development and evolution of the migratory neural crest: a gene regulatory perspective. Curr. Opin. Genet. Dev. 2006;16:360–366. doi: 10.1016/j.gde.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Meulemans D, Jones M, Bronner-Fraser M. Ancient Evolutionary Origin of the Neural Crest Gene Regulatory Network. Devel Cell. 2007;13:405–420. doi: 10.1016/j.devcel.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Schultz R, Berkowitz EC, Pease DC. The electron microscopy of the lamprey spinal cord. Journal of Morphology. 1956;98:251–274. [Google Scholar]
- Shigetani Y, Sugahara F, Kawakami Y, Murakami Y, Hirano S, Kuratani S. Heterotopic shift of epithelial-mesenchymal interactions in vertebrate jaw evolution. Science. 2002;296:1316–1319. doi: 10.1126/science.1068310. [DOI] [PubMed] [Google Scholar]
- Shimeld SM, Donoghue PCJ. Evolutionary crossroads in developmental biology: cyclostomes (lamprey and hagfish) Development (Cambridge, England) 2012;139:2091–2099. doi: 10.1242/dev.074716. [DOI] [PubMed] [Google Scholar]
- Shipley AE. On some points in the development of Petromyzon fluviatilis. Quart. J. of Microscop. Sci. 27:1–46. [Google Scholar]
- Smith JJ, Antonacci F, Eichler EE, Amemiya CT. Programmed loss of millions of base pairs from a vertebrate genome. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11212–11217. doi: 10.1073/pnas.0902358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Kuraku S, Holt C, Sauka-Spengler T, Jiang N, Campbell MS, Yandell MD, Manousaki T, Meyer A, Bloom OE, et al. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat Genet. 2013;45:415–421. doi: 10.1038/ng.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, Stuart AB, Sauka-Spengler T, Clifton SW. Development and analysis of a germline BAC resource for the sea lamprey, a vertebrate that undergoes substantial chromatin diminution. Chromosoma. 2010 doi: 10.1007/s00412-010-0263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y. Normal stages of development in the lamprey, Lampetra reissneri (Dybowski) Zool. Sci. 1988;5:109–118. [Google Scholar]
- Tretjakoff D. Das periphere Nervensystem des Flussneunauges. Zeitschrift fur Wissenschaftliche Zoologie. 1927;129:359–452. [Google Scholar]
- Yamazaki Y, Fukutomi N, Takeda K, Iwata A. Embryonic Development of the Pacific Lamprey, Entosphenus tridentatus. Zool. Sci. 2003;20:1095–1098. doi: 10.2108/zsj.20.1095. [DOI] [PubMed] [Google Scholar]
- Young JZ. Memoirs: The Autonomic Nervous System of Selachians. Quart. J. of Microscop. Sci. 1933;75:571–624. [Google Scholar]
- Yu J-KS. The evolutionary origin of the vertebrate neural crest and its developmental gene regulatory network--insights from amphioxus. Zoology (Jena) 2010;113:1–9. doi: 10.1016/j.zool.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yu J-K, Meulemans D, McKeown SJ, Bronner-Fraser M. Insights from the amphioxus genome on the origin of vertebrate neural crest. Genome Res. 2008;18:1127–1132. doi: 10.1101/gr.076208.108. [DOI] [PMC free article] [PubMed] [Google Scholar]