Abstract
Background and Aims
Non-invasive predictors identifying subjects with compensated liver disease at highest risk for transitioning to a decompensated state are lacking. We hypothesized that liver shear stiffness as measured by magnetic resonance elastography is an important non-invasive predictor of hepatic decompensation.
Methods
Among patients with advanced fibrosis undergoing magnetic resonance elastography (2007–11), a baseline cohort and follow up cohort (compensated liver disease) were established. Cause specific cox proportional hazards analysis adjusting for competing risks was utilized to determine the association between elevated liver shear stiffness and development of decompensation (hepatic encephalopathy, ascites, variceal bleeding).
Results
In the baseline cohort (n=430), subjects with decompensated liver disease had a significantly higher mean liver shear stiffness (6.8 kPa, IQR 4.9–8.5) as compared to subjects with compensated liver disease (5.2 kPa, IQR 4.1–6.8). After adjustment for Model for End Stage Liver Disease score, hepatitis C, age, gender, albumin, and platelet count, the mean liver shear stiffness (OR=1.13, 95%CI 1.03–1.27) was an independently associated with decompensated cirrhosis at baseline. Over a median follow up of 27 months (n=167), 7.2% of subjects with compensated disease experienced hepatic decompensation. In the follow up cohort, the hazard of hepatic decompensation was 1.42 (95% CI 1.16 –1.75) per unit increase in liver shear stiffness over time. The hazard of hepatic decompensation was 4.96 (95% CI 1.4–17.0, p=0.019) for a subject with compensated disease and mean LSS value ≥ 5.8 kPa as compared to an individual with compensated disease and lower mean LSS values.
Conclusion
Baseline liver shear stiffness assessed by magnetic resonance elastography is independently associated with decompensated liver disease.
Keywords: non invasive, outcomes, natural history, prognosis, cirrhosis
INTRODUCTION
Patients affected by compensated and decompensated cirrhosis are known to have disparate clinical outcomes. As compared to the general population, individuals with compensated cirrhosis have a 5 fold increase, whereas patients with decompensated disease have a 10 fold increase in mortality.[1] Overall survival is lower at 1 year (75% vs. 87%) and 5 years (45% vs. 67%) for persons with decompensated cirrhosis as compared to compensated cirrhosis. The probability of transitioning from a compensated to decompensated state varies between 4% to 12% per year.[2–4] Given that a majority of deaths in patients with compensated cirrhosis are due to progression to a decompensated state and the development of its ensuing complications, the ability to predict decompensation is important. If patients with compensated liver disease at the highest risk of decompensation can be identified, it may be possible to institute enhanced surveillance and prophylactic measures for this patient subset.
However, objective tests that predict the risk of transition to a decompensated state are lacking. Hepatic venous pressure gradient (HVPG), as an indirect measure of portal pressure across the liver, is a potential marker of this transition.[5, 6] However, the measurement of HVPG is invasive, subject to operator variability and not readily available in clinical practice. Furthermore, it is unclear whether it completely captures the risk of decompensation.[7] Non-invasive methods that assess liver stiffness, namely ultrasound based transient elastography (TE) or magnetic resonance elastography (MRE), are predictive of cirrhosis and the presence of clinically significant portal hypertension at baseline.[8] However, it is unclear whether these methods can identify compensated patients at risk for clinical decompensation.[9] The role of liver stiffness as a potential predictor of hepatic decompensation as measured by TE has been recently examined.[10–12] However, to date a heterogeneous group of patients with variable degrees of underlying fibrosis has been studied, with concomitant liver biopsies available in only a minority of patients. Hence, the role of elastography in the group at highest risk (namely persons with advanced fibrosis) is unknown.[10, 11] The technical specifications of TE may also limit its utility in patients that are obese or among individuals with ascites.[13]
The use of magnetic resonance elastography (MRE) as a predictor of hepatic decompensation has not been studied. In a recent analysis, MRE had a significantly higher diagnostic accuracy as compared to ultrasound based elastography for staging liver fibrosis.[14] The presence of ascites or obesity is also not a limiting factor with MRE and the frequency of complete examinations may be higher as compared with ultrasound-based approaches. Thus, we hypothesized that liver stiffness as measured by MRE is an important non-invasive predictor of hepatic decompensation in patients with compensated liver disease.
METHODS
The aims of the study were to (1) assess the baseline relationship between elevated liver shear stiffness and presence of decompensated liver disease and (2) assess whether elevated liver shear stiffness among persons with compensated liver disease can predict the development of future decompensated cirrhosis.
Magnetic Resonance Elastography
MRE is a commercially available technique for quantitatively assessing liver shear stiffness. The premise of the examination is based on the observation that fibrosis is associated with elevated liver stiffness and significantly different from normal liver parenchyma. It is analogous to physical examination where a “stiff or hard” liver on palpation potentially signifies the presence of advanced fibrosis. It typically adds less than 5 minutes when incorporated as part of a standard abdominal MRI exam. MRE measures the mechanical property of liver tissue by transmitting mechanical waves into the parenchyma and quantifying stiffness based on wave propagation and velocity. A pneumatic passive driver is placed over the lower chest and upper abdomen overlying the right lobe of the liver at the level of xiphisternum. This driver transmits mechanical waves at 60Hz which are transferred from an active driver component placed outside the scanning room. The active and passive drivers are connected to each other by a 7.6m long plastic tube. The mechanical waves induce propagating shear waves within the liver and are imaged by using a specialized MRI sequence (MR Elastography sequence). The data is processed using inversion algorithms to generate quantitative images or elastograms that are representative of the liver's mechanical properties. Mean stiffness values (in kilopascals, kPa) are measured in regions of interest within the liver.[15, 16]
Subjects
We examined all consecutive patients that underwent an MRE at Mayo Clinic Rochester between 2007 and 2011 with follow up through September 2012. Though the database was created and outcomes registered concurrent with subjects getting MRE, the analysis was a retrospective analysis. A majority of the examinations took place in the outpatient setting and occurred at the physician's discretion and was not based on a priori clinical decision rules. The most common reasons for MRE included follow up of known cirrhosis, exploration of abnormal liver function tests, follow up of fibrosis status after initial diagnosis and/or initiation of disease-specific treatment, and evaluation of liver mass.
Of all patients that underwent MRE, subjects with either a clinical diagnosis of cirrhosis or biopsy proven advanced fibrosis (stage 3 or 4) were examined. This group formed the baseline cohort for determining the level of association between LSS and the presence of compensated or decompensated disease. The clinical stage of cirrhosis for individual subjects was assigned using an accepted classification system as proposed by D'Amico: stage 1(absence of esophageal varices and of ascites), stage 2 (presence of non-bleeding esophageal varices without ascites), stage 3 (ascites with or without non bleeding esophageal varices) and stage 4 (variceal bleeding with or without ascites).[3]
From all patients in the baseline cohort, we then selected subjects with biopsy proven advanced fibrosis (stage 3 or 4) and excluded patients based on the following criteria: a) presence of HCC and b) and hepatic decompensation or liver transplantation at baseline to create a cohort of patients with compensated liver disease (follow up cohort). The follow up cohort was limited to persons with biopsy proven advanced fibrosis to enrich the group with those at highest risk of decompensation. In patients with multiple examinations over the 4 years study period, only the baseline examination was considered and examinations/patients without paired biopsies were excluded.
Statistical analysis
The primary variable of interest was liver shear stiffness (LSS) as measured by MRE. The primary outcome was either the presence (baseline cohort) or development (follow up cohort) of hepatic decompensation, defined as the presence of ascites, hepatic encephalopathy, or variceal bleeding. A diagnosis of decompensation was based on clinical assessment and not simply on radiological findings (e.g. trace ascites on imaging). Though more than one complication was possible in an individual patient (e.g. variceal bleeding and hepatic encephalopathy), each subject was only counted once for having an event of interest. The follow up cohort was followed until the last clinical visit, development of decompensated disease, death, liver transplantation, or end of the study period (September 2012).
Comparisons between continuous variables were made using Student t -test or Wilcoxon two sample test while dichotomous variables were compared using Chi square or Fisher's exact test. Differences in liver stiffness among persons with compensated and decompensated liver disease in the baseline cohort were compared. In the baseline cohort, logistic regression analysis was used to assess the association between elevated LSS and the presence of hepatic decompensation adjusted for age, sex, diagnosis, Model for End Stage Liver Disease (MELD) score, albumin, ALT, body mass index (BMI) and platelet count.
In the follow up cohort, given that death or liver transplantation may be competing events and the occurrence of either event may preclude or alter the probability of decompensation, competing risks analysis was utilized to assess the association between elevated LSS and development of hepatic decompensation.[17, 18] Cumulative incidence estimates for decompensation were generated. Cause specific Cox proportional hazards analysis was utilized to assess the association between elevated LSS and the time to developing hepatic decompensation after accounting for competing events. Time dependent ROC analysis were completed using the nearest neighbor estimation method with a span of lambda(n)=0.05. To select an optimal threshold value for liver stiffness, we considered the Younden J index to optimize the predictive ability of the cut-off points by time point while giving equal weights to sensitivity and specificity. Subsequently, cumulative incidence curves of decompensation where death and liver transplantation are competing risks were generated stratified by the optimal cutoff point. A non parametric estimation of cumulative incidence function and the comparison between the two groups in the presence of competing risks was considered and tested using the Gray's test.[19] Time-dependent ROC curve analysis was performed with R software, version 3.0.1 and with the “survivalROC” package. The rest of the analysis with SAS 9.3. The study was approved by the Mayo Clinic Institutional Review Board.
RESULTS
Baseline cohort
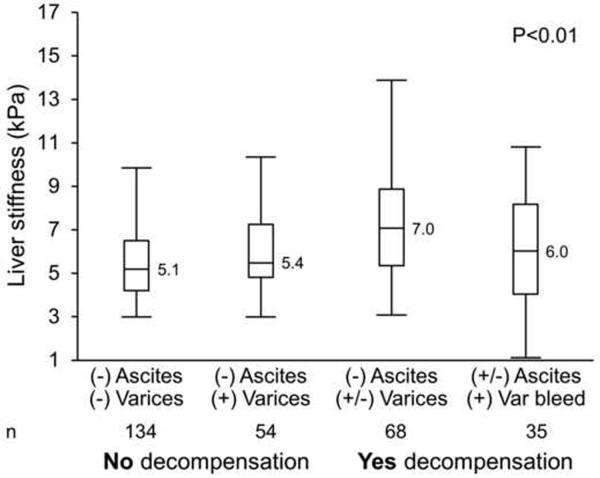
Between 2007 and 2011, there were 430 patients with advanced fibrosis and/or a clinical diagnosis of cirrhosis and comprised the baseline cohort. Of these individuals, a total of 230 (53.4 %) subjects had biopsy-proven advanced fibrosis. In a majority of the cases the biopsy was performed before the MRE (median 11 months, interquartile range, IQR 0.03–46.7). In 36 cases, the biopsy was done after the MRE (median, 0.6 months, IQR 0.06–1.6). At baseline, 288 (68%) subjects did not have evidence of hepatic decompensation while 135 (32%) subjects met criteria for hepatic decompensation. Table 1 describes the characteristics of patients with and without decompensation. Among subjects with hepatic decompensation, a total of 37 patients (9%) had a current or previous history of variceal bleeding, 110 patients (26%) had ascites and 35 patients (8%) had hepatic encephalopathy. Subjects with decompensated liver disease had a significantly higher median LSS value (6.8 kPa, interquartile range IQR 4.9–8.5) as compared to those with compensated cirrhosis or advanced fibrosis (5.2 kPa, IQR 4.1–6.8). There was a significant difference (p<0.001) in mean LSS by D'Amico clinical stage as well (stage 1: 5.1 kPa (IQR 4.1–6.4), stage 2: 5.4 kPa (IQR 4.8–7.2), stage 3: 7 kPa (IQR 5.3–8.8), stage 4: 6 kPa (IQR 4.0–8.2)) although a linear trend across clinical stages 3 and 4 was not observed (Fig. 1).
Table 1.
Baseline demographics of all subjects in baseline cohort (biopsy proven stage 3 or 4 or clinical diagnosis of cirrhosis) and follow up cohort (biopsy proven stage 3 or 4 fibrosis subjects with compensated disease).
| Cohort | Baseline | Follow up | ||||
|---|---|---|---|---|---|---|
| Decompensation | Overall | No | Yes | Overall | No | Yes |
| N | 430 | 288 | 135 | 167 | 155 | 12 |
| Age (yrs) | 59 (51, 68) | 59 (51, 68) | 60 (51,68) | 55 (47, 64) | 55 (47,64) | 60 (49,69) |
| Female | 40% | 41% | 37% | 45% | 56% | 50% |
| Diagnosis: HCV | 22.7% | 25.7% | 14.8% | 32.2% | 31.6% | 41.7% |
| Alcohol | 9.5% | 7.9% | 13.3% | 3.9% | 4.2% | 0 |
| NAFLD | 25.5% | 26.7% | 23.7% | 23.2% | 22.9% | 27.3% |
| Cholestatic | 8.6% | 10.1% | 5.9% | 13.5% | 13.9% | 9.1% |
| Other | 33.6% | 29.5% | 42.2% | 27.1% | 27.8% | 18.2% |
| ALT | 46 (30, 81) | 52 (32, 97) | 41 (26,67) | 68 (34,121) | 56 (39,82) | |
| MELD | 7 (6, 10) | 6 (6, 8) | 9 (7, 12) | 6 (6,6) | 6 (6, 6) | 6 (6, 6) |
| Bilirubin mg/dL | 1 (0.6,1.6) | 0.8 (0.5–1.2) | 1.6 (1.0–2.4) | 0.7 (0.4, 1) | 0.7 (0.4–1.0) | 1.1 (0.8–1.4) |
| BMI | 29.0 (25,34) | 29.2 (25,34) | 28.5 (24,34) | 28.8 (26, 35) | 28.6 (25, 35) | 30.8 (27, 37) |
| Platelet K/uL | 131 (87, 187) | 140 (98, 204) | 104 (68, 160) | 170 (126, 239) | 172 (129, 237) | 117 (81, 214) |
| Albumin g/dL | 3.8 (3.4, 4.3) | 4.1 (3.6, 4.4) | 3.5 (3.1 3.8) | 4.2 (3.9, 4.4) | 4.3 (3.9, 4.4) | 4 (3.6, 4.3) |
Characteristics by presence or absence of decompensation are also provided.
HCV: hepatitis C; MELD: model for end stage liver disease; Median and interquartile range provided
Figure 1.
Association between liver stiffness and clinical stages of liver disease (D'Amico et al) among subjects with cirrhosis in the baseline cohort.
Similar results were obtained when only patients with biopsy proven stage 4 fibrosis were considered (n=116). Subjects with decompensated biopsy proven stage 4 fibrosis had a significantly higher median LSS value (6.2 kPa, IQR 5.2–8.6) as compared to those with compensated cirrhosis (5.1 kPa, IQR 4.1–6.4). There was a significant difference (p<0.001) in mean LSS by D'Amico clinical stage as well ((stage 1: 4.5 kPa (IQR 3.8–6.03), stage 2: 6.5 kPa (IQR 5.3–7.3), stage 3: 8 kPa (IQR 5.6–8.8), stage 4: 6 kPa (IQR 4.2–8.6)).
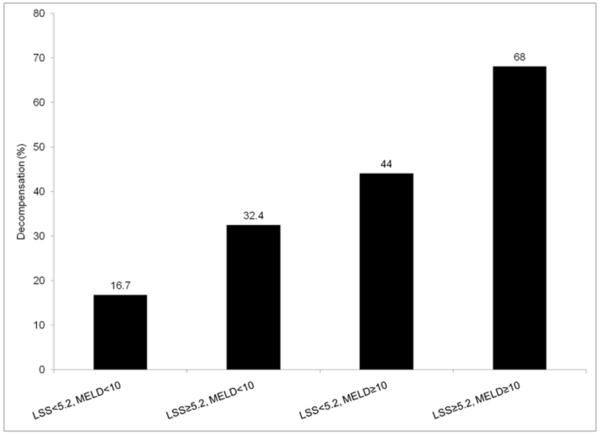
We next examined the association between MELD score and median LSS value (5.2 kPa, IQR 4.1–6.8) for decompensated liver disease in the baseline cohort (Fig 2). The presence of hepatic decompensation at baseline ranged from 16.7% (MELD score less than 10 and LSS <5.2kPa) to 68% (MELD score 10 and LSS 5.5kPa). Following adjustment for MELD score (odds ratio, OR=1.13, 95% confidence interval, CI 1.06–1.21), chronic hepatitis C (OR=2.59, 95% CI 1.32–5.33), age, gender, albumin, and platelet count, mean LSS was independently associated with decompensated cirrhosis at baseline (OR=1.13, 95% CI 1.03–1.27) (Table 2). There was no difference in the magnitude of effect after adjusting for ALT or BMI.
Figure 2.
Percent of subjects with decompensation by MELD score and liver stiffness (LSS) among subjects with cirrhosis in the baseline cohort
MELD: model for end stage liver disease; LSS: Liver Shear Stiffness
Table 2.
Predictors of presence of decompensation in the baseline cohort (biopsy proven stage 3 or 4 or clinical diagnosis of cirrhosis) on multivariable analysis
| Variable | Odds Ratio | 95% CI |
|---|---|---|
| Liver stiffness (per unit) | 1.13 | 1.03–1.27 |
| MELD score (per unit) | 1.13 | 1.06–1.21 |
| Age (per year) | 0.99 | 0.97–1.01 |
| Serum Albumin (per g/dL) | 0.77 | 0.65–0.90 |
| Platelets (per K/uL) | 0.99 | 0.99–1.00 |
| Diagnosis: HCV | 2.59 | 1.32–5.33 |
| Gender: Female | 0.77 | 0.44–1.32 |
HCV: hepatitis C; MELD: model for end stage liver disease
Follow up cohort
In the follow up cohort, there were 167 subjects with compensated liver disease and biopsy proven advanced fibrosis (57% stage 3 and 43% stage 4). Over a median follow up of 27 months (range 0–65 months), 7% (n=12) of patients experienced hepatic decompensation (ascites in 7 subjects, hepatic encephalopathy in 3 subjects). Of these 41.7% (n=5) events were seen in patients that were stage 3 fibrosis.
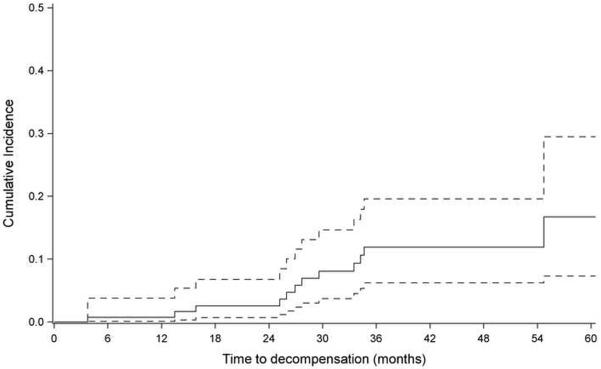
Figure 3 shows the cumulative incidence of decompensation after accounting for competing events (death or liver transplantation). The hazard of hepatic decompensation was 1.42 (95% CI 1.16 –1.75) per unit increase in LSS over time. When limited to stage 4 fibrosis, the hazard ratio of hepatic decompensation was 1.65 (95% CI 1.22–2.27) per unit increase in LSS over time.
Figure 3.
Cumulative incidence of development of decompensation in the follow up cohort after accounting for competing events (death or liver transplantation). 95% Confidence interval (dash line) is also provided.
Given the small number of events, we were unable to conduct a valid multivariable logistic regression analysis. However, elevated mean LSS was an independent predictor of hepatic decompensation among persons with compensated liver disease independent of MELD score and hepatitis C status on separate bivariate analyses after accounting for competing risks (Table 3).
Table 3.
Predictors of development of decompensation in the follow up cohort (biopsy proven stage 3 or 4 fibrosis subjects with compensated disease) on separate bivariate analyses.
| Model | Variable | Decompensation Hazard ratio (95% CI) | p |
|---|---|---|---|
| Model 1 | Liver stiffness | 1.91 (1.21–3.00) | 0.01 |
| MELD score | 0.44 (0.15–1.29) | 0.13 | |
| Model 2 | Liver stiffness | 1.41 (1.15–1.72) | 0.001 |
| Age | 1.01 (0.96–1.06) | 0.68 | |
| Model 3 | Liver stiffness | 1.27 (0.99–1.62) | 0.02 |
| Serum Albumin | 0.31 (0.06–1.63) | 0.15 | |
| Model 4 | Liver stiffness | 1.42 (1.16–1.73) | 0.001 |
| Diagnosis: HCV | 1.65 (0.50–5.51) | 0.71 |
HCV: hepatitis C; MELD: model for end stage liver disease
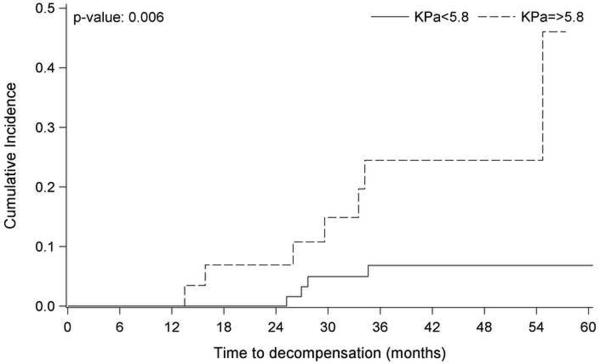
Finally, we examined the cumulative incidence of decompensation by the optimal cutoff for liver stiffness, 5.8 kPa. Cumulative incidence of liver decompensation in subjects with LSS above the optimum cutoff (kPa 5.8) was significantly higher than that in the group below the optimum cutoff (p=0.006). The cumulative incidence rate was significantly higher in subjects with kPa 5.8 at two years (6.9%; 95% CI, 1.2–20.0 versus 0.0%; 95% CI, 0.0-0.0) and three years (19.6%; CI, 6.8–37.4 versus 6.8%; CI, 2.2–15.3) (Fig 4). Whereas, using a univariate Cox proportional hazard model, with liver stiffness categorized with the optimal cutoff of 5.8 kPa, the hazard ratio of hepatic decompensation was 4.96 (95% CI 1.4–17.0, p=0.019) for a subject with compensated liver disease and mean LSS value 5.8 kPa as compared to an individual with compensated liver disease and lower mean LSS values.(Fig 4)
Figure 4.
Cumulative incidence of development of decompensation in the follow up cohort after accounting for competing events (death or liver transplantation) with a liver shear stiffness less than or greater than 5.8kPa.
DISCUSSION
In this study, we report that LSS assessed by MRE is associated with hepatic decompensation at baseline and is an independent predictor for the development of hepatic decompensation in patients with compensated liver disease and advanced fibrosis. Specifically, an elevated liver stiffness appears to help identify subjects likely to develop future hepatic decompensation. Over a median follow up of 27 months, subjects with compensated liver disease and mean liver LSS values above 5.8 kPa had a 5 fold higher risk of transitioning to decompensated cirrhosis as compared to subjects with compensated liver disease and lower mean LSS values. Given that the development of decompensated cirrhosis represents an ominous milestone in the natural history of cirrhosis, the ability of a non-invasive technique, i.e. MRE, to potentially capture this risk of transition in patients at highest risk (all with advanced fibrosis) is encouraging.
Increased fibrosis leads to intrahepatic resistance and correlates with portal hypertension. LSS as measured by MRE is likely one of many factors identified as a predictor of decompensation which include HVPG, MELD, obesity and thickness of fibrous septa separating cirrhotic nodules.[5, 20–22] The advantage offered by MRE is that liver shear stiffness as a likely reflection of advanced fibrosis is measured across the entire liver and may therefore be able to capture the risk of decompensation. One however notes that there is likely a dynamic component to decompensation (e.g. presence of infection, development of renal failure due to extrahepatic factors) that is unlikely to be captured by any mathematical model or single objective measurement. There may be a point beyond which complications of portal hypertension may develop from mechanisms that are not completely explained by accumulation of fibrosis or effectively captured by elastography.[7] For example, at certain stages of disease, there may be a poor correlation with liver stiffness due to the overarching influence of extrahepatic factors or an imbalance between splanchnic vasodilatation and active intrahepatic resistance.[13, 20, 22–24].
We anticipate that elastography, specifically MRE may have an increasing role in the management of patients with liver disease. For example, if patients at highest risk of decompensation are identified, one may speculate that closer follow up may be helpful. MRE may also help discern which one of a pair of individuals with compensated liver disease may be at higher risk of decompensation with disease specific therapy (e.g. antiviral therapy for HCV). Its role as an adjunct to HVPG remains unknown. Alternatively, a lower liver stiffness may prompt longer periods of follow up. Further studies including the role of serial LSS measurements in prognosis and identifying the natural history of compensated cirrhosis are obviously needed.
Our study has several strengths. We were able to examine one of the largest experiences with MRE and concomitant liver biopsies in a heterogeneous outpatient population. Specifically, we examined subjects with advanced fibrosis and cirrhosis, the group of patients with liver disease at highest risk of decompensation. We were able to successfully examine a large group of obese persons with a wide range of BMI and individuals with ascites with a low rate of incomplete examinations.
Despite a median follow up of 27 months, the frequency of episodes related to hepatic decompensation was low. However, the numbers of patients progressing to decompensated cirrhosis fall within the estimates of annual progression as noted in prior natural history studies (approximately 5–7% per year).[2–4] Further, the technology is novel enough (introduced into clinical practice in 2007) where longer periods of follow up are not available. We did not examine the role of serial MRE in patients with liver disease nor did we examine the effect of treatment (for example successful antiviral therapy for hepatitis C, alcohol abstinence, weight loss) within separate subsets of cirrhotics by their underlying disease. Serial MRE was only available in a subset of patients with cirrhosis. Further, repeat biopsies were not available in a large enough group of patients to confidently comment on the effect of treatment and possible regression of disease. However, the aim of our study was to discern whether a singular value of liver stiffness at baseline was adequate enough to provide prognostic information. Other limitations of MRE include the potential for falsely elevated stiffness values in the setting of acute inflammation, cholestasis, and/or congestion/edema. Of note, the association between elevated liver stiffness and decompensation was independent of ALT as a potential a reflection of inflammation. A majority of the examinations took place at the physician's discretion and we were unable to compare characteristics of patients that underwent an MRE with those that did not. This selection may have introduced a bias that may accentuate the purported association between elevated liver stiffness and decompensation. We included patients with stage 3 fibrosis in enumeration of patients with compensated disease. We feel that it is helpful to examine patients with stage 3 fibrosis as a subset of them may have cirrhosis that may be missed on biopsy due to either sampling bias or as a result under staging between different pathologists.[25] As a matter of fact, 40% of decompensation events were observed in patients that were stage 3 fibrosis. Patients with decompensated cirrhosis had higher median values of LSS as compared with patients with compensated liver disease. However, there was an important overlap between groups. Therefore, though absolute values of liver stiffness that separates compensated versus decompensated disease may not be generalizable, the presence of elevated liver stiffness in subjects with compensated disease should be of concern. The predictive ability of liver stiffness, albeit by transient elastography has also recently been published which is in line with our findings.[12]
In summary, liver stiffness as measured MRE appears to have a promising role in the management of patients with chronic liver disease. In this study, we demonstrate the ability of MRE to potentially identify patients at high risk of decompensation. These data suggest that MRE may evolve its role from diagnostic test to surveillance procedure to actively help in the management of subjects at highest need for vigilance.
Acknowledgments
Financial Support: This study was supported by a NIH grants T32 DK07198(SKA), KL2 TR000136 (JAT), RC1EB10393 (JAT), and R01 EB001981 (RLE) for design and conduct of the study, collection, management, and analysis of the data.
List of Abbreviations
- LSS
Liver shear stiffness
- MRE
magnetic resonance elastography
- HCV
hepatitis C
- MELD
model for end stage liver disease
- CI
confidence interval
- OR
Odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Mayo Clinic and RLE have intellectual property rights and a financial interest in MRE technology. Mayo Clinic and RLE hold equity and RLE serves as CEO of Resoundant, Inc. None of the other authors have conflicts of interest or any specific financial interests relevant to the subject of this manuscript.
REFERENCES
- [1].Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: A population-based cohort study. Liver International. 2012;32:79–84. doi: 10.1111/j.1478-3231.2011.02517.x. [DOI] [PubMed] [Google Scholar]
- [2].Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: A cohort study. Alimentary Pharmacology and Therapeutics. 2010;32:1343–1350. doi: 10.1111/j.1365-2036.2010.04473.x. [DOI] [PubMed] [Google Scholar]
- [3].D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. Journal of hepatology. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- [4].Schuppan D, Afdhal NH. Liver cirrhosis. The Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- [6].Ripoll C, Lastra P, Rincon D, Catalina V, Banares R. Comparison of MELD, HVPG, and their changes to predict clinically relevant endpoints in cirrhosis. Scand J Gastroenterol. 2012;47:204–211. doi: 10.3109/00365521.2011.645500. [DOI] [PubMed] [Google Scholar]
- [7].Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293–1302. e1294. doi: 10.1053/j.gastro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- [8].Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Berzigotti A, Ashkenazi E, Reverter E, Abraldes JG, Bosch J. Noninvasive diagnostic and prognostic evaluation of liver cirrhosis and portal hypertension. Disease Markers. 2011;31:129–138. doi: 10.3233/DMA-2011-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Robic MA, Procopet B, Métivier S, Péron JM, Selves J, Vinel JP, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: A prospective study. Journal of hepatology. 2011;55:1017–1024. doi: 10.1016/j.jhep.2011.01.051. [DOI] [PubMed] [Google Scholar]
- [11].Corpechot C, Carrat F, Poujol-Robert A, Gaouar F, Wendum D, Chazouillères O, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56:198–208. doi: 10.1002/hep.25599. [DOI] [PubMed] [Google Scholar]
- [12].Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, et al. Liver Stiffness Measurements are Associated with Risk of Decompensation, Liver Cancer, and Death in Patients with Chronic Liver Diseases: a Systematic Review and Meta-Analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013 doi: 10.1016/j.cgh.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Llop E, Berzigotti A, Reig M, Erice E, Reverter E, Seijo S, et al. Assessment of portal hypertension by transient elastography in patients with compensated cirrhosis and potentially resectable liver tumors. Journal of hepatology. 2012;56:103–108. doi: 10.1016/j.jhep.2011.06.027. [DOI] [PubMed] [Google Scholar]
- [14].Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- [15].Glaser KJ, Manduca A, Ehman RL. Review of MR elastography applications and recent developments. J Magn Reson Imaging. 2012;36:757–774. doi: 10.1002/jmri.23597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology. 2008;47:332–342. doi: 10.1002/hep.21972. [DOI] [PubMed] [Google Scholar]
- [17].Kim WR, Therneau TM, Benson JT, Kremers WK, Rosen CB, Gores GJ, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology. 2006;43:345–351. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]
- [18].Fine J, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- [19].Gray R. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- [20].Sethasine S, Jain D, Groszmann RJ, Garcia-Tsao G. Quantitative histological-hemodynamic correlations in cirrhosis. Hepatology. 2012;55:1146–1153. doi: 10.1002/hep.24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Berzigotti A, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Morillas R, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54:555–561. doi: 10.1002/hep.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Garcia-Tsao G, Friedman S, Iredale J, Pinzani M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445–1449. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- [24].Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, et al. Measurement of Spleen Stiffness to Evaluate Portal Hypertension and the Presence of Esophageal Varices in Patients With HCV-Related Cirrhosis. Gastroenterology. 2012;143:646–654. doi: 10.1053/j.gastro.2012.05.035. [DOI] [PubMed] [Google Scholar]
- [25].Robert M, Sofair AN, Thomas A, Bell B, Bialek S, Corless C, et al. A comparison of hepatopathologists' and community pathologists' review of liver biopsy specimens from patients with hepatitis C. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2009;7:335–338. doi: 10.1016/j.cgh.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]