Abstract
Objectives
In tissue regeneration research, the term “bioactivity” was initially used to describe the resistance to removal of a biomaterial from host tissues after intraosseous implantation. Hydraulic calcium silicate cements (HCSCs) are putatively accepted as bioactive materials, as exemplified by the increasing number of publications reporting that these cements produce an apatite-rich surface layer after they contact simulated body fluids.
Methods
In this review, the same definitions employed for establishing in vitro and in vivo bioactivity in glass–ceramics, and the proposed mechanisms involved in these phenomena are used as blueprints for investigating whether HCSCs are bioactive.
Results
The literature abounds with evidence that HCSCs exhibit in vitro bioactivity; however, there is a general lack of stringent methodologies for characterizing the calcium phosphate phases precipitated on HCSCs. Although in vivo bioactivity has been demonstrated for some HCSCs, a fibrous connective tissue layer is frequently identified along the bone–cement interface that is reminiscent of the responses observed in bioinert materials, without accompanying clarifications to account for such observations.
Conclusions
As bone-bonding is not predictably achieved, there is insufficient scientific evidence to substantiate that HCSCs are indeed bioactive. Objective appraisal criteria should be developed for more accurately defining the bioactivity profiles of HCSCs designed for clinical use.
Keywords: Bioactivity, Calcium silicate, Hydraulic cement, In vitro, In vivo
1. Introduction
A bioactive material may be broadly defined as “one which has been designed to induce specific biological activity”.1 Based on this generic definition, biologically active materials may include those that promote tissue regeneration by adhesion to soft and hard tissues of the human body, those that possess cell-instructive and molecular signalling properties via functionalized ligands or incorporating growth factors for regulating cell proliferation, migration, differentiation, protein expression and mineralization processes. Other bioactive materials include those that are designed for biosensing via physicochemical interactions, those that contain recognition sites for cleavage of enzymes involved in cell functions, and those that possess antimicrobial or immunoregulatory activities by incorporating antimicrobial agents or molecules that mimic natural host-defense peptides.2–6 Along the same line of thought, bioactive materials may also include those that incorporate bioactive peptides with antithrombotic, antihypertensive, opioid or antioxidative properties for controlled release.7
Prior to the adoption of this contemporary interpretation of bioactivity, scientists in the field of tissue regeneration have been using a more focused definition of “bioactivity” to describe the resistance of a calcium phosphosilicate glass to be removed from the host hard and soft tissues, after it was implanted in femurs and muscles in a rat model.8 Interfacial bonding between the implant and living tissues has subsequently been observed in other synthetic calcium phosphate ceramics, silicate-based, borate-based and phosphate-based glasses.9,10 A bioactive material, as defined by Hench and coworkers, is one that elicits a specific biological response at the interface of the material, which results in the formation of a bond between living tissues and the material.11 A feature commonly identified from these materials is a time-dependent kinetic modification of the material’s surface via the formation of a carbonated apatite surface layer following its implantation in vivo.12,13
The tissue regeneration definition of bioactivity has undergone a subtle paradigm drift, after the feature of in vivo carbonated apatite formation14 was found to be reproducible in vitro by immersing the material in a simulated body fluid (SBF) designed to mimic human blood plasma.15 Thus, according to Kokubo and Takadama, a bioactive material is one on which bone-like carbonated apatite will form selectively after it is immersed in a serum-like solution.16 Over the years, the scientific community at large has putatively accepted this paradigm drift, with the assumption that demonstration of “in vitro bioactivity” is the indirect equivalent of affirming a material’s bone-bonding potential. Although in vitro bioactivity evaluation is appealing because of its simplicity and rapidity in data generation, a recent review cautioned the lack of adequate scientific evidence to support the assumption that a material that initiates the deposition of calcium phosphate salts on its surface after immersion in SBF will bond directly to bone following intraosseous implantation.17 For example, a host of sol–gel reaction-derived metallic oxides, including SiO2, TiO2, ZrO2, Nb2O5 and Ta2O5 were found to possess in vitro bioactivity after immersing in simulated body fluid18–22; however, the ability of these metallic oxide gels to bond to bone in vivo has not been demonstrated.
The introduction of hydraulic calcium (alumino) silicate cements has provided clinicians with alternative biomaterials for dentine replacement, pulp capping, pulpotomy, creation of apical barriers in teeth with open apices, repair of root perforation and resorptive defects, as well as orthograde or retrograde root canal fillings.23–26 Among their many desirable properties, hydraulic calcium silicate cements (HCSCs) have been described as possessing bioactive properties that influence their surrounding environments.24 A discussion of bioactivity based on its generic definition is beyond the scope of this review. Rather, the bioactivity of HCSCs will be discussed from a tissue regeneration perspective.
2. Biologically inactive versus bioactive inorganic materials
When a biomaterial is implanted in the human body, the host tissue reacts towards the implant in different ways depending on the tissue response along the implant surface. Accordingly, a biomaterial may be classified into 4 types based on their tissue responses: nearly inert, porous, resorbable or bioactive. 27 As HCSCs are non-resorbable and after setting, do not possess pores that are large enough for ingrowth of bone or blood vessels, only the tissue responses of nearly inert and bioactive materials will be described.
No material implanted in living tissues is completely inert. Thus, the term “bioinert” is designated to any material which, when implanted into the human body, elicits minimal interaction with its surrounding tissues. Examples of these materials are stainless steel, titanium, alumina, partially stabilized zirconia and ultrahigh molecular weight polyethylene. Following implantation of a foreign material into the body, the material’s surface is immediately coated with proteins derived from blood and interstitial fluids. It is through this layer of adsorbed proteins that the cells sense foreign surfaces.28 In response, the body’s defense mechanism will stimulate the formation of a non-adherent fibrous capsule around the implant in an attempt to isolate it from the surrounding tissue. The thickness of this protective fibrous capsule depends on the chemical reactivity of the implanted material, and on the motion and fit of the material at the interface.29 Because the interface is not chemically or biologically bonded, micro-movement of the implant will result in progressive thickening of the non-adherent fibrous capsule and eventually leads to functional deterioration of the implanted material.
By contrast, a bioactive material creates an environment compatible with osteogenesis, and in some cases, compatible with soft tissues30 by developing a natural bonding interface between living and non-living materials. With the exception of calcite (calcium carbonate) and b-tricalcium phosphate, which are examples of resorbable bioceramics that bond directly to living bone,31 interfacial bonding of other bioactive materials with bone is initiated via ion–exchange reactions between the bioactive implant and surrounding body fluids. This results in the formation of a biologically active carbonated apatite layer on the implant surface that is chemically and crystallographically equivalent to the mineral phase in bone.32 Human plasma is supersaturated with respect to calcium and phosphate ions. The presence of certain functional groups, such as silanol (Si–OH) on the material surface induces nucleation of carbonated apatite crystallites from the amorphous calcium phosphate that is deposited over the initially formed silica gel layer. For bone, interfacial bonding occurs because of the rapid turnover of bone, as well as the biological equivalence of the carbonated apatite deposits with the inorganic portion of bone. This enables a collagen matrix to be deposited by osteoblasts over the carbonated apatite layer. Subsequent mineralization of the collagen fibrils results in bonding of the living tissues to the implanted material.27 For bonding to soft tissues, collagen fibrils are chemisorbed on the porous silica gel layer via electrostatic, ionic and/or hydrogen bonding.33,34
The first bioactive glass–ceramic invented and the most extensively studied was the SiO2–Na2O–CaO–P2O5 melt-derived quaternary glass system invented by Dr. Hench,35 commonly known as 45S5 Bioglass® (USBiomaterials Corp., Alachua, FL, USA). Based on this formulation and subsequently developed bioactive glass formulations, the ability of these materials to bond to bone tissue was thought to occur in 11 stages (Table 1). These stages represent the combined results of the surface chemical reactivity of bioactive glasses in physiological media (Stages 1–5), and the body’s healing and regenerative responses (Stages 6–11).27 Reaction stages 1–5 in bioactive glasses lead to rapid release of soluble ionic species and formation of a porous hydrated silica gel and polycrystalline carbonated apatite bi-layer on the glass surface. These reaction layers enhance the adsorption of proteins and growth factors (Stage 6), influence the length of time macrophages are required to clear the surgical site of debris for tissue repair (Stage 7), promote attachment (Stage 8) and proliferation and differentiation of osteoblasts from mesenchymal stem cells (Stage 9). Deposition of an extracellular collagen matrix (Stage 10) and subsequent mineralization of the collagen matrix deposited by osteoblasts (Stage 11) follow, ultimately resulting in mature osteocytes encased in a collagen-carbonated apatite matrix.
Table 1.
Reaction stages in 45S5 Bioglass® with increasing time.
| Stage | Reaction event |
Response | |
|---|---|---|---|
| 1 | Sodium and calcium hydrogen ion exchange on glass surface | Chemical Occurs in vitro or in vivo |
|
| 2 | Dissolution of surface silica and formation of surface silanol | ||
| 3 | Condensation and repolymerization of silanol to form SiO2-rich surface laver | ||
| 4 | Precipitation of amorphous calcium phosphate on the silica gel surface layer | ||
| 5 | Nucleation and crystallization of amorphous calcium phosphate to carbonated apatite | ||
| 6 | Protein adsorption (growth factors, etc) to the surface carbonated apatite layer | Biological Occurs only in vivo |
|
| 7 | Action of macrophages to remove debris from surgical site | ||
| 8 | Attachment of mesenchymal stem cells on bioactive surface | ||
| 9 | Differentiation of stem cells to form bone growing cells, osteoblasts | ||
| 10 | Generation of extracellular matrix by osteoblasts to form bone | ||
| 11 | Mineralisation of extracellular matrix to enclose bone cells, osteocytes |
It has been shown that fibroblasts do not spread and proliferate on bioactive glass surfaces, contrary to what occurs on the surface of bioinert materials.36 The exact mechanism is not clear, but may be due to selective adsorption of serum proteins on the surface of bioactive materials. Bioactive glass containing a calcium phosphate-rich layer was found to preferentially adsorb fibronectin, which contains the integrin-binding arginine–glycine–aspartic acid (RGD) amino acid sequence for enhanced osteoblast adhesion.37 Other researchers observed that the configuration of absorbed fibronectin was different depending on the type of surface exposed by the biomaterial. A specific fibronectin conformation present on bioactive glasses that reacted with simulated body fluid to form a surface CaP amorphous layer was found to induce very strong osteoblast adhesion.38 This is important, since mesenchymal stem cells take time to migrate from their niches to a surgical site and arrive later than fibroblasts. If fibroblasts proliferate, a non-adherent fibrous capsule forms, which inhibits interfacial bonding between the implanted material and the host tissue. When fibroblasts remain ‘quiescent’ along the surface of bioactive glass, new bone can be produced upon differentiation of the mesenchymal stem cells and endothelial progenitor cells into osteoblasts and capillary wall, respectively.
A bioactivity index (BI) was introduced to rank the level of bioactivity of a specific material.39 This index is defined as the time taken for more than 50% of the interface to bond to bone (t0.5) and is represented by IB = 100/t0.5bb. The presence of silica was found to be crucial for a material to exhibit bioactivity. This is due to the partial dissolution of the material in an alkaline environment, which releases Si4+ ions to form the silica gel surface layer in Stages 2 and 3, and creates silanol groups to act as sites for the nucleation of carbonated apatite in Stage 5 (Table 1). Nevertheless, incorporation of increasing concentrations of silica in the glass resulted in a diminished rate of bioactivity. Complete loss of bone-bonding ability occurred with further increases in SiO2 concentration, the exact concentration of which was dependent upon whether the glasses were melt-derived (>60 mol%)40 or sol–gel reaction-derived (>90 mol%).41 The presence of P2O5 and Na2O was initially thought to be essential for glass-ceramics to be bioactive. However, sol–gel derived CaO–SiO2 binary calcium silicate gel glasses were also found to bond firmly to bone after intraosseous implantation.42,43 Thus, absence of phosphate from most HCSCs does not preclude these cements from exhibiting bioactive behaviour. Conversely, partial substitution of CaO with Al2O3 in the binary CaO–SiO2 glass composition to produce ternary CaO–Al2O3–SiO2 glasses resulted in an Al2O3 concentration-dependent reduction/inhibition of the ability of these glass systems to form a calcium phosphate surface layer after their exposure to SBF.44 Addition of as little as 3 mass% Al2O3 completely inhibited the bone-bonding ability of bioactive glass.45 The inhibitory effect of Al3+ on bone-bonding was attributed to an increased resistance of the bioactive glass to ion exchange surface reactions, to the precipitation of the multivalent ions as oxides, hydroxides or carbonates, and to the shift of isoelectric point of the surface from negative to positive at physiological pH.46 As tricalcium aluminate is a component of many HCSCs, the potential effect of aluminium on the bioactivity of these cements will be discussed in subsequent sections.
Release of inorganic ions by bioactive glasses may trigger intracellular responses.47 Of the elements released by bioactive glasses, Si is known to be an essential element for metabolic processes associated with calcification of bone tissues48 and induction of the apatite precipitation.49 Dietary intake of Si has been shown to increase bone mineral density.50 Orthosilicic acid stimulates type I collage synthesis and osteoblast differentiation in human osteoblast-like cells in vitro.51–53 Inorganic monomeric and polymeric silica/silicate has been shown to increase the expression of osteoprotegerin in osteogenic cells, and modulate the cross-talk between osteoblasts and osteoclasts. Osteoprotegerin is a decoy receptor for the receptor activator of nuclear factor kappa B ligand (RANKL). Osteoprotegerin binding to RANKL on osteoblast/stromal cells, blocks the RANKL-RANK ligand interaction between osteoblast/stromal cells and osteoclast precursors. This has the effect of inhibiting the differentiation of osteoclast precursors into mature osteoclasts. Thus, inorganic silica/silicate has the potential to stimulate osteogenesis by inhibiting osteoclast growth and differentiation.54 Release of calcium ions favours osteoblast proliferation, differentiation and extracellular matrix mineralization,55 activates Ca-sensing receptors in osteoblasts, and increases expression of growth factors such as insulin-like growth factor-I (IGF-I) or IGF-II.56,57 Additional extracellular responses may be produced by adsorption of plasma-derived transforming growth factor-β1 on the surface silica gel layer. Bioactive glasses have also been shown to promote angiogenesis by stimulating the secretion of vascular endothelial growth factor by human fibroblasts.58–61
The information in this section represents only the tip of an iceberg on the humongous amount of research published on the bioactivity of glass and glass–ceramics. Nevertheless, the information provides the background for evaluating the bioactivity of HCSCs.
3. In vitro bioactivity of HCSCs
3.1. Direct demonstration of in vitro bioactivity
Of all the HCSCs available to date, the most well known and most thoroughly investigated is clinker-derived Portland cement. The latter is composed of different phases, including tricalcium silicate, dicalcium silicate, tricalcium aluminate, tetracalcium aluminoferrite and calcium sulphate. The first HCSC patented for endodontic applications is mineral trioxide aggregate (MTA),62 which contains all the aforementioned mineral phases, as well as bismuth oxide as a radiopacifier.63 The aluminoferrite phase in the grey version of MTA is absent from the white version of MTA.64
Although bioactivity is not as esteemed property for industrial applications of Portland cements, in vitro bioactivity of white Portland cement has been reported after it was immersed in SBF for 7 days.65 Using a combination of X-ray diffraction (XRD) and Fourier transform-infrared spectroscopy (FT-IR), the authors reported formation of a layer of “hydroxyapatite” on the surface of white Portland cement after immersion of the set cement in SBF. Dissolution of portlandite (calcium hydroxide) and formation of calcite (calcium carbonate; reaction product of calcium hydroxide with atmospheric carbon dioxide) were also observed on contact of set white Portland cement with SBF. In another study, set white Portland cement was immersed in phosphate-containing fluid and the resultant calcium phosphate phase was examined using XRD and FT-IR, in combination with scanning electron microscopy, transmission electron microscopy and electron diffraction.66 The authors reported that the initial calcium phosphate phase formed was amorphous calcium phosphate (ACP). This precursor phase was subsequently transformed into calcium-deficient, poorly crystalline, B-type apatite (i.e. the PO43− groups in apatite being substituted by CO32−). The authors concluded that the in vitro bioactivity of MTA materials is likely to be attributed to their Portland cement component. Identification of the ACP phase is of biological significance because the latter is the key intermediate calcium phosphate precursor phase that precedes biological apatite formation in osteogenesis.67–69 As will be discussed below, identification of ACP precursors bridges the missing link in the sequence of stages that contribute to the in vitro bioactivity of HCSCs. In addition, by using the contemporary principles of biomineralization, strategies have been developed with the use of polyanionic acid analogues of noncollagenous proteins for stabilizing the ACP precursor phase to prevent its premature transformation into carbonated apatite. The resultant polyanionic acid-stabilized ACP precursors have been used experimentally for biomimetic intrafibrillar mineralization of collagen fibrils in demineralized dentine.70 Such a technology has potential applications in remineralization of hybrid layers created by dentine adhesives to prevent their degradation by matrix metalloproteinases and cathepsin K,71 as well as in the remineralization of dentinal caries.72
The two major components of Portland cements, tricalcium silicate and dicalcium silicate, have been produced separately using sol-gel reactions to create experimental, phase-pure tricalcium silicate73,74 and dicalcium silicate cements.75 These phase-pure calcium silicate cements also demonstrated in vitro bioactivity by the precipitation of apatite crystallites on the cement surface after immersion in SBF. These results have led to the preparation of experiment bioactive tricalcium silicate76,77 and dicalcium silicate78 cements for endodontic applications. The use of these experimental calcium silicate cements should alleviate potential concerns regarding aluminium-induced neurotoxicity79–81 that may arise with the clinical use of clinker-derived tricalcium aluminate-containing HCSCs.
By and large, the in vitro bioactivity observed in Portland cements and phase-pure calcium silicate cements are recapitulated in MTA and the MTA-like clan of commercially available or experimental HCSCs designed for restorative and endodontic uses.82–101,78,102,103 These calcium phosphate reaction products were observed after the cements were immersed in SBF based on Kokubo’s formula or its modification, 15–17 Dulbecco’s phosphate-buffered saline, Hank’s balanced salt solution, or simply phosphate-containing fluid, with different degrees of reactivity. The ultimate reaction product formed on the surface of the set cements was, according to most of those publications, “hydroxyapatite”.
3.2. Mechanism of action
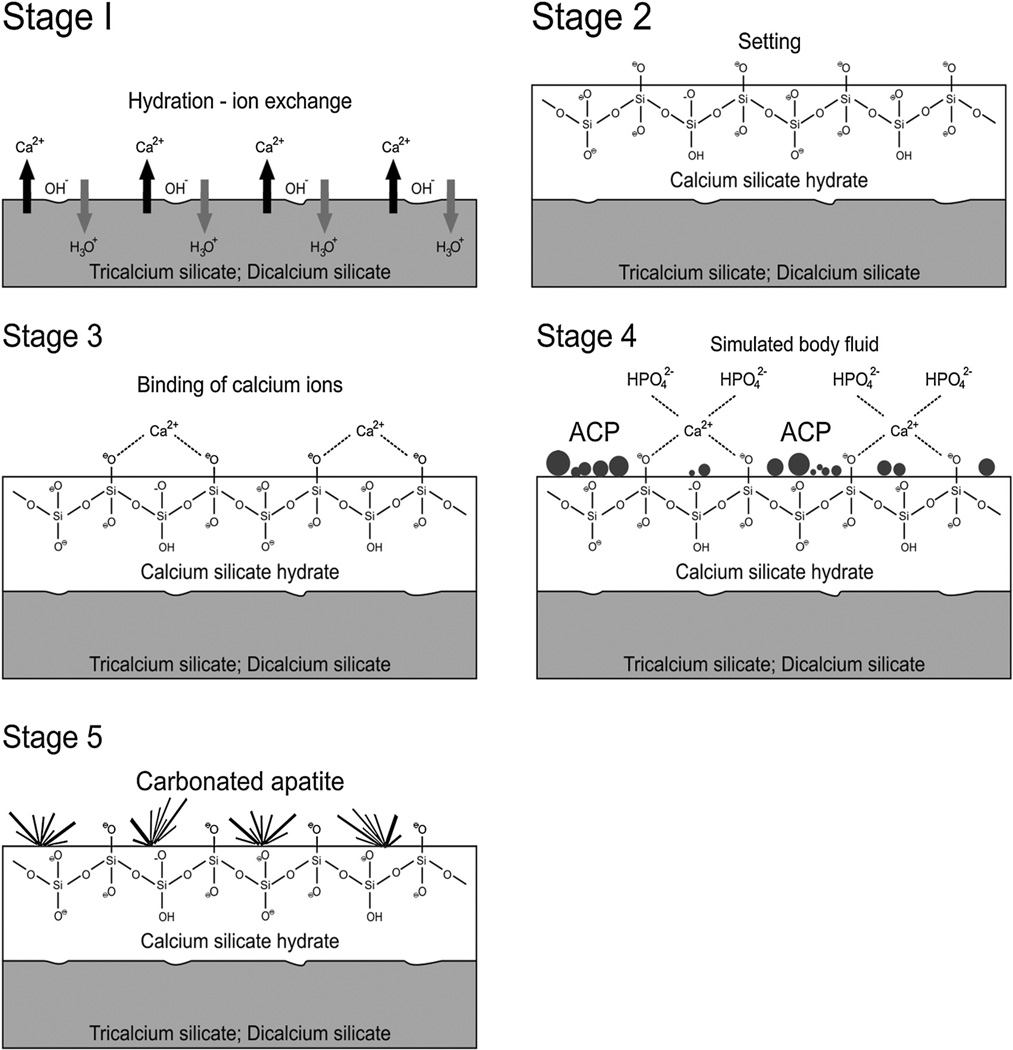
The in vitro bioactivity of MTA and MTA-like materials is tissue-independent and may proceed in stages that parallel those proposed for bioactive glasses. For comparative purposes, a similar sequence of events will be used in this discussion. These stages are schematically represented in Fig. 1.
Fig. 1.
A schematic of the five sequential stages of events that contribute to the manifestation of in vitro bioactivity of hydraulic calcium silicate cements after the latter is immersed in simulated body fluid (not to scale). ACP: amorphous calcium phosphate.
Stage 1: Hydrolysis and ion exchange. Ion exchange occurs following hydration of the calcium silicate particles, with rapid exchange of Ca2+ with H+ or H3O+ ions from the aqueous mixing solution to form a solid–iquid interface.92 Reaction of Ca2+ ions with OH− ions derived from water results in the formation of calcium hydroxide (portlandite)104 that creates a highly alkaline environment. Although these reactions occur almost immediately after cement hydration, continuous release of Ca2+ and SiO32− after initial setting, together with the release of minor amounts of Al3+, Fe3+ and SO42−, depending on the type of material, result in the formation of other inorganic mineral phases.
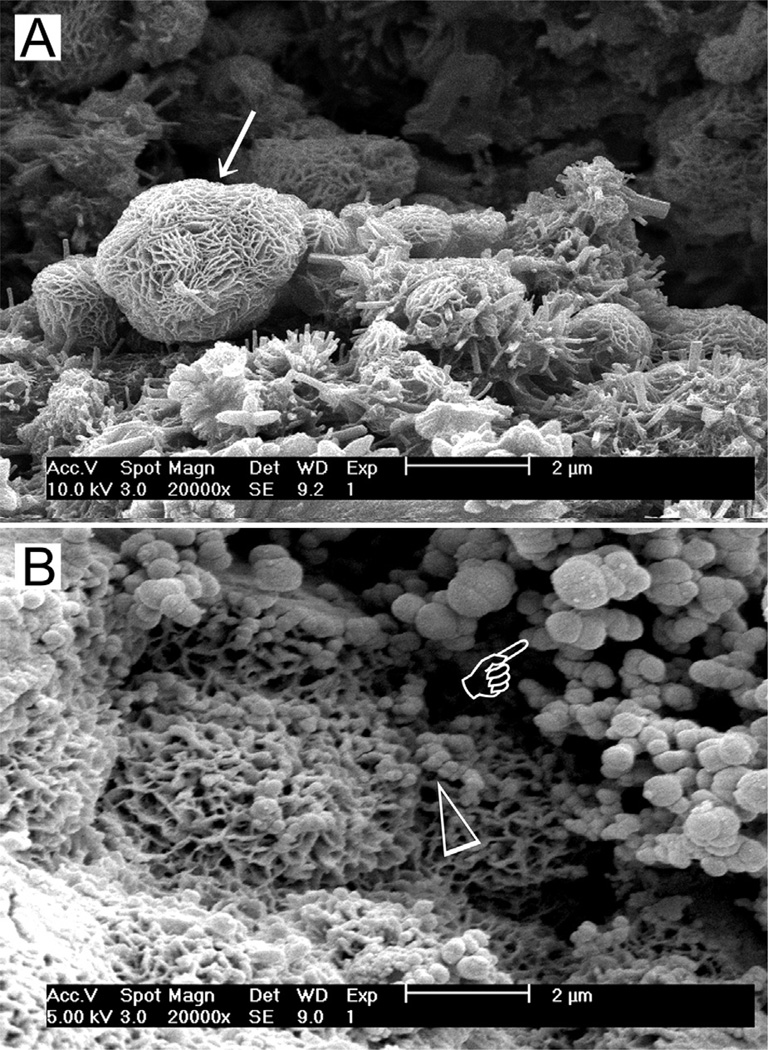
Stage 2: Formation of calcium silicate hydrate. Cation exchange increases the hydroxyl concentration of the solution. The surfaces of the calcium silicate particles are attacked by OH− ions in solution, resulting in hydrolysis of SiO44− group in an alkaline environment. The result is the formation of an amorphous calcium silicate hydrate phase on the surface of the mineral particles (Fig. 2a). Calcium silicate hydrate is a non-stoichiometric, porous, water-containing silicate gel layer105 containing silanol (Si–OH) groups that forms the main binding phase in a set cement matrix.
Fig. 2.
(A) Scanning electron microscopy (SEM) image of the surface of set white MTA powder showing formation of a calcium silicate hydrate layer (arrow) over the surface of the mineral particles after hydration of the powder. (B) SEM image of the surface of set white MTA powder after immersion in phosphate-containing fluid for 8 h. Amorphous calcium phosphate is deposited over the surface of the calcium silicate hydrate reaction phase in the form of spherule clusters (pointer). Some of the smaller amorphous calcium phosphate spherules are dispersed within the calcium silicate hydrate phase.
Stage 3: Binding of calcium silicate hydrate with calcium ions. Deprotonation of silanol groups in the calcium silicate hydrate phase at alkaline pH produces a negatively charged surface with BBSiO− functional groups106,107:
≡SiOH + H2O ↔ ≡SiO− + H3O+
This negatively charged surface attracts cations released into solution, such as Ca2+, via electrostatic interaction to decrease the total energy of the system, resulting in an increase of cations on the set cement surface:
≡SiO− + Ca2+ → ≡SiO− ⋯ Ca2+
This region, consisting of a charged surface and an equal but opposite charge in the solution, is called an electric double layer on which other substances may deposit under suitable conditions.
Stage 4: Precipitation of ACP. When the set calcium silicate cement is immersed in a phosphate-containing solution, which contains hydrolyzed HPO42− ions, electrostatic interaction occurs between the HPO42− ions and Ca2+ ions on the calcium silicate hydrate surface.
H2O + PO43− ↔ HPO42− + OH−
≡SiO− ⋯ Ca2+ + HPO42− → ≡SiO− ⋯ Ca2+ ⋯ HPO42−
Continued release of calcium ions from the set cement into the phosphate-containing solution leads to supersaturation of Ca2+ and HPO42− ions in the solution, which in turn results in the formation of an ACP precursor phase in the solution. These initially formed subnanometer-sized ACP precursors are known as pre-nucleation clusters and have an average diameter of 0.87 ± 0.2 nm.108 They remain relative stable in the phosphate-containing solution in the absence of a nucleation-inducing surface. In the presence of a nucleation-inducing surface, aggregation of the prenucleation clusters leads to their densification near the cement surface, producing a “dense liquid” CaP-rich phase.109 Coalescence of the densified prenucleation clusters subsequent results in the deposition of globular ACP on the set cement surface (Fig. 2b), with the general formula Ca9(PO4)6−x(HPO4)x(OH)x.110 Apart from the observation of ACP formation in Portland cement,68 ACP in the form of aggregated spherulites was also reported after ProRoot MTA (Dentsply Tulsa Dental Specialties, Tulsa, OK, USA), MTA Angelus (Angelus Soluções Odontológicas, Londrina, PR, Brazil), MTA Branco (Angelus) and MTA BIO (Angelus) were immersed in phosphate-buffered saline or Hank’s balanced salt solution.86,103
Stage 5: Nucleation and transformation of amorphous calcium phosphate into carbonated apatite. In the presence of a nucleation-inducing CSH surface (≡SiO−⋯Ca2+⋯ HPO42−), the ACP undergoes phase transformation over time into carbonated apatite111,112 (Fig. 3 a and b). This transformation occurs via an octacalcium phosphate (OCP) intermediate phase Ca8H2(PO4)6·5H2O.113–116 While it is indisputable that apatite represents the endpoint of this phase transformation, the morphology of the calcium phosphate phases presented in the endodontic literature on in vitro bioactivity of hydraulic calcium silicate cements is highly variable, ranging from acicular, lath-like, petal-like, plate-like, globular structures to needle-shaped,82–101,78,102,103 with different Ca/P molar ratios (ranging from 1.33 to 1.67) (Fig. 4). Although it is possible that some of these represent calcium-deficient apatite with variable degrees of lattice substitutions by cations such as Na+, Mg2+ and anions such as Cl−, it is likely some of these crystalline morphologies represent the intermediate OCP phase described in classic calcium phosphate characterization studies. Formation of an intermediate OCP phase by MTA or MTA-like materials was sparsely discussed in the endodontic literature except in a couple of studies.86,103 Indiscriminate use of the term “hydroxyapatite” (HA) in most of these endodontic studies is reflected by the inadequate chemo-analytical techniques employed for characterizing the calcium phosphate precipitates. The so-formed apatite is not stoichiometric HA but is carbonated apatite, which represents the biologic apatite found in bone, cartilage enamel and dentine.117,118 Accurate characterization of calcium phosphate phases requires the simultaneous use of XRD and FT-IR; the latter may be further complemented with micro-Raman spectroscopy.119–121 It should be emphasized that CaP mineral phase information derived from XRD cannot be substituted by the peak designations derived from the combined use of FT-IR and micro-Raman spectroscopy. Even with the use of all these techniques, differentiation between octacalcium phosphate and apatite is extremely difficult,122–124 and often required high resolution transmission electron microscopy of the crystalline lattice planes to determine the length of the unit cell axes and their crystallographic orientation.125 The crystal structure of OCP has been described as an alternative stacking of apatite layers and hydrated layers along its [1 0 0] direction. 122,126 The similarity of the apatite layer in OCP and the structure in HA provide geometrically favourable conditions for phase transformation from OCP to carbonated apatite. In a model of OCP–HA transformation, it has been suggested that transformation of OCP to HA occurs through epitaxial growth of HA on the OCP surface along the OCP[1 0 0]/HA[1 0 0] and OCP [0 0 1]/HA[1 0 0] crystalline planes.123 Transformation of OCP to HA may be accomplished in solution through dissolution–precipitation, or occur by solid-state transformation via one of the two processes127:
5Ca8H2(PO4)6·5H2O → 4Ca10(PO4)6(OH)2+6H3PO4+17H2O
Ca8H2(PO4)6·5H2O + 2Ca2+ → Ca10(PO4)6(OH)2 + 3H2O + 4H+
The first reaction represents solid-state transformation and may occur in experiments that examined desiccated calcium silicate cements after they are immersed in phosphate-containing fluids. The second reaction requires an additional supply of calcium ions and is more likely to occur in solutions containing calcium. Such a scenario may be found when there is continuous leaching of calcium ions from the set HCSCs after they are immersed in phosphate-containing fluids or serum.
Fig. 3.
(A) SEM image of the surface of hydrated white MTA powder after immersion in phosphate-containing fluid for 168 h. A crystalline calcium phosphate mineral phase (open arrow) is scattered around the calcium silicate hydrate-coated MTA mineral particles (energy dispersive X-ray analysis data not shown). Amorphous calcium phosphate spherules can no longer be observed. (B) High magnification image of the calcium phosphate mineral phase in (A). These crystallites have short a-axis and b-axis but long c-axis, forming needle-shaped crystallites with hexagonal cross sections (open arrowhead).
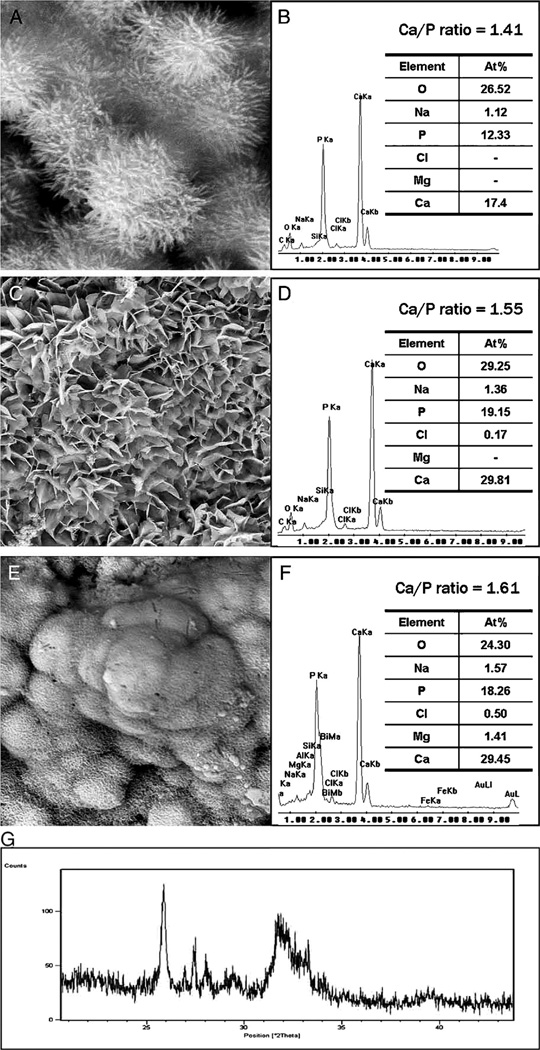
Fig. 4.
Characterization of precipitates formed by MTA BIO after 2 months of immersion in phosphate-buffered saline. (A) SEM image showing the acicular nature of spherules (original magnification, 8000×). (B) Energy dispersive X-ray (EDAX) spectrum for precipitates in (A) and semi-quantitative chemical composition showing their Ca/P molar ratio. (C) SEM image showing petal-like precipitates (original magnification, 1000×). (D) EDAX spectrum for precipitates in (C) revealed a greater Ca/P molar ratio and lattice substitution of Na and Cl. (E) SEM image of compact lath-like precipitates (original magnification, 1000×). (F) Semi-quantitative analysis of the EDAX data derived from (E) indicates that the precipitates have a Ca/P molar ratio of 1.61 with lattice substitution of Na, Cl, and Mg. (G) X-ray diffraction pattern of the calcium phosphate precipitates obtained after 2 months of immersion in phosphate-buffered saline, revealing the presence of poorly crystalline apatite.
Reproduced from Reyes-Carmona et al.86, with permission from the publisher.
Taken together, evidence is abundant in the literature showing that HCSCs exhibit in vitro activity. However, these studies were not performed as rigorously as those examining bioactive glasses. Although there have been attempts to compare the in vitro bioactivity of different commercially available or experimental HCSCs, most of the work was qualitative in nature. In particular, there was no adoption of a matrix such as the use of an in vitro version of the Bioactive Index, in systematically quantifying the percentage of the cement surface covered by calcium phosphate salts, or the variation in thickness of the apatite or apatite-like mineral layer over time.
4. In vivo bioactivity of HCSCs
As discussed in the introductory section, scientific evidence is lacking to support the assumption that a biomaterial that initiates the deposition of calcium phosphate salts on its surface after immersion in SBF will bond directly to bone following placement of the material in a surgical site.17 Thus, it is appropriate to review the in vivo bioactive potential of HCSCs following their surgical implantation in appropriate animal models. Understandably, HCSCs are not designed for complete filling of a surgical wound. However, it is important to see if the in vitro bioactivity reported for Portland cement and other HCSCs marketed for clinical use can be reproduced in vivo. Specifically, it is pertinent to identify if these materials can bond directly to bone without encasement by a fibrous connective tissue, the latter being the property of a bioinert material.
Because biocompatibility is not the subject of discussion in this review, studies that involved subcutaneous implantation of set HCSCs will be excluded from the discussion. Likewise, histological studies that involved testing of HCSCs as a root-end filling material will be excluded, as the tissue response involves migration of fibroblasts from the adjacent periodontal ligament to produce a layer of cementum over the material. Thus, only quantitative histological studies that involved the placement of HCSCs as intraosseous implants will be examined. Of those studies, four studies128–131 that involved placement of HCSC-loaded polyethylene tubes into extraction sockets in a rat model were also excluded, as those implants were perceived to fit rather loosely within the surgical site. The results from the six studies selected based on these inclusion and exclusion criteria are summarized in Table 2.
Table 2.
Studies on in vivo bioactivity (bone-bonding responses) hydraulic calcium silicate cements following intraosseous implantation.
| Reference | Material investigated |
Animal model |
Surgical site |
Number of intra-osseous implants |
Time period (days) |
Delivery Method |
Results |
||
|---|---|---|---|---|---|---|---|---|---|
| New bone apposition in direct contact with material Number (%) |
New bone separated from material by fibrous connective tissue Number (%) |
Mixed (incomplete direct bone contact) Number (%) |
|||||||
| 132 | Grey MTA | Guinea pig | Mandible | 5 | 60 | Teflon cup | 1 (20%) | 4 (80%) | NA |
| 133 | Grey MTA | Guinea pig | Mandible | 10 | 70 | Teflon cup | 1 (10%) | 9 (90%) | 0 (0%) |
| Tibia | 11 | 80 | 5 (45.45%) | 5 (45.45%) | 1 (9.1%) | ||||
| 134 | Grey MTA | Rat | Parietal bone | 20 | 15 | Freshly mixed; direct placement | 0 (0%)a | 20 (100%)c | 0 (0%)b |
| 18 | 30 | 0 (0%)a | 14 (77.8%)c | 4 (22.2%)b | |||||
| 18 | 60 | 2 (11.2%)a | 8 (44.4%)c | 8 (44.4%)b | |||||
| 135 | Grey MTA | Guinea pig | Mandible | 13 | 14 | Teflon cup | 8 (81.5%) | 5 (38.5%) | NA |
| 13 | 84 | 7 (53.8%) | 6 (46.2%) | ||||||
| Portland cement | 13 | 14 | 7 (53.8%) | 6 (46.2%) | |||||
| 13 | 84 | 10 (76.9%) | 3 (23.1%) | ||||||
| 136 | Grey MTA | Guinea pig | Mandible | 10 | 28 | Teflon cup | Unspecified specimen numbers–fibrous capsule (absent/slight), new bone in close contact with material (extensive) for both time periods. Negative control (empty Teflon cup)–thin fibrous capsule for both time periods. | ||
| 12 | 84 | ||||||||
| 137d | Grey MTA | Rat | Femur | 15 | 7 | Freshly mixed; direct placement | 15 (100%)c | ||
| 15 | 28 | 15 (100%)b | |||||||
| 15 | 56 | 15 (100%)a | |||||||
Defined as extensive (complete coverage or "bridging" of the material surface with bone).
Defined as moderate (at least 50% of the material surface partially covered with bone).
Defined as slight (occasional islands of osteogenesis over the material surface; less than 25% of the material surface covered with bone).
Results questionable - negative control groups (no material placement in implant cavity) for each time period exhibited the same results as the experimental groups.
Although in vitro bioactivity has been reported for most of the HCSCs available for clinical use, in vivo evaluation of the bone-bonding potential of these cements was almost exclusive focused on grey MTA (ProRoot MTA, Dentsply Tulsa Dental Specialties).132–137 Although a Bioactivity Index similar to that utilized in in vivo bioactive glass research has not been employed to quantify the time required for more than 50% of the cement interface to bond to bone, there were attempts in some studies to classify the bone apposition responses in terms of the percentage of exposed cement surface area that is in direct contact with new bone,134,137 or the temporal changes in bone apposition responses prior to the sacrifice of the animals.134–137
With the exception of one study in which the results are questionable due to the same bioactivity responses elicited by grey MTA and the negative control (no material placement in intraosseous site),137 the rest of the studies were unanimous in demonstrating direct apposition of bone to grey MTA is possible without the new bone being separated from the material by a fibrous capsule. However, such a hallmark of in vivo bioactivity could not be predictably achieved in all the five studies.132–136 For example, in a guinea pig model employed by Torabinejad et al.,133 90% of the grey MTA specimens that were implanted in the mandible were incapable of direct bonding to bone, with the histological manifestation of fibrous connective tissue between the newly formed bone and the set cement. In the same study, 45.5% of the specimens implanted in the tibia were incapable of direct bonding to bone, while 9.1% of the specimens demonstrated incomplete direct bone contact with intervening fibrous tissues. These unfavourable results could not have been attributed to the displacement of the implanted cup containing the tested material into the surrounding tissues, as those specimens could not be sectioned satisfactorily and would not have been reported.133 Likewise, these unfavourable results could not be accounted for by implanting grey MTA in a bioinert Teflon cup, as similar results were achieved when freshly mixed grey MTA was directly placed into the intraosseous site.134 In any scientific discipline that involves hypothesis testing, a hypothesis can seldom be accepted if 90%, or even 50% of the results are contrary to what is proposed. Yet, in a subsequent comprehensive review by those authors, MTA was described as bioactive based on its ability to form an apatite layer on its surface when it comes in contact with physiological fluids in vivo or with SBFs in vitro.25 In the age of evidence-based endodontics,138 it is worth pondering the question raised by Bohner and Lemaitre in a leading opinion biomaterials paper: “Can bioactivity be tested in vitro with simulated body fluid solution?”.17
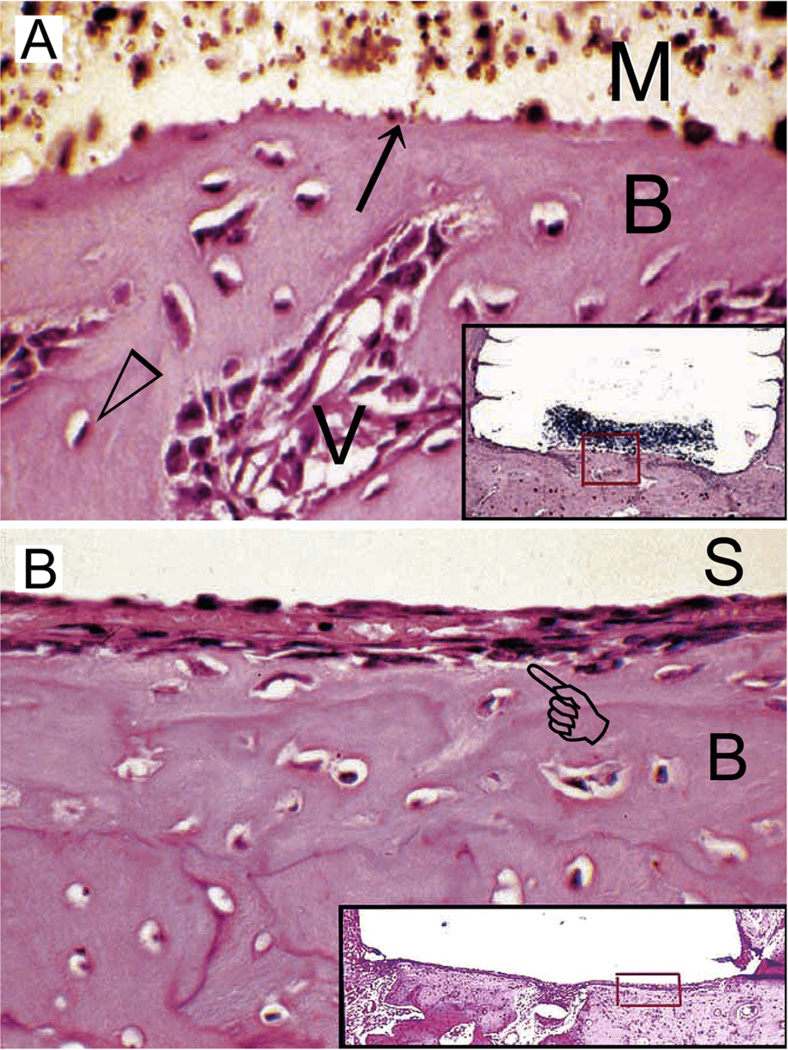
Similar results were achieved in another study that compared the in vivo bone-bonding ability of grey MTA versus Portland cement in a guinea pig model.135 The study concluded that there was no statistical significance between a clinical-grade and an industrial-grade HCSC in terms of their ability to bond to bone. In that study, the authors used grooved Teflon cups for filling the HCSCs to prevent dislodgement of the implants from the intraosseous sites. They also examined the biological responses after two time periods, 14 days and 84 days. For both Portland cement and grey MTA, both direct contact of the cement with new bone (Fig. 5A), and the presence of a thin layer of fibrous connective tissue between the cement and new bone (Fig. 5B) were observed. Although not explicitly mentioned in that paper, an important result was obtained after re-analysis of the data presented by comparing the responses of each material over the two time periods. That is, there is no statistically significant increase in specimens exhibiting direct bone-cement contact with time, for either grey MTA or Portland cement (Fisher exact tests, p > 0.05).
Fig. 5.
(A) Light microscopy image of the tissue response following implantation of grey MTA (ProRoot MTA) in an intraosseous site for 2 weeks. The material-hard tissue interface (arrow) shows new bone (B) apposition in direct contact with the material (M). Open arrowhead: osteocyte; V: capillary blood vessel. Inset: grey MTA-filled Teflon tube (removed, space left behind) and adjacent tissues showing remnants of grey MTA and the location (red box) from which the high magnification image is derived (haematoxylin–eosin stained section; original magnification, 250×). (B) Light microscopy image of the tissue response following implantation of grey MTA in another intraosseous site for 2 weeks. The grey MTA was lost during histological preparation (S). The bone tissue (B) has healed well, but a thin layer of fibrous connective tissue (pointer) separates the newly formed bone from direct contact with the implanted grey MTA. Inset: grey MTA-filled Teflon tube (dislodged) and adjacent tissues showing the location (red box) from which the high magnification image is derived (haematoxylin-eosin stained section; original magnification, 250×). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Reproduced from Saidon et al.135 with permission from the publisher; images cropped and labelled with permission from Dr. Kamran Safavi).
Since direct bone apposition occurs in grey MTA and Portland cement intraosseous implants, it is envisaged that the in vivo stages of bioactivity (i.e. stages 6–11; Table 1) that were described for bioactive glasses should be recapitulated in these HCSCs, as these stages represent the body’s physiological healing and regeneration processes. Adsorption of proteins on the apatite-coated cement surface (Stage 6) should enable the mesenchymal stem cells that migrate into the bony defect to “see” a bone-like surface complete with organic components, and not a foreign material. There is only one study in the endodontic literature that attempted to analyze the surfaces of grey MTA and white MTA when these cements were allowed to set in the presence of foetal bovine serum.84 This would have been the perfect opportunity to analyze the type of proteins/growth factors that preferentially attached to the set cement surfaces. Unfortunately, the study only analyzed the surface inorganic chemical composition of the set cement and nothing was performed to characterize the protein components. The effect of macrophages on clearing the debris around the implanted material for tissue repair (Stage 7), being part of the host inflammatory response, has been shown in many studies.128,129,135
Inasmuch as the in vivo bone-bonding potential of grey MTA was unpredictably achieved, there was no explanation in the studies involved with regard to why this is so. Thus, it is logical to ask why a non-adherent fibrous capsule is sometimes formed that prevents direct contact of the newly formed bone with the set cement. One of the reasons is probably due to the initial high alkalinity66 of the intraosseous site following implantation of grey MTA. Although part of the calcium hydroxide formed in the initiate hydrolysis of tricalcium silicate and late hydrolysis of dicalcium silicate is deposited as crystalline portlandite, saturated aqueous calcium hydroxide is also present as “pore solution” within the bulk of the set hydrated cement.139,140 Leaching of this “pore solution” results in coagulation necrosis of the tissues in contact with the material, and causes an initial mild to moderate inflammatory response that may result in the formation of granulation tissue by fibroblasts on resolution of the inflammation. To date, no study has attempted to correlate the extent of inflammation around the intraosseous grey MTA implants with the subsequent ability of the grey MTA to bond directly to bone.
The major cations that leach from set grey MTA was reported to be (in parts per million): Ca 176.7 ± 3.3, Si 13.4 ± 0.6; Bi 6.1 ± 0.5; Fe 2.5 ± 0.4; Al 2.3 ± 0.2, and Mg 1.0 ± 0.1.82 The beneficial effects of Ca and Si ions on osteogenesis have already been described in the section on bioactive glasses. It is envisaged that leaching of these cations from set grey MTA and Portland cement will exert similar beneficial effects on osteogenesis and contribute to the in vivo bioactivity of these HCSCs. It was also mentioned in the section on bioactive glasses that addition of as little as 3 mass% Al2O3 completely inhibited the bioactivity of bioactive glass.45 Portland cement contains 4.7 mass% Al2O3, with the calculated constitution of tricalcium aluminate and tetracalcium aluminoferrite being 7.9 mass% and 8.1 mass%, respectively.26 Although some of the leached Al ions may be incorporated into the calcium silicate hydrate phase of set calcium silicate cements,141–143 part of the leach ions may still exist in solution, which may have an adverse effect on the in vivo bioactivity of grey MTA and Portland cement. To date, no information is available on the in vivo bioactivity of other commercially available HCSCs. To test whether aluminium has a detrimental effect on the in vivo bioactivity of HCSCs, it is necessary to compare the bone-bonding potential of white MTA with the corresponding grey versions of the cement, because tricalcium aluminate is negligible, and tetracalcium aluminoferrite is virtually absent from white MTA.64,144 Future studies should also examine the in vivo bioactivity of aluminium-free, sol–gel reaction-derived HCSCs and compare the results with aluminium-containing, clinker-derived HCSCs.
For the other cations released by grey MTA, magnesium has been shown to stimulate adhesion of osteoblastic cells to implant surfaces by interacting with integrins of osteoblast cells which are responsible for cell adhesion.145,146 By contrast, iron has an inhibitory effect on bone morphogenetic protein 2-induced osteoblastogenesis.147 Addition of 20 mass% Bi2O3 to a dicalcium silicate cement resulted in lower cell proliferation, differentiation and formation of calcium deposits by MG63 human osetosarcoma cells in cell culture experiments.148 Of the minor inorganic phases formed on the cement surface following hydration, the potential effects of ettringite and calcium monosulphate aluminate hydrate on osteogenesis is unknown. Calcite, which is frequently formed on the cement surface, has been shown to bond directly to bone without the formation of a carbonated apatite surface layer.31
It has been mentioned in the section on bioactive glasses that fibroblasts do not spread and proliferate on bioactive glass surfaces, contrary to what occurs on the surface of bioinert materials.36 Although set MTA provides an excellent surface for the attachment of bone-forming cells or osteoblast-like cells,149–154 mesenchymal stem cells take 24–72 h to migrate from their stem cell niches to the surgical site before they can attach to the surface of the intraosseous implant (Stage 8). Unlike bioactive glass, MTA surfaces also provide a highly favourable environment for fibroblasts to attach and proliferate.155–158 Thus, at least, theoretically, there is a competition between fibroblasts and osteogenic cells for attachment to the protein-coated calcium silicate cement surface in vivo. In a more recent study on the attachment and proliferation of fibroblasts and osteoblasts on calcium silicate cements, it was found that human periodontal ligament fibroblasts and human osteoblasts attach and proliferate well on MTA-based materials. However, the osteoblasts exhibited lower proliferation rates compared with fibroblasts.159 Because the two cell lines require different growth media in cell culture environments to support osteogenic differentiation of the osteoblasts, experiments on the fibroblasts and osteoblasts were performed separately. The study may be repeated by culturing both cell lines in the same medium and without stimulating osteogenic differentiation of the osteoblasts. Quantifying the cell type that preferentially attaches to the surface of MTA-based materials should provide an in-depth appreciation of whether competition between fibroblasts and osteoblasts for attachment is responsible for unpredictable bond-bonding following intraosseous implantation of HCSCs. That said, it does not mean that demonstration of a fibrous capsule over the set cement surface is an indication of the suboptimal clinical effectiveness of these HCSCs. Rather, attachment of periodontal ligament fibroblasts to the cement surface provides a means for regenerating the periodontal ligament and deposition of cementum, as discussed in detail in a recent systematic review.160 Formation of cementum provides the justification for the clinical superiority in using HCSCs as clinical root-end filling materials.
5. Conclusion
Hydraulic calcium silicate cements have been reported and promoted as bioactive materials based on their ability to produce apatite after interacting with phosphate ions derived from physiological or SBFs. As this attribute was first observed in a SiO2–Na2O–CaO–P2O5 quaternary bioactive glass, the same definitions employed for establishing in vitro bioactivity and in vivo bioactivity in glass or glass–ceramic systems, and the proposed mechanisms involved in these phenomena are used as blueprints for reviewing whether these activities are identifiable in HCSCs. As far as in vitro bioactivity is concerned, all papers published on this phenomenon clearly demonstrated that some forms of calcium phosphate deposition on the surface of HCSCs after these materials were immersed in SBFs or phosphate-containing fluids. It is likely that these calcium phosphate deposits represent the amorphous or crystalline precursors of carbonated apatite, or carbonated apatite per se, depending on the condition and timing upon which the specimens were examined, and the techniques employed for analyzing these inorganic precipitations. In this regard, the phenomenon of in vitro bioactivity of HCSCs is indisputable. However, studies performed on HCSCs lack the robustness that was demonstrated in similar studies performed on bioactive glasses, in terms of quantifying the spatiotemporal events associated with the activity.
Based on the same definition adopted for bioactive glass and glass–ceramics, in vivo bioactivity has been demonstrated for at least grey MTA and Portland cement. However, a fibrous connective tissue layer is frequently observed along the newly formed bone-cement interface that is reminiscent of the responses observed in bioinert materials. The composition, constitutional phases and hydration characteristics of clinker-derived HCSCs are much more complex compared to a quaternary or even a ternary melt-derived bioactive glass system. The contribution of early and late hydration of different constitutional phases in clinker-derived HCSCs, to the rate and extent of carbonated apatite formation has not been established. Data is also lacking on the potential in vivo bioactivity of other HCSCs such as white MTA and MTA-like materials, as well as phase-pure HCSCs. In matters of style, it is tempting to swim with the current by endorsing that HCSCs exhibit predictable in vivo bioactive behaviour akin to those observed in 45S5 bioactive glass. In matters of principle, however, one has to acknowledge that there is presently insufficient scientific evidence to support this assumption. More importantly, the parameters responsible for this uncertainty have not been recognized. Even though this assumption may be valid, the current methods generally employed by the endodontic community for validating this assumption leaves room for improvement in terms of understanding how the composition (including types of opacifier), hydration phases and alkalinity of different HCSCs may influence the predictability of their in vivo bone-bonding responses. In addition, universally acceptable criteria are lacking for objective appraisal of the relative in vivo bioactivity of different HCSCs. As such, the term “bioactivity” is used rather ambiguously in studies on these cements. Appraisal criteria should be developed by reputable organizations such as the International Organization for Standardization (ISO) or the American Society for Testing and Materials (ASTM), to enable manufacturers and scientists to accurately define the bioactivity profiles of novel HCSCs developed for restorative and endodontic applications.
Supplementary Material
Acknowledgments
This work was supported by grant R01 DE015306-06 from NIDCR (PI. David H Pashley), grant 81130078 (PI. Jihua Chen) and grant 81300898 (PI. Kai Jiao) from the National Nature Science Foundation of China.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jdent.2013.12.015.
REFERENCES
- 1.Williams DF, editor. Definitions in biomaterials. Amsterdam/ Oxford/New York/Tokyo: Elsevier; 1987. [Google Scholar]
- 2.Hubbell JA. Bioactive biomaterials. Current Opinion in Biotechnology. 1999;10:123–129. doi: 10.1016/s0958-1669(99)80021-4. [DOI] [PubMed] [Google Scholar]
- 3.Asefa T, Duncan CT, Sharma KK. Recent advances in nanostructured chemosensors and biosensors. Analyst. 2009;134:1980–1990. doi: 10.1039/b911965p. [DOI] [PubMed] [Google Scholar]
- 4.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34:8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 5.Maia FR, Bidarra SJ, Granja PL, Barrias CC. Functionalization of biomaterials with small osteoinductive moieties. Acta Biomaterialia. 2013;9:8773–8789. doi: 10.1016/j.actbio.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Cado G, Aslam R, Séon L, Garnier T, Fabre R, Parat A, et al. Self-defensive biomaterial coating against bacteria and yeasts: polysaccharide multilayer film with embedded antimicrobial peptide. Advanced Functional Materials. 2013;23:4801–4809. [Google Scholar]
- 7.Sharma S, Singh R, Rana S. Bioactive peptides: a review. International Journal of Bioautomation. 2011;15:223–250. [Google Scholar]
- 8.Hench LL, Paschall HA. Histochemical responses at a biomaterial’s interface. Journal of Biomedical Materials Research. 1974;8:49–64. doi: 10.1002/jbm.820080307. [DOI] [PubMed] [Google Scholar]
- 9.Hench LL, Splinter RJ, Allen WC, Greenlee TK. Bonding mechanisms at the interface of ceramic prosthetic materials. Journal of Biomedical Materials Research. 1971;5:117–141. [Google Scholar]
- 10.Neo M, Nakamura T, Ohtsuki C, Kokubo T, Yamamuro T. Apatite formation on three kinds of bioactive material at an early stage in vivo: a comparative study by transmission electron microscopy. Journal of Biomedical Materials Research. 1993;27:999–1006. doi: 10.1002/jbm.820270805. [DOI] [PubMed] [Google Scholar]
- 11.Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF, et al. Bioactive glass in tissue engineering. Acta Biomaterialia. 2011;7:2355–2373. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hench LL, Ethridge EC. Biomaterials—an interfacial approach. New York: Academic Press; 1982. [Google Scholar]
- 13.Davies JE, editor. The bone–biomaterial interface. Toronto: University of Toronto Press; 1993. [Google Scholar]
- 14.Kitsugi T, Nakamura T, Yamamuro T, Kokubo T, Shibuya T, Takagi M. SEM-EPMA observation of three types of apatite containing glass ceramics implanted in bone: the variance of a Ca,P-rich layer. Journal of Biomedical Materials Research. 1987;21:1255–1271. doi: 10.1002/jbm.820211008. [DOI] [PubMed] [Google Scholar]
- 15.Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure change in bioactive glass–ceramic A–W. Journal of Biomedical Materials Research. 1990;24:721–734. doi: 10.1002/jbm.820240607. [DOI] [PubMed] [Google Scholar]
- 16.Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Bohner M, Lemaitre J. Can bioactivity be tested in vitro with SBF solution? Biomaterials. 2009;30:2175–2179. doi: 10.1016/j.biomaterials.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Cho SB, Nakanishi K, Kokubo T, Soga N, Ohtsuki C, Nakamura T. Apatite formation on silica gel in simulated body fluid: its dependence on structures of silica gels prepared in different media. Journal of Biomedical Materials Research. 1996;33:145–151. doi: 10.1002/(SICI)1097-4636(199623)33:3<145::AID-JBM4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Peltola T, Pätsi M, Rahiala H, Kangasniemi I, Yli-Urpo A. Calcium phosphate induction by sol–gel-derived titania coatings on titanium substrates in vitro. Journal of Biomedical Materials Research. 1998;41:504–510. doi: 10.1002/(sici)1097-4636(19980905)41:3<504::aid-jbm22>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 20.Uchida M, Kim HM, Kokubo T, Nakamura T. Bonelike apatite formation induced on zirconia gel in simulated body fluid and its modified solutions. Journal of the American Ceramic Society. 2001;84:2041–2044. [Google Scholar]
- 21.Miyazaki T, Kim HM, Kokubo T, Ohtsuki C, Kato H, Nakamura T. Apatite-forming ability of niobium oxide gels in a simulated body fluid. Journal of the Ceramic Society of Japan. 2001;109:929–933. [Google Scholar]
- 22.Miyazaki T, Kim HM, Kokubo T, Kato H, Nakamura T. Induction and acceleration of bonelike apatite formation on tantalum oxide gel in simulated body fluid. Journal of Sol–Gel Science and Technology. 2001;21:83–88. [Google Scholar]
- 23.Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dental Materials. 2008;24:149–164. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review. Part I. Chemical, physical, and antibacterial properties. Journal of Endodontics. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review. Part III. Clinical applications, drawbacks, and mechanism of action. Journal of Endodontics. 2010;36:400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Darvell BW, Wu RC. MTA—an hydraulic silicate cement: review update and setting reaction. Dental Materials. 2011;27:407–422. doi: 10.1016/j.dental.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Bioceramics Hench LL. From concept to clinic. Journal of the American Ceramic Society. 1991;74:1487–1510. [Google Scholar]
- 28.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial–cell interactions by adsorbed proteins: a review. Tissue Engineering. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 29.Cao W, Hench LL. Bioactive materials. Ceramics International. 1996;22:493–507. [Google Scholar]
- 30.Wilson J, Noletti D. Bonding of soft tissues to Bioglass®. In: Yamamuro T, Hench LL, Wilson J, editors. Handbook of bioactive ceramics, vol. 1. Bioactive glasses and glass–ceramics. Boca Raton: CRC Press; 1990. [Google Scholar]
- 31.Neo M, Kotani S, Fujita Y, Nakamura T, Yamamuro T, Bando Y, et al. Differences in ceramic–bone interface between surface-active ceramics and resorbable ceramics: a study by scanning and transmission electron microscopy. Journal of Biomedical Materials Research. 1992;26:255–267. doi: 10.1002/jbm.820260210. [DOI] [PubMed] [Google Scholar]
- 32.Greenspan DC. Bioactive glass: mechanism of bone bonding. Tandläkartidningen Ǻrk. 1999;91 [Google Scholar]
- 33.Zhong JP, LaTorre GP, Hench LL. The kinetics of bioactive ceramics. Part VII. Binding of collagen to hydroxyapatite and bioactive glass. In: Andersson OH, Yli-Urpo A, editors. Proceedings of the 7th international symposium on ceramics in medicine: bioceramics. Vol. 7. Oxford: Butterworth-Heinemann; 1994. pp. 61–66. [Google Scholar]
- 34.Merwin GE, Atkins JS, Wilson J, Hench LL. Comparison of ossicular replacement materials in a mouse ear model. Otolaryngology—Head and Neck Surgery. 1982;90:461–469. doi: 10.1177/019459988209000417. [DOI] [PubMed] [Google Scholar]
- 35.Hench LL. The story of Bioglass®. Journal of Materials Science – Materials in Medicine. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 36.Seitz TL, Noonan KD, Hench LL, Noonan NE. Effect of fibronectin on the adhesion of an established cell line to a surface reactive biomaterial. Journal of Biomedical Materials Research. 1982;16:195–207. doi: 10.1002/jbm.820160303. [DOI] [PubMed] [Google Scholar]
- 37.García AJ, Ducheyne P, Boettiger D. Effect of surface reaction stage on fibronectin-mediated adhesion of osteoblast-like cells to bioactive glass. Journal of Biomedical Materials Research. 1998;40:48–56. doi: 10.1002/(sici)1097-4636(199804)40:1<48::aid-jbm6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 38.El-Ghannam A, Ducheyne P, Shapiro IM. Effect of serum proteins on osteoblast adhesion to surface-modified bioactive glass and hydroxyapatite. Journal of Orthopaedic Research. 1999;17:340–345. doi: 10.1002/jor.1100170307. [DOI] [PubMed] [Google Scholar]
- 39.Hench LL. Ducheyne P, Lemons JE, editors. Bioactive ceramics. Bioceramics: materials characteristics versus in vivo behavior. Annals of the New York Academy of Sciences. 1988:523, 554. [PubMed] [Google Scholar]
- 40.Hench LL, Anderson ÖH. Bioactive glasses. In: Hench LL, Wilson J, editors. An introduction of bioceramics. London: World Scientific; 1993. pp. 41–62. [Google Scholar]
- 41.Li R, Clark AE, Hench LL. An investigation of bioactive glass powders by sol–gel processing. Journal of Applied Biomaterials. 1991;2:231–239. doi: 10.1002/jab.770020403. [DOI] [PubMed] [Google Scholar]
- 42.Saravanapavan P, Jones JR, Pryce RS, Hench LL. Bioactivity of gel–glass powders in the CaO–SiO2 system: a comparison with ternary (CaO–P2O5–SiO2) and quaternary glasses (SiO2–CaO–P2O5–Na2O) Journal of Biomedical Materials Research A. 2003;66:110–119. doi: 10.1002/jbm.a.10532. [DOI] [PubMed] [Google Scholar]
- 43.Saravanapavan P, Jones JR, Verrier S, Beilby R, Shirtliff VJ, Hench LL, et al. Binary CaO–SiO2 gel–glasses for biomedical applications. Bio-Medical Materials and Engineering. 2004;14:467–486. [PubMed] [Google Scholar]
- 44.Branda F, Arcobello-Varlese F, Costantini A, Luciani G. Effect of the substitution of M2O3 (M = La, Y, In, Ga, Al) for CaO on the bioactivity of 2.5CaO × 2SiO2 glass. Biomaterials. 2002;23:711–716. doi: 10.1016/s0142-9612(01)00173-9. [DOI] [PubMed] [Google Scholar]
- 45.Greenspan DC, Hench LL. Chemical and mechanical behavior of bioglass-coated alumina. Journal of Biomedical Materials Research. 1976;10:503–509. doi: 10.1002/jbm.820100405. [DOI] [PubMed] [Google Scholar]
- 46.Zhong J, Latorre G, Greenspan D, Hench LL. Alumina inhibitory effect on the formation of hydroxyapatite. In: Wilson J, Greenspan D, Hench LL, editors. Proceedings of the 8th international symposium on ceramics in medicine: bioceramics. Vol. 8. Oxford: Butterworth-Heinemann; 1995. pp. 489–492. [Google Scholar]
- 47.Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass–ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Carlisle EM. Silicon: a possible factor in bone calcification. Science. 1970;167:279–280. doi: 10.1126/science.167.3916.279. [DOI] [PubMed] [Google Scholar]
- 49.Damen JJM, Ten Cate JM. Silica-induced precipitation of calcium phosphate in the presence of inhibitors of hydroxyapatite formation. Journal of Dental Research. 1992;71:453–457. doi: 10.1177/00220345920710030601. [DOI] [PubMed] [Google Scholar]
- 50.Jugdaohsingh R, Tucker KL, Qiao N, Cupples LA, Kiel DP, Powell JJ. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham Offspring cohort. Journal of Bone and Mineral Research. 2004;19:297–307. doi: 10.1359/JBMR.0301225. [DOI] [PubMed] [Google Scholar]
- 51.Silver IA, Deas J, Erecińska M. Interactions of bioactive glasses with osteoblasts in vitro: effects of 45S5 Bioglass, and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomaterials. 2001;22:175–185. doi: 10.1016/s0142-9612(00)00173-3. [DOI] [PubMed] [Google Scholar]
- 52.Bosetti M, Zanardi L, Hench L, Type CM. I collagen production by osteoblast-like cells cultured in contact with different bioactive glasses. Journal of Biomedical Materials Research A. 2003;64:189–195. doi: 10.1002/jbm.a.10415. [DOI] [PubMed] [Google Scholar]
- 53.Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HFJ, Evans BAJ, Thompson RPH, et al. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127–135. doi: 10.1016/s8756-3282(02)00950-x. [DOI] [PubMed] [Google Scholar]
- 54.Schröder HC, Wang XH, Wiens M, Diehl-Seifert B, Kropf K, Schloßmacher U, et al. Silicate modulates the cross-talk between osteoblasts (SaOS-2) and osteoclasts (RAW 264.7 cells): inhibition of osteoclast growth and differentiation. Journal of Cellular Biochemistry. 2012;113:3197–3206. doi: 10.1002/jcb.24196. [DOI] [PubMed] [Google Scholar]
- 55.Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, et al. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials. 2005;26:4847–4855. doi: 10.1016/j.biomaterials.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Marie PJ. The calcium-sensing receptor in bone cells: a potential therapeutic target in osteoporosis. Bone. 2010;46:571–576. doi: 10.1016/j.bone.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 57.Valerio P, Pereira MM, Goes AM, Leite MF. Effects of extracellular calcium concentration on the glutamate release by bioactive glass (BG60S) preincubated osteoblasts. Biomedical Materials. 2009;4:045011. doi: 10.1088/1748-6041/4/4/045011. [DOI] [PubMed] [Google Scholar]
- 58.Leu A, Stieger SM, Dayton P, Ferrara KW, Leach JK. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Engineering Part A. 2009;15:877–885. doi: 10.1089/ten.tea.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorustovich AA, Roether JA, Boccaccini AR. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Engineering Part B Reviews. 2010;16:199–207. doi: 10.1089/ten.TEB.2009.0416. [DOI] [PubMed] [Google Scholar]
- 60.Gerhardt LC, Widdows KL, Erol MM, Burch CW, Sanz-Herrera JA, Ochoa I, et al. The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials. 2011;32:4096–4108. doi: 10.1016/j.biomaterials.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 61.Gerhardt LC, Widdows KL, Erol MM, Nandakumar A, Roqan IS, Ansari T, et al. Neocellularization and neovascularization of nanosized bioactive glass-coated decellularized trabecular bone scaffolds. Journal of Biomedical Materials Research A. 2013;101:827–841. doi: 10.1002/jbm.a.34373. [DOI] [PubMed] [Google Scholar]
- 62.Torabinejad M, White DJ. Tooth filling material and method of use. 5415547. US Patent No. 1993
- 63.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis R, Pitt Ford TR. The constitution of mineral trioxide aggregate. Dental Materials. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Primus CM. Dental material. 7892342. US Patent No. 2011
- 65.Coleman NJ, Nicholson JW, Awosanya K. A preliminary investigation of the in vitro bioactivity of white Portland cement. Cement and Concrete Research. 2007;37:1518–1523. [Google Scholar]
- 66.Tay FR, Pashley DH, Rueggeberg FA, Loushine RJ, Weller RN. Calcium phosphate phase transformation produced by the interaction of the Portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. Journal of Endodontics. 2007;33:1347–1351. doi: 10.1016/j.joen.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 67.Mahamid J, Sharir A, Addadi L, Weiner S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proceedings of the National Academy of Sciences United States of America. 2008;105:12748–1253. doi: 10.1073/pnas.0803354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahamid J, Aichmayer B, Shimoni E, Ziblat R, Li C, Siegel S, et al. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proceedings of the National Academy of Sciences United States of America. 2010;107:6316–6321. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahamid J, Addadi L, Weiner S. Crystallization pathways in bone. Cells Tissues Organs. 2011;194:92–97. doi: 10.1159/000324229. [DOI] [PubMed] [Google Scholar]
- 70.Niu LN, Zhang W, Pashley DH, Breschi L, Mao J, Chen JH, et al. Biomimetic remineralization of dentin. Dental Materials. 2013 doi: 10.1016/j.dental.2013.07.013. pii: S0109-5641(13)00175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. Strategies to prevent hydrolytic degradation of the hybrid layer—a review. Dental Materials. 2013;29:999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi YP, Li N, Niu LN, Primus CM, Ling JQ, Pashley DH, et al. Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate. Acta Biomaterialia. 2012;8:836–842. doi: 10.1016/j.actbio.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao W, Wang J, Zhai W, Wang Z, Chang J. The self-setting properties and in vitro bioactivity of tricalcium silicate. Biomaterials. 2005;26:6113–6121. doi: 10.1016/j.biomaterials.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 74.Wan XH, Chang CK, Mao DL, Jiang L, Li M. Preparation and in vitro bioactivities of calcium silicate nanophase materials. Materials Science Engineering C. 2005;25:455–461. [Google Scholar]
- 75.Gou Z, Chang J, Zhai W, Wang J. Study on the self-setting property and the in vitro bioactivity of beta-Ca2SiO4. Journal of Biomedical Materials Research B: Applied Biomaterials. 2005;73:244–251. doi: 10.1002/jbm.b.30203. [DOI] [PubMed] [Google Scholar]
- 76.Chen CC, Ho CC, David Chen CH, Wang WC, Ding SJ. In vitro bioactivity and biocompatibility of dicalcium silicate cements for endodontic use. Journal of Endodontics. 2009;35:1554–1557. doi: 10.1016/j.joen.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 77.Formosa LM, Mallia B, Bull T, Camilleri J. The microstructure and surface morphology of radiopaque tricalcium silicate cement exposed to different curing conditions. Dental Materials. 2012;28:584–595. doi: 10.1016/j.dental.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 78.Camilleri J, Sorrentino F, Damidot D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dental Materials. 2013;29:580–593. doi: 10.1016/j.dental.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 79.Shaw CA, Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunologic Research. 2013;56:304–316. doi: 10.1007/s12026-013-8403-1. [DOI] [PubMed] [Google Scholar]
- 80.Han S, Lemire J, Appanna VP, Auger C, Castonguay Z, Appanna VD. How aluminum, an intracellular ROS generator promotes hepatic and neurological diseases: the metabolic tale. Cell Biology and Toxicology. 2013;29:75–84. doi: 10.1007/s10565-013-9239-0. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L, Jin C, Liu Q, Lu X, Wu S, Yang J, et al. Effects of subchronic aluminum exposure on spatial memory, ultrastructure and L-LTP of hippocampus in rats. Journal Toxicology Science. 2013;38:255–268. doi: 10.2131/jts.38.255. [DOI] [PubMed] [Google Scholar]
- 82.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. Journal of Endodontics. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 83.Bozeman TB, Lemon RR, Eleazer PD. Elemental analysis of crystal precipitate from gray and white MTA. Journal of Endodontics. 2006;32:425–428. doi: 10.1016/j.joen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Tingey MC, Bush P, Levine MS. Analysis of mineral trioxide aggregate surface when set in the presence of fetal bovine serum. Journal of Endodontics. 2008;34:45–49. doi: 10.1016/j.joen.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 85.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Australian Endodontic Journal. 2009;35:147–152. doi: 10.1111/j.1747-4477.2008.00137.x. [DOI] [PubMed] [Google Scholar]
- 86.Reyes-Carmona JF, Felippe MS, Felippe WT. Biomineralization ability and interaction of mineral trioxide aggregate and white Portland cement with dentin in a phosphate-containing fluid. Journal of Endodontics. 2009;35:731–736. doi: 10.1016/j.joen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 87.Taddei P, Tinti A, Gandolfi MG, Rossi PL, Prati C. Ageing of calcium silicate cements for endodontic use in simulated body fluids: a micro-Raman study. Journal of Raman Spectroscopy. 2009;40:1858–1866. [Google Scholar]
- 88.Taddei P, Tinti A, Gandolfi MG, Rossi PL, Prati C. Vibrational study on the bioactivity of Portland cement-based materials for endodontic use. Journal of Molecular Structure. 2009;924:548–554. [Google Scholar]
- 89.Han L, Okiji T, Okawa S. Morphological and chemical analysis of different precipitates on mineral trioxide aggregate immersed in different fluids. Dental Materials Journal. 2010;29:512–517. doi: 10.4012/dmj.2009-133. [DOI] [PubMed] [Google Scholar]
- 90.Gandolfi MG, Ciapetti G, Taddei P, Perut F, Tinti A, Cardoso MV, et al. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dental Materials. 2010;26:974–992. doi: 10.1016/j.dental.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 91.Gandolfi MG, Taddei P, Tinti A, De Stefano Dorigo E, Rossi PL, Prati C. Kinetics of apatite formation on a calcium-silicate cement for root-end filling during ageing in physiological-like phosphate solutions. Clinical Oral Investigation. 2010;14:659–668. doi: 10.1007/s00784-009-0356-3. [DOI] [PubMed] [Google Scholar]
- 92.Gandolfi MG, Taddei P, Tinti A, Prati C. Apatite-forming ability (bioactivity) of ProRoot MTA. International Endodontic Journal. 2010;43:917–929. doi: 10.1111/j.1365-2591.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- 93.Gandolfi MG, Van Landuyt K, Taddei P, Modena E, Van Meerbeek B, Prati C. Environmental scanning electron microscopy connected with energy dispersive X-ray analysis and Raman techniques to study ProRoot mineral trioxide aggregate and calcium silicate cements in wet conditions and in real time. Journal of Endodontics. 2010;36:851–857. doi: 10.1016/j.joen.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Budiraharjo R, Neoh KG, Kang ET, Kishen A. Bioactivity of novel carboxymethyl chitosan scaffold incorporating MTA in a tooth model. International Endodontic Journal. 2010;43:930–939. doi: 10.1111/j.1365-2591.2010.01771.x. [DOI] [PubMed] [Google Scholar]
- 95.Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. International Endodontic Journal. 2011;44:1081–1087. doi: 10.1111/j.1365-2591.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- 96.Gandolfi MG, Taddei P, Siboni F, Modena E, Ginebra MP, Prati C. Fluoride-containing nanoporous calcium-silicate MTA cements for endodontics and oral surgery: early fluorapatite formation in a phosphate-containing solution. International Endodontic Journal. 2011;44:938–949. doi: 10.1111/j.1365-2591.2011.01907.x. [DOI] [PubMed] [Google Scholar]
- 97.Gandolfi MG, Taddei P, Siboni F, Modena E, Ciapetti G, Prati C. Development of the foremost light-curable calciumsilicate MTA cement as root-end in oral surgery, chemical– physical properties, bioactivity and biological behavior. Dental Materials. 2011;27:e134–e157. doi: 10.1016/j.dental.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 98.Gandolfi MG, Taddei P, Tinti A, De Stefano Dorigo E, Prati C. Alpha-TCP improves the apatite-formation ability of calcium-silicate hydraulic cement soaked in phosphate solutions. Materials Science Engineering C. 2011;31:1412–1422. [Google Scholar]
- 99.Bird DC, Komabayashi T, Guo L, Opperman LA, Spears R. In vitro evaluation of dentinal tubule penetration and biomineralization ability of a new root-end filling material. Journal of Endodontics. 2012;38:1093–1096. doi: 10.1016/j.joen.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shokouhinejad N, Nekoofar MH, Razmi H, Sajadi S, Davies TE, Saghiri MA, et al. Bioactivity of EndoSequence root repair material and bioaggregate. International Endodontic Journal. 2012;45:1127–1134. doi: 10.1111/j.1365-2591.2012.02083.x. [DOI] [PubMed] [Google Scholar]
- 101.Formosa LM, Mallia B, Camilleri J. The chemical properties of light- and chemical-curing composites with mineral trioxide aggregate filler. Dental Materials. 2013;29:e11–e19. doi: 10.1016/j.dental.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 102.Han L, Okiji T. Bioactivity evaluation of three calcium silicate-based endodontic materials. International Endodontic Journal. 2013;46:808–814. doi: 10.1111/iej.12062. [DOI] [PubMed] [Google Scholar]
- 103.Gandolfi MG, Taddei P, Modena E, Siboni F, Prati C. Biointeractivity-related versus chemi/physisorption-related apatite precursor-forming ability of current root end filling materials. Journal of Biomedical Materials Research B: Applied Biomaterrials. 2013;101:1107–1123. doi: 10.1002/jbm.b.32920. [DOI] [PubMed] [Google Scholar]
- 104.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. International Endodontic Journal. 2007;40:462–470. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 105.Richardson G. The calcium silicate hydrates. Cement and Concrete Research. 2008;38:137–158. [Google Scholar]
- 106.Labbez C, Jönsson B, Pochard I, Nonat A, Cabane C. Surface charge density and electrokinetic potential of highly charged minerals: experiments and Monte Carlo simulations on calcium silicate hydrate. Journal of Physical Chemistry B. 2006;110:9219–9230. doi: 10.1021/jp057096+. [DOI] [PubMed] [Google Scholar]
- 107.Liu X, Ding C, Chu PK. Mechanism of apatite formation on wollastonite coatings in simulated body fluids. Biomaterials. 2004;25:1755–1761. doi: 10.1016/j.biomaterials.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 108.Dey A, Bomans PH, Müller FA, Will J, Frederik PM, de With G, et al. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nature Materials. 2010;9:1010–1014. doi: 10.1038/nmat2900. [DOI] [PubMed] [Google Scholar]
- 109.Dorvee JR, Veis A. Water in the formation of biogenic minerals: peeling away the hydration layers. Journal of Structural Biology. 2013;183:278–303. doi: 10.1016/j.jsb.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Combes C, Rey C. Amorphous calcium phosphates: synthesis, properties and uses in biomaterials. Acta Biomaterialia. 2010;6:3362–3378. doi: 10.1016/j.actbio.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 111.Eanes ED. Amorphous calcium phosphate. Monographs in Oral Science. 2001;18:130–147. doi: 10.1159/000061652. [DOI] [PubMed] [Google Scholar]
- 112.Pan H, Liu XY, Tang R, Xu HY. Mystery of the transformation from amorphous calcium phosphate to hydroxyapatite. Chemical Communications. 2010;46:7415–7417. doi: 10.1039/c0cc00971g. [DOI] [PubMed] [Google Scholar]
- 113.Eidelman N, Chow LC, Brown WE. Calcium phosphate phase transformations in serum. Calcified Tissue International. 1987;41:18–26. doi: 10.1007/BF02555126. [DOI] [PubMed] [Google Scholar]
- 114.Bodier-Houllé P, Steuer P, Voegel JC, Cuisinier FJ. First experimental evidence for human dentine crystal formation involving conversion of octacalcium phosphate to hydroxyapatite. Acta Crystallographica Section D: Biological Crystallography. 1998;54:1377–1381. doi: 10.1107/s0907444998005769. [DOI] [PubMed] [Google Scholar]
- 115.Biological LeGeros RZ. synthetic apatites. In: Brown PW, Constantz B, editors. Hydroxyapatite and related material. CRC Press: Boca Raton; 1994. pp. 3–28. [Google Scholar]
- 116.Tseng YH, Mou CY, Chan JC. Solid-state NMR study of the transformation of octacalcium phosphate to hydroxyapatite: a mechanistic model for central dark line formation. Journal of the American Chemical Society. 2006;128:6909–6918. doi: 10.1021/ja060336u. [DOI] [PubMed] [Google Scholar]
- 117.Chickerur NS, Tung MS, Brown WE. A mechanism for incorporation of carbonate into apatite. Calcified Tissue International. 1980;32:55–62. doi: 10.1007/BF02408521. [DOI] [PubMed] [Google Scholar]
- 118.Dorozhkin SV. Calcium orthophosphates: occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter. 2011;1:121–164. doi: 10.4161/biom.18790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.LeGeros RZ. Calcium phosphate in oral biology and medicine. Basel, Switzerland: Karger; 1991. [PubMed] [Google Scholar]
- 120.Fowler BO, Marković M, Brown WE. Octacalcium phosphate, 3. Infrared and Raman vibrational spectra. Chemistry of Materials. 1993;5:1417–1423. [Google Scholar]
- 121.Marković M, Fowler BO, Tung MS. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. Journal of Research of the National Institute of Standards and Technology. 2004;109:553–568. doi: 10.6028/jres.109.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown WE. Octacalcium phosphate and hydroxyapatite: crystal structure of octacalcium phosphate. Nature. 1962;196:1048–1050. [Google Scholar]
- 123.Brown WE, Smith JP, Lehr JR, Frazier AW. Octacalcium phosphate and hydroxyapatite: crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature. 1962;196:1050–1055. [Google Scholar]
- 124.Taves DR. Similarity of octacalcium phosphate and hydroxyapatite structures. Nature. 1963;200:1312–1313. [Google Scholar]
- 125.Xin Rl Leng Y, Wang N. In situ TEM examinations of octacalcium phosphate to hydroxyapatite transformation. Journal of Crystal Growth. 2006;289:339–344. [Google Scholar]
- 126.Matthew M, Takagi S. Structure of biological minerals in dental research. Journal of Research of the National Institute of Standards and Technology. 2001;106:1035–1044. doi: 10.6028/jres.106.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elliot JC. Studies in inorganic chemistry 18: structure and chemistry of the apatites and other calcium orthophosphates. Amsterdam: Elsevier; 1994. [Google Scholar]
- 128.Cintra LT, de Moraes IG, Estrada BP, Gomes-Filho JE, Bramante CM, Garcia RB, et al. Evaluation of the tissue response to MTA and MBPC: microscopic analysis of implants in alveolar bone of rats. Journal of Endodontics. 2006;32:556–559. doi: 10.1016/j.joen.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 129.Cintra LT, Bernabé PF, de Moraes IG, Gomes-Filho JE, Okamoto T, Consolaro A, et al. Evaluation of subcutaneous and alveolar implantation surgical sites in the study of the biological properties of root-end filling endodontic materials. Journal of Applied Oral Science. 2010;18:75–82. doi: 10.1590/S1678-77572010000100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gomes-Filho JE, de Moraes Costa MT, Cintra LT, Lodi CS, Duarte PC, Okamoto R, et al. Evaluation of alveolar socket response to Angelus MTA and experimental light-cure MTA. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics. 2010;110:e93–e97. doi: 10.1016/j.tripleo.2010.05.065. [DOI] [PubMed] [Google Scholar]
- 131.Gomes-Filho JE, de Moraes Costa MM, Cintra LT, Duarte PC, Takamiya AS, Lodi CS, et al. Evaluation of rat alveolar bone response to Angelus MTA or experimental light-cured mineral trioxide aggregate using fluorochromes. Journal of Endodontics. 2011;37:250–254. doi: 10.1016/j.joen.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 132.Torabinejad M, Hong CU, Pitt Ford TR, Kaiyawasam SP. Tissue reaction to implanted super-EBA and mineral trioxide aggregate in the mandible of guinea pigs: a preliminary report. Journal of Endodontics. 1995;21:569–571. doi: 10.1016/s0099-2399(06)80987-8. [DOI] [PubMed] [Google Scholar]
- 133.Torabinejad M, Ford TR, Abedi HR, Kariyawasam SP, Tang HM. Tissue reaction to implanted root-end filling materials in the tibia and mandible of guinea pigs. Journal of Endodontics. 1998;24:468–471. doi: 10.1016/s0099-2399(98)80048-4. [DOI] [PubMed] [Google Scholar]
- 134.Moretton TR, Brown CE, Jr, Legan JJ, Kafrawy AH. Tissue reactions after subcutaneous and intraosseous implantation of mineral trioxide aggregate and ethoxybenzoic acid cement. Journal of Biomedical Materials Research. 2000;52:528–533. doi: 10.1002/1097-4636(20001205)52:3<528::aid-jbm11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 135.Saidon J, He J, Zhu Q, Safavi K, Spångberg LS. Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics. 2003;95:483–489. doi: 10.1067/moe.2003.20. [DOI] [PubMed] [Google Scholar]
- 136.Sousa CJ, Loyola AM, Versiani MA, Biffi JC, Oliveira RP, Pascon EA. A comparative histological evaluation of the biocompatibility of materials used in apical surgery. International Endodontic Journal. 2004;37:738–748. doi: 10.1111/j.1365-2591.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 137.Rahimi S, Mokhtari H, Shahi S, Kazemi A, Asgary S, Eghbal MJ, et al. Osseous reaction to implantation of two endodontic cements: mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM) Medicina Oral Patología Oral y Cirugía Bucal. 2012;17:e907–e911. doi: 10.4317/medoral.18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Torabinejad M, Bahjri K. Essential elements of evidenced-based endodontics: steps involved in conducting clinical research. Journal of Endodontics. 2005;31:563–569. doi: 10.1097/01.don.0000164137.28104.2f. [DOI] [PubMed] [Google Scholar]
- 139.Brouwers HJH, van Eijk RJ. Alkali concentrations of pore solution in hydrating OPC. Cement and Concrete Research. 2003;33:191–196. [Google Scholar]
- 140.Chen W, Brouwers HJH. Alkali binding in hydrated Portland cement paste. Cement and Concrete Research. 2010;40:716–722. [Google Scholar]
- 141.Richardson IG, Brough AR, Brydson R, Groves GW, Dobson CM. Location of aluminum in substituted calcium silicate hydrate (C-S-H) gels as determined by 29Si and 27Al NMR and EELS. Journal of the American Ceramic Society. 1995;76:2285–2288. [Google Scholar]
- 142.Andersen MD, Jakobsen HJ, Skibsted J. Incorporation of aluminum in the calcium silicate hydrate (C-S-H) of hydrated Portland cements: a high-field 27Al and 29Si MAS NMR investigation. Inorganic Chemistry. 2003;42:2280–2287. doi: 10.1021/ic020607b. [DOI] [PubMed] [Google Scholar]
- 143.Pardal X, Pochard I, Nonat A. Experimental study of Si–Al substitution in calcium-silicate-hydrate (C-S-H) prepared under equilibrium conditions. Cement and Concrete Research. 2009;39:637–653. [Google Scholar]
- 144.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dental Materials. 2005;21:297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 145.Zreiqat H, Howlett CR, Zannettino A, Evans P, Schulze-Tanzil G, Knabe C, et al. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. Journal of Biomedical Materials Research. 2002;62:175–184. doi: 10.1002/jbm.10270. [DOI] [PubMed] [Google Scholar]
- 146.Yamasaki Y, Yoshida Y, Okazaki M, Shimazu A, Uchida T, Kubo T, et al. Synthesis of functionally graded MgCO3 apatite accelerating osteoblast adhesion. Journal of Biomedical Materials Research. 2002;62:99–105. doi: 10.1002/jbm.10220. [DOI] [PubMed] [Google Scholar]
- 147.Yang Q, Jian J, Abramson SB, Huang X. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. Journal of Bone and Mineral Research. 2011;26:1188–1196. doi: 10.1002/jbmr.337. [DOI] [PubMed] [Google Scholar]
- 148.Chiang TY, Wei CK, Ding SJ. Effects of bismuth oxide on physicochemical properties and osteogenic activity of dicalcium silicate cements. Journal of Medical Biology Engineering. 2013 http://dx.doi.org/10.5305/jmbe.1386. [Google Scholar]
- 149.Koh ET, McDonald F, Pitt Ford TR, Torabinejad M. Cellular response to mineral trioxide aggregate. Journal of Endodontics. 1998;24:543–547. doi: 10.1016/S0099-2399(98)80074-5. [DOI] [PubMed] [Google Scholar]
- 150.Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. Journal of Biomedical Materials Research. 1997;37:432–439. doi: 10.1002/(sici)1097-4636(19971205)37:3<432::aid-jbm14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 151.Mitchell PJ, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999;20:167–173. doi: 10.1016/s0142-9612(98)00157-4. [DOI] [PubMed] [Google Scholar]
- 152.Zhu Q, Haglund R, Safavi KE, Spångberg LS. Adhesion of human osteoblasts on root-end filling materials. Journal of Endodontics. 2000;26:404–406. doi: 10.1097/00004770-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 153.Nakayama A, Ogiso B, Tanabe N, Takeichi O, Matsuzaka K, Inoue T. Behaviour of bone marrow osteoblast-like cells on mineral trioxide aggregate: morphology and expression of type I collagen and bone-related protein mRNAs. International Endodontic Journal. 2005;38:203–210. doi: 10.1111/j.1365-2591.2004.00917.x. [DOI] [PubMed] [Google Scholar]
- 154.Al-Rabeab E, Perinpanayagam H, MacFarland D. Human alveolar bone cells interact with ProRoot and tooth-colored MTA. Journal of Endodontics. 2006;32:872–875. doi: 10.1016/j.joen.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 155.Bonson S, Jeansonne BG, Lallier TE. Root-end filling materials alter fibroblast differentiation. Journal of Dental Research. 2004;83:408–413. doi: 10.1177/154405910408300511. [DOI] [PubMed] [Google Scholar]
- 156.Balto HA. Attachment and morphological behavior of human periodontal ligament fibroblasts to mineral trioxide aggregate: a scanning electron microscope study. Journal of Endodontics. 2004;30:25–29. doi: 10.1097/00004770-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 157.Samara A, Sarri Y, Stravopodis D, Tzanetakis GN, Kontakiotis EG, Anastasiadou E. A comparative study of the effects of three root-end filling materials on proliferation and adherence of human periodontal ligament fibroblasts. Journal of Endodontics. 2011;37:865–870. doi: 10.1016/j.joen.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 158.Hakki SS, Bozkurt SB, Ozcopur B, Purali N, Belli S. Periodontal ligament fibroblast response to root perforations restored with different materials: a laboratory study. International Endodontic Journal. 2012;45:240–248. doi: 10.1111/j.1365-2591.2011.01968.x. [DOI] [PubMed] [Google Scholar]
- 159.Willershausen I, Wolf T, Kasaj A, Weyer V, Willershausen B, Marroquin BB. Influence of a bioceramic root end material and mineral trioxide aggregates on fibroblasts and osteoblasts. Archives of Oral Biology. 2013;58:1232–1237. doi: 10.1016/j.archoralbio.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 160.Katsamakis S, Slot DE, Van der Sluis LWM, Van der Weijden F. Histological responses of the periodontium to MTA: a systematic review. Journal of Clinical Periotondology. 2013;40:334–344. doi: 10.1111/jcpe.12058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.