Abstract
Antibiotic-associated infection with the bacterial pathogen Clostridium difficile is a major cause of morbidity and increased healthcare costs. C difficile infection (CDI) follows disruption of the indigenous gut microbiota by antibiotics. Antibiotics create an environment within the intestine that promotes C difficile spore germination, vegetative growth, and toxin production, leading to epithelial damage and colitis. Studies of patients with CDI and animal models have shown that the indigenous microbiota can inhibit expansion and persistence of C difficile. Although the specific mechanisms of these processes are not known, they are likely to interfere with key aspects of the pathogen’s physiology, including spore germination and competitive growth. Increasing our understanding of how the intestinal microbiota manage C difficile could lead to better means of controlling this important nosocomial pathogen.
Keywords: Colonization resistance, C difficile, microbiome, microbial ecology, antibiotics
Introduction
The fulfillment of Koch’s postulates for C difficile in 1977 was one of the first formal recognitions of a microbiome-related disease. Although C difficile itself fit into the mold of a typical bacterial pathogen, C difficile infection (CDI) was unique in that previous antibiotic treatment of the patient was necessary for development of the full disease phenotype. We review C difficile as a pathogenic organism and discuss the basic epidemiologic features CDI, as well as its molecular pathogenesis. Research into the developing story of how C difficile interacts with the indigenous intestinal microbiota has provided important insights into the role of the microbiome in human health and disease.
C difficile and Antibiotic-associated Colitis
C difficile is a Gram-positive, anaerobic, spore-forming bacterium that was first isolated from the feces of healthy infants1. Interestingly, the organism appears to be highly prevalent in infants, who rarely demonstrate any clinical signs of infection with fully virulent strains2, 3. As the child and subsequently the microbiome matures, C difficile is no longer readily detected; in the healthy adult population, asymptomatic colonization with this organism is considered to be a rare event3, 4. Rates of colonization are increased primarily among adults with frequent healthcare-associated contact and patients in chronic-care facilities4.
The pathogenesis of CDI involves production of 2 members of the family of large clostridial toxins: TcdA and TcdB, which are products of genes located with a pathogenicity locus. 5–7 In C difficile, these toxins are large proteins with glycosyltransferase activities targeting the host GTPases Rho, Rac, and Cdc42, which regulate actin8, 9. Administration of purified toxin in model systems, including ligated ileal loops, is sufficient to replicate the intestinal damage that is characteristic of CDI10. Furthermore, there is evidence that these toxins have systemic effects on the host11. Although TcdA and TcdB are the most well-studied virulence factors of C difficile, other putative virulence factors have also been described12. A subset of C difficile strains produce an additional toxin, often referred to as binary toxin, that is related to iota toxin from C perfringens13, 14. Recent interest in binary toxin is likely related to the fact that the recent epidemic strains of C difficile, exemplified by the NAP1/027/BI strain, often produce this putative virulence factor15.
The epidemiology of CDI has been characterized by increases in incidence and severity that began around the year 200016, 17. It was recently estimated that CDI is the most common health care-associated infection, producing estimated annual hospital costs of more than $3 billion 18. The increase in virulence has been associated with the increasing isolation of epidemic strains related to NAP1/027/BI15, 19. There has been debate as to whether epidemic strains have enhanced virulence properties that result in more severe disease or if these strains simply cause more cases of CDI without causing worse patient outcomes 20. However, the overall increase in incidence and severity of CDI in the past decade has spurred research into C difficile pathogenesis. During the same period of time, the growing interest in the role of the indigenous microbiota in human health and disease, exemplified by projects such as the National Institutes of Health’s Human Microbiome Project21, 22 and the European MetaHIT consortium,23 has produced a scientific environment well-suited for the study of CDI. There is now much interest in the interactions between this pathogen in the intestinal microbiome.
The Microbiota Affects C difficile Invasion
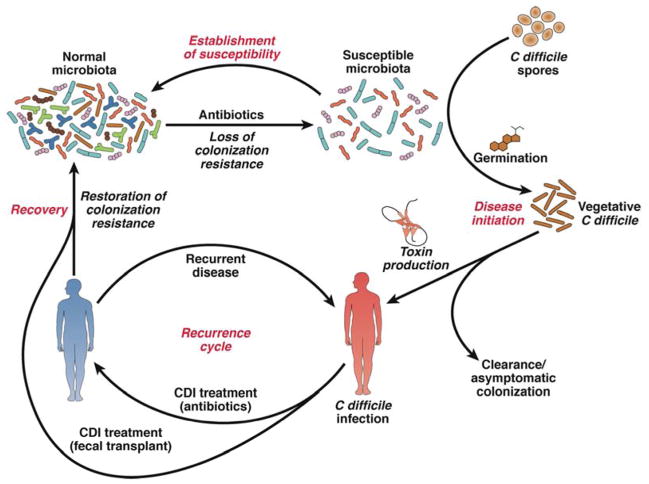
The concept that the indigenous gut microbiota mediates some form of resistance against colonization by bacterial pathogens was proposed long before C difficile was associated with antibiotic-associated pseudomembranous colitis24. Productive infection of animals with enteric bacterial pathogens often requires pre-exposure to antibiotics 25–27. However, until recently, the specific mechanisms by which the indigenous microbiome could prevent colonization by pathogenic organisms were not of widespread scientific interest. The requirement for antibiotic treatment before infection with a pathogen was simply regarded as a technical hurdle to overcome in order to investigate specific interactions between the pathogen in the host. However, increasing interest in the role of the indigenous microbiome in maintaining intestinal homeostasis has focused attention on its interactions with pathogens. The development of tractable animal models for studying CDI and the concurrent application of experimental microbial ecology to host-associated bacterial communities has led to multiple reports of how they influence the development of CDI and its complications. Work in experimental models of CDI and with clinical material from patients has indicated that C difficile, the intestinal microbiota, and other host factors form a complex system; specific alterations to this system can promote pathogenesis of CDI (Figure 1).
Figure 1. Cycle of CDI.
Antibiotic administration alters the indigenous intestinal microbiota, producing an environment that permits germination of C difficile spores and expansion of the pathogen. C difficile produces toxins that cause colitis and other symptoms. Antibiotics directed against C difficile can decrease the load of the pathogen and toxin production. Returning the microbiota to a state of colonization resistance cures CDI. However, if the microbiota is unable to restore resistance to colonization by C difficile patients have recurring CDI. In certain cases, repeat courses of anti-C difficile antibiotic therapy can eradicate the pathogen. In other cases, therapeutic restoration of a diverse microbiota via FMT is required to overcome CDI.
Studies in animals and humans have demonstrated that antibiotics have profound and, in some cases, long-lasting effects on the community structure of the gut microbiota28, 29. Antibiotics affect not only the overall size of the gut bacterial community, but also the composition of the community (they alter proportions of specific bacterial species). However, these effects do not necessarily occur together30. These effects on community structure are presumed to alter functions of the community,31 and for purposes of this discussion, might reduce resistance to CDI.
The hamster model of CDI was initially used to study interactions among C. difficile, the microbiota, and host tissues32, but mouse models are increasing in use. In the hamster model of CDI, clindamycin is the standard antibiotic used to generate a susceptible state33. A recent study followed the effect of clindamycin on the gut microbiota of hamsters associated with experimental CDI34. In the mouse models of disease, a variety of antibiotics have been shown to lead to loss of resistance to C difficile colonization35–38. Additionally, specific effects of antibiotics on the mouse gut microbiome have been described, allowing researchers to investigate how specific members of the microbiome mediate resistance to colonization39, 40.
Recent interest in mouse models of CDI began with a 2008 report in which Chen et al. used a cocktail of 5 antibiotics, followed by a single dose of clindamycin, to render mice susceptible to colonization by C difficile and subsequent development of a severe colitis35. A subsequent study by a separate group, using this same model, associated susceptibility with a loss of the normal members of the cecal community (mainly members of the family Lachnospiraceae) and a relative increase in the abundance of Enterobacteriaceae37. Furthermore, the severity of disease was related to the relative dynamics of these bacterial families, with more severe outcomes associated with a failure of Lachnospiraceae to return and a continued dominance with Enterobacteriaceae. Formal tests of the relative roles of these organisms in mediating colonization resistance were accomplished by monocolonization of germ-free mice with murine representatives of each of these groups of bacteria39. Colonization with a murine E coli isolate had no effect on the ability of C difficile to colonize the intestine and induce colitis, whereas a Lachnospiraceae isolate significantly reduced the levels of colonization by C difficile and the severity of colitis.
Then, Lawley et al. demonstrated that administration of clindamycin permitted long-term, non-fatal colonization of mice with C difficile 40. They identified 6 phylogenetically diverse species of bacteria that when administered together could restore colonization resistance and prevent chronic carriage of C difficile. It is interesting to note that clindamycin has not been reported to disrupt resistance against C difficile colonization in all genetically identical mice. Two studies reported that clindamycin can overcome colonization resistance in C57BL/6 mice38, 40, whereas a third group only achieved transient (2–3 day) colonization of the same strain of mice with C difficile following an identical dose of clindamycin37. Baseline differences in microbiota of mice from different colonies might cause these differences in results. Comparative microbiome analysis of these different groups of mice could provide additional information about the roles of specific groups of bacteria in resistance to colonization with C difficile.
Experimental infection of humans with C difficile is not ethical, but studies of the microbiomes of patients with naturally occurring C difficile infection have provided information that correlates with findings from animal studies. Antibiotics alter the structure of microbial communities29, and fecal samples from patients who developed CDI after antibiotic therapy have decreased diversity and other changes in the microbiota, compared to patients who did not develop CDI34. The intestinal microbiome of patients with recurrent disease has lower diversity than patients without CDI or those whose CDI was successfully treated and did not recur 41. Furthermore, patients with primary or recurrent symptomatic CDI have fecal microbiomes that differ in composition from patients with asymptomatic colonization by C difficile4. This observation makes it clear why fecal transplantation is so successful in the treatment of recalcitrant CDI (see FMT review by Alex Khoruts and Elaine Petrof in this same issue).
Microbiome Structure and Function in Resistance to C difficile Colonization
Although disruption of the healthy fecal microbiota can create an ecologic environment in which C difficile can flourish, little is understood about the changes in the environmental and microbial communities that occur in the intestine. Host and microbial community factors are directly involved in C difficile invasion of a dysbiotic intestinal community. C difficile selects specific environments for expansion and colonization, which appear to occur via complementary mechanisms. Development of CDI may involve altered bile acid metabolism and competition among microbes for nutrients, although other mechanisms by which the indigenous microbiota resists colonization by pathogenic bacteria exist 42.
Bile Salts in C difficile Spore Germination and Vegetative Growth
Germination of C difficile spores can be stimulated by a complex mixture of bile salts. The mechanisms by which individual bile salts and amino acids affect spore germination have only recently been elucidated 43–46. Interestingly, the primary bile acids cholate and chenodeoxycholate have different effects on the spore germination process. The conjugated and deconjugated forms of cholate, along with the amino acid glycine, function together to promote germination of C difficile spores in vitro, whereas chenodeoxycholate is a potent inhibitor of germination 44, 45. Importantly, physiologically relevant levels of these bile acids are sufficient to affect germination in vitro. Chenodeoxycholate competitively inhibits germination at a concentration ~10-fold lower than the stimulatory concentration of cholate. So, under normal physiological conditions, chenodeoxycholate suppresses C difficile invasion in vivo.
Cholate and chenodeoxycholate are metabolized into the secondary bile acids deoxycholate and lithocholate, respectively. In line with the effects of primary bile acids on spore germination, deoxycholate stimulates germination whereas lithocholate is a potent inhibitor. However, deoxycholate is toxic to vegetative C difficile; germination in the presence of this compound leads to quick death of bacteria. Therefore, an environment in which cholate levels are markedly higher than these other primary and secondary bile acids would support C difficile germination and expansion.
Administration of antibiotics causes a large shift in the bile acid pool in the cecum of mice, which can disrupt this balance and lead to spore germination (see Figure 2). 47, 48. Cholate is not detectable in the cecum of mice with a normal microbiota, but antibiotics increase its levels while levels of secondary bile acids remain low. So, C difficile spores are not expected to germinate until they are induced to do so by changes in concentrations of bile acids in the intestine.
Figure 2. Bile Acids Affect the Ability of C difficile to Colonize the Intestinal Tract.
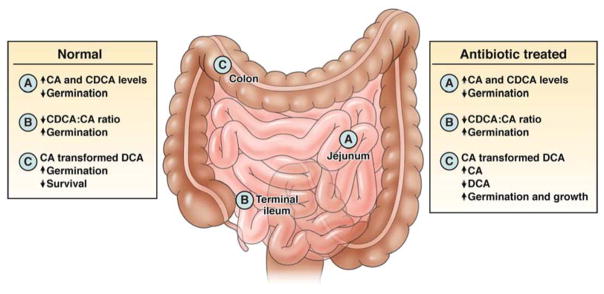
In the healthy microbiota, levels of cholate (CA) and chenodeoxycholate (CDCA) are high in the small intestine, and chenodeoxycholate inhibits germination. Because cholate is less well absorbed than chenodeoxycholate in the terminal ileum, the net effect is an increased ratio of cholate: chenodeoxycholate, resulting in increased spore germination 49–51. As vegetative C difficile cells move into the colon, they are exposed to the toxic effects of deoxycholate (DCA), further preventing C difficile from gaining a foothold in the colon. When the microbiota is disrupted with antibiotics bacterial transformation of primary bile acids (such as CA to DCA) can be greatly suppressed. In this case CA levels remain high while bacterial transformation of CA to DCA does not occur. This results in increased CA mediated spore germination and a loss of inhibitory effects of deoxycholate on C difficile growth. The net effect is the expansion of this pathogen will readily occur in the colon.
Until this past year, only in vitro studies had shown that bile acids were able to impact the germination of C difficile spores. Recently, researchers have shown that bile acid derivatives that inhibit spore germination in vitro reduce the ability of C difficle to colonize the intestines of mice52. Furthermore, in an elegant genetic screen, Sorg et al. identified a putative protease (CspC) that is required for spore germination in vitro 53 and could be a long-sought after spore germination receptor. cspC mutants of C difficle cannot germinate in the presence of cholate and glycine, and are unable to colonize and cause disease in hamsters. Therefore, the ability to sense bile is critical for C difficle colonization of the intestine. These findings support a role for bile metabolism in C difficile colonization of the intestine.
Ecologic Niche Expansion: Availability of Food Sources
The intestinal microbiota can also impact colonization by pathogens such as C difficile by providing competition for resources. Antibiotic disruption of the intestinal microbiota alters sources of nutrients in at least 2 ways. First, by reducing the diversity of the intestinal microbiota, antibiotics decrease competition for limited resources and free up previously unavailable ecological niches. Second, bacterial cell lysis releases carbon sources that the remaining community can consume. Despite the large number of C difficile genomes that have been sequenced, there have been relatively few studies of the metabolic networks used by C difficile to colonize the intestine. In vitro studies have shown that C difficile can grow on simple sugars, amino acids, peptides, and complex carbohydrates 54–57.
Wilson and Perini used continuous flow cultures to investigate whether a complex microbiota suppresses C difficile infection by competing for available resources 58. In growth medium formulated from feces of germ-free mice, cultivation of the cecal microbiota from hamsters gave rise to a community that not only resisted C difficile invasion but could also displace an already established C difficile infection. When hamster cecal communities were grown on a rich synthetic medium, they lost their ability to resist C difficile, although they were still diverse. To investigate what components of the germ-free mouse fecal medium promoted expansion of intestinal bacteria that suppresses C difficile, the authors added back individual carbon sources that were major components of this medium. Interestingly, they found that components of mucin (sialic acids, N-acetylglucosamine, and N-acetylneuraminic acid, which are abundant in the fecal medium) fully restored the ability to suppress C difficile to communities cultured in rich medium.
In support of these findings, C difficile was recently reported to use host-derived sialic acids to proliferate in the intestines of mice 59. C difficile was found to be able to break down sialic acids and use them as a carbon source; mutants C difficile that lacked this activity had a 4-fold reduction in colony-forming units/g of feces. Although these results show that C difficile can metabolize sialic acids to reach high densities in the intestine, additional factors are likely to be required for expansion of C difficile in the intestine.
Rolf Freter proposed a chemostat model for pathogen colonization, based on observations made while studying the persistence of individual microbes in a complex microbiota 60. This model might explain how C difficile persists in the intestine after antibiotic treatment and how competing microbes could displace C difficile during bacteriotherapy treatment 61. For example, if 2 organisms are in direct competition for a limited nutrient, the organism that can most efficiently use this nutrient will outcompete the other. This model also predicts that when one organism already fully occupies an intestinal niche, it is difficult for other organisms to invade.
In support of this idea, colonization of hamsters with a non-toxigenic strain of C difficile strain before with a toxigenic strain can completely prevent C difficile-associated colitis 62, 63. These findings indicate that the non-toxigenic strain occupies a niche cannot be taken over by a new strain. In a prospective study, patients who were followed until they developed CDI were found to have reduced proportions of the family Clostridiales Incertae Sedis XI just prior to infection, compared with individuals who did not develop CDI64. C difficile is a member of the family Clostridiales Incertae Sensu XI—loss of the Clostridiales Incertae Sedis XI could create a niche that C difficile is able to occupy. However, due to other alterations in the intestinal microbiota of these patients, we cannot conclude that loss of these microbes is directly responsible for the subsequent C difficile colonization.
Therapeutic Interventions with Defined Microbial Communities
Administration of defined microbes, usually single-strain probiotics, has had only limited success in ameliorating the symptoms of CDI. This is not surprising given recent animal and human studies associating large-scale alterations in microbial communities with antibiotic use and CDI. FMT is becoming more accepted for the treatment of recurrent CDI (reviewed by Khoruts and Vercoe, this issue). Despite its success, the undefined and complex nature of stool makes it difficult to envision widespread FMT therapy for individuals other than those with severe recurrent CDI.
Defined communities of microbes have been shown to be effective against recurrent CDI in patients and animal models. Interestingly, the 3 defined communities that are effective against recurrent CDI are remarkably simple, compared to the diversity of the intestinal microbiota. In addition, each community shares few organisms at the genus level, indicating there will be many combinations that can suppress CDI. Tvede and Rask-Madsen reported that a cocktail of 10 facultative aerobes and anaerobes was effective against recurrent CDI in 5 patients65. They noted that all of the patients were deficient in Bacteroides before the cocktail was administered, and then afterward the genus was detected in fecal samples from the patients. Petrof et al. reported using a 33-strain combination of microbes cultured from human fecal samples to treat 2 patients with recurrent CDI 66. The strains were selected based primarily on their antibiotic susceptibility and safety, while the researchers attempted to generate as much taxonomic diversity as possible. Both patients recovered from CDI, although it is not clear this was a direct result of population of the intestine with the defined microbes or an indirect result of stimulation of the indigenous microbiota. Sequencing analysis of microbial communities from fecal samples of the patients indicated that the 33 strains administered to the patients were gradually lost.
Although Petrof et al. only included 2 patients in their trial, the concept that the indigenous microbiota can be altered through addition of microbes was supported by the effects of a 6 species community of microbes administered to a mouse model of CDI 40. Interestingly, none of the 6 strains appeared to be able to colonize the mice over long time periods, but a single administration of these microbes increased the microbial diversity in the intestines of mice. It therefore appears that the defined communities might act by stimulating a suppressed indigenous microbiota.
Conclusions
The ability of FMT to cure recurrent CDI in up to 95% of patients provides support for strategies aimed at restoring the diversity of the intestinal microbiota. The efficacy of fecal communities from a variety of donors with diverse microbiotas, indicates that many microbial community structures will be able to prevent or treat CDI. For most cases, the fecal material for transplantation spends a significant amount of time being prepared under aerobic conditions, thus it is likely that a substantial proportion of the microbes being administered in FMTs do not remain viable. Studies are underway to understand how feces,, as well as defined microbial communities, are able to interfere with CDI. It is also critical to understand not only how these communities suppress C difficile invasion, but how the implantation of new communities may affect other aspects of human health. Studies have linked the intestinal microbiota with diseases such as diabetes, colon cancer, obesity, and atherosclerosis. If we use the microbiota to treat disease, we may inadvertently increase the risk of others. Continued research into the roles of the intestinal microbiome in health and disease might lead to ways to manipulate this community for benefit while minimizing unintended consequences.
Acknowledgments
Research Funding: AI090871 (VBY), AI090872 (RAB)
Footnotes
Conflicts of Interest: VBY is a recipient of an ASPIRE research award.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Hall IC, O’Toole E. Intestinal flora in new-born infants with the description of a new pathogic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390–402. [Google Scholar]
- 2.Thompson CM, Jr, Gilligan PH, Fisher MC, et al. Clostridium difficile cytotoxin in a pediatric population. Am J Dis Child. 1983;137:271–4. doi: 10.1001/archpedi.1983.02140290057015. [DOI] [PubMed] [Google Scholar]
- 3.Rousseau C, Poilane I, De Pontual L, et al. Clostridium difficile carriage in healthy infants in the community: a potential reservoir for pathogenic strains. Clin Infect Dis. 2012;55:1209–15. doi: 10.1093/cid/cis637. [DOI] [PubMed] [Google Scholar]
- 4.Rea MC, O’Sullivan O, Shanahan F, et al. Clostridium difficile carriage in elderly subjects and associated changes in the intestinal microbiota. J Clin Microbiol. 2012;50:867–75. doi: 10.1128/JCM.05176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voth DE, Ballard JD. Clostridium difficile Toxins: Mechanism of Action and Role in Disease. Clin Microbiol Rev. 2005;18:247–63. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pruitt RN, Lacy DB. Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol. 2012;2:28. doi: 10.3389/fcimb.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen A. Clostridium difficile toxins: mediators of inflammation. J Innate Immun. 2012;4:149–58. doi: 10.1159/000332946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht G, Pothoulakis C, LaMont JT, et al. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82:1516–24. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Just I, Fritz G, Aktories K, et al. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem. 1994;269:10706–12. [PubMed] [Google Scholar]
- 10.Kelly CP, Becker S, Linevsky JK, et al. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J Clin Invest. 1994;93:1257–65. doi: 10.1172/JCI117080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamm EE, Voth DE, Ballard JD. Identification of Clostridium difficile toxin B cardiotoxicity using a zebrafish embryo model of intoxication. Proc Natl Acad Sci U S A. 2006;103:14176–81. doi: 10.1073/pnas.0604725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vedantam G, Clark A, Chu M, et al. Clostridium difficile infection: Toxins and non-toxin virulence factors, and their contributions to disease establishment and host response. Gut Microbes. 2012:3. doi: 10.4161/gmic.19399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rupnik M, Grabnar M, Geric B. Binary toxin producing Clostridium difficile strains. Anaerobe. 2003;9:289–94. doi: 10.1016/j.anaerobe.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Stiles BG, Wigelsworth DJ, Popoff MR, et al. Clostridial binary toxins: iota and C2 family portraits. Front Cell Infect Microbiol. 2011;1:11. doi: 10.3389/fcimb.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 16.Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466–72. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–49. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubberke E. Clostridium difficile infection: the scope of the problem. J Hosp Med. 2012;7 (Suppl 3):S1–4. doi: 10.1002/jhm.1916. [DOI] [PubMed] [Google Scholar]
- 19.Goorhuis A, Bakker D, Corver J, et al. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin Infect Dis. 2008;47:1162–70. doi: 10.1086/592257. [DOI] [PubMed] [Google Scholar]
- 20.Walk ST, Micic D, Jain R, et al. Clostridium difficile ribotype does not predict severe infection. Clin Infect Dis. 2012 doi: 10.1093/cid/cis786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proctor LM. The Human Microbiome Project in 2011 and beyond. Cell Host Microbe. 2011;10:287–91. doi: 10.1016/j.chom.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Human Microbiome Project C. A framework for human microbiome research. Nature. 2012;486:215–21. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freter R. The fatal enteric cholera infection in the guinea pig, achieved by inhibition of normal enteric flora. J Infect Dis. 1955;97:57–65. doi: 10.1093/infdis/97.1.57. [DOI] [PubMed] [Google Scholar]
- 25.Bohnhoff M, Drake BL, Miller CP. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc Soc Exp Biol Med. 1954;86:132–7. doi: 10.3181/00379727-86-21030. [DOI] [PubMed] [Google Scholar]
- 26.Wadolkowski EA, Laux DC, Cohen PS. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of growth in mucus. Infect Immun. 1988;56:1030–5. doi: 10.1128/iai.56.5.1030-1035.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadolkowski EA, Burris JA, O’Brien AD. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1990;58:2438–45. doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonopoulos DA, Huse SM, Morrison HG, et al. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun. 2009;77:2367–75. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson CJ, Young VB. Antibiotic administration alters the community structure of the gastrointestinal micobiota. Gut Microbes. 2010;1:279–284. doi: 10.4161/gmic.1.4.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson CJ, Bohannan BJ, Young VB. From structure to function: the ecology of host-associated microbial communities. Microbiol Mol Biol Rev. 2010;74:453–76. doi: 10.1128/MMBR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartlett JG, Onderdonk AB, Cisneros RL, et al. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977;136:701–5. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- 33.Lusk RH, Fekety R, Silva J, et al. Clindamycin-induced enterocolitis in hamsters. J Infect Dis. 1978;137:464–75. doi: 10.1093/infdis/137.4.464. [DOI] [PubMed] [Google Scholar]
- 34.Peterfreund GL, Vandivier LE, Sinha R, et al. Succession in the gut microbiome following antibiotic and antibody therapies for Clostridium difficile. PLoS One. 2012;7:e46966. doi: 10.1371/journal.pone.0046966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Katchar K, Goldsmith JD, et al. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008;135:1984–92. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Theriot CM, Koumpouras CC, Carlson PE, et al. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes. 2011;2 doi: 10.4161/gmic.19142. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reeves AE, Theriot CM, Bergin IL, et al. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes. 2011;2:145–58. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buffie CG, Jarchum I, Equinda M, et al. Profound Alterations of Intestinal Microbiota following a Single Dose of Clindamycin Results in Sustained Susceptibility to Clostridium difficile-Induced Colitis. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeves AE, Koenigsknecht MJ, Bergin IL, et al. Suppression of Clostridium difficile in the Gastrointestinal Tract of Germ-Free Mice Inoculated with a Murine Lachnospiraceae Isolate. Infect Immun. 2012 doi: 10.1128/IAI.00647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 42.Britton RA, Young VB. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 2012 doi: 10.1016/j.tim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howerton A, Ramirez N, Abel-Santos E. Mapping interactions between germinants and Clostridium difficile spores. J Bacteriol. 2011;193:274–82. doi: 10.1128/JB.00980-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorg JA, Sonenshein AL. Bile salts and glycine as co-germinants for Clostridium difficile spores. J Bacteriol. 2008 doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorg JA, Sonenshein AL. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J Bacteriol. 2009;191:1115–7. doi: 10.1128/JB.01260-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson KH. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J Clin Microbiol. 1983;18:1017–9. doi: 10.1128/jcm.18.4.1017-1019.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antunes LC, Finlay BB. A comparative analysis of the effect of antibiotic treatment and enteric infection on intestinal homeostasis. Gut Microbes. 2011;2:105–8. doi: 10.4161/gmic.2.2.15610. [DOI] [PubMed] [Google Scholar]
- 48.Giel JL, Sorg JA, Sonenshein AL, et al. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One. 2010;5:e8740. doi: 10.1371/journal.pone.0008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krag E, Phillips SF. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. J Clin Invest. 1974;53:1686–94. doi: 10.1172/JCI107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mekhjian HS, Phillips SF, Hofmann AF. Colonic absorption of unconjugated bile acids: perfusion studies in man. Dig Dis Sci. 1979;24:545–50. doi: 10.1007/BF01489324. [DOI] [PubMed] [Google Scholar]
- 51.Schiff ER, Small NC, Dietschy JM. Characterization of the kinetics of the passive and active transport mechanisms for bile acid absorption in the small intestine and colon of the rat. J Clin Invest. 1972;51:1351–62. doi: 10.1172/JCI106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howerton A, Patra M, Abel-Santos E. A new strategy for the prevention of Clostridium difficile infection. J Infect Dis. 2013;207:1498–504. doi: 10.1093/infdis/jit068. [DOI] [PubMed] [Google Scholar]
- 53.Francis MB, Allen CA, Shrestha R, et al. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog. 2013;9:e1003356. doi: 10.1371/journal.ppat.1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouillaut L, Self WT, Sonenshein AL. Proline-dependent regulation of Clostridium difficile Stickland metabolism. J Bacteriol. 2013;195:844–54. doi: 10.1128/JB.01492-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson S, Calos M, Myers A, et al. Analysis of proline reduction in the nosocomial pathogen Clostridium difficile. J Bacteriol. 2006;188:8487–95. doi: 10.1128/JB.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karasawa T, Ikoma S, Yamakawa K, et al. A defined growth medium for Clostridium difficile. Microbiology. 1995;141 (Pt 2):371–5. doi: 10.1099/13500872-141-2-371. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura S, Nakashio S, Yamakawa K, et al. Carbohydrate fermentation by Clostridium difficile. Microbiol Immunol. 1982;26:107–11. doi: 10.1111/j.1348-0421.1982.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 58.Wilson KH, Perini F. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect Immun. 1988;56:2610–4. doi: 10.1128/iai.56.10.2610-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng KM, Ferreyra JA, Higginbottom SK, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502:96–9. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freter R, Brickner H, Botney M, et al. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983;39:676–85. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilson KH. The microecology of Clostridium difficile. Clin Infect Dis. 1993;16 (Suppl 4):S214–8. doi: 10.1093/clinids/16.supplement_4.s214. [DOI] [PubMed] [Google Scholar]
- 62.Merrigan M, Sambol S, Johnson S, et al. Susceptibility of hamsters to human pathogenic Clostridium difficile strain B1 following clindamycin, ampicillin or ceftriaxone administration. Anaerobe. 2003;9:91–5. doi: 10.1016/S1075-9964(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 63.Sambol SP, Merrigan MM, Tang JK, et al. Colonization for the prevention of Clostridium difficile disease in hamsters. J Infect Dis. 2002;186:1781–9. doi: 10.1086/345676. [DOI] [PubMed] [Google Scholar]
- 64.Vincent C, Stephens DA, Loo VG, et al. Reductions in intestinal Clostridiales precede the development of nosocomial Clostridium difficile infection. Microbiome. 2013:1. doi: 10.1186/2049-2618-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;1:1156–60. doi: 10.1016/s0140-6736(89)92749-9. [DOI] [PubMed] [Google Scholar]
- 66.Petrof EO, Gloor GB, Vanner SJ, et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013:1. doi: 10.1186/2049-2618-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]