Abstract
Major depressive disorder (MDD) is a recurrent mood disorder. The high rate of recurrence of MDD suggests the presence of stable vulnerability factors that place individuals with a history of major depression at an increased risk for the onset of another episode. Previous research has linked the remitted state, and therefore increased vulnerability for depressive relapse, with difficulties in the use of pleasant autobiographical memories to repair sad mood. In the present study, we examined the neural correlates of these difficulties. Groups of 16 currently euthymic, remitted depressed individuals and 16 healthy (control) women underwent functional magnetic resonance imaging (fMRI) during sad mood induction and during recovery from a sad mood state through recall of mood-incongruent positive autobiographical memories. Sad mood was induced in participants by using film clips; participants then recalled positive autobiographical memories, a procedure previously shown to repair negative affect. During both the sad mood induction and automatic mood regulation, control participants exhibited activation in the left ventrolateral prefrontal cortex (vlPFC) and cuneus; in contrast, remitted participants exhibited a decrease in activation in these regions. Furthermore, exploratory analyses revealed that reduced activation levels during mood regulation predicted a worsening of depressive symptoms at a 20-month follow-up assessment. These findings highlight a dynamic role of the vlPFC and cuneus in the experience and modulation of emotional states and suggest that functional anomalies of these brain regions are associated with a history of, and vulnerability to, depression.
Keywords: Ventral prefrontal cortex, Depression, Affect, Mood, Emotion regulation, fMRI, Autobiographical memory
Impairment in the regulation of negative emotions plays a significant role in the onset and maintenance of major depressive disorder (MDD). Indeed, individuals with a history of depression have been posited to differ from their nondepressed counterparts not in the degree to which they become sad but, rather, by an inability to repair or regulate their moods once they do become sad (Teasdale, 1988). Cognitive reappraisal represents an effective strategy in the effortful control of negative emotional states. Another, more automatic, process that has received increasing theoretical and empirical attention is the recall of positive autobiographical memories. Indeed, whereas most research conducted on the impact of mood on cognitive functioning and information processing has focused on mood congruency hypotheses, which posit that positive and negative moods should facilitate the recall of positive and negative information, respectively, several investigators have now reported the opposite effects, that people show an automatic tendency to retrieve pleasant thoughts and memories during the experience of negative mood states (Erber & Erber, 1994; Josephson, Singer, & Salovey, 1996; Parrott & Sabini, 1990; Rusting & DeHart, 2000). This process of recalling positive autobiographical events is thought to occur as part of an automatic attempt to regulate or reverse unpleasant mood (Erber & Erber, 1994; Josephson et al., 1996; Parrott & Sabini, 1990; Rusting & DeHart, 2000).
Given this literature, individual differences in the ability to access mood-incongruent memories should be associated with the ability to regulate negative mood. The available evidence, however, indicates that currently depressed participants, and participants with a history of depressive episodes but who are not currently depressed, do not differ from never-disordered individuals in their ability to recall positive autobiographical memories during a negative mood state, but rather, in their capacity to utilize positive memories to alleviate negative affect (Joormann & Siemer, 2004; Joormann, Siemer, & Gotlib, 2007). That is, in prior studies, the positivity of autobiographical memories did not distinguish never-depressed from recurrently depressed individuals; rather, these groups were differentiated by the inability of formerly depressed individuals to use positive memories to alleviate sad mood. Therefore, difficulty in using positive autobiographical recall to reduce negative mood may represent a stable, trait-related factor that contributes to the high rate of recurrence in this debilitating disorder. Indeed, nearly 80 % of depressed individuals will experience more than one major depressive episode over the course of their lifetime (e.g., Boland & Keller, 2009), often within the first 2 years of recovery (Keller & Shapiro, 1981).
Despite important findings implicating a role of impairments in automatic mood regulation in vulnerability for depression, the neural mechanisms underlying the difficulties experienced by formerly depressed individuals in using positive autobiographical recall to repair negative mood states have not yet been elucidated. Although researchers are beginning to clarify the neural correlates of the successful retrieval of autobiographical and self-relevant information in nondisordered individuals (Cavanna, 2007; Cooney, Joorman, Atlas, Eugène, & Gotlib, 2007; Spreng & Grady, 2010; Spreng, Mar, & Kim, 2009), the neural basis of remitted individuals’ difficulty in using mood-incongruent thoughts to improve sad mood is unknown. Given the high recurrence rate for depression, as well as clear difficulties in the ability of remitted depressed individuals to regulate sad mood, it is critical that we gain a better understanding of the neural dysfunction underlying automatic emotion regulation in recurrent depression.
Neuroimaging investigations of the regulation of affect have typically examined activation patterns occurring during effortful attempts at regulating sad mood (e.g., cognitive reappraisal), and have documented activations in subregions of the frontal lobe, including ventro- and dorsolateral prefrontal cortex, as well as in the anterior cingulate cortices (Buhle et al., 2013). Other studies that have also examined simultaneous activity in ventral limbic areas (e.g., amygdala) have found negative correlations in activations between these regions (Buhle et al., 2013), providing support for a fronto-limbic network model of the regulation of emotion. The extant literature on the neuroimaging of individuals who are experiencing an acute episode of depression suggests that dysfunction of prefrontal cortices contributes to emotion regulatory difficulties in this disorder (Rive et al., 2013). Whether this dysfunction persists during the implementation of more automatic attempts at mood repair (e.g., recall of positive autobiographical memories), however, or is sustained following recovery from acute episodes of depression, is not yet understood. Furthermore, no studies have examined whether MDD-related anomalies in activation underlying mood regulatory processes can be used to predict recurrence of depression in vulnerable individuals (e.g., remitted depressed populations).
Importantly, using a design similar to the one utilized in this study, Cooney et al. (2007) demonstrated that the process of using mood-incongruent recall of autobiographical memories to repair sad mood in nondisordered adults is associated with activation in specific areas of frontolimbic emotion regulatory networks, including the ventral prefrontal (i.e., Brodmann’s areas [BA] 47 and 11) and subgenual anterior cingulate (sgACC; BA 25) cortices. Given evidence for the specific involvement of these regions in the recall of positive autobiographical memories following the induction of a sad mood state (Cooney et al., 2007), in addition to findings showing that depressed individuals exhibit both anomalous activity in these regions during the processing of negative emotional material (Rive et al., 2013) and decreased prefrontal function in the recall of general autobiographical events (Whalley, Rugg, & Brewin, 2012), we hypothesized that participants with recurrent depression would exhibit reduced activation in these brain regions during the recall of positive memories following the induction of a negative mood state. Furthermore, given recent evidence that patterns of neural activation during affective tasks may be useful in predicting illness course (Canli et al., 2005; Foland-Ross et al., 2013), as well as prior work documenting that a majority of individuals who experience recurrent episodes of depression will develop a depressive relapse within the first 2 years of recovery (Keller & Shapiro, 1981), we examined whether anomalous activation observed during the recall of positive information in depression is associated with change in depressive symptoms an average of 20 months later.
Method
Participants
The study was approved by Stanford University’s institutional review board, and each participant provided written informed consent. Participants were recruited through advertisements posted in numerous locations (e.g., internet bulletin boards, university kiosks, supermarkets). The Structured Clinical Interview for the DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1996) was administered to all participants in order to assess current and lifetime diagnoses for anxiety, mood disorders, psychotic symptoms, alcohol and substance use, and somatoform and eating disorders. Participants who met the DSM-IV criteria for at least two previous major depressive episodes in their lives but did not meet the diagnostic criteria for current MDD or any other Axis I disorder were included in the depressed group, and participants with no current or past Axis I disorder were included in the control (CTL) group. All of the participants were fluent in English, had no history of head trauma, and were paid $25/h for their participation.
To assess the current severity of depressive symptoms, participants were administered the Beck Depression Inventory–II (BDI; Beck, Steer, & Brown, 1996) on the day of scanning (T1). All individuals were required to obtain scores in the minimal symptom range (<14) on the Beck Depression Inventory-II at T1. Participants also completed the BDI-II at follow-up (T2), an average of 20 months following the initial diagnostic and scan assessments.
Sixteen individuals with remitted depression (RMD) and 16 never-disordered CTL participants met the inclusion criteria and were included in this study (descriptive statistics can be found in Table 1). Seven of the depressed participants were taking one or more psychotropic medications at the time of scanning (buproprion, N = 1; anticonvulsant, N = 2; SSRI, N = 7), and three were receiving talk (psycho)therapy.
Table 1.
Demographic characteristics and mood ratings
| CTL (N = 16) | RMD (N = 16) | ||
|---|---|---|---|
| Age | 47.2 (4.3) | 44.7 (6.5) | t(30) = 1.26, p = .22 |
| BDI-II | 2.3 (3.2) | 6.1 (4.1) | t(26.7) = –2.74, p = .01 |
| BAI | 4.1 (4.2) | 5.1 (7.5) | t(30) = –0.46, p = .65 |
| College educated (%) | 83.3 | 61.1 | χ2(5) = 6.88, p = .23 |
| Ethnicity (% Caucasian) | 50.0 | 87.5 | χ2(4) = 6.59, p = .09 |
| Medicated (%) | – | 43.8 | – |
| Talk therapy (%) | – | 18.8 | |
| MDD Episodes | – | 4.5 (2.1) | – |
| Mood, Baseline | 2.38 (1.15) | 2.63 (1.09) | t(30) = 0.85, p > .05 |
| Mood, Positive Recall 1 | 2.81 (1.11) | 2.56 (0.89) | t(30) = 0.70, p > .05 |
| Mood, Mood Elaboration | 1.58 (0.52) | 1.56 (0.53) | t(19) = 0.12, p > .05 |
| Mood, Positive Recall 2 | 2.87 (1.13) | 2.69 (1.08) | t(29) = 0.45, p > .05 |
CTL = healthy controls;
RMD = remitted depressed participants; BDI-II = Beck Depression Inventory–II at baseline (Time 1) scan assessment;
BAI = Beck Anxiety Inventory; standard deviations are presented in parentheses.
Mood repair task
The mood repair task used during scanning has been described previously (Joormann, Cooney, Henry, & Gotlib, 2012). Briefly, this task consisted of four separate, 1-min-long scans. In the first, participants focused on a fixation cross (Baseline). In the second, participants saw a screen with a prompt asking them to recall “a happy, positive memory from your high school years that made you feel good” (Positive Recall 1). Following this, while the scanner was off, participants were told that they were about to watch a short film clip, and they were instructed to get into the feeling of the clip as intensely as possible and to try to imagine what they would feel if they were in this situation. They then viewed a sad 4-min film clip that depicted a young girl dying of cancer and her parents’ reaction to her imminent death. Immediately after they watched the film clip, participants heard instructions delivered over headphones asking them to think of other times that they may have experienced something similar and to focus on the feelings associated with that event for approximately 2 min. Scanning then proceeded for 1 min as participants saw a screen asking them to elaborate their sad mood by “really getting into the feeling” generated by the sad film clip and instructions (Mood Elaboration). Finally, participants were prompted to recall a second (separate) positive autobiographical memory for 1 min (Positive Recall 2). Importantly, positioning positive recall epochs both before and after the Mood Elaboration condition allowed us to differentiate between activation occurring during the recall of positive autobiographical memories more generally (Positive Recall 1) versus activations uniquely associated with the recall of mood-incongruent autobiographical memories (i.e., positive autobiographical memories recalled during a sad mood: Positive Recall 2). Before and after each scan, participants were asked to use a button box to rate their current mood on a four-point visual analog scale (1 = very sad, 4 = very happy). In order to minimize demand characteristics, participants were not told that this task assessed mood regulation, but were told instead that the task examined their ability to recall personal memories. Participants generated all memories at the time of scanning.
Image acquisition
Blood-oxygen-level-dependent (BOLD) data were acquired with a 1.5-T strength General Electric Signa MR scanner (Milwaukee, WI), using a spiral pulse sequence (Glover & Law, 2001; 24 axial slices, in-plane resolution 3.75 × 3.75 mm, gap = 0 mm, repetition time [TR] = 2,000 ms, echo time [TE] = 40 ms, flip angle [FA] = 90°). A structural T1-weighted volume was acquired coplanar to functional scans (29 axial slices, in-plane resolution 3.75 × 3.75 mm, gap = 0 mm, TR = 400 ms, TE = 14 ms, FA = 90°).
Behavioral data analysis
Mood ratings, acquired directly following each of the Baseline, Mood Elaboration, Positive Recall 1, and Positive Recall 2 conditions were analyzed using a mixed-design analysis of variance (ANOVA). The effects of the between-subjects (Diagnostic Group) and within-subjects (Condition) factors were modeled in order to assess the main effects of each variable and their interactions.
fMRI data analysis: Whole brain
All preprocessing and analyses were conducted with the Analysis of Functional Neural Images software (AFNI; Cox, 1996). Voxel time-series data were concatenated, slice time corrected, and corrected for motion (Fourier interpolation, two-pass), excluding participants who moved more than 1.5 mm. Data were spatially smoothed with a 3.75-mm Gaussian smoothing kernel, and high-pass filtered (0.008 Hz, 120 s). Prior to analysis, functional images were co-registered to anatomical images.
To compare indexes of neural activation occurring during Positive Recall 2 to that during Mood Elaboration and Positive Recall 1, the preprocessed time-series data for each participant were analyzed with a voxel-wise mixed-model ANOVA using the AFNI program 3dANOVA3, with Subject entered as a repeated factor. To control for multiple comparisons in this voxel-wise assessment, we conducted 10,000 Monte Carlo simulations using AFNI’s AlphaSim (Ward, 2000) and calculated, with an image-defined Gaussian filter calculated by AFNI’s 3dFWHM, that an uncorrected single-voxel significance threshold of p < .05 and a cluster threshold of 25 voxels would be necessary to hold a corrected family-wise Type I error at p < .05. Multifactor effects in clusters resulting from the omnibus test were decomposed by extracting parameter estimates (proportional to fMRI signal change) of BOLD signal response for each condition, separately for each cluster. To decompose significant interactions, we examined whether the RMD and CTL groups differed in activation changes occurring between Mood Elaboration versus Positive Recall 1 (to understand group effects related to the induction of sad mood), Positive Recall 2 versus Mood Elaboration (to understand group effects related to the repair of sad mood), and Positive Recall 2 versus Positive Recall 1 (to understand group effects related to the mood-incongruent recall of positive autobiographical memories). Additional analyses examining group differences in activation during the general recall of positive autobiographical memories (Positive Recall 1 vs. Baseline) are presented in the supplemental materials.
fMRI data analysis: Predictors of symptom change
To elucidate which of the activations that were identified in our whole-brain ANOVA predicted longitudinal symptom change, contrast coefficients of activation for each region, and for each of the conditions identified in the ANOVA as showing a main effect of group or condition or an interaction of group and condition, were regressed against BDI-II change scores (BDIT2 − BDIT1) across participants, controlling for initial symptom severity (BDIT1) and for the time duration (in months) between the T1 and T2 assessments.
Results
Participant characteristics
Demographic and clinical characteristics of the participants are presented in Table 1. The RMD and CTL groups did not differ in age [t(30) = 1.26, p = .219], in BAI scores [t(30) = −0.464, p = .646], or in socioeconomic status, as measured by house-hold income [χ2(4) = 5.173, p = .270], ethnicity [χ2(4) = 6.59, p = .086], and level of education [χ2(5) = 6.88, p = .230]. RMD participants had slightly, but significantly, higher scores on the BDI-II than did CTL participants (CTL: M = 2.25, SD = 3.19; RMD: M = 6.13, SD = 4.05), t(26.7) = −2.737, p = .011. Importantly, however, the BDI-II scores of the participants in both groups were well below the cutoff of 14 used to indicate the presence of minimal depressive symptoms.
Thirteen (81.3 %) of the CTL and 16 (100 %) of the RMD participants were reassessed at T2, an average of 20.6 ± 15.0 months later. The two groups did not differ in the lengths of the interval between T1 and T2 [t(23) = 0.18, p = .86]. With respect to treatment change from T1 to T2, three RMD participants and two CTL participants reported a change in medication. One additional RMD participant reported a change in talk therapy. Given evidence linking pharmacological and psychosocial treatment with BDI scores (e.g., Lustman, Freedland, Griffith, & Clouse, 2000), these six participants were excluded from analyses of the neural predictors of symptom change. Analyses of the remaining 23 participants showed that BDI-II scores were not significantly different between T1 and T2 within either the CTL [t(10) = 1.83, p = .10] or the RMD [t(10) = −0.26, p = .80] groups, or across both groups [t(22) = −1.49, p = .15]. The mean absolute change in BDI-II scores for the entire sample, however, was significantly greater than zero [t(22) = 5.66, p < .001].
Behavioral data
Because of technical difficulties with the recordings, data concerning mood ratings were missing for some of the participants in both groups for the Mood Elaboration phase (CTL, 25 %; RMD, 44 %). A two-way (group [CTL, RSK] by condition [Positive Recall 1, Mood Elaboration, Positive Recall 2]) repeated measures ANOVA conducted on the mood ratings collected during scanning yielded a significant main effect of condition, F(3, 54) = 20.40, p < .001, but no significant main effect of group, F(1, 29) < 1, or interaction of group and condition, F(3, 54) = 3.27. Follow-up paired t tests indicated that participants’ (positive) mood ratings decreased significantly after the negative mood induction and increased after recalling the second positive autobiographical memory (t s > 4.26, ps < .001), indicating that the sad mood induction was successful for both the CTL and RMD groups and that recalling positive memories improved participants’ mood states.
Imaging data: Whole-brain analyses
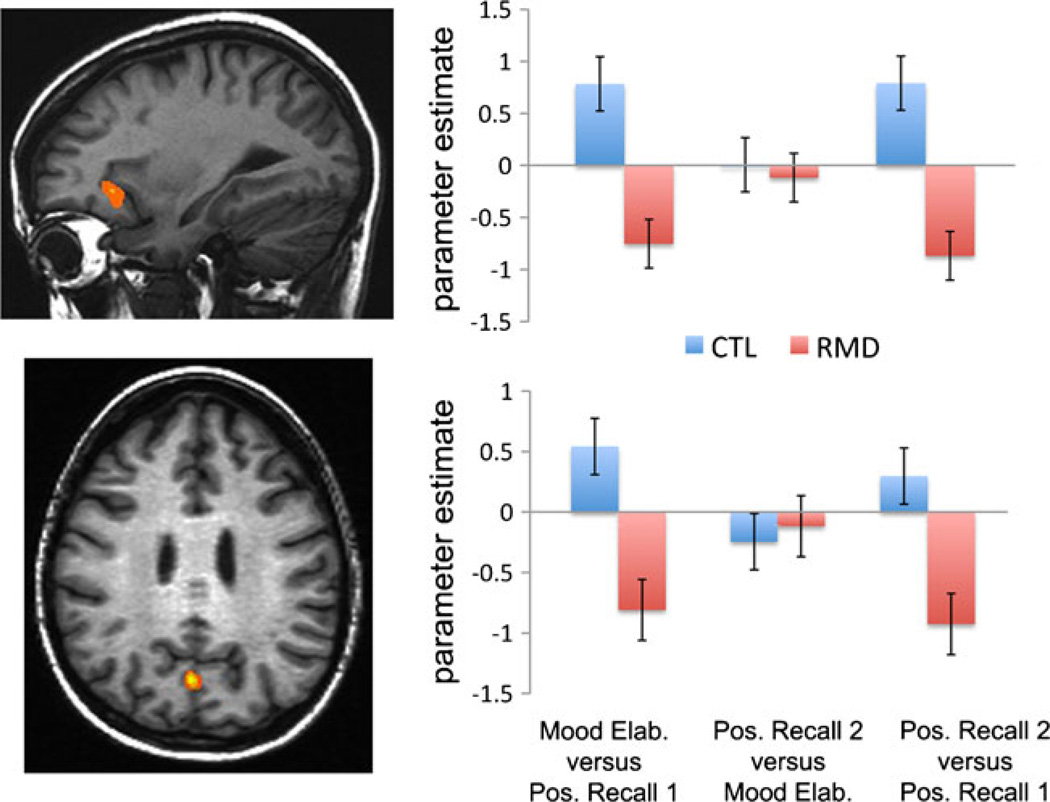
The voxel-wise ANOVA yielded significant effects in two brain regions: a frontal cluster centered in the ventrolateral prefrontal cortex (vlPFC; BA 47, k = 62 voxels, x, y, z coordinates of peak voxel: −22, −19, 1), and a cluster encompassing the left cuneus and precuneus (k = 26 voxels, x, y, z coordinates of peak voxel: −4, −67, 31; see Fig. 1). Follow-up analyses yielded no main effects of condition or group, Fs < 1.80, ps > 1.9; however, we did find a significant interaction of group and condition in both the vlPFC, F(2, 90) = 23.14, p < .001, and cuneus, F(2, 90) = 9.442, p < .001. Decomposition of the significant interaction effects indicated that whereas CTLs showed a significant increase in activation of the vlPFC and cuneus during the induction of a sad mood state [Mood Elaboration vs. Positive Recall 1; vlPFC: t(15) = −2.20, p = .044; cuneus: t(15) = 3.83, p = .002], RMDs showed a decrease in these regions [vlPFC: t (15) = 2.63, p = .019; cuneus: t (15) = 2.44, p = .028].Moreover, whereas CTLs demonstrated an increase in vlPFC activation during the recall of positive autobiographical memories following the induction of a sad mood state (Positive Recall 2 vs. Positive Recall 1), t(15) = −3.79, p = .002, RMDs showed a decrease, t(15) = 3.16, p = .006. Finally, whereas CTLs did not exhibit a significant change in cuneus activation during this contrast, t (15) = −1.11, p = .285, RMDs demonstrated a significant decrease in cuneus activation, t (15) = 2.55, p = .022. We observed no significant interaction of group and condition in either region during the repair of sad mood, using the contrast of Positive Recall 2 versus Mood Elaboration (both ts < 1.12, ps > .282).
Fig. 1.
Corrected statistical significant maps showing significant effects in the ventrolateral prefrontal cortex (vlPFC; top left) and cuneus (bottom left). Estimates of the percentages of signal change extracted from these two clusters (right) illustrate that activation changes occurring during the induction of sad mood (Mood Elaboration vs. Positive Recall 1) and the recall of mood-incongruent autobiographical memories (Positive Recall 2 vs. Positive Recall 1) varied as a function of group. Mood Elab. = Mood Elaboration; Pos. Recall 1 = Positive Recall 1; Pos. Recall 2 = Positive Recall 2; CTL = healthy controls; RMD = remitted depressed. Error bars represent standard errors
Associations with medication and prior depressive episodes
Given the narrow range of medication type within our RMD sample, we examined the effect of current medication status (medicated vs. unmedicated) on the interaction of group and condition by entering this factor as a variable in the univariate GLM. Medication status was not a significant predictor of activation in either the vlPFC, F(1, 89) = 0.379, p = .540, or cuneus, F(1, 89) = 0.772, p = .382. Further, even after including medication status as a variable in the model, our original finding of an interaction of group and condition was highly significant in both regions, Fs > 9.42, ps < .001.
Given that our primary analyses yielded an interaction of group and condition (but no main effects of condition or group), we assessed associations between number of previous episodes of depression and activations from the contrasts corresponding to the induction of sad mood (Mood Elaboration vs. Positive Recall 1), the repair of sad mood (Positive Recall 2 vs. Mood Elaboration), and mood-incongruent recall of positive autobiographical memories (Positive Recall 2 vs. Positive Recall 1). No significant correlations were obtained in these analyses for either brain region, rs < .22, ps > .362.
Associations with symptom change
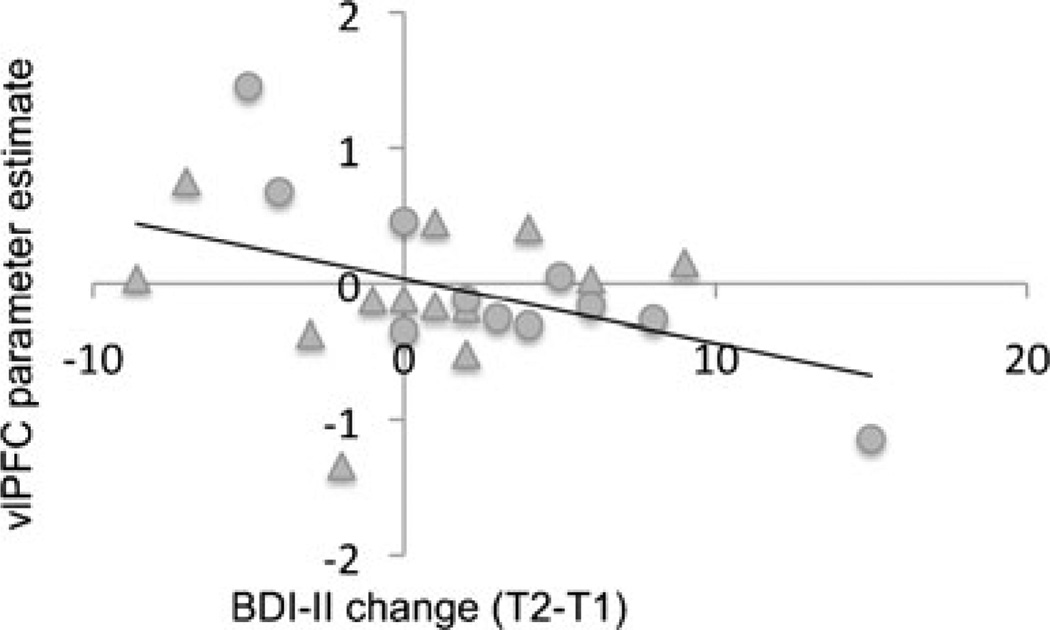
Linear regression analyses revealed that, across the sample of RMD and CTL participants whose treatment remained unchanged between T1 and T2, a significant inverse association was present between vlPFC activation during the contrast of Positive Recall 2 and Mood Elaboration and change in BDI-II scores [t(18) = −2.225, p = .037; see Fig. 2]. This finding remained significant after controlling for diagnosis. No other significant associations arose between neural activation and symptom change.
Fig. 2.
Scatterplot showing the inverse relation between activation in the left ventrolateral prefrontal cortex (vlPFC) at Time 1 and subsequent change in depressive symptoms. All values are unadjusted. Circles indicate never-depressed participants, and triangles represent remitted depressed participants. T1 = Time 1; T2 = Time 2; BDI-II = Beck Depression Inventory–II. Parameter estimates represent single-subject activation values, extracted from the vlPFC cluster identified using the contrast of Positive Recall 2 versus Mood Elaboration
Discussion
Approximately 80 % of depressed individuals will experience more than one major depressive episode over the course of their lifetime (e.g., Boland & Keller, 2009). More than 50% of these individuals will experience a depressive relapse within 2 years following recovery (Keller & Shapiro, 1981). This high rate of recurrence almost certainly indicates the presence of stable vulnerability factors that place individuals with a history of major depression at an increased risk for the subsequent onset of another episode. Previous research has linked the remitted state, and thus increased vulnerability for depressive relapse, with difficulties in the use of pleasant autobiographical memories to repair sad mood (Joormann et al., 2007). The present study was designed to delineate the neural correlates of these difficulties. The results of this study showed that, consistent with our hypothesis, the process of retrieving a positive autobiographical memory following the induction of a sad mood in remitted depressed adults was associated with abnormalities in activation of the left vlPFC. In addition, during this retrieval/repair process, we also found depression-related abnormalities in activation of the cuneus. More specifically, whereas remitted depressed individuals showed a decrease in activation of the vlPFC and cuneus during the mood-incongruent recall of positive autobiographical memories (Positive Recall 2 vs. Positive Recall 1), never-depressed participants showed the opposite pattern.
The two regions identified in our voxel-wise analyses—the left vlPFC and cuneus—are well documented as being involved in the retrieval of autobiographical memories in unselected samples (Cavanna, 2007; Spreng & Grady, 2010; Spreng et al., 2009; Svoboda, McKinnon, & Levine, 2006). The left vlPFC in particular represents a core hub of the autobiographical memory network (Svoboda et al., 2006); in a study of nondisordered participants, Cooney et al. (2007) found that down-regulating negative affect using positive autobiographical recall was directly associated with functioning in this brain area. The present investigation is the first to examine the neural correlates of this process in individuals with a history of depression. Our finding of reduced activation in the vlPFC and cuneus in remitted depressed adults during the recall of a positive autobiographical memory following the induction of a sad mood state suggests that aberrant functioning in these areas of the brain might underlie the trait-based difficulties in automatic (i.e., noneffortful) emotion regulatory processes that have been documented in this population (Joormann et al., 2007).
Although our findings of increased vlPFC activation during the recall of positive autobiographical memories in never-disordered CTL participants is consistent with that reported in previous investigations (Badre & Wagner, 2007), it is important to note that the neuroimaging literature also implicates a role for this region in the effortful control of negative emotion (e.g., cognitive reappraisal; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008), as well as in other aspects of basic cognitive control, including inhibition (Wager et al., 2005), task switching (Badre & Wagner, 2007), and the allocation of attention (Levy & Wagner, 2011). Involvement of the vlPFC across both emotional and nonemotional aspects of cognition is perhaps not surprising, given the dependence of emotion regulation on cognitive control mechanisms (Joormann & D’Avanzato, 2010). Effective reappraisal, for example, is reliant on a person’s ability to override automatic biases in cognition (e.g., attention, interpretation) that would otherwise lead to unwanted appraisals of the emotion-eliciting cues and to sustained negative affect. Replacing automatic appraisals with alternative evaluations of the situation thus requires cognitive control (Siemer & Reisenzein, 2007). Few studies have directly investigated the relation among variations in cognitive control, emotional experience, and vlPFC function in depression. Therefore, elucidating the links among these variables in individuals with a history of MDD is an important goal for future research.
Although the primary goal of this study was to assess neural dysfunction in remitted depressed individuals during mood-incongruent autobiographical recall in the service of recovery from a sad mood state, reduced activation in the vlPFC and cuneus was also observed in remitted depressed individuals as they experienced sad mood. Previous research examining the neural correlates of negative mood induction has implicated the vlPFC in the experience of sad mood (Cooney et al., 2007; Lévesque et al., 2003). Furthermore, both the left vlPFC and cuneus have been documented to be involved in the recall of sad autobiographical memories (Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003; Young et al., 2011), a process that has been used previously to induce a negative mood (Damasio et al., 2000; Harrison et al., 2008). In this context, one interpretation of the present findings is that remitted depressed participants exhibited less activation of the vlPFC and cuneus during mood elaboration because they were less able to engage cognitively with the task than were their never-depressed counterparts. Thus, they may have experienced difficulties trying to put themselves in the situation depicted in the film and trying to remember similar events from their lives. Because we did not collect information on participants’ subjective reports of task performance, including the valence and arousal levels of the positive memories that they had recalled during scanning, we could not address this possibility directly.
In addition to the patterns of group differences that emerged during mood-incongruent recall and sad mood elaboration, exploratory analyses revealed associations between neural activation in the left vlPFC and depressive symptoms assessed an average of 20 months later. Specifically, decreased activation in the left vlPFC during the contrast of Positive Recall 2 versus Mood Elaboration predicted a worsening of depressive symptoms at follow-up. These findings add to a literature documenting the potential efficacy of using patterns of neural activation to predict the course of illness (Canli et al., 2005; Foland-Ross et al., 2013). Indeed, they are the first findings to suggest that greater brain activity in the vlPFC during the specific process of regulating a sad mood state is beneficial to long-term outcome. If replicated, these findings are important in suggesting that individuals with less activation in this region may require stronger interventions than do individuals who show more robust activation.
Similar prospective analyses have helped to identify clinically meaningful neural indicators of treatment response in MDD. Previous studies have documented metabolism-based biomarkers of treatment success such as the insula (McGrath et al., 2013) and sgACC (Siegle, Carter, & Thase, 2006). Combining this literature with the present findings, an important next step in understanding the predictive utility of neural activation in the course of depressive illness could involve identification of neural patterns that predict which interventions are effective in preventing recurrence in remitted individuals. Researchers should additionally examine the utility of cognitive retraining in boosting vlPFC function; prior literature documents the existence of programs that are effective at boosting the mood-altering effects of positive autobiographical memory retrieval in depression (e.g., Werner-Seidler & Moulds, 2012). Whether vlPFC function increases with this training and with training-related decreases in negative mood remains to be explored.
Interestingly, the results of our prospective analysis of symptom change were restricted both anatomically (to the vlPFC region) and functionally (to the Positive Recall 2 vs. Mood Elaboration contrast). The specificity of this finding follows prior work documenting vlPFC as a prime facilitator of the down-regulation of negative emotional states (Torrisi, Lieberman, Bookheimer, & Altshuler, 2013) and suggests, despite its involvement in a number of different cognitive processes (as we discussed above), that the degree to which individuals are able to activate the vlPFC during the specific process of repairing mood, rather than vlPFC functioning level more generally, is what is relevant to the illness course. Certainly, future investigations that test this formulation more explicitly would be helpful in clarifying the nature of brain function in the context of symptom prediction.
By requiring participants to recall complex emotional experiences from their own lives, the task used in the present study has high ecological validity. Indeed, consistent with behavioral studies investigating the use of pleasant memory recall as a form of emotion regulation (Joormann et al., 2012; Joormann & Siemer, 2004; Joormann et al., 2007; Josephson et al., 1996), mood-incongruent autobiographical recall in the present study was associated with a decrease in sad mood, even though participants were not instructed to regulate affect. Thus, mood ratings may not be as susceptible to demand effects as are tasks in which participants are asked explicitly to use a specific strategy to decrease negative affect (e.g., cognitive reappraisal). Certainly, however, a number of diverse cognitive and emotional processes are engaged in autobiographical recall, which limits our ability to tightly control for variations in the strategies that people used both during the mood elaboration and mood regulation phases of the task. In addition, whereas previous research has found differences between never-depressed and remitted depressed individuals’ ability to regulate sad mood using positive memories (Joormann et al., 2007), we found no such pattern. This absence of a group effect may have been due to a lack of sensitivity of our four-point mood measure, or to a lack of statistical power for this variable, for which some data were missing. It is also possible, however, that fMRI data are more sensitive than are participant reports in detecting disturbances in the mood-altering effects of mood-incongruent recall. Group differences in activation occurring during the first positive memory recall may have resulted in variations in baseline activation for the mood elaboration condition; the present study design, however, did not permit us to investigate this possibility directly. Finally, it is important to note that, whereas major depression frequently tends to co-occurs with forms of anxiety (Brown, Campbell, Lehman, Grisham, & Mancill, 2001), participants in our remitted sample had no comorbid diagnoses. Future studies, therefore, will be needed to examine whether our findings may be influenced by current and lifetime diagnoses for anxiety.
In conclusion, the present study is important in beginning to elucidate the neural correlates of both the instantiation of a negative mood state and the subsequent recovery from that mood state in individuals with a history of depression who are not currently in an episode. It is also the first study to identify activation of the vlPFC during automatic mood regulation as a potential predictor of long-term outcome. Our findings of the involvement of the vlPFC and cuneus in both the induction of sad mood and the recall of positive material following a sad mood indicate that flexible recruitment of these regions in the service of experiencing and regulating both negative and positive mood states is critical to healthy emotional functioning, and that their dysfunction may contribute to vulnerability for depressed mood. Finally, the significant associations that we obtained between the level of vlPFC activation during the repair of a sad mood state and change in depressive symptoms indicate that patterns of neural activation may be useful in identifying individuals who are prone to depressive relapse. The results that we obtained offer new insights into possible mechanisms that impede recovery from sad mood in vulnerable individuals; future studies that investigate more systematically and explicitly the impact of this dysfunction on vulnerability for relapse will have important implications for understanding the course of MDD.
Acknowledgments
This research was supported by National Institute of Mental Health (Grant Nos. MH59259 and MH74849 awarded to I.H.G., MH090617 to L.C.F.-R., and MH60655 to J.J.), the Brain and Behavior Research Foundation (via NARSAD Young Investigator Awards to L.C.F.-R. and J.J.), and the Hope for Depression Research Foundation (awarded to I.H.G. and L.C.F.-R.).
Footnotes
The authors declare that they have no competing financial interests.
Contributor Information
Lara C. Foland-Ross, Email: lfolandross@stanford.edu, Stanford University, Stanford, CA, USA; Stanford Mood and Anxiety Disorders Laboratory, Department of Psychology, Stanford University, Jordan Hall, Building 420, 450 Serra Mall, Stanford, CA 94305, USA.
Rebecca E. Cooney, Stanford University, Stanford, CA, USA
Jutta Joormann, University of Miami, Miami, FL, USA.
Melissa L. Henry, Stanford University, Stanford, CA, USA
Ian H. Gotlib, Stanford University, Stanford, CA, USA
References
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory–II. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- Boland RJ, Keller MB. Course and outcome of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd ed. New York, NY: Guilford; 2009. pp. 23–43. [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Ochsner KN. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, Gotlib IH. Amygdala reactivity to emotional faces predicts improvement in major depression. NeuroReport. 2005;22:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- Cavanna AE. The precuneus and consciousness. CNS Spectrums. 2007;12:545–552. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joorman J, Atlas LY, Eugène F, Gotlib IH. Remembering the good times: Neural correlates of affect regulation. NeuroReport. 2007;18:1771–1774. doi: 10.1097/WNR.0b013e3282f16db4. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI, Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Erber R, Erber MW. Beyond mood and social judgment: Mood-incongruent recall and mood regulation. European Journal of Social Psychology. 1994;24:1–24. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders—clinician version (SCID-CV) Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- Foland-Ross LC, Hamilton JP, Sacchet MD, Furman DJ, Sherdell L, Gotlib IH. Activation of medial prefrontal and posterior cingulate cortices during encoding of negative material predicts symptom worsening in major depression. 2013 doi: 10.1097/WNR.0000000000000095. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yücel M. Modulation of brain resting-state networks by sad mood induction. PLoS ONE. 2008;3:e1794. doi: 10.1371/journal.pone.0001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. Journal of Abnormal Psychology. 2012;121:61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, D’Avanzato C. Emotion regulation in depression: Examining the role of cognitive processes. Cognition and Emotion. 2010;24:913–939. [Google Scholar]
- Joormann J, Siemer M. Memory accessibility, mood regulation, and dysphoria: Difficulties in repairing sad mood with happy memories? Journal of Abnormal Psychology. 2004;113:179–188. doi: 10.1037/0021-843X.113.2.179. [DOI] [PubMed] [Google Scholar]
- Joormann J, Siemer M, Gotlib IH. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. Journal of Abnormal Psychology. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Josephson BR, Singer JA, Salovey P. Mood regulation and memory: Repairing sad moods with happy memories. Cognition and Emotion. 1996;10:437–444. [Google Scholar]
- Keller MB, Shapiro RW. Major depressive disorder: Initial results from a one-year prospective naturalistic follow-up study. Journal of Nervous and Mental Disease. 1981;169:761–768. [PubMed] [Google Scholar]
- Lévesque J, Eugène F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: A randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23:618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott WG, Sabini J. Mood and memory under natural conditions: Evidence for mood-incongruent recall. Journal of Personality and Social Psychology. 1990;59:321–336. [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder: A systematic review of neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2013 doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rusting CL, DeHart T. Retrieving positive memories to regulate negative mood: Consequences for mood-congruent memory. Journal of Personality and Social Psychology. 2000;78:737–752. doi: 10.1037//0022-3514.78.4.737. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Siemer M, Reisenzein R. Appraisals and emotions: Can you have one without the other? Emotion. 2007;7:26–29. doi: 10.1037/1528-3542.7.1.26. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience. 2010;22:1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD. Cognitive vulnerability to persistent depression. Cognition and Emotion. 1988;2:247–274. [Google Scholar]
- Torrisi SJ, Lieberman MD, Bookheimer SY, Altshuler LL. Advancing understanding of affect labeling with dynamic causal modeling. NeuroImage. 2013;82:481–488. doi: 10.1016/j.neuroimage.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 from http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim.
- Whalley MG, Rugg MD, Brewin CR. Autobiographical memory in depression: An fMRI study. Psychiatric Research. 2012;201:98–106. doi: 10.1016/j.pscychresns.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Young KD, Erickson K, Nugent AC, Fromm SJ, Mallinger AG, Furey ML, Drevets WC. Functional anatomy of autobiographical memory recall deficits in depression. Psychological Medicine. 2011;42:345–357. doi: 10.1017/S0033291711001371. [DOI] [PMC free article] [PubMed] [Google Scholar]