Abstract
Background
Many animal models have been developed to study bronchopulmonary dysplasia (BPD). The preterm rabbit is a low-cost, easy-to-handle model, but it has a high mortality rate in response to the high oxygen concentrations used to induce lung injury. The aim of this study was to compare the mortality rates of two models of hyperoxia-induced lung injury in preterm rabbits.
Methods
Pregnant New Zealand white rabbits were subjected to caesarean section on gestational day 28 or 29 (full term = 31 days). The premature rabbits in the 28-day gestation group were exposed to room air or FiO2 ≥95%, and the rabbits in the 29-day gestation group were exposed to room air or FiO2 = 80% for 11 days. The mean linear intercept (Lm), internal surface area (ISA), number of alveoli, septal thickness and proportion of elastic and collagen fibers were quantified.
Results
The survival rates in the 29-day groups were improved compared with the 28-day groups. Hyperoxia impaired the normal development of the lung, as demonstrated by an increase in the Lm, the septal thickness and the proportion of elastic fibers. Hyperoxia also decreased the ISA, the number of alveoli and the proportion of collagen fibers in the 28-day oxygen-exposed group compared with the control 28-day group. A reduced number of alveoli was found in the 29-day oxygen exposed animals compared with the control 29-day group.
Conclusions
The 29-day preterm rabbits had a reduced mortality rate compared with the 28-day preterm rabbits and maintained a reduction in the alveoli number, which is comparable to BPD in humans.
Introduction
Bronchopulmonary dysplasia (BPD) is a multifactorial disorder with a pathogenesis that includes prematurity, the requirement for mechanical ventilation, inadequate antioxidant defenses and activation of the inflammatory response through several mechanisms. The most important mechanism for activating the inflammatory response is oxygen exposure [1], but other mechanisms can result in decreased alveolarization [2]–[7].
Due to this complex pathogenesis, many experimental models have been developed by exposing the immature lung to injuries induced by hyperoxia, mechanical stretch, inflammation and genetic modification [8], [9], [10]. These BPD models, included rats, lambs and baboons were essential tools for obtaining a better understanding of this disease [11]–[17]. Rodents are a well suited to model BPD given that the newborn rodent is born during the saccular stage of lung development. It was shown that the exposure of newborn mice to pure oxygen for one week resulted in pulmonary edema and hemorrhage, followed by a chronic repair phase, characterized by fibroblast proliferation and collagen deposition [18]. Oxygen exposure in rodents not only induces lung injury but also disrupts lung structure, affecting alveolarization and vascularization [19]. Although the premature rat model subjected to hyperoxia is advantageous due to its ease of manipulation and lower cost compared with larger-animal models [20], their main disadvantage is the small body size, which is a restriction for the study of lung mechanics and the alveolar and total lung surfactant pool in premature newborns [11]. Furthermore the oxygen concentrations used in most studies exceed the levels of supplementation currently applied to the preterm infant and lack the oxygen fluctuations that are clinically observed in preterm infants, which could potentially induce differences in molecular signaling not reproduced in the experimental setting [21].
Experimental larger-animal models including the preterm lamb and baboons allowed the use of mechanical ventilation for prolonged time, resulting in the creation of novel animal models of BPD [14]–[16]. Preterm baboons and lambs mechanically ventilated and exposed to maternal glucocorticoids and surfactant treatment, allowed the development of lung histopathologic findings of evolving neonatal chronic lung disease, similar to what occurs in preterm infants with BPD [4], [7], [22], [23]. These characteristics include widening and reduction of the alveoli number, a decrease of the alveolar surface area, presence of capillary dimorphism, increase in alveolar septal thickness and a modification in density and disposition of the elastic and collagen fibers, with persistent muscularization of pulmonary arterioles, and increased muscularization of bronchioles [9], [24], [25]. The preterm baboon program ended almost a decade ago, due to the high cost and ethical concerns, leaving the preterm lamb model as the only large animal, physiological model of neonatal chronic lung disease [26]. Advantage of both models is that they reproduce the clinical setting of preterm birth, respiratory failure, and prolonged ventilation support with oxygen-rich gas for days or weeks [27], [28]. Advantages of the preterm baboon model were the close phylogeny of baboons to humans and the preterm baboons were more immature than preterm lambs. An advantage of the preterm lamb model is that the fetal lambs are larger (2–3 kg body weight) and therefore, more amenable to physiological studies and repeated blood sampling. Disadvantages of both models are that they are expensive and require 24 h critical care.
The use of rabbits as animal models to study the effects of hyperoxia on the developing lung has been limited to lung or macrophage cultures exposed to oxygen. In vivo studies with rabbits following premature birth has been restricted to short periods of hyperoxia ranging from 20 hours to 4 days [29], [30].
In a previous study, pregnant rabbits were inoculated with Escherichia coli on the 29th day of gestation, and ceftriaxone therapy was initiated 8 hours after inoculation and continued for a period of 8 days. Although these animals remained under observation for a prolonged period (8 days), the prenatal infection was used to induce lung injury. These studies [10] support the central hypothesis of the current study of using premature rabbits born at 29 days of gestation for an experimental model of BPD.
Premature rabbits born at 28 days of gestation have been used in a model of BPD that was initially developed at our institution. This model was based on long periods of exposure to high oxygen concentrations (FiO2 ≥95%). However, the mortality rate was high in these studies [6], [31].
The aim of the present investigation was to evaluate the effects of prolonged exposure to oxygen on the mortality and lung development of premature rabbits by comparing 2 models of lung injury based on different gestational ages and oxygen concentrations: 28-days gestation preterm rabbits exposed to FiO2 ≥95% and 29-days gestation preterm rabbits exposed to FiO2 = 80%.
Methods
The experimental protocol was approved by the Ethics Committee for the Analysis of Research Projects at the Teaching Hospital of the University of São Paulo's School of Medicine (CAPPesq - project number 487/07).
On the 28th or 29th day of gestation (full term = 31 days), pregnant New Zealand white rabbits were subjected to cesarean sections following sedation with ketamine and acepromazine (10 mg/kg and 0.1 mg/kg, respectively, given intramuscularly (IM) and a spinal block using 2% Marcaine and xylocaine (1∶1, vol:vol)).
The abdominal and uterine walls were sectioned, and the fetuses were removed according to their location in the uterine horns (proximal or distal) and identified by the order of their removal. Sequential numbering by marker pen on the dorsal region was used to identify the animals to minimize the effect of the uterine position on the birth weight [32].
The first animal from each litter was randomly assigned to a study group immediately after birth. The groups were composed of animals exposed to room air or animals exposed to oxygen concentrations equal to 80% or ≥95%. Based on the allocation of the first animal from each litter, the animals were alternately allocated to the study groups. Four study groups were created: 28 days of gestation exposed to room air (Air 28), 28 days of gestation exposed to FiO2 ≥95% (O2 28), 29 days of gestation exposed to room air (Air 29) and 29 days of gestation exposed to FiO2 = 80% (O2 29).
The animals were maintained in premature infant incubators (C-86, Fanem LTDA, São Paulo, Brazil) on trays coated with a thin layer of autoclaved sawdust (sterile wood shavings) that was changed twice per day to keep the animals in a clean, dry environment. The incubator temperature was maintained between 30 and 32°C and was controlled by an electronic thermometer. The animals were weighed daily prior to the first feeding of the day (analytical scale TR 403; Denver Instrument Company, USA).
The animals were fed with a milk formula similar to natural rabbit milk, consisting of 5 g Neocate (Support), 5 g caseical (Support) and 15 g triglyceryl CM AGE (Support) in 100 ml milk. One drop of Vitanove vitamin complex was added to the formula starting on the third day of life.
The diet volume was 100 ml/kg on the first day of life, 150 ml/kg on the second day of life and 200 ml/kg from the third to the eleventh day. Food was administered at 12-hour intervals.
Warm humidified oxygen was supplied at a concentration of 80% or ≥95% to the animals in the O2 28 and O2 29 groups, respectively. The oxygen was delivered by neonatal nebulizers (Intermed, São Paulo, Brazil) through a multiperforated sealed acrylic chamber to prevent the accumulation of carbon dioxide (CO2). The oxygen concentrations were controlled using a room oxygen analyzer (Dixtal, São Paulo, Brazil).
The animals were sacrificed by exsanguination through sectioning of the abdominal aorta after achieving deep sedation with pentobarbital (25 mg/kg given intraperitoneally) on the 11th day after birth.
For the histological analysis of the lungs, a tracheostomy was performed with the insertion of a metal cannula (1 mm in diameter) immediately after sacrifice. The lungs were inflated to a pressure of 30 cmH2O [33]; after one minute, the trachea was ligated using a 0-0-cotton thread, followed by dissection and removal of the lungs and assessment of the lung volume. The slide preparation involved 1 mm thick sagittal cuts of the distal portion of the right inferior lobe. The author responsible for the morphometric analyses was blinded to the groups.
The histology slides were stained with hematoxylin and eosin for morphometric analysis, modified Weigert's resorcin and orcein for the analysis of elastic fibers and Picrosirius for the analysis of collagen fibers.
All analyses were performed by the same author, at the same time. A Nikon E600 microscope coupled to a 100-point grid of 50 lines was used for the morphometric analysis, and a minimum of 10 microscopic fields were examined per animal at 100× magnification. The morphometric analysis of the slides was performed by 2 blinded investigators.
Lung development was assessed by determining the mean linear intercept (Lm), the internal alveolar surface area (ISA), the number of alveoli, the thickening of the inter-alveolar septum and the proportion of elastic and collagen fibers.
The same 100-point grid of 50 lines was used for the analysis of Lm, considering the intersection of each of the 50 lines in the grid with an alveolar wall as 1 intercept. The Lm was calculated by the following formula: Lm = total length of the lines/number of alveolar intercepts.
The ISA represents the alveolar surface that is available for gas exchange and was determined according to the following formula: ISA = 4 V/Lm, where V = the fixed lung volume at a trans-tracheal pressure of 30 cmH2O multiplied by the parenchymal fraction. The parenchymal fraction was calculated using the same grid by counting the points that overlaid the lung tissue and excluding those that overlaid blood vessels and bronchi larger than 2 mm in diameter. The number of points was divided by 100. The following formula was used: V = (total lung volume x mean number of points)/100.
The number of alveoli was determined by a blinded investigator using an image analyzer (Image-Pro Plus 4.5, Media Cybernetics INC, USA). Lung sections stained by hematoxylin and eosin were photographed at 100× magnification, and 10 fields (images) were used for each slide.
The thickness of the inter-alveolar septum was determined manually with an image analyzer (Image-Pro Plus 4.5, Media Cybernetics INC, USA) by a blinded investigator. The assessment was performed on one slide per animal that was stained by Picrosirius, and 4 to 8 septa were assessed using 4 photographs per slide at 200× magnification.
The proportion of elastic and collagen fibers in the lung tissue was assessed by a single blinded investigator using an image analyzer (Image-Pro Plus 4.5, Media Cybernetics INC, USA). The lung sections stained by resorcin and orcein (for elastic fibers) and Picrosirius (for collagen fibers) [21] were photographed at 200× magnification, and 20 fields (images) were examined for each slide. The proportion of elastic and collagen fibers was determined by dividing the area of elastic or collagen fibers by the total area minus the area of the airspace.
The survival analyses were performed using the Kaplan-Meier method. Continuous variables were analyzed using one-way ANOVA with the Student-Newman-Keuls test used as a discriminatory post-hoc test. The Kruskal-Wallis one-way analysis of variance by ranks test was used as a nonparametric data test when the data failed to meet the prerequisites for a normal distribution and similar variance. Dunn's method was used as a discriminatory post-hoc test. A level of significance of 0.05 was adopted. SigmaPlot 11.0 (Systat Software, Inc. Chicago, USA) was used for the statistical analysis.
Results
Animal Survival
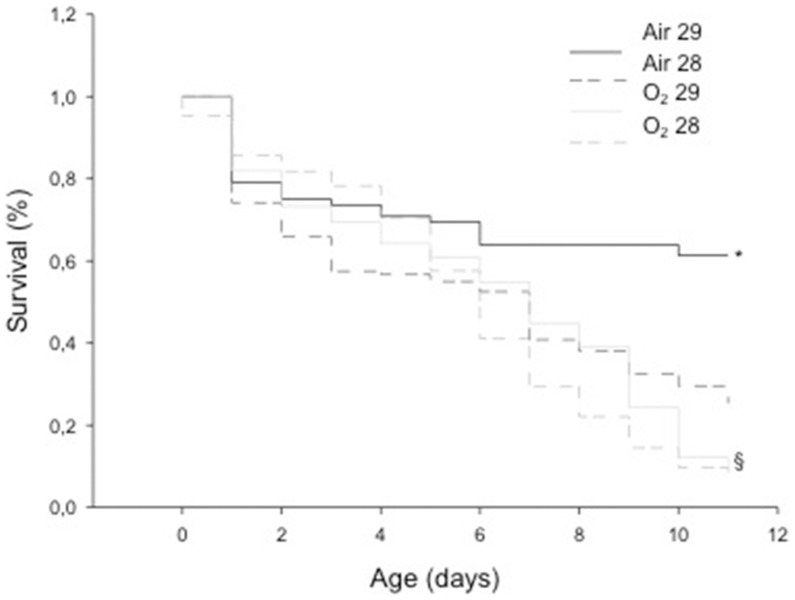
The total number of litters used in this experiment was 65 for the 28-day gestational age pups and 40 for the 29-day gestational age pups, with a mean number of pups/litter of 5.2±2.8 (mean ± sd). The animals in the Air 29 group exhibited higher survival rates compared with the Air 28, O2 28 and O2 29 groups. The survival rate observed in the animals from the O2 29 group was higher than that in the animals from the O2 28 group (Figure 1).
Figure 1. Kaplan-Meier survival curves – A comparison between the groups ‘room air’ and ‘oxygen’ is shown with animals born at 28 (dashed lines) and 29 days of gestation (solid lines).
A higher survival rate was observed in both Air Groups (*, p<0.05 vs Oxygen Groups) and a higher survival rate among O2 29 animals vs O2 28 Group (§, p<0.05)
Morphometric Analysis of the Lungs
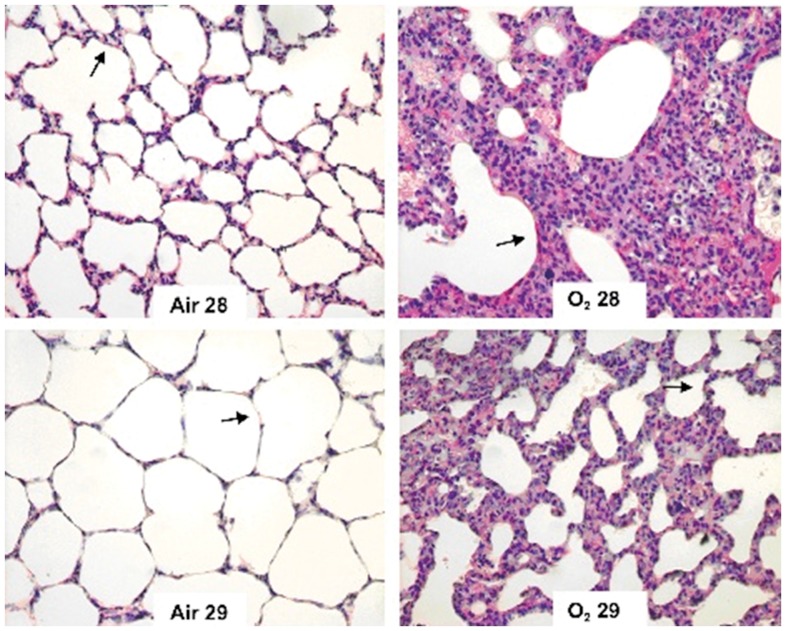
The normal development of the lungs was blocked by hyperoxia, as demonstrated by an increased Lm in the O2 28 and O2 29 groups, representing a larger alveolar size, compared with the Air 28 and Air 29 groups. This difference was significant only in the first group. A decrease in the ISA was observed in both groups exposed to oxygen (O2 28 and O2 29) compared with the corresponding groups kept in room air (Air 28 and Air 29), although this difference was significant only in the first group. A significantly decreased number of alveoli and an increased thickness of the inter-alveolar septum were noted in the groups subjected to hyperoxia compared with those kept in room air, thus confirming the interrupted alveolar development secondary to oxygen exposure (Table 1).
Table 1. The values of Lm and ISA and the number of alveoli and septal thickening are shown in the groups.
| Air 28 | O2 28 | Air 29 | O2 29 | |
| (n = 17) | (n = 17) | (n = 8) | (n = 8) | |
| Lm (µm) 1 | 60.5±18.2* | 85.3±27.3 | 74.3±10.6 | 86.4±14.9 |
| ISA (µm2)2 | 0.033±0.014* | 0.031±0.014 | 0.049±0.020 | 0.044±0.022 |
| Alveoli number (per field)3 | 32.02±11.29# | 17.91±8.73 | 30.24±8.46# | 21.44±4.93 |
| Septal thickening (µm)4 | 3.90±1.40# | 19.08±16.8§ | 3.84±1.09 | 13.59±6.68 |
| Lung volume (ml)5 | 2.63±1.83 | 1.93±0.80 | 3.90±1.27 | 2.81±1.54 |
*p<0.05 vs. all other groups; #p<0.05 vs. O2; §p<0.05 vs. Air 28 and Air 29.
: Power of performed test with alpha = 0,050∶0,975; 2: Power of performed test with alpha = 0,050∶1,000; 3: Power of performed test with alpha = 0,050∶1,000; 4: Power of performed test with alpha = 0,050∶1,000; 5: Power of performed test with alpha = 0,050∶0,087.
Proportion of Elastic and Collagen Fibers
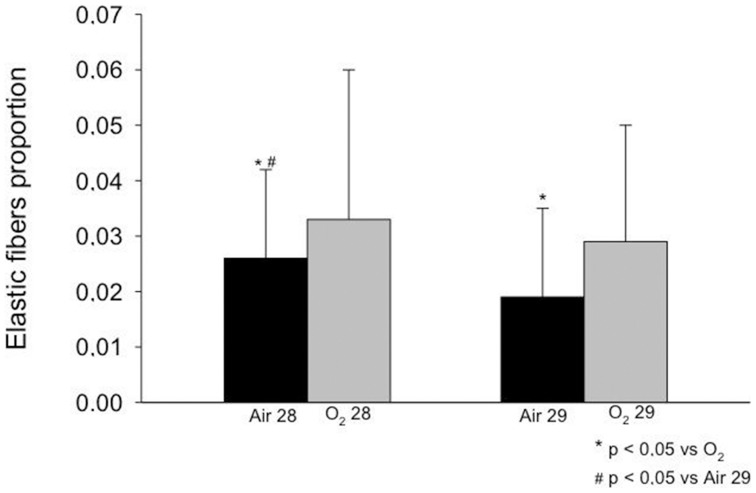
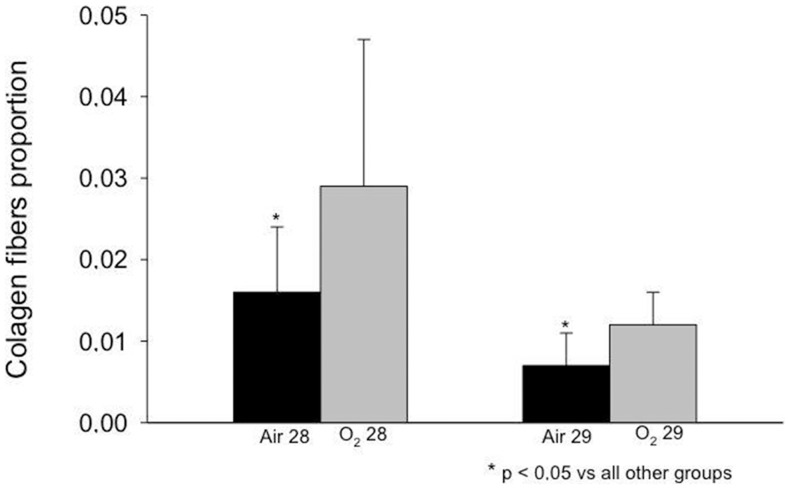
The elastic and collagen fiber proportion increased in the O2 29 group compared with the Air 29 group (Figures 2 and 3). A similar increase in the fiber proportion was observed in the animals in the O2 28 group compared with the Air 28 group (Figures 2 and 3). When both groups of animals born at 28 days were compared with the groups of animals born at 29 days, the elastic and collagen fiber proportion in the 29-day groups was lower than in the 28-day groups. Disorganization of the elastic and collagen fibers was observed in animals that had been exposed to oxygen (Figures 4 and 5, respectively).
Figure 2. Elastic fibers proportion observed in the study groups.
A higher proportion of elastic fibers were observed in both oxygen exposed groups compared with room air at the same gestational age (p<0.05) and a higher proportion of elastic fibers was observed among more immature animals exposed to room air (p<0.05).
Figure 3. Collagen fibers proportion observed in the study groups.
A higher proportion of collagen fibers were observed in both oxygen exposed groups compared with room air at the same gestational age (p<0.05)
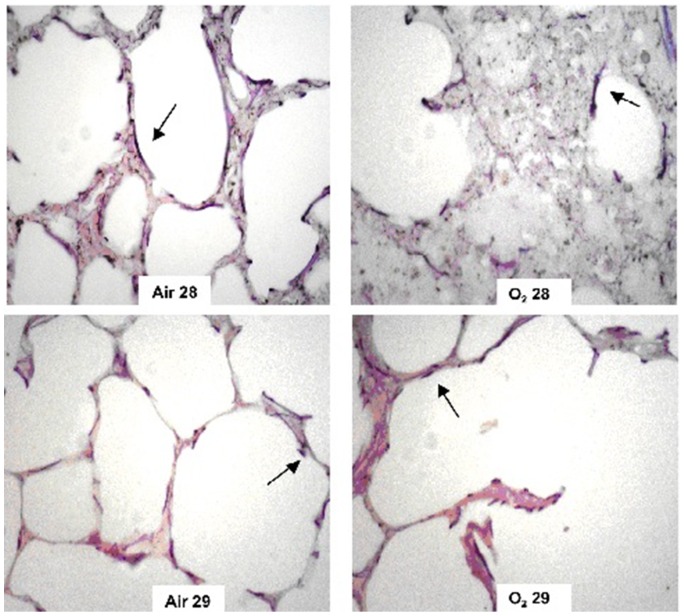
Figure 4. Distribution of elastic fibers (arrows) in the study groups.
Oxygen exposed animals (right panels, upper and lower) showed an intense disorganization of elastic fibers compared to animals from the Air 28 and Air 29 group (left panels, upper and lower).
Figure 5. Distribution of collagen fibers (arrows) in the study groups.
Animals exposed to oxygen showed an increased proportion of collagen fibers compared to those exposed to room air.
Discussion
The use of premature rabbits born at 29 days of gestation and subjected to an oxygen concentration of 80% resulted in a reduced number of alveoli and a trend in other histopathological findings, comparable with BPD characteristics in humans. These findings were similar to those observed in animals born at 28 days of gestation and subjected to an oxygen concentration ≥95%, though with a higher survival rate. A small difference of one day in the gestational age caused differences (comparable to hyperoxia) in the morphometric analysis of the lungs.
The use of premature rabbits exposed to hyperoxia as an additional experimental model to study BPD has the advantage of a lower cost, when compared with the baboon or preterm lamb model, which require intensive care for 24 hours, and a structure of complex and expensive laboratory not available in many research centers [15], [34]. Furthermore, the size of the premature rabbit (approximately 33±5 g), which is larger than the preterm mouse or rat, allows the use of a number of injuries, including hyperoxia, mechanical ventilation [35], and inflammation [36], that make this model suitable not only to better understand different molecular pathways which result in impaired lung development, with disruption of the entire process of alveolarization, but also the pathways that mediate lung repair and regeneration after injury. Future refinement and modification of this current animal model to better mimic the human condition is important, including to limit the levels of O2 used to cause lung injury to those currently used in preterm infants, as well as to allow fluctuations of O2 levels [37] and the use of therapies more similar to current standard of care (steroids, surfactant, etc.) [38].
This animal model shows close concordance to human beings in terms of the stages of lung development [30], [32] because the alveolar phase of rabbit development begins at the 28th day of gestation, for a full term of 31–33 days. This alveolar phase continues for a long period after birth, with a dramatic increase during the first 2 weeks of postnatal life; therefore, there may be critical differences between species in the early control of alveolarization and, thus, the pathways leading to lung injury [32], [39]. With 29 days of gestation, most of the alveolarization has not occurred, which makes this timing an acceptable model for BPD in humans, although with a lower mortality rate. This model was previously used to evaluate the impact of postnatal nutrition on lung growth and development [40].
The decision to use oxygen as an aggravating factor was based on the ease of implementing this model and on previous studies demonstrating that oxygen alone can trigger the cascade of inflammatory events that interrupt alveolar development. Rat lungs exposed to 100% oxygen until death or 85% oxygen for 7 days exhibit proliferation and hypertrophy of type II epithelial cells, decreased surface area and capillary volume and increased superoxide dismutase activity [18], [41]. Premature newborns are particularly sensitive to the deleterious effects of oxygen [11]. The ability of preterm infants to manage free radicals found in the plasma is low and declines during the early postnatal period [8], [42].
Our results show that survival was higher among animals born at 29 days of gestation compared with those born at 28 days of gestation among both animals exposed to oxygen and those subjected to room air. The higher survival rate in the former group may hinder the practical use of this experimental model [31]. A 67% mortality rate was observed in rats exposed to a FiO2 ≥95% for 14 days compared with rats exposed to room air (the mortality rate at room air was 33%) [11].
To characterize pulmonary lesions caused by hyperoxia, we studied the major changes that occur in BPD in humans, including changes in the alveolar size, the ISA, the number of alveoli, the thickness of the alveolar septum and the proportion of elastic and collagen fibers in the pulmonary parenchyma [13].
The size and distance between the alveolar walls is well represented in a simplified analysis through determining the Lm. In this study, hyperoxia modified the normal development of the lung, as demonstrated by an increase in the Lm. A similar evaluation of the interruption of lung development was performed in premature rats treated with dexamethasone or saline solution 12 hours before birth and randomized to hyperoxia (≥95%) or room air immediately after birth for 7 to 12 days [11]. Another similar evaluation was performed in premature baboons subjected to mechanical ventilation and hyperoxia [15].
A study conducted on 8 children with fatal cases of BPD also found an increased Lm, demonstrating the importance of this parameter in characterizing the disease [23].
In a model previously developed at our institution, premature rabbit lungs exposed to hyperoxia (≥95%) for 7 or 11 days exhibited increases in Lm compared with the room air group [6], [31]. In the present study, the O2 28 group showed histopathological findings that are consistent with the majority of studies in the literature, which report a 24% increase in Lm associated with oxygen exposure. This increase represents an increase in alveolar size secondary to impaired lung development, and the O2 29 group showed a similar trend with a 16% increase [6], [11], [23], [31].
The ISA calculation reflects the area of the alveolar-capillary membrane and the surface area that is available for performing gas exchange [43], [44]. This study demonstrated a decreased ISA in the groups that had been exposed to oxygen (O2 28 and O2 29) compared with the groups subjected to room air, although significance was reached only in the O2 28 group. Several authors have studied the effects of the impaired lung development observed in BPD on the ISA. Rats exposed to hyperoxia (95%) for 7 days exhibit a decreased ISA compared with rats exposed to room air [11]. Baboons treated with exogenous surfactant after birth and exposed to oxygen and mechanical ventilation show a 60% decrease in the ISA [15].
The autopsies of newborn infants with BPD have indicated a reduced ISA [23], confirming the importance of analyzing this parameter in addition to Lm in experimental models of this disease.
The reduced number of alveoli in the lungs consequent to impaired lung development is a central finding in BPD. Rats exposed to FiO2 60% exhibit a 50% reduction in the number of alveoli [45]. Interrupted alveolar development has been demonstrated in premature baboons (at 71% gestation) that developed lung injury following ventilation without volutrauma [43] and in baboons after premature birth and mechanical ventilation with 80–100% FiO2 for 3 weeks [16], [17]. A study on premature lambs subjected to ventilation for 3 or 4 weeks revealed reduced alveolar septation and fewer alveoli compared with animals mechanically ventilated after birth at term [13].
Newborn mice exposed to room air or 85% oxygen for 28 days exhibit decreased alveolar septation, an increased number of terminal air spaces and increased pulmonary fibrosis in oxygen group. These findings are evident after 7 days of oxygen exposure and are more pronounced at 28 days [46].
The autopsies of premature infants with BPD demonstrated a reduced number, area and perimeter of alveoli. In this study, the finding of a reduced number of alveoli in the groups subjected to hyperoxia demonstrates that animals born at 29 days of gestation and exposed to 80% oxygen for 11 days had reduced mortality and reproduced this central characteristic of BPD [47].
Regarding the thickness of the alveolar septum, an increase was noted in the O2 28 and O2 29 groups compared with the Air 28 and Air 29 groups, although similar to the ISA, significance was reached only in the O2 28 group. Increased thickness of the alveolar septum has been described in newborn infants with fatal cases of BPD [23]. Several studies have confirmed the above-described findings in humans regarding the thickness of the alveolar septum and its relationship with BPD [16], [17].
The effect of BPD on the proportion of elastic fibers in the premature lung has been extensively studied. This study found an intense disorganization and increased proportion of elastic fibers in animals from the O2 28 group compared with the Air 28 group. The proportion of elastic fibers was smaller in the 29-day groups (Air and O2) than in the 28-day groups. Some authors have described an increased deposition of elastic fibers in the alveolar walls and an increased and abnormal distribution of elastin in the terminal respiratory units among the pathological findings of BPD in children [15], [48], [49]. Other authors have reported that the elastic tissue in children with BPD progressively increases with age [16]. Increased thickness, tortuosity and an irregular distribution of elastic fibers have been observed in children with BPD [23]. Increased elastic fiber content, uneven lung inflation, inflammation and edema have also been observed in premature lambs following prolonged mechanical ventilation for 3 to 4 weeks [13].
Regarding the deposition of collagen fibers, a quantitative increase and maturation of collagen fibers in the parenchyma occurs during normal lung development. The concentration and organization of collagen content is altered in experimental models of BPD. The proportion of collagen fibers relative to the lung parenchyma in response to oxygen exposure remains controversial in the literature. An increased deposition of collagen fibers was reported in children who are at risk for developing fatal cases of BPD [16]. A larger deposition of type I collagen fibers was found in the alveolar walls [48].
In the present study, the proportion of collagen fibers was increased in animals exposed to oxygen compared with those exposed to room air.
The major limitation of this study is that it was an experimental laboratory investigation and does not precisely reproduce the pathophysiology of BPD as it occurs in clinical practice. The lesions found in premature newborns usually occur due to mechanical ventilation with FiO2 values from 35% to 45%. This study reproduced BPD by maximizing the effects of oxygen toxicity, using a substantially higher level of FiO2 (80% and 95%), which the newborn infant does not experience. Another limitation of this study is that it did not investigate gestational age and oxygen concentration separately but determined the association of these two variables.
Understanding the pathophysiological mechanisms of BPD is vital for developing effective treatments for this disease. Hence, it is important to develop experimental models that enable researchers to better reproduce the pathology, to understand BPD more completely and to test new treatment methods.
This study has described an animal model that was developed to study BPD based on lung immaturity (29-day gestation) and oxygen exposure (80%), which can facilitate new studies involving oxygen-induced tissue injury. We have reproduced anatomopathological lesions comparable to those found in BPD in a simple and inexpensive manner, using only hyperoxia. We stress that this experimental animal model of oxygen lung injury is not superior to those that already exist, but it has technical features (including a lower cost and size of preterm animals [33±5 g]) that make it an additional option for studying BPD.
In conclusion, lung injury induced by prolonged oxygen exposure in premature rabbits born at 29 days of gestation and exposed to FiO2 = 80% resulted in a reduced number of alveoli and a trend in other histopathological findings comparable with those of BPD in humans. This group of animals had a higher survival rate compared with the group of animals born at 28 days of gestation.
Acknowledgments
We thank Marcelo S. Santos, the laboratory technician who took care of and fed the premature rabbits.
Funding Statement
The authors have no support or funding to report.
References
- 1. Saugstad OD (2010) Oxygen and oxidative stress in bronchopulmonary dysplasia. J Perinatol Med U S A 38: 571–577. [DOI] [PubMed] [Google Scholar]
- 2. Speer CP (2003) Inflammation and bronchopulmonary dysplasia. Seminars in Neonatology U S A 08: 29–38. [DOI] [PubMed] [Google Scholar]
- 3. Bancalari E, Claure N, Sosenko IRS (2003) Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Seminars in Neonatology USA 08: 63–71. [DOI] [PubMed] [Google Scholar]
- 4. Parker TA, Abman SH (2003) The pulmonary circulation in bronchopulmonary dysplasia. Seminars in Neonatology U S A 08: 51–62. [DOI] [PubMed] [Google Scholar]
- 5. Chess PR, D'Angio CT, Pryhuber GS, Maniscalco WM (2006) Pathogenesis of Bronchopulmonary Dysplasia. Semin Perinato U S A 30: 171–178. [DOI] [PubMed] [Google Scholar]
- 6. Mascaretti RS, Mataloun MMGB, Dolhnikoff M, Rebello CM (2009) Lung morphometry, collagen and elastin content: changes after hyperoxic exposure in preterm rabbits. Clinics B R A 64: 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deakins KM. Bronchopulmonary Dysplasia (2009) Respiratory Care U S A. 54: 1252–1262. [PubMed] [Google Scholar]
- 8. Chang M, Bany-Mohammed F, Kenney MC, Beharry KD (2013) Effects ofa superoxid dismutase mimetic on biomarkers of lung angiogenesis and alveolarization during hyperoxia with intermittent hypoxia. Am J Transl Res U S A 5: 594–607. [PMC free article] [PubMed] [Google Scholar]
- 9. Coalson JJ, Kuehl TJ, Escobedo MB, Hilliard JL, Smith F, et al. (1982) A baboon model of bronchopulmonary dysplasia II. pathologic features. Exp Mol Pathol U S A 37: 335–350. [DOI] [PubMed] [Google Scholar]
- 10. Guen GLC, Denis C, Franco-Montoya ML, Jarry A, Delacourt C, et al. (2008) Antenatal infection in the rabbit impairs post-natal growth and lung alveolarisation. Eur Respir J U K 32: 1520–1528. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Martinez MA, Frank L (1997) Prenatal dexamethasone administration to premature rats exposed to prolonged hyperoxia: a new rat model of pulmonary fibrosis (bronchopulmonary dysplasia). J Pediatr B R A 130: 409–416. [DOI] [PubMed] [Google Scholar]
- 12. Crapo JD, Barry BE, Foscue HA, Shelburne J (1980) Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses oxygen. Am Rev Respir Dis U S A 122: 123–143. [DOI] [PubMed] [Google Scholar]
- 13. Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, et al. (1999) Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med U S A 159: 945–958. [DOI] [PubMed] [Google Scholar]
- 14. Bland RD, Albertine KH, Pierce RA, Starcher BC (2003) Impaired alveolar development and abnormal lung elastin in preterm lambs with chronic lung injury: Potential benefits of retinol treatment. Biol Neonate U S A 84: 101–112. [DOI] [PubMed] [Google Scholar]
- 15. Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA (1999) Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med U S A 160: 1333–1346. [DOI] [PubMed] [Google Scholar]
- 16. Coalson JJ, Winter V, deLemos RA (1995) Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med U S A 152: 640–646. [DOI] [PubMed] [Google Scholar]
- 17. Coalson JJ, Winter VT, Gerstmann DR, Idell S, King RJ, et al. (1992) Pathophysiologic, morphometric, and biochemical studies of the premature baboon with bronchopulmonary dysplasia. Am Rev Respir Diseas U S A 145: 872–881. [DOI] [PubMed] [Google Scholar]
- 18. Bonikos DS, Bensch KG, Ludwin SK, Northway WH Jr (1975) Oxygen toxicity in the newborn: the effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice. Lab Invest U S A 32: 619–635. [PubMed] [Google Scholar]
- 19. Randell SH, Mercer RR, Young SL (1990) Neonatal hyperoxia alters the pulmonary alveolar and capillary structure of 40-day-old rats. Am J Pathol USA 136: 1259–1266. [PMC free article] [PubMed] [Google Scholar]
- 20. Albertine KH (2013) Progress in understanding the pathogenesis of BPD using the baboon and sheep models. Sem Perinatol U S A 37: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lim K, Wheeler KI, Gale TJ, Jackson HD, Kihlstrand JF, et al. (2014) Oxygen saturation targeting in preterm infants receiving continuous positive airway pressure J Pediatr U S A. 164: 730–736. [DOI] [PubMed] [Google Scholar]
- 22. Coalson JJ (2006) Pathology of bronchopulmonary dysplasia. Semin Perinatol U S A 30: 179–84. [DOI] [PubMed] [Google Scholar]
- 23. Margraf LR, Tomashefski JF Jr, Bruce MC, Dahms BB (1991) Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis USA 143: 391–400. [DOI] [PubMed] [Google Scholar]
- 24. Pierce RA, Albertine KH, Starcher BC (1997) Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol Lung Cell Mol Physiol U S A 272: L452–460. [DOI] [PubMed] [Google Scholar]
- 25. Bland RD, Ling CY, Albertine KH (2003) Pulmonary vascular dysfunction in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol U S A 285: L76–L85. [DOI] [PubMed] [Google Scholar]
- 26.Moore S (2011) A discussion of ethical issues in the case of baby fae. JCCC Honors Journal 11, Article 2. Available at: http://scholarspace.jccc.edu/honors_journal/vol2/iss2/2. Acessed 25 January 2014.
- 27. MacRitchie AN, Albertine KH, Sun J (2001) Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol U S A 281: L1011–1020. [DOI] [PubMed] [Google Scholar]
- 28. Bland RD, Albertine KH, Carlton DP (2005) Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med U S A 172: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frank L, Sosenko IR (1991) Failure of premature rabbits to increase antioxidant enzymes during hyperoxic exposure: increased susceptibility to pulmonary oxygen toxicity compared with term rabbits. Pediatr Res U S A 29: 292–296. [DOI] [PubMed] [Google Scholar]
- 30. Kovar J, Sly PD, Willet KE (2002) Postnatal alveolar development of the rabbit. J Appl Physiol U S A 93: 629–635. [DOI] [PubMed] [Google Scholar]
- 31. Mataloun MMGB, Rebello CM, Mascaretti RS, Dohlnikoff M, Leone CR (2006) Pulmonary responses to nutritional restriction and hyperoxia in premature rabbits. J Pediatr B R A 82: 179–185. [DOI] [PubMed] [Google Scholar]
- 32. Karnak I, Muftuoglu S, Cakar N, Cahit Tanyel F (1999) Organ growth and lung maturation in rabbit fetuses. Res Exp Med U S A 198: 277–287. [DOI] [PubMed] [Google Scholar]
- 33. Lum H, Mitzner W (1987) A species comparison of alveolar size and surface forces. J Appl Physiol U S A 62: 1865–1871. [DOI] [PubMed] [Google Scholar]
- 34. Thomson MA, Yoder BA, Winter VT, Martin H, Catland D, et al. (2004) Treatment of immature baboons for 28 days with early nasal continuous positive airway pressure. Am J Respir Crit Care Med U S A 169: 1054–1062. [DOI] [PubMed] [Google Scholar]
- 35. Nilsson R (1982) The artificially ventilated preterm rabbit neonate as experimental model of hyaline membrane disease. Acta Anaesthesiol Scand UK 26: 89–103. [DOI] [PubMed] [Google Scholar]
- 36. Speer CP (2006) Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med U S A 11: 354–362. [DOI] [PubMed] [Google Scholar]
- 37. Huang Y, Kempen MB, Munck AB, Swagemakers S, Driegen S, et al. (2012) Hypoxia-inducible factor 2a plays a critical role in the formation of alveoli and surfactant. Am J Respir Cell Mol Biol USA 46: 224–232. [DOI] [PubMed] [Google Scholar]
- 38. Walsh MC, Yao Q, Horbar JD, Carpenter JH, Lee SK, et al. (2006) Changes in the use of postnatal steroids for bronchopulmonary dysplasia in 3 large neonatal networks. Pediatrics U S A 118: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 39. Kikkawa Y, Kaibara M, Motoyama EK, Orzalesi MM, Cook CD (1971) Morphologic development of fetal rabbit lung and its acceleration with cortisol. Am J Pathol U S A 64: 423–442. [PMC free article] [PubMed] [Google Scholar]
- 40. Mataloun MMGB, Leone CR, Mascaretti RS, Dolhnikoff M, Rebello CM (2009) Effect of postnatal malnutrition on hyperoxia induced newborn lung development. Braz J Med Biol Res B R A 42: 606–613. [DOI] [PubMed] [Google Scholar]
- 41. Bremer F, Bendix I, Prager S, Van de Looji Y, Reinboth BS, et al. (2012) Interaction of Inflammation and Hyperoxia in a Rat Model of Neonatal White Matter Damage. PLOS ONE U S A 7: e49023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weinberger B, Laskin DL, Heck DE, Laskin JD (2002) Oxygen toxicity in premature infants. Toxicol Appl Pharmacol U S A 181: 60–67. [DOI] [PubMed] [Google Scholar]
- 43. Dunnil MS (1962) Quantitative methods in the study of pulmonary pathology. Thorax U K 17: 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thurlbeck WM (1967) The internal surface area of nonemphysematous lungs. Am Rev Respir Dis U S A 95: 765–773. [DOI] [PubMed] [Google Scholar]
- 45. Johnson BH, Yi M, Masood A, Belcastro R, Li J, et al. (2009) A critical role for the IL-1 receptor in lung injury induced in neonatal rats by 60% O2. Pediatr Res U S A 66: 260–265. [DOI] [PubMed] [Google Scholar]
- 46. Warner BB, Stuart LA, Papes RA, Wispé JR (1998) Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol USA 275: 110–117. [DOI] [PubMed] [Google Scholar]
- 47. Okamoto CT, Bahr JA, Silva LLG, Noronha L (2009) Histopathological and morphometric analysis in the diagnosis of “new” broncopulmonary dysplasia and clinical and pathological comparison with the classic form of the disease. J Bras Patol Med Lab B R A 45: 155–160. [Google Scholar]
- 48. Coalson JJ (2003) Pathology of new bronchopulmonary dysplasia. Seminars in Neonatology U S A 08: 73–81. [DOI] [PubMed] [Google Scholar]
- 49. Thibeault DW, Marbry SM, Ikechukww IE, Troug WE (2003) Collagen scaffolding during development and its deformation with chronic lung disease. Pediatrics U S A 111: 766–776. [DOI] [PubMed] [Google Scholar]