Abstract
Overweight and obesity are rapidly becoming a central public health challenge around the world. Previous studies have suggested that elevated Body Mass Index (BMI) might be associated with structural changes in both gray and white matter, but this association is still not well understood. The present study aimed to investigate the relationship between BMI and brain structure with a relatively large sample of young adults (N = 336) in a small age range (20 ± 1 years). VBM results showed significant negative correlations between BMI and Gray Matter Volumes (GMV) in the MCC, left OFC, and left VMPFC. There was also a significant negative correlation between BMI and white matter integrity as indexed by fractional anisotropy (FA) in bilateral cingulum. Further tractography analysis showed a significant negative correlation between BMI and the number of fibers passing the MCC region. Regression analysis showed that gray matter and white matter in these regions both contributed to the variance of BMI. These results remained significant even when analysis was restricted to the subjects with normal-weights. Finally, we found that decision making ability (as assessed by the Iowa Gambling Task) mediated the association between the structure of the MCC (a region responsible for impulse control and decision making) and BMI. These results shed light on the structural neural basis of weight variations.
Keywords: Obesity, Overweight, Midcingulate cortex (MCC), Cingulum, Voxel-based Morphometry (VBM), Diffusion Tensor Imaging (DTI), Tractography, the Iowa Gambling Task (IGT)
Introduction
Overweight and obesity are rapidly becoming a central public health challenge around the world. In the United States, nearly 65% of adults are overweight or obese (Stein and Colditz 2004). In populous China, even though the percentage of overweight and obese adults is modest (30% of adults, Wang et al. 2007b), their absolute number of over 200 million (Wu 2006) is staggering. Overweight and obesity are associated with increased risk for cardiovascular/metabolic diseases, as well as several common adult cancers (Renehan et al. 2008). Because the fundamental cause of overweight and obesity is an energy imbalance between calories consumed and calories expended, the solution to this problem appears very simple: eat in moderation and engage in regular physical activity. However, this commonsense advice is difficult to follow for many people, and the reasons involved are not clear.
There is mounting evidence that the inability to resist calorie-rich and highly appetitive food represents a special case of addiction behavior (Kelley and Berridge 2002; Rolls 2007; Trinko et al. 2007; Volkow et al. 2008). Similar to drug addiction, the loss of willpower to resist food temptation and the development of eating habits that can lead to obesity may be explained by a hypo-functioning impulse control and decision-making system in the cingulate cortex and prefrontal cortex (Bechara 2005; Noël et al. 2013). Studies (see Table S1 and Supplemental Text for a summary) have suggested that elevated BMI is associated with structural changes in both gray matter (Gunstad et al. 2008; Horstmann et al. 2011; Pannacciulli et al. 2006; Raji et al. 2010; Taki et al. 2008; Walther et al. 2010) and white matter (Pannacciulli et al. 2006; Raji et al. 2010; Walther et al. 2010; Mueller et al. 2011; Stanek et al. 2010; Xu et al. 2011). However, the results are mixed and the exact mechanism is still poorly understood. More importantly, studies of young adults are especially needed because this is the critical period for establishing good eating habits and hence for effective interventions. Nevertheless, due to the relatively delayed maturation of the prefrontal cortex (Giedd et al. 1999; Reiss et al. 1996; Sowell et al. 2003), adolescents and young adults are particularly vulnerable to disadvantageous decision making in their food choices. Studies have also suggested that healthy adolescents do not show the normal adult pattern of prefrontal responses to high calorie food images (Killgore and Yurgelun-Todd 2005), and their orbitofrontal response to high-calorie food images predicts BMI (Killgore and Yurgelun-Todd 2005). As a result, these populations are known to be engaged in unhealthy food choices (Anding et al. 2001; Johnston et al. 1998), and their regulation of food choices may be particularly challenged by the environments at school or university campuses where unhealthy food options are prominent.
The present study aimed to investigate the relationship between BMI and brain structure in a relatively large sample of normal Chinese young adults (N = 336) in a very narrow age range (20 ± 1 years). We employed voxel-based morphometry (VBM) to analyze gray matter volume (GMV), track-based spatial statistics (TBSS) to analyze white matter integrity (fractional anisotropy [FA]), and TrackVis to analyze fiber tractography. In addition, the Iowa Gambling Task (IGT) was used to assess the subjects’ decision making capacity, which might serve as one of the cognitive mechanisms linking brain structure to BMI. We hypothesized that BMI would be inversely correlated with the IGT performance as well as with the gray and white matter structures in the brain regions responsible for decision making and impulse control.
Methods
Participants
Participants of this study were 336 (141 males, 195 females) healthy Chinese college students (18–24 years old, mean age = 20.38 years, SD = 1.00). They had normal or corrected-to-normal vision, and had no history of neurological or psychiatric problems according to self-report. None of them was identified to have alcohol or nicotine dependence according to the Alcohol Use Disorders Identification Test (Saunders et al. 1993) and the Fagerström Test for Nicotine Dependence (Heatherton et al. 1991). They also scored within the normal range on the Beck Depression Inventory (Beck et al. 1996) and Beck Anxiety Inventory (Beck and Steer 1990). The Chinese version of these tests was administered by a trained research assistant. Informed written consent was obtained from all participants. The study was approved by the Beijing Normal University (BNU) Institutional Review Board.
BMI Measurement
Subjects reported their weight and height in a self-report questionnaire, and their BMIs were calculated as weight (kg) / [height (m)]2. Self-reported data on weight and height have been used by previous large-scale studies on body mass (van Strien et al. 2010; Edwards et al. 2012; Yang et al. 2010; Lim et al. 2011; Strauss 1999; Kuczmarski et al. 2001; Goodman et al. 2000) and proved to be reliable in calculating BMI, with a high correlation (r = .92) between BMI calculated from self-reports and that from actual measurements (Goodman et al. 2000). Furthermore, all BNU students including all of our participants were given an annual physical examination at the beginning of the academic year in September and they were informed of their height and weight. Self-report data on height and weight were collected in December.
The IGT
A computerized version of the IGT (Bechara et al. 2000b) was used in the present study. It was designed to assess decision making under ambiguity and risk (Bechara et al. 1994; Bechara et al. 1997; Bechara et al. 2000b; Bechara et al. 2005). To motivate subjects, they were informed that the amount of their winning would be converted into real money. Subjects were asked to select one card at a time (100 trials in total) from one of the four decks (labeled A, B, C, and D). As described in previous studies (Bechara et al. 2000b; He et al. 2010; He et al. 2012; Koritzky et al. 2013) and the IGT manual (PAR, Inc.), two of the decks were disadvantageous because they yielded high immediate gain but a greater loss in the long run (i.e., net loss of 250 yuan on average over 10 cards), and two decks were advantageous because they yielded lower immediate gain but a smaller loss in the long run (i.e., net gain of 250 yuan on average over 10 cards). The IGT score [calculated by subtracting the total number of selections of the disadvantageous decks (A and B) from the total number of selections of the advantageous decks (C and D)] for the first 40 and last 60 trials were calculated to represent performance in decision under ambiguity and decision under risk respectively (Bechara et al. 1997). Higher IGT scores indicated superior performance.
MRI Protocol
One high-resolution structural MRI measurement and one diffusion tensor procedure were performed on each subject in a half hour MRI session on a 3T Siemens MAGNETOM Trio system (Siemens Medical Systems, Iselin, NJ) with Total Imaging Matrix (TIM) at BNU Imaging Center for Brain Research. A T1-weighted 3D-Magnetization Prepared RApid Gradient Echo (MPRAGE) sequence was used to cover the whole brain (TR/TE = 2530/3.39 ms, flip angel = 7°, matrix = 256 × 256, 128 sagittal slices, 1.33 mm thickness). The diffusion-tensor data for each subject were acquired using a diffusion-weighted, single-shot, spin-echo, EPI sequence (TR/TE = 7200/104ms, matrix = 128 × 128, 49 axial slices, 2.5 mm slice thickness, b-value = 1000 s/mm2) in 64 directions. A dual spin-echo technique combined with bipolar gradients was employed to minimize the geometric distortion induced by eddy currents.
VBM Analysis
Structural MRI data were analyzed with FSL-VBM, an optimized voxel-based morphometry analysis toolbox (Ashburner and Friston 2000; Good et al. 2001) implemented in FSL (Smith et al. 2004). This approach requires no prior information about the location of possible differences in gray matter, and has been proven to be not operator-dependent. First, structural images were extracted using BET (Smith 2002). Next, tissue-type segmentation was carried out using FAST4 (Zhang et al. 2001). The resulting gray-matter partial volume images were then aligned to the gray-matter template in the MNI152 standard space using the affine registration tool FLIRT (Jenkinson and Smith 2001; Jenkinson et al. 2002), followed by nonlinear registration using FNIRT (Andersson et al. 2007b, a), which used a b-spline representation of the registration warp field (Rueckert et al. 1999). The spatially normalized images were then averaged to create a study-specific template, to which the native gray matter images were registered again using both linear and nonlinear algorithms as described above. The registered partial volume images were then modulated by dividing them with the Jacobian of the warp field to correct for local expansion or contraction. The modulated segmented images, which represent the GMV, were then smoothed with an isotropic Gaussian kernel with a 3 mm standard deviation. Finally, a voxel-wise general linear model was used to examine the correlation between the resulting gray matter images and BMI. Non-parametric permutation methods (Randomise v2.1 in FSL) were used for inference on statistic maps (Nichols and Holmes 2002). The null distribution at each voxel was constructed using 10,000 random permutations of the data. Threshold-free cluster enhancement (TFCE) was used to correct for multiple comparisons across the whole brain. The mean GMV in each significant cluster was then extracted for each individual.
TBSS Analysis
The DTI data were processed by FMRIB’s Diffusion Toolbox (FDT) implemented in FSL. Diffusion data were corrected for eddy currents and possible head motion. Images were then skull-stripped (Smith 2002), aligned to MNI space using FNIRT (Andersson et al. 2007b, a), and resampled to 1 mm3. FA was reconstructed by fitting a diffusion tensor model at each voxel. Voxelwise statistical analysis of the FA data was carried out using TBSS (Smith et al. 2006), part of FSL. The mean FA image was created and thinned to create a mean FA skeleton that represented the centers of all tracts common to the group. Each subject’s aligned FA data were then projected onto this skeleton and the resulting data were fed into voxelwise cross-subject statistics. Finally, the correlation between the resulting skeletonized FA images and BMI was computed using non-parametric permutation methods (Randomise v2.1 in FSL, Nichols and Holmes 2002). The null distribution at each voxel was constructed using 10,000 random permutations of the data. TFCE was used to correct for multiple comparisons across the whole brain. The mean FA value in each significant cluster was then extracted for each individual.
Tractography Analysis
FDT-preprocessed DTI data were then fed into Diffusion Toolkit (version 0.6.2.1, Wang et al. 2007a) and TrackVis (version 0.5.2.1, both available at http://www.trackvis.org/) to do fiber tracking. TrackVis performed automatic fiber tracking to reconstruct fibers across the whole WM by tracking fibers from each voxel in the brain. Basically, fibers were reconstructed by TrackVis along the principal eigenvector of each voxel’s diffusion tensor. Tracking termination criteria were angle > 45° and FA < 0.2. Fiber tracking was performed successively in each subject’s native space. The cluster in the MCC region revealed by VBM analysis was back-projected to each subject’s native space and served as the seed region for fiber tracking. Fiber tracking was done only in the MCC because the other two regions (i.e., left OFC and left VMPFC) revealed by VBM were relatively small for fiber tracking. The number of fibers passing this region with length > 5 mm was counted and recorded for each subject. At the same time, all fibers with length > 5 mm in the whole brain were also counted and recorded, and served as a covariate for later analysis. BMI was then correlated with the number of fibers passing the MCC region while excluding the influence of total number of tracks.
Mediation Analysis
To test the hypothesis that brain structure affects decision making (IGT) which in turn affects BMI, a simple mediation analysis was performed as described by Preacher and Hayes (2008, 2004). SPSS macro INDIRECT (http://www.afhayes.com/public/indirect.zip) was used to estimate the path coefficients and generate bootstrap confidence intervals for the total and specific indirect effects of independent variable (X) on dependent variable (Y) through the mediator variable (M). The size and significance of the indirect effects were reported as suggested by Rucker et al. (2011).
Results
The mean BMI for our sample was 20.4 kg/m2 (SD = 2.2), ranging from 16.3 to 27.8. According to WHO BMI classification, there were 70 (54 female) underweight participants (BMI < 18.5), 251 (136 female) normal weight participants (18.5 ≤ BMI < 25), and 15 (5 female) overweight participants (BMI ≥ 25). The BMI distribution in the present study was comparable with that in other studies of Chinese college students (Sakamaki et al. 2005; Ge 1997). Males (21.01 ± 2.28) had significantly higher BMI than females (19.92 ± 2.05; t(334) = 4.57, p < .01), consistent with previous findings in healthy young Chinese (Lei et al. 2005).
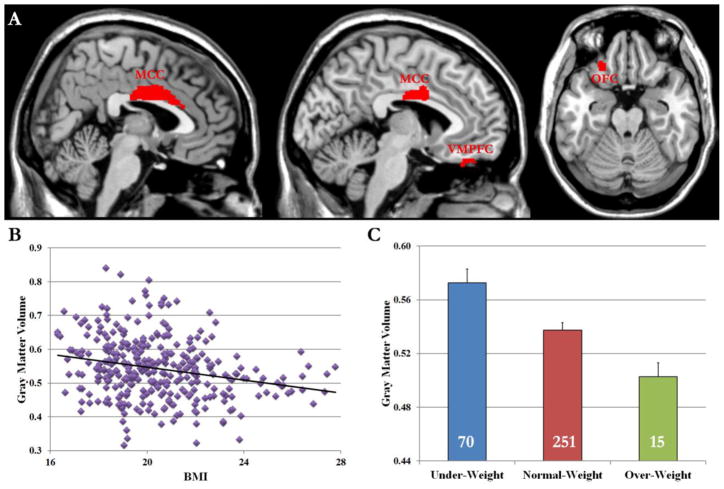
VBM analysis revealed that BMI was negatively correlated with GMV in the midcingulate cortex (MCC, local maxima in MNI coordinates x, y, z = 0, −10, 32, Table 1 and Fig. 1A). Further analysis suggested that male (r(141) = −.263, p < .01) and female (r(195) = −.185, p < .01) participants showed similar, negative correlations. The correlation (Fig. 1B) remained significant when we limited the sample to the normal-weight group (r (251) = −.18, p < .01). The GMV in the MCC region was highest in underweight group, lowest in the over-weight group, and intermediate in the normal-weight group (Fig. 1C). Two small clusters of gray matter in the left orbital frontal cortex (OFC, MNI coordinates −24, 30, −24), and the left ventromedial prefrontal cortex (VMPFC, MNI coordinates −6, 36, −26) also showed negative correlations with BMI in the VBM analysis (Table 1).
Table 1.
Summary of VBM and TBSS results
| Brain region | Voxels | MNI x | MNI y | MNI z | TFCE corrected p |
|---|---|---|---|---|---|
| VBM results for gray matter | |||||
| MCC | 629 | 0 | −10 | 32 | .009 |
| L OFC | 74 | −24 | 30 | −24 | .030 |
| L VMPFC | 25 | −6 | 36 | −26 | .040 |
| TBSS results for white matter | |||||
| R Cingulum | 194 | 17 | 24 | 22 | .008 |
| L Cingulum | 139 | −10 | 14 | 24 | .004 |
MNI: Montreal Neurological Institute coordinates; TFCE: Threshold-free cluster enhancement; VBM: Voxel-based morphometry; MCC: Midcingulate cortex; L: Left; OFC: Orbitofrontal cortex; VMPFC: Ventromedial prefrontal cortex; TBSS: Tract-based spatial statistics.
Fig. 1.
(A) VBM analysis revealed that the GMVs in the MCC, left VMPFC, and left OFC regions were negatively correlated with BMI. (B) Scatter plot shows the pattern of correlation between GMV in the MCC and BMI. (C) ROI analysis suggested that GMV in the MCC region was highest in the under-weight group, lowest in the over-weight group, and intermediate in the normal-weight group. The number on each bar denotes the number of subjects in each group. Error bars indicate standard errors.
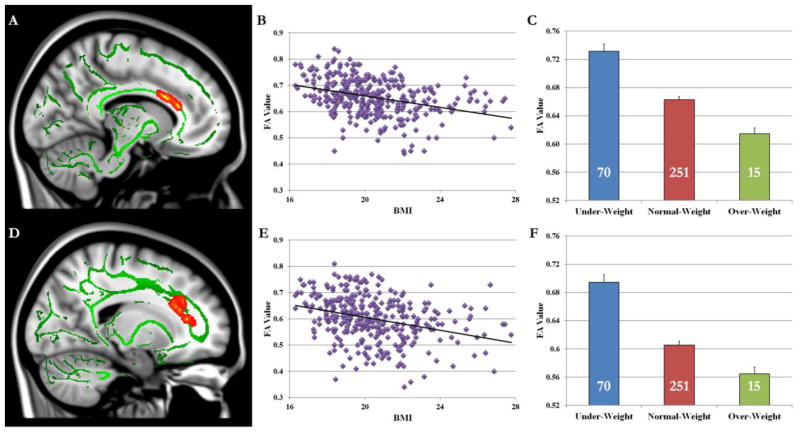
TBSS analysis showed that BMI was negatively correlated with white matter integrity (i.e., FA value) in the left cingulum (MNI coordinates −10, 14, 24, Table 1 and Fig. 2A) and right cingulum (MNI coordinates 17, 24, 22, Table 1 and Fig. 2D). Further analysis revealed that male and female participants showed similar results in both left (male: r(141) = −.342, p < .01; female: r(195) = −.315, p < .01) and right (male: r(141) =−.314, p < .01; female: r(195) = −.272, p < .01) cingulum. These correlations (Fig. 2B and Fig. 2E) remained significant when we limited the sample to normal-weight subjects (left cingulum: r (251) = −.21, p < .001; right cingulum: r (251) = −.22, p < .001). FA value in both cingulum regions (Fig. 2C and Fig. 2F) was highest in the under-weight group, lowest in the over-weight group, and intermediate in the normal-weight group.
Fig. 2.
TBSS analysis revealed that FA values in both left (A) and right (D) cingulum were negatively correlated with BMI. Scatter plots show the pattern of correlations between FA values in left (B) and right (E) cingulum and BMI. ROI analysis suggested that FA values in left (C) and right (F) cingulum were highest in the under-weight group, lowest in the over-weight group, and intermediate in the normal-weight group. The number on each bar denotes the number of subjects in each group. Error bars indicate standard errors.
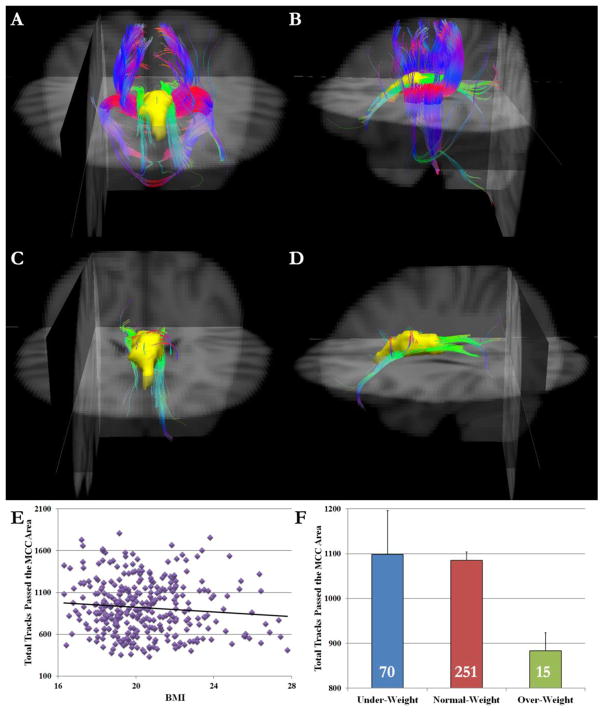
To better understand our TBSS results, white matter tractography was carried out for each subject using the identified MCC region as the seed region. Individuals differed greatly in the number of tracks passing the MCC region. Whereas some participants showed many tracks passing the MCC region (Fig. 3A and Fig. 3B), others showed only a few tracks (Fig. 3C and Fig. 3D). There was no gender difference in how many tracks passed the MCC region (t(334) = 1.03, p = .30). Results showed that BMI was negatively correlated with the total tracks passing the MCC seed region after controlling for the total number of tracks of the whole brain (partial r(333) = −.164, p < .01, Fig. 3E). The correlation remained significant when we limited our sample to the normal-weight subjects (r(248) = −.110, p < .05). As showed in Fig. 3F, the overweight group showed fewer tracks passing the MCC region than both the under-weight (t(83) = −2.18, p < .05) and normal-weight groups (t(264) = −2.20, p < .05), with no difference between the latter two groups (t(319) = .84, p = .40).
Fig. 3.
Anterior (A) and left lateral view (B) of a sample participant who had many tracks passing the MCC region. In contrast, (C) and (D) show a sample participant who had few tracks passing the MCC region. The MCC ROI is shown in yellow. Red indicates the medio-lateral plane, green the dorso-ventral orientation, and blue the rostro-caudal direction. Background images are in radiological orientation. (E) Scatter plot shows the negative correlation pattern between BMI and total tracks that passed the MCC region. (F) The overweight group showed fewer tracks passing the MCC region than both under-weight and normal-weight groups. The number on each bar denotes the number of subjects in each group. Error bars indicate standard errors.
Further regression analysis suggested that all three factors described above (MCC GMV, bilateral cingulum FA, and number of tracks passing the MCC region) made unique contributions to BMI in our sample (F(4,331) = 23.79, p < .001, R2 = .22). The regression coefficients were all negative: The MCC GMV (β = −.20, t = −4.06, p < .001), left (β = −.24, t = −3.94, p < .001) and right cingulum FA (β = −.20, t = −3.33, p = .001), and the number of tracks passing the MCC region (β = −.13, t = −2.66, p = .008). Results were similar even when we limited the sample to the normal-weight subjects (F(4,246) = 21.76, p < .001, R2 = .19).
Finally, BMI was negatively correlated with the IGT score in the first 40 trials (i.e., decisions under ambiguity; r(336) = −.13, p < .05) but not with IGT score in the last 60 trials (i.e., decisions under risk; r(336) = .01, p = .85). The correlation between BMI and IGT score in the first 40 trials was significant even when the sample was limited to the normal-weight group (r(251) = −.11, p < .05). These results were compatible with a previous report (Horstmann et al. 2011). Mediation analysis suggested that the IGT score in the first 40 trials mediated the association between GMV in the MCC region and BMI (indirect effect = −.23, p < .05) as well as between the number of white matter tracks passing this region and BMI (indirect effect = −.27, p < .05).
Discussion
In this study, we found moderate but statistically significant correlations between BMI and GMV in the MCC region as well as GMVs in the left OFC and VMPFC. White matter analysis also revealed significant negative correlations between BMI and white matter integrity (FA) in bilateral cingulum. Further tractography analysis showed a significant negative correlation between BMI and number of fibers passing the MCC region. Furthermore, gray matter and white matter both contributed to the variance of BMI. Finally, BMI was inversely correlated with IGT performance, and the IGT performance mediated the correlation between MCC morphormetry and BMI. Taken together, these results suggested that both gray and white matter structures in brain regions responsible for decision making and impulse control contributed to individual difference in BMI. The results remained significant when we only considered the normal-weight group, so the results of the present study shed light on the structural neural basis of normal variations in BMI as well as overweight.
The IGT is a task with very salient immediate rewards conflicting with the achievement of long-term goals, which is similar to everyday choice between tasty food (i.e., rich in fat and sugar) and healthy food. We found a negative correlation between BMI and IGT score in the first 40 trials. In the early phase of IGT (approximately the first 40 trials), subjects do not have yet explicit knowledge about the contingencies in the task (Brand et al. 2007). At this stage, the decisions are made under ambiguity and are largely guided by implicit information, as opposed to the decisions during the later trials of the task, namely decisions under risk (Brand et al. 2007). Studies have shown that this earlier stage (but not the later stage, i.e., decision making under risk) was related to problem gambling severity (Brevers et al. 2012), and 5-HTTLPR genotype (He et al. 2010). In line with a previous study (Brogan et al. 2011), our result suggest that people who are overweight tended to show deficits in decision making and impulse control ability. This finding supports the functional relevance of the observed correlation between brain structure and BMI. We found that BMI was inversely correlated with GMV in the MCC, OFC, and VMPFC, all of which have been shown to be important in decision making and impulse control based on lesion studies (Bechara 2005; Bechara et al. 2000a; Bechara and Damasio 2005; Bechara et al. 1994) and neuroimaging studies (Li et al. 2009; Xue et al. 2009). In the context of eating behavior, poor decision making and impulse control may facilitate overeating, especially when faced with a constant supply of highly palatable food.
As mentioned above, we found that the GMVs in the MCC, OFC, and VMPFC regions were negatively correlated with BMI. The MCC, along with the anterior cingulate cortex (ACC), have long been suggested to play a vital role in impulse control from both monkey and human studies (for reviews, see Shackman et al. 2011; Cole et al. 2009). The revealed regions are located at the ventral MCC, both anterior and posterior MCC according to the cingulate 4 region model (Yu et al. 2011; Destrieux et al. 2010; Palomero-Gallagher et al. 2009; Vogt 2009; Vogt 2005). These regions have been demonstrated to be involved in many different functions, including negative affect, pain, and cognitive control (see Shackman et al. 2011 for a review). The MCC is also the main hub for the impulse control network, which connects to the OFC and VMPFC - two key regions known to be important for neural mechanisms of decision making. The impulse control system controls the basic impulses generated by food cues and allows more flexible pursuit of long-term goals. Studies have suggested that obese subjects did poorly in impulse control tasks (Elias et al. 2003; Horstmann et al. 2011). They also showed deficits in decision making tested by the IGT (Brogan et al. 2011; Brogan et al. 2010). Functional MRI studies also showed that lean individuals had less activity in the impulse control/decision making network than obese individuals in response to food cues (Batterink et al. 2010; Le et al. 2006; Le et al. 2007). Consistent with these studies, the present study found BMI inversely correlated with GMV in regions important for impulse control (i.e., the MCC) and decision making (i.e., left OFC and VMPFC) in healthy Chinese young adults, suggesting that the same impulse control and decision making deficits may have emerged even before they became obese.
While gray matter reflects the neuronal cell bodies, white matter reflects myelinated axon tracts connecting different neurons. In the present study, the TBSS results revealed that BMI was inversely correlated with FA value in bilateral cingulum, which is a medial associative bundle that runs within the cingulate gyrus courses rostral, dorsal and caudal to the corpus callosum (Catani and Thiebaut de Schotten 2008). Anatomically, it contains fibers connecting the anterior temporal gyrus, orbitofrontal cortex, medial frontal cortex, parietal lobe, occipital lobe, and temporal lobe to different portions of the cingulate cortex (Catani and Thiebaut de Schotten 2008). Previous studies suggested that the cingulum connected several brain regions sub-serving neural mechanisms of attention, memory and emotion (Rudrauf et al. 2008). Using the MCC region as an ROI, DTI tractography analysis showed the number of fibers passing the MCC region was inversely correlated with BMI. The major difference between tracks of low- and high-BMI individuals are in the connections between the MCC and subcortical regions (Figure 3), suggesting somewhat fewer connections (i.e., neuronal axons) between cortical and subcortical regions in higher BMI individuals. Fewer such axons might lead to weaker control over subcortical regions. Consistent with this finding, studies have suggested that obese and overweight individuals are unable to suppress the urge to consume high-calorie food (Kelley and Berridge 2002; Rolls 2007; Trinko et al. 2007; Volkow et al. 2008). The failure of this impulse control system in obese and overweight individuals might be driven by the hyperactive subcortical impulsive neural system to instigate behavioral response to food.
The present study had several limitations. First, height and weight were self-reported rather than measured. Although other large-scale studies also used self-reported data (van Strien et al. 2010; Edwards et al. 2012; Yang et al. 2010; Lim et al. 2011; Strauss 1999; Kuczmarski et al. 2001; Goodman et al. 2000) and there is a high correlation (r = .92) between BMI calculated from self-reports and that from actual measurements (Goodman et al. 2000), it would still be better to measure weight and height during the experiment. Second, BMI is only a rough measure of body fat. Recent VBM studies have shown the complexity of obesity-related brain abnormalities (Kurth et al. 2012; Weise et al. 2013) and the resulting limitation of BMI as a surrogate of obesity. Third, the present study only looked at the GMV. Future studies should also consider cortical thickness in addition to GMV to better understand how gray matter influences BMI (Erpelding et al. 2012).
The use of a normal healthy sample to investigate the neuroanatomical basis of BMI is both a strength and a weakness. On the positive side, this study suggested that even within the normal range of BMI, there is a small but significant gradual change in gray and white matter information in the MCC region. However, the BMI in the present study had a narrow range with only 15 participants being overweight and none being obese. These results may or may not be generalizable to other populations (e.g., clinical samples, other ethnic groups).
In conclusion, the present study investigated the correlation between BMI and brain structure in a large sample of Chinese young adults. We found that BMI was inversely correlated with the IGT score in the first 40 trials; GMV in the MCC, left OFC, and left VMPFC; white matter integrity in bilateral cingulum; and white matter tracks connecting MCC and subcortical regions. Moreover, IGT score mediated the correlation between brain structure and BMI. Taken together, these results suggested that the gray and white matter in the MCC region, which is responsible for decision making and impulsive control, both made small but significant contributions to BMI in healthy Chinese college students. These results shed light on the neuroanatomical basis of individual differences in BMI.
Supplementary Material
Acknowledgments
This research was supported by the 111 Project (B07008) to Qi Dong, the National Science Foundation of China (31130025) to Gui Xue, and by research grants from NIDA R01DA023051 and NCI R01CA152062 to Antoine Bechara. We would also like to thank all lab members who helped with data collection.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB technical report TR07JA1. 2007a from wwwfmriboxacuk/analysis/techrep.
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2. 2007b from wwwfmriboxacuk/analysis/techrep.
- Anding JD, Suminski RR, Boss L. Dietary intake, body mass index, exercise, and alcohol: are college women following the dietary guidelines for Americans? Journal of American College Health. 2001;49 (4):167–171. doi: 10.1080/07448480109596299. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Voxel-based morphometry--the methods. Neuroimage. 2000;11 (6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52 (4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8 (11):1458–1463. doi: 10.1038/nn1584. nn1584 [pii] [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52 (2):336–372. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50 (1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000a;10 (3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275 (5304):1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9 (4):159–162. doi: 10.1016/j.tics.2005.02.002. discussion 162–154 :S1364-6613(05)00033-1 [pii] [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000b;123 ( Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Beck Depression Inventory—II M. San Antonio. TX: The Psychological Corporation; 1996. [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck anxiety inventory. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. J Clin Exp Neuropsychol. 2007;29 (1):86–99. doi: 10.1080/13803390500507196. TKJ8J7U371025W5P [pii] [DOI] [PubMed] [Google Scholar]
- Brevers D, Cleeremans A, Goudriaan AE, Bechara A, Kornreich C, Verbanck P, Noel X. Decision making under ambiguity but not under risk is related to problem gambling severity. Psychiatry Res. 2012;200 (2–3):568–574. doi: 10.1016/j.psychres.2012.03.053. [DOI] [PubMed] [Google Scholar]
- Brogan A, Hevey D, O’Callaghan G, Yoder R, O’Shea D. Impaired decision making among morbidly obese adults. Journal of psychosomatic research. 2011;70 (2):189–196. doi: 10.1016/j.jpsychores.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Brogan A, Hevey D, Pigntti R. Anorexia, bulimia, and obesity: shared decision making deficits on the Iowa Gambling Task (IGT) Journal of the International Neuropsychological Society. 2010;16 (4):711. doi: 10.1017/S1355617710000354. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44 (8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Cole MW, Yeung N, Freiwald WA, Botvinick M. Cingulate cortex: Diverging data from humans and monkeys. Trends in neurosciences. 2009;32 (11):566–574. doi: 10.1016/j.tins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53 (1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TL, Velez Edwards DR, Villegas R, Cohen SS, Buchowski MS, Fowke JH, Schlundt D, Long JR, Cai Q, Zheng W, Shu XO, Hargreaves MK, Jeffrey S, Williams SM, Signorello LB, Blot WJ, Matthews CE. HTR1B, ADIPOR1, PPARGC1A, and CYP19A1 and obesity in a cohort of Caucasians and African Americans: an evaluation of gene-environment interactions and candidate genes. Am J Epidemiol. 2012;175 (1):11–21. doi: 10.1093/aje/kwr272. kwr272 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias M, Elias P, Sullivan L, Wolf P, D’agostino R. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. International journal of obesity. 2003;27 (2):260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153 (8):1602–1609. doi: 10.1016/j.pain.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Ge K. Body mass index of young Chinese adults. Asia Pacific J Clin Nutr. 1997;6 (3):175–179. [PubMed] [Google Scholar]
- Giedd J, Blumenthal J, Jeffries N, Castellanos F, Liu H, Zijdenbos A, Paus T, Evans A, Rapoport J. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2:861–862. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Good C, Johnsrude I, Ashburner J, Henson R, Friston K, Frackowiak R. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14 (1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106 (1):52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E. Relationship between body mass index and brain volume in healthy adults. The International journal of neuroscience. 2008;118 (11):1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- He Q, Xue G, Chen C, Lu Z, Dong Q, Lei X, Ding N, Li J, Li H, Chen C, Li J, Moyzis RK, Bechara A. Serotonin transporter gene-linked polymorphic region (5-HTTLPR) influences decision making under ambiguity and risk in a large Chinese sample. Neuropharmacology. 2010;59 (6):518–526. doi: 10.1016/j.neuropharm.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Xue G, Chen C, Lu ZL, Lei X, Liu Y, Li J, Zhu B, Moyzis RK, Dong Q, Bechara A. COMT Val158Met polymorphism interacts with stressful life events and parental warmth to influence decision making. Scientific Reports. 2012;2:677. doi: 10.1038/srep00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86 (9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Horstmann A, Busse FP, Mathar D, Muller K, Lepsien J, Schlogl H, Kabisch S, Kratzsch J, Neumann J, Stumvoll M, Villringer A, Pleger B. Obesity-Related Differences between Women and Men in Brain Structure and Goal-Directed Behavior. Front Hum Neurosci. 2011;5:58. doi: 10.3389/fnhum.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17 (2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5 (2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnston CS, Solomon RE, Corte C. Vitamin C status of a campus population: college students get a C minus. Journal of American College Health. 1998;46 (5):209–213. doi: 10.1080/07448489809600224. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. The Journal of Neuroscience. 2002;22 (9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. Neuroreport. 2005;16 (8):859–863. doi: 10.1097/00001756-200505310-00016. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Developmental psychobiology. 2005;47 (4):377–397. doi: 10.1002/dev.20099. [DOI] [PubMed] [Google Scholar]
- Koritzky G, He Q, Xue G, Wong S, Xiao L, Bechara A. Processing of time within the prefrontal cortex: Recent time engages posterior areas whereas distant time engages anterior areas. NeuroImage. 2013;72:280–286. doi: 10.1016/j.neuroimage.2013.01.056. [DOI] [PubMed] [Google Scholar]
- Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Journal of the American Dietetic Association. 2001;101 (1):28. doi: 10.1016/S0002-8223(01)00008-6. [DOI] [PubMed] [Google Scholar]
- Kurth F, Levitt JG, Phillips OR, Luders E, Woods RP, Mazziotta JC, Toga AW, Narr KL. Relationships between gray matter, body mass index, and waist circumference in healthy adults. Human Brain Mapping. 2012 doi: 10.1002/hbm.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DSNT, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, Krakoff J. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. The American journal of clinical nutrition. 2006;84 (4):725–731. doi: 10.1093/ajcn/84.4.725. [DOI] [PubMed] [Google Scholar]
- Le DSNT, Pannacciulli N, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Krakoff J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. The American journal of clinical nutrition. 2007;86 (3):573–579. doi: 10.1093/ajcn/86.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, Liu M, Chen X, Deng F, Lv J, Jian W, Xu H, Tan L, Yang Y, Wang Y. Relationship of total body fatness and five anthropometric indices in Chinese aged 20–40 years: different effects of age and gender. European journal of clinical nutrition. 2005;60 (4):511–518. doi: 10.1038/sj.ejcn.1602345. [DOI] [PubMed] [Google Scholar]
- Li X, Lu Z-L, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Human Brain Mapping. 2009;31 (3):410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JS, Son HK, Park SK, Jacobs DR, Jr, Lee DH. Inverse associations between long-term weight change and serum concentrations of persistent organic pollutants. Int J Obes (Lond) 2011;35 (5):744–747. doi: 10.1038/ijo.2010.188. [DOI] [PubMed] [Google Scholar]
- Mueller K, Anwander A, Möller HE, Horstmann A, Lepsien J, Busse F, Mohammadi S, Schroeter ML, Stumvoll M, Villringer A. Sex-dependent influences of obesity on cerebral white matter investigated by diffusion-tensor imaging. PLoS One. 2011;6 (4):e18544. doi: 10.1371/journal.pone.0018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Holmes A. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15 (1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Current opinion in neurobiology. 2013 doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. Receptor architecture of human cingulate cortex: Evaluation of the four-region neurobiological model. Human brain mapping. 2009;30 (8):2336–2355. doi: 10.1002/hbm.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le DSNT, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31 (4):1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers. 2004;36 (4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior research methods. 2008;40 (3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31 (3):353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children A volumetric imaging study. Brain. 1996;119 (5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. The Lancet. 2008;371 (9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- Rolls E. Understanding the mechanisms of food intake and obesity. Obesity Reviews. 2007;8 (s1):67–72. doi: 10.1111/j.1467-789X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Rucker DD, Preacher KJ, Tormala ZL, Petty RE. Mediation analysis in social psychology: Current practices and new recommendations. Social and Personality Psychology Compass. 2011;5 (6):359–371. [Google Scholar]
- Rudrauf D, Mehta S, Grabowski TJ. Disconnection’s renaissance takes shape: Formal incorporation in group-level lesion studies. Cortex. 2008;44 (8):1084–1096. doi: 10.1016/j.cortex.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. Medical Imaging, IEEE Transactions on. 1999;18 (8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Sakamaki R, Toyama K, Amamoto R, Liu C-J, Shinfuku N. Nutritional knowledge, food habits and health attitude of Chinese university students -a cross sectional study. Nutrition Journal. 2005;4 (1):4. doi: 10.1186/1475-2891-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, Delafuente JR, Grant M. Development of the Alcohol-Use Disorders Identification Test (Audit) - Who Collaborative Project on Early Detection of Persons with Harmful Alcohol-Consumption .2. Addiction. 1993;88 (6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12 (3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17 (3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols T, Mackay C, Watkins K, Ciccarelli O, Cader M, Matthews P. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31 (4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen-Berg H, Bannister P, De Luca M, Drobnjak I, Flitney D. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature neuroscience. 2003;6 (3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, Gunstad JJ. Obesity Is Associated With Reduced White Matter Integrity in Otherwise Healthy Adults*. Obesity. 2010;19 (3):500–504. doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- Stein CJ, Colditz GA. The epidemic of obesity. Journal of Clinical Endocrinology & Metabolism. 2004;89 (6):2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- Strauss RS. Comparison of measured and self-reported weight and height in a cross-sectional sample of young adolescents. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1999;23 (8):904. doi: 10.1038/sj.ijo.0800971. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity. 2008;16 (1):119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- Trinko R, Sears RM, Guarnieri DJ, DiLeone RJ. Neural mechanisms underlying obesity and drug addiction. Physiology & behavior. 2007;91 (5):499. doi: 10.1016/j.physbeh.2007.01.001. [DOI] [PubMed] [Google Scholar]
- van Strien T, van der Zwaluw CS, Engels RC. Emotional eating in adolescents: a gene (SLC6A4/5-HTT) - depressive feelings interaction analysis. J Psychiatr Res. 2010;44 (15):1035–1042. doi: 10.1016/j.jpsychires.2010.03.012. S0022-3956(10)00087-7 [pii] [DOI] [PubMed] [Google Scholar]
- Vogt B. Cingulate neurobiology and disease. Oxford University Press; 2009. [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6 (7):533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363 (1507):3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31 (7):1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen A, Wedeen V. Diffusion toolkit: a software package for diffusion imaging data processing and tractography. 2007a:3720. [Google Scholar]
- Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007b;31 (1):177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- Weise CM, Thiyyagura P, Reiman EM, Chen K, Krakoff J. Fat-Free Body Mass but not Fat Mass is Associated with Reduced Gray Matter Volume of Cortical Brain Regions Implicated in Autonomic and Homeostatic Regulation. NeuroImage. 2013;64:712–721. doi: 10.1016/j.neuroimage.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Overweight and obesity in China. Bmj. 2006;333 (7564):362–363. doi: 10.1136/bmj.333.7564.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li Y, Lin H, Sinha R, Potenza MN. Body mass index correlates negatively with white matter integrity in the fornix and corpus callosum: A diffusion tensor imaging study. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cereb Cortex. 2009;19 (5):1019–1027. doi: 10.1093/cercor/bhn147. bhn147 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, Madden PA, Heath AC, Martin NG, Montgomery GW, Goddard ME, Visscher PM. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42 (7):565–569. doi: 10.1038/ng.608. ng.608 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Zhou Y, Liu Y, Jiang T, Dong H, Zhang Y, Walter M. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage. 2011;54 (4):2571–2581. doi: 10.1016/j.neuroimage.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20 (1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.