Abstract
Background & Aims
Fibroblast Growth Factors (FGFs) promote the proliferation and survival of hepatic progenitor cells (HPCs) via AKT-dependent β-catenin activation. Moreover, the emergence of hepatocytes expressing the HPC marker A6 during 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)-induced liver injury is mediated partly by FGF and β-catenin signaling. Herein, we investigate the role of FGF signaling and AKT-mediated β-catenin activation in acute DDC liver injury.
Methods
Transgenic mice were fed DDC chow for 14 days concurrent with either Fgf10 over-expression or inhibition of FGF signaling via expression of soluble dominant-negative FGF Receptor (R)-2IIIb.
Results
After 14 days of DDC treatment, there was an increase in periportal cells expressing FGFR1, FGFR2, and AKT-activated phospho-Serine 552 (pSer552) β-CATENIN in association with up-regulation of genes encoding FGFR2IIIb ligands, Fgf7, Fgf10, and Fgf22. In response to Fgf10 over-expression, there was an increase in the number of pSer552-β-CATENIN(positive)+ive periportal cells as well as cells co-positive for A6 and hepatocyte marker, Hepatocyte Nuclear Factor-4α (HNF4α). A similar expansion of A6+ive cells was observed after Fgf10 over-expression with regular chow and after partial hepatectomy during ethanol toxicity. Inhibition of FGF signaling increased the periportal A6+iveHNF4α+ive cell population while reducing centrolobular A6+ive HNF4α+ive cells. AKT inhibition with Wortmannin attenuated FGF10-mediated A6+iveHNF4α+ive cell expansion. In vitro analyses using FGF10 treated HepG2 cells demonstrated AKT-mediated β-CATENIN activation but not enhanced cell migration.
Conclusion
During acute DDC treatment, FGF signaling promotes the expansion of A6-expressing liver cells partly via AKT-dependent activation of β-CATENIN expansion of A6+ive periportal cells and possibly by reprogramming of centrolobular hepatocytes.
Keywords: Fibroblast Growth Factor 10, hepatic progenitor cells, DDC, beta-catenin
Introduction
Human diseases such as congenital biliary atresia and primary biliary cirrhosis are marked pathologically with periportal fibrosis surrounding expanding biliary ductular reactions, the cells of which exhibit characteristics consistent with those of epithelial progenitor and stem cells [1, 2]. Treatment of rodents with 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) induces an analogous fibrosis with ductular reactions populated with hepatic progenitor cells (HPCs) expressing SOX9 [3], 1C3 [3], PROMININ-1 (CD133) [4], and A6 [5]. Recent lineage tracing studies suggest that the HPC transdifferentiation toward a hepatocyte cell fate during DDC injury is negligible [6-8]. Instead, hepatocytes undergo some degree of reprogramming or transdifferentiation with de novo expression of A6, SOX9, CK19, and OPN, which are conventionally considered HPC or biliary epithelial cell (BEC) markers [9].
Fibroblast Growth Factor (FGF) signaling regulates hepatogenesis [10-12], progenitor cell expansion, and liver regeneration [13, 14]. The FGF family comprises 22 polypeptide ligands that bind to 4 promiscuous tyrosine kinase FGF receptors (FGFR), each expressed as two isoforms [15]. FGFRs are principally located in the cell membrane although nuclear localization has been described [16, 17]. We previously demonstrated that during early hepatogenesis, FGF10, expressed by embryonic mesenchymal hepatic stellate cells, promotes HPC proliferation via β-catenin activation [10]. Postnatally, hepatocyte proliferation following liver injury is regulated in part by activation of FGF signaling via FGFR2IIIb [14]. During DDC-induced liver injury, mesenchymal cell expression of FGF7 is known to regulate HPC expansion, and over-expression of Fgf7 reduces hepatocyte damage and cholestatic liver injury [13].
Wnt/β-catenin signaling has been implicated in HPC-mediated liver regeneration [18, 19]. Binding of the Wnt ligand to Frizzled receptor leads to dephosphorylation, activation, and nuclear translocation of the transcriptional regulator β-CATENIN. Using ex vivo embryonic liver cultures, Sekhon et al showed that FGF signaling promotes β-catenin-mediated proliferation of hepatoblasts [20]. Activation of β-CATENIN can also occur non-canonically via receptor tyrosine kinase (RTK) activation through AKT-dependent [21, 22] and Protein Kinase A (PKA) mediated [23] phosphorylation of β-CATENIN at Serine-552 (pSer552-β-CATENIN). FGF signaling promotes HPC proliferation in vitro via AKT-dependent β-CATENIN activation [24]. Postnatal HPC proliferation induced by DDC treatment is mediated in part via β-catenin activation through increased expression of Wnt ligands [19]. Hyper-activation of liver-specific β-catenin during chronic DDC-induced liver injury leads to increased expansion of A6-expressing hepatocytes in association with improved hepatic repair and resolution of cholestasis [25].
In this study, we further investigate the role of FGF signaling in the emergence and expansion of A6+ive cells during DDC-induced liver injury. We also demonstrate a link between FGF signaling and β-catenin activation during acute DDC liver injury, during which the initial expansion of A6+ive cells is induced, analogous to what is observed in embryonic liver development.
Materials and Methods
Experimental Animals and Procedures
Six-week old, C57BL/6J (wild-type, WT) male mice (Jackson Laboratories) were fed either a standard diet or 0.1% DDC diet (Test Diet, Richmond) up to 14 days. Inducible transgenic and littermate control mice were given water with 1% doxycycline (Clontech) 2 days prior to and throughout DDC treatment. CMVcre;Rosa26rtTA/−;tet(O)-sFGFR2-IIIb+/− mice (dnFGFR) exhibited induced ubiquitous expression of dominant-negative soluble FGFR2-IIIb as confirmed by qPCR (Supp. Fig. 1B) [26]. CMVcre;Rosa26rtTA/−;tet(O)-Fgf10+/− mice (Fgf10-induced) exhibited induced Fgf10 over-expression in uninjured, and DDC treated mice [27]. In a separate experiment, Fgf10-induced mice were given daily intra-peritoneal injections of the AKT inhibitor, Wortmannin (Fgf10+Wort, 0.7 mg/kg) or vehicle solution (Fgf10+Vehicle) in DMSO with 1× phosphate buffered saline (PBS) (Sigma-Aldrich), two days prior to and throughout DDC treatment. Fgf10 was also induced two days prior to and throughout 70% partial hepatectomy (PHx) combined with ethanol (EtOH) gavage (1g/kg, every 12 hrs pre- and post-PHx). All procedures were done in compliance with the IACUC of Children’s Hospital Los Angeles/Saban Research Institute guidelines for use of laboratory animals.
Tissue Collection
After carbon dioxide euthanasia, 1× PBS was flushed through the portal vein. Portions of the right lobe were collected for histology and total RNA. Single cell suspension of whole liver and non-hepatocyte fractions were obtained by mechanical and enzymatic digestion followed by serial centrifugation [4] to enrich the non-hepatocyte fractions (NHF). qPCR for Albumin and Hnf4 was performed to confirm depletion of hepatocytes from the NHF (Supp Figure. 1A).
Immunohistochemistry and Immunofluorescence Analysis
Collected tissues were fixed in 4% paraformaldehyde (PFA) or 30% sucrose for 4 hours. 5μm sections of paraffin-embedded and OCT-embedded tissues were utilized for immunostaining (Supp. Table 1). Hematoxylin & Eosin (H&E) was utilized for morphologic analyses. Quantification of A6+iveHNF4α+ive cells per lobular region was performed by separating each lobule into periportal (0-96μm), mid (97-194μm), and central regions (195-290μm) (Figure 2E). Immunohistochemistry was performed with the Dako EnVision+ Dual Link System-HRP (DAB+) kit (Dako). Immunofluorescence (IF) images were taken using a Leica DM5500B microscope (Leica). All IF quantifications were performed on at least three images per animal with three animals per treatment (n=3).
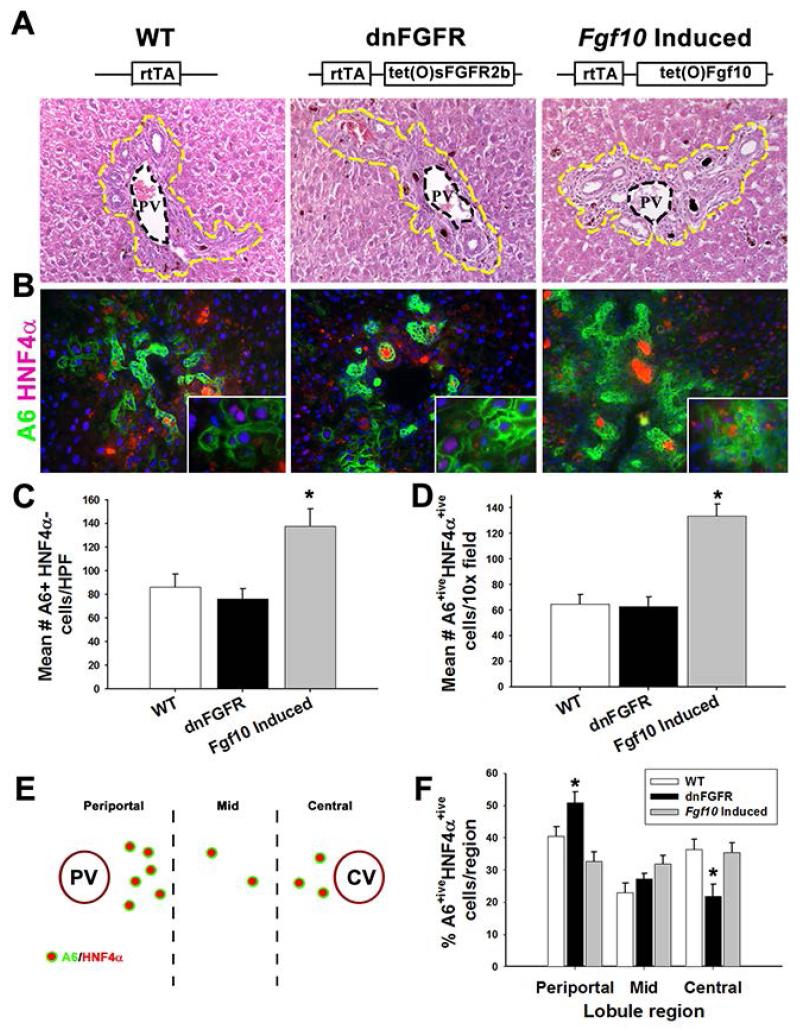
Fig. 2. FGF signaling regulates A6 expression in HNF4α+ive hepatocytes.
(A) WT, dnFGR, and Fgf10-induced H&E staining of periportal regions (20×, yellow dashed line indicates periportal cells). (B) IF staining for A6 (green), and HNF4α (red) (20× with 40× inset). (C) Quantification of the mean number of A6+iveHNF4α−ive cells per high powered field (HPF). (D) Quantification of mean number of A6+iveHNF4α+ive cells per HPF. (E) Schematic for quantification of A6+iveHNF4α+ive cells lobular region. (F) Quantification of percent of A6+iveHNF4α+ive cells per lobular region. Statistical significance was determined by ANOVA with Fisher’s post-hoc test (n=3, * p<0.05).
Quantitative PCR Analysis of Gene Expression
Total RNA was isolated with the Qiagen RNeasy Mini kit (Qiagen). Complementary DNA (cDNA) was prepared with iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCR (qPCR) was performed using the Light-Cycler Taqman Master (Roche Applied Science) and probes from the Universal Probe Library (Roche Applied Science) against intron spanning, gene specific primers (Supp. Table 2). 18s was selected as the optimal internal control for all analyses.
Cell Migration Assays
HepG2 cells were cultured in DMEM with 1% L-Glutamine, 1% Pen/Strep, and 10% Fetal Bovine Serum (Gibco). Scratch assays in serum free conditions were used to determine the dose response of HepG2 cell migration to recombinant FGF10 (rFGF10) (n=8). Images were taken at 0, 24, and 48 hours post scratch and the width determined by three measurements per image. An optimal dose of rFGF10 (10ng/mL) was determined from a generated standard curve for trans-well cell migration assays. 40,000 cells were cultured in 8 μm pore trans-well inserts (BD Falcon) for 24 hours and stained with crystal violet to quantify cell migration by cell counting and spectrophotometer analysis (Victor3, Perkin Elmer). Western blot analysis was performed as previously described to confirm signaling pathway activation in rFGF10 treated HepG2 cells (Supp. Table 1) [24].
Statistical Analysis
ANOVA-Post hoc Fisher’s PLSD test or Mann-Whitney Rank Sum was performed using Statview (SAS Institute, Inc.) to calculate statistical significance (p<0.05).
Results
Expression of FGF ligands and receptors is up-regulated during acute DDC liver injury
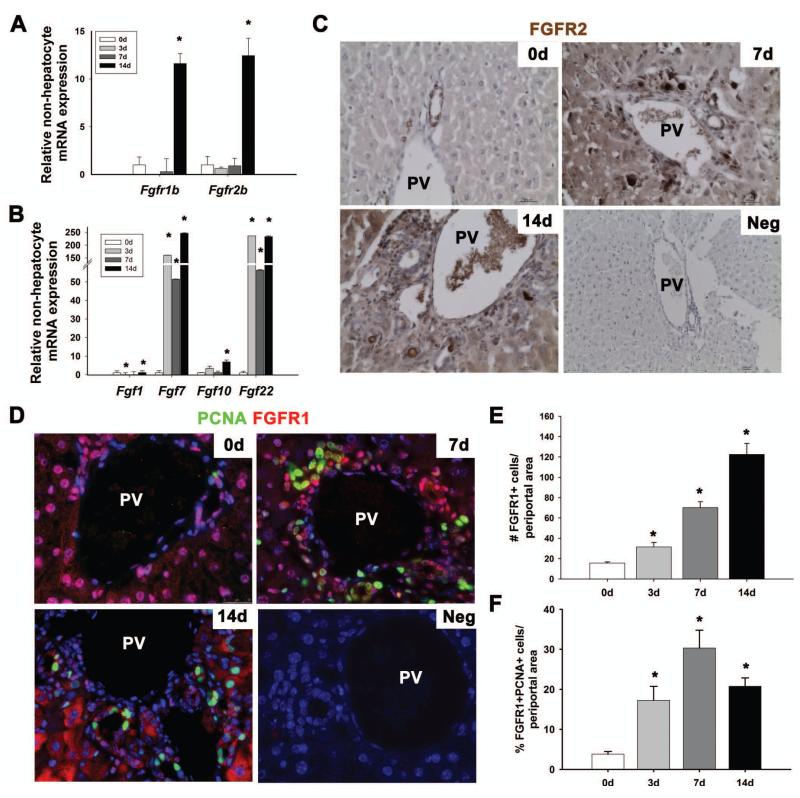
FGF ligands expressed by mesenchymal cells regulate HPCs and liver regeneration during chemically induced liver injury [13, 16, 17]. Thus, qPCR was performed on RNA extracted from a density centrifugation-enriched, non-hepatocyte cell fraction (NHF) and from whole liver tissue in order to assess FGF ligand and receptor gene expression during acute DDC injury. Partial enrichment of small non-hepatocyte cells was validated by qPCR with a marked reduction in expression of hepatocyte genes Albumin and Hnf4α relative to the hepatocyte cell fraction (Supp. Figure 1A) [4]. Within the first 14 days of DDC treatment, we observed 11.6-fold and 12.4-fold increases in Fgfr1IIIb and Fgfr2IIIb expression, respectively, in the NHF (Figure 1A, p<0.05), indicating FGFR activation as previously described [24]. FGF1, FGF2, FGF3, and FGF10 possess high binding affinity for FGFR1IIIb while FGF1, FGF3, FGF7, FGF10, and FGF22 exhibit high binding affinity for FGFR2IIIb [28]. qPCR was performed in lieu of western blot analyses given the lack of reliable antibodies against FGF ligands. Fgf3 expression was not detected by qPCR. Fgf10 expression was significantly up-regulated 14 days after DDC injury while Fgf7 and Fgf22 were upregulated throughout the entire observation period (Figure 1B, p<0.05). Fgf1 expression was transiently down-regulated at 3 days and significantly upregulated by 14 days of DDC treatment (Figure 1B, p<0.05). In contrast, gene expression profiles from whole liver samples for Fgfr2IIIb and all Fgf’s, with the exception of Fgf10, demonstrated no significant upregulation, which indicates that these genes are predominantly expressed by cells within the NHF (Supp. Figure 2A, B, p<0.05).
Fig. 1. FGF signaling is up-regulated during DDC injury.
Relative expression levels of Fgfr1IIIb and Fgfr2IIIb (A) and Fgf1, Fgf7, Fgf10, and Fgf22 (B) was performed on the NHF. (C) Immunohistochemistry for FGFR2 (brown) in the periportal region at 0d, 7d, and 14d DDC injury (20×) and representative negative control (10×). (D) Immunofluorescence staining for PCNA (green) and FGFR1 (red) at 0d, 7d, and 14d DDC injury and representative negative control (40×). (E) Quantification of the number of FGFR1+ive periportal cells at 0d, 3d, 7d, and 14d DDC injury. (F) Quantification of the % of FGFR1+ivePCNA+ive periportal cells. Statistical significance was determined by Mann-Whitney Rank Sum test (n=3, *p<0.05).
Using non-isoform specific antibodies, we observed increased nuclear, cytoplasmic, and membrane-bound FGFR1 and FGFR2 in periportal cells and hepatocytes at 7d and 14d DDC injury (Fig. 1C,D). There was an increase in the fraction of FGFR1+ivePCNA+ive cells in the periportal region throughout acute DDC treatment (Fig. 1D-F, p<0.05). FGFR1 was expressed by CK19+ive biliary epithelial cells, and small periportal HNF4α+ive hepatocytes but not by αSMA+ive mesenchymal cells (Supp. Figure 2C-E). Thus, the majority of proliferating FGFR+ive periportal cells are either biliary epithelial cells or hepatocytes in acute DDC injury.
Fgf10 promotes expression of A6 in HNF4α+ive hepatocytes
In order to assess the effects of FGF signaling on DDC liver injury, transgenic mouse models were utilized to inhibit FGF signaling with dominant-negative FGFR2b (dnFGFR) mice and to hyper-activate FGF signaling with Fgf10 over-expressing mice (Figure 2A). After 14 days, there were no observable differences in liver-to-body weight ratios, serum AST, ALT levels (Table 1), or fibrosis amongst WT, dnFGFR and Fgf10-induced animals (Supp. Figure 3A). There was, however, a significant reduction in total serum bilirubin levels in Fgf10-induced mice similar to that observed by Takase et al with Fgf7 over-expression [13].
Table 1. Effects of dnFGFR and Fgf10 over-expression on DDC liver injury Treatment.
| Treatment | Liver/Body Weight Ratio |
AST (U/L) | ALT (U/L) | Total Bilirubin (mg/dL) |
|---|---|---|---|---|
| WT | 0.08±0.003 | 2171.7± 230.3 | 2336.8± 342.5 | 17.4± 3.6 |
| dnFGFR | 0.07±0.005 | 2245.2 ± 112.2 | 2404.3± 458.0 | 15.5± 2.8 |
| Fgf10 Induced | 0.08±0.002 | 1446.5± 383.8 | 2588.8± 639.0 | 5.2± 1.2* |
Values are given as mean ± standard error of mean (SEM). Statistical analysis was determined using ANOVA with Fisher’s post-hoc test.
denotes P<0.05 compared to WT and dnFGFR.
Abbreviations: AST, asparatate transaminase; ALT, alanine aminotransferase.
Using A6, a putative HPC marker, and HNF4α as a marker of hepatocyte differentiation, we observed an increase in the number of nonbiliary A6+iveHNF4α+ive cells near the portal vein after Fgf10 over-expression for 14 days in uninjured mice (Supp. Figure 4A). We also observed a marked increase in nonbiliary A6+iveCK19−ive cells with Fgf10 over-expression in 14 day DDC-treated livers (Supp. Figure 3C). Specifically there were increases in both A6+iveHNF4α−ive cells and A6+iveHNF4α+ive hepatocytes in Fgf10-induced livers (Figure 2B-D, p<0.0001 and p<0.05 respectively) without a significant change in the relative distribution of cells across the periportal, mid, and central regions of the hepatic lobule (Figure 2E-F, Supp. Figure 3B). A similar increase in periportal A6+iveHNF4α+ive cells was observed with Fgf10 over-expression in the setting of HPC expansion associated with acute ethanol-induced impairment of hepatocyte proliferation [29-31] following partial hepatectomy (Supp. Figure 4B). Conversely, inhibition of FGF signaling during DDC treatment did not affect the number of A6+iveHNF4α−ive or A6+ive HNF4α+ive cells compared to WT (Figure 2C-D). However, dnFGFR mice exhibited altered intralobular distribution of A6+iveHNF4α+ive cells with an increase in the number of A6+iveHNF4α+ive cells in the periportal region and a reduction in the cells in the central region relative to WT and Fgf10-induced mice (Figure 2F, p<0.05).
We next sought to determine if FGF signaling might enhance migration of hepatocytes, throughout the liver lobule. Given the lack of options for in vivo lineage tracing of A6+ive cells, we evaluated the effect of FGF signaling on cell migration in vitro. Scratch assay on HepG2 cells, a transformed human hepatocyte cell line which expresses HPC markers and FGFRs, determined the dose responsiveness of these cells to recombinant (r) FGF10 (Supp. Figure 5A-B). Although HepG2 cells are known to exhibit evidence of elevated levels of basal activation of β-catenin at baseline [32], we detected an increase in protein levels of pAKT and pSer552-β-catenin within 30 minutes after media supplementation with 10 ng/mL of rFGF10 (Supp. Figure 5C,D). Thus, despite high basal activation of β-catenin, HepG2 cells are still responsive to FGF10. We then performed Boyden chamber trans-well migration assays ± rFGF10, and observed no significant change in HepG2 cell migration compared to controls (Supp. Figure 5E,F). Thus, either FGF signaling does not regulate HPC-like hepatocyte migration or it does so via a non-AKT-mediated β-catenin pathway.
FGF signaling mediates periportal cell proliferation and AKT-dependent β-catenin activation
Centrolobular cell proliferation, as assessed by phospho-Histone-H3 (pHH3) staining, was not significantly different amongst treatment groups (data not shown). However, proliferation of the periportal cells (Figure 3A) exclusive of large hepatocytes but inclusive of A6+ive cells, BECs, mesenchymal cells, and small HNF4α+ive hepatocytes, was reduced in dnFGFR livers and increased with Fgf10 over-expression compared to WT (Figure 3A-B, p<0.01). Given that β-catenin activation regulates the expansion of the A6+ive cell population with DDC injury and that FGF promotes AKT-dependent β-catenin activation of embryonic and tumor initiating HPC in vitro, we sought to determine if FGF signaling induces AKT-mediated β-catenin activation in DDC liver injury. We observed a ~10% increase in the fraction of pSer552-β-catenin+ive periportal cells in Fgf10-induced livers compared to WT (Figure 3C-D, p<0.0001). In contrast, there was a marked reduction in the fraction of pSer552-β-catenin+ive periportal cells in dnFGFR livers compared to WT and Fgf10-induced livers (Figure 3C-D, p<0.0001, and p<0.01 respectively). Collectively, these observations indicate that FGF signaling promotes AKT-dependent β-catenin activation in periportal cells during the initial two weeks of DDC-induced liver injury.
Fig. 3. FGF signaling regulates periportal cell proliferation and AKT-dependent β-catenin phosphorylation.
(A) IF staining for cell proliferation by phospho-Histone H3 (red) (pHH3) and DAPI (blue) (40×). (B) Quantification of the percent of pHisH3+ive periportal cells. (C) IF staining for pSer552-β-catenin+ive (red) cells with DAPI (blue) per periportal area (40×). (D) Quantification of the percent of pSer552-β-catenin+ive periportal cells. White dashed lines delineate periportal cell population. Statistical significance was determined by ANOVA with Fisher’s post-hoc test (n=3, * p<0.05, # p<0.0001).
Inhibition of PI3K signaling disrupts A6+iveHNF4α+ive cell expansion
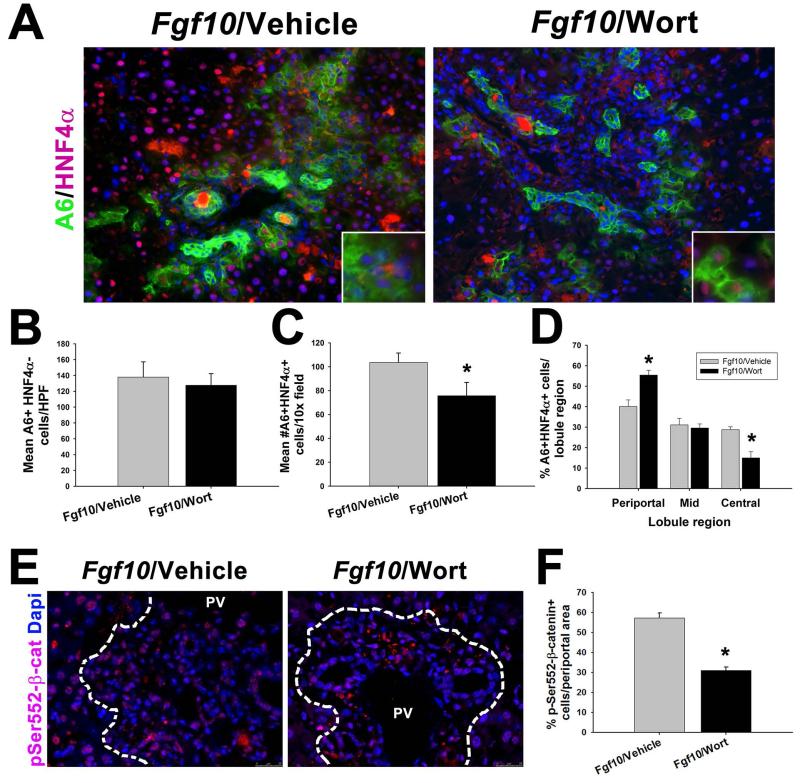
To further validate the role of FGF signaling in β-catenin-dependent expansion of A6+ive cells during acute DDC liver injury, Fgf10-induced animals were injected daily with the AKT inhibitor Wortmannin (Fgf10+Wort). No significant difference was observed with serum AST, ALT, total bilirubin, and fibrosis between Fgf10+Wort and Fgf10+vehicle controls (Table 2, Supp. Figure 6A). Fgf10+Wort treatment did not affect the expansion of the A6+iveHNF4α−ive cell population (Figure 4A-B) but significantly reduced the number of A6+iveHNF4α+ive cells compared to controls (Figure 4C, p<0.05). The distribution of A6+iveHNF4α+ive cells within the hepatic lobule was also altered, in a pattern similar to that seen in dnFGFR livers in which the percent of cells near the portal vein is greater than that near the central vein (Figure 4D, Supp. Figure 6B, p<0.05). Collectively these data indicate that inhibition of FGF/AKT signaling disrupts hepatocyte expression of A6+ive cells, an indication of hepatocyte transdifferentiation towards biliary cells as seen with DDC injury.
Table 2. Effects of pAKT inhibition with Fgf10 over-expression on DDC liver injury.
| Treatment | Liver/Body Weight Ratio |
AST (U/L) | ALT (U/L) | Total Bilirubin (mg/dL) |
|---|---|---|---|---|
| Fgf10/Vehicle | 0.07± 0.004 | 1173.0± 315.8 | 2238.3± 15.5 | 4.6± 1.6 |
| Fgf10/Wort | 0.11± 0.028 | 1604.7± 282.8 | 1987.7± 439.1 | 6.7± 2.1 |
Values are given as mean ± standard error of mean (SEM). Statistical analysis was determined using the Mann-Whitney Rank Sum test. Abbreviations: AST, asparatate transaminase; ALT, alanine aminotransferase.
Fig. 4. Wortmannin inhibition of Fgf10 induced livers disrupts the A6+HNF4α+ cell differentiation and migration.
(A) IF staining for A6 (green), and HNF4α (red) (20× with 40× inset). (B) Quantification of mean number of A6+ive HNF4α−ive cells per HPF. (C) Quantification of average number of A6+iveHNF4α+ive cells per HPF. (D) Quantification of % of A6+iveHNF4α+ive cells per lobular regions. (E) IF staining for pSer552-β-catenin+ive (red) cells with DAPI (blue) (40×). (F) Quantification of the % of pSer552-β-catenin+ive periportal cells. Statistical significance was determined by Mann-Whitney Rank Sum test (n=3, * p<0.05).
IF analysis of AKT-dependent β-catenin activation revealed a ~30% reduction in the percentage of pSer552-β-catenin+ive periportal cells in the Fgf10+Wort livers compared to Fgf10+Vehicle (Fig. 4F, p<0.05). We also observed a 50% reduction in periportal cell proliferation in Fgf10+Wort compared to Fgf10+Vehicle (Supp. Figure 6C,D, p<0.01). These data indicate that AKT-dependent β-catenin activation plays a key role in regulating the A6+iveHNF4α+ive cell population during cholestatic liver injury.
Discussion
FGF signaling is a key regulator of endoderm specification toward a liver-specific fate [11] as well as embryonic HPC survival and proliferation [10, 24]. Postnatally, FGF signaling regulates liver regeneration following injury [13, 14, 33]. In this study, we show that FGF signaling promotes the initial expansion of a DDC-induced periportal A6+ive HNF4α−ive cell and A6+iveHNF4α+ive hepatocyte populations in association with AKT-mediated activation of β-catenin.
Takase et al. observed that FGF7, expressed by the periportal Thy1+ive mesenchymal cells, induces the expansion of periportal HPCs during adult cholestatic liver injury [13]. We observed a similar increase in A6+iveHNF4α−ive cell expansion with over-expression of Fgf10, a member of the FGF7 ligand sub-family [34]. Knockout of Fgf7 in the liver leads to impairment of CK19+ive HPC proliferation during DDC injury and is lethal in 80% of mice by 8 weeks compared to nearly zero mortality in WT mice [13]. We utilized a sublethal inhibition of FGF signaling during acute DDC treatment and observed a reduction in the number of centrolobular A6+iveHNF4α+ive cells. Inhibition of AKT by Wortmannin in Fgf10-induced mice similarly disrupted hepatocyte expression of A6 consistent with previous observations of reduced A6+ive cell expansion with decreased phosphoinositide 3-kinase (PI3K)/AKT activation, knockout of c-met, the HGF Receptor gene, [35] or liver-specific conditional knockout of β-catenin [18]. Our in vitro data indicated a lack of enhanced hepatocyte migration with rFGF10 treatment. Although we did not observe migration of HepG2 cells in response to FGF10 treatment, it does not fully preclude the possibility of FGF-mediated cell migration. Our observations are consistent with the emergence of A6+ive hepatocytes as a result of hepatocyte reprogramming and not downstream migration of A6+ive HPCs.
Using hepatocyte-specific, adeno-associated virus vector-mediated Cre expression, Malato et al demonstrated minimal progenitor cell contribution to the population of newly formed hepatocytes with DDC injury [8]. Instead, Yanger et al showed evidence of hepatocyte reprogramming/transdifferentiation to BECs during DDC injury under the regulation of Notch signaling [9]. The authors also demonstrated that hepatocytes, which express BEC markers such as A6, OPN, and SOX9, acquire apical-basal polarity after 3 weeks of DDC injury [9]. In this study, we observed hepatocyte expression of A6 during acute DDC injury is partly regulated by Fgf10 and β-catenin signaling. This increase in A6 expressing hepatocytes was also associated with a significant increase in cell proliferation, consistent with the findings of Yanger et al that hepatocyte reprogramming is associated with high cellular turnover [36]. It is also reasonable to speculate that FGF signaling may also be a regulator of hepatocyte reprogramming to BECs, although further studies would be required to prove this. We also observed A6 expression by hepatocytes after Fgf10 over-expression in the absence of injury and during alcohol-impaired liver regeneration after partial hepatectomy, thus, implying a more generalizable role for FGF signaling in hepatocyte reprogramming.
β-catenin activation promotes the proliferation of a variety of cells in the liver, including HPCs. Increased Wnt ligand expression with subsequent downstream β-catenin activation promotes the proliferation of DDC-induced HPCs [19]. Additionally, liver-specific expression and activation of β-catenin increases the population of A6-expressing hepatocytes throughout the hepatic lobule and improved liver function with chronic DDC treatment [25]. FGF signaling induces β-catenin activation in embryonic HPC in vitro [10, 20] in part via downstream activation of AKT [24]. In this study, we observed increased AKT-mediated β-catenin activation and cell proliferation in the periportal region with two weeks of in vivo Fgf10 over-expression concurrent with DDC treatment. We extrapolate that FGF signaling regulates the initial expansion of the A6+iveHNF4α−ive HPCs. Despite the increase in pSer552-β-catenin+ive periportal cells and A6-expressing hepatocytes seen with Fgf10 over-expression, there was no improvement in hepatocyte injury as assessed by serum AST and ALT consistent with observations by Thompson et al, in which excessive β-catenin expression and activation did not improve serum biochemistry markers for liver injury during the initial two weeks of DDC treatment [25]. While the role of hepatocyte reprogramming in liver regeneration remains unclear, the authors observed improved liver function tests with hyperactivation of β-catenin at 150 days indicating that these cells may improve liver regeneration long term. Given this finding, we speculate that prolonged Fgf10 over-expression, were it feasible, may similarly ameliorate liver injury with prolonged DDC treatment. Unfortunately, the utility of the our transgenic model of ubiquitous over-expression of Fgf10 is limited to less than 3 weeks due to gastrointestinal complications which limit mouse viability [36]. Liver-specific Fgf10 over-expression has not been performed. In our study, inhibition of FGF signaling through soluble FGFR2b expression and Wortmannin inhibition resulted in a decrease in pSer552-β-catenin+ive periportal cells without reducing the A6+iveHNF4α−ive cell population. Taken together, these data suggest that AKT-mediated β-catenin activation is sufficient but not necessary to expand this cell population. Retention of pSer552-β-catenin+ive periportal cells may be due to incomplete inhibition of FGF signaling with dominant-negative FGFR2IIIb expression or it may be due to PKA-mediated phosphorylation of β-catenin at Serine-552, which was not analyzed in this study [23]. Hence, our data are consistent with reprogramming of hepatocytes with A6 expression towards a biliary epithelial phenotype during DDC-induced liver injury.
In conclusion, we demonstrate that FGF signaling, partly through AKT-mediated β-catenin activation, regulates the expansion of the A6+ive HPCs and A6+iveHNF4α+ive hepatocytes. Further investigation into the transdifferentiation of hepatocytes into BECs and their function in response to injury will provide a better understanding of liver regeneration.
Supplementary Material
Acknowledgements
We thank Dr. Henri R. Ford, Frederic Sala, Dr. Hide Tsukamoto, and Dr. David Warburton for their support and stimulating feedback on this project. We also thank Dr. Valentina Factor, Dr. Joshua Friedman, and Dr. Linheng Li for their generous gifts of antibodies to perform this study.
Supported in part by the National Institutes of Health Grants K08 AAA01690 (K.W.) and the Franklin Martin Award from the American College of Surgeons (K.W.)
Abbreviations
- HPCs
hepatic progenitor cells
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- CD133
Prominin-1
- FGF
Fibroblast Growth Factors
- FGFR
Fibroblast Growth Factor Receptor
- RTK
receptor tyrosine kinase
- WT
wild type
- dnFGFR
dominant negative Fibroblast Growth Factor Receptor
- PCNA
Proliferating Cell Nuclear Antigen
- NHF
non-hepatocyte fraction
- PFA
paraformaldehyde
- H&E
hematoxylin and eosin
- cDNA
complementary DNA
- qPCR
quantitative PCR
- HGF
Hepatocyte Growth Factor
- HNF4α
Hepatocyte Nuclear Factor-4α
- GSK3β
glycogen synthase kinase-3 beta
- PI3K
Phosphoinositide 3-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors of this manuscript have no conflicts of interest to disclose.
References
- [1].Stamp LA, Braxton DR, Wu J, Akopian V, Hasegawa K, Chandrasoma PT, et al. The GCTM-5 Epitope Associated with the Mucin-Like Glycoprotein FCGBP Marks Progenitor Cells in Tissues of Endodermal Origin. Stem Cells. 2012;30:1999–2009. doi: 10.1002/stem.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45:1091–1096. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- [3].Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, et al. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. 2011;25:1193–1203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rountree CB, Barsky L, Ge S, Zhu J, Senadheera S, Crooks GM. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25:2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- [5].Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632–640. doi: 10.1053/jhep.2003.50104. [DOI] [PubMed] [Google Scholar]
- [6].Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- [7].Espanol-Suner R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575. doi: 10.1053/j.gastro.2012.08.024. e1567. [DOI] [PubMed] [Google Scholar]
- [8].Malato Y, Naqvi S, Schurmann N, Ng R, Wang B, Zape J, et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. The Journal of clinical investigation. 2011;121:4850–4860. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes & development. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, et al. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology. 2007;46:1187–1197. doi: 10.1002/hep.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Calmont A, Wandzioch E, Tremblay KD, Minowada G, Kaestner KH, Martin GR, et al. An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev Cell. 2006;11:339–348. doi: 10.1016/j.devcel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- [12].Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science (New York, NY. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- [13].Takase HM, Itoh T, Ino S, Wang T, Koji T, Akira S, et al. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev. 2013;27:169–181. doi: 10.1101/gad.204776.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bohm F, Speicher T, Hellerbrand C, Dickson C, Partanen JM, Ornitz DM, et al. FGF receptors 1 and 2 control chemically induced injury and compound detoxification in regenerating livers of mice. Gastroenterology. 2010;139:1385–1396. doi: 10.1053/j.gastro.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. Journal of biochemistry. 149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chioni AM, Grose R. FGFR1 cleavage and nuclear translocation regulates breast cancer cell behavior. J Cell Biol. 2012;197:801–817. doi: 10.1083/jcb.201108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Reilly JF, Maher PA. Importin beta-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J Cell Biol. 2001;152:1307–1312. doi: 10.1083/jcb.152.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- [19].Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R, et al. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology. 2007;133:1579–1591. doi: 10.1053/j.gastro.2007.08.036. [DOI] [PubMed] [Google Scholar]
- [20].Sekhon SS, Tan X, Micsenyi A, Bowen WC, Monga SP. Fibroblast growth factor enriches the embryonic liver cultures for hepatic progenitors. Am J Pathol. 2004;164:2229–2240. doi: 10.1016/S0002-9440(10)63779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krejci P, Aklian A, Kaucka M, Sevcikova E, Prochazkova J, Masek JK, et al. Receptor tyrosine kinases activate canonical WNT/beta-catenin signaling via MAP kinase/LRP6 pathway and direct beta-catenin phosphorylation. PloS one. 2012;7:e35826. doi: 10.1371/journal.pone.0035826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- [24].Mavila N, James D, Utley S, Cu N, Coblens O, Mak K, et al. Fibroblast Growth Factor Receptor-Mediated Activation of AKT-beta-Catenin-CBP Pathway Regulates Survival and Proliferation of Murine Hepatoblasts and Hepatic Tumor Initiating Stem Cells. PloS one. 2012;7:e50401. doi: 10.1371/journal.pone.0050401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thompson MD, Awuah P, Singh S, Monga SP. Disparate cellular basis of improved liver repair in beta-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Am J Pathol. 2010;177:1812–1822. doi: 10.2353/ajpath.2010.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Celli G, LaRochelle WJ, Mackem S, Sharp R, Merlino G. Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. Embo J. 1998;17:1642–1655. doi: 10.1093/emboj/17.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Clark JC, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT, et al. FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. Am J Physiol Lung Cell Mol Physiol. 2001;280:L705–715. doi: 10.1152/ajplung.2001.280.4.L705. [DOI] [PubMed] [Google Scholar]
- [28].Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- [29].Chen J, Kunos G, Gao B. Ethanol rapidly inhibits IL-6-activated STAT3 and C/EBP mRNA expression in freshly isolated rat hepatocytes. FEBS Lett. 1999;457:162–168. doi: 10.1016/s0014-5793(99)01031-5. [DOI] [PubMed] [Google Scholar]
- [30].Yang SQ, Lin HZ, Yin M, Albrecht JH, Diehl AM. Effects of chronic ethanol consumption on cytokine regulation of liver regeneration. Am J Physiol. 1998;275:G696–704. doi: 10.1152/ajpgi.1998.275.4.G696. [DOI] [PubMed] [Google Scholar]
- [31].Zhang BH, Farrell GC. Chronic ethanol consumption disrupts complexation between EGF receptor and phospholipase C-gamma1: relevance to impaired hepatocyte proliferation. Biochem Biophys Res Commun. 1999;257:89–94. doi: 10.1006/bbrc.1999.0403. [DOI] [PubMed] [Google Scholar]
- [32].de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tsai SM, Wang WP. Expression and function of fibroblast growth factor (FGF) 7 during liver regeneration. Cell Physiol Biochem. 2011;27:641–652. doi: 10.1159/000330073. [DOI] [PubMed] [Google Scholar]
- [34].Igarashi M, Finch PW, Aaronson SA. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7) J Biol Chem. 1998;273:13230–13235. doi: 10.1074/jbc.273.21.13230. [DOI] [PubMed] [Google Scholar]
- [35].Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M, et al. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55:1215–1226. doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Speer AL, Al Alam D, Sala FG, Ford HR, Bellusci S, Grikscheit TC. Fibroblast growth factor 10-fibroblast growth factor receptor 2b mediated signaling is not required for adult glandular stomach homeostasis. PloS one. 2012;7:e49127. doi: 10.1371/journal.pone.0049127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.