Abstract
The low stiffness of reconstituted collagen hydrogels has limited their use as scaffolds for engineering implantable tissues. Although chemical crosslinking has been used to stiffen collagen and protect it against enzymatic degradation in vivo, it remains unclear how crosslinking alters the vascularization of collagen hydrogels. In this study, we examine how the crosslinking agents genipin and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) alter vascular stability and function in microfluidic type I collagen gels in vitro. Under moderate perfusion (~10 dyn/cm2 shear stress), tubes of blood endothelial cells exhibited indistinguishable stability and barrier function in untreated and crosslinked scaffolds. Surprisingly, under low perfusion (~5 dyn/cm2 shear stress) or nearly zero transmural pressure, microvessels in crosslinked scaffolds remained stable, while those in untreated gels rapidly delaminated and became poorly perfused. Similarly, tubes of lymphatic endothelial cells under intermittent flow were more stable in crosslinked gels than in untreated ones. These effects correlated well with the degree of mechanical stiffening, as predicted by analysis of fracture energies at the cell-scaffold interface. This work demonstrates that crosslinking of collagen scaffolds does not hinder normal endothelial cell physiology; instead, crosslinked scaffolds promote vascular stability. Thus, routine crosslinking of scaffolds may assist in vascularization of engineered tissues.
Keywords: microvascular tissue engineering, stiffness, vascular physiology, extracellular matrix, perfusion
INTRODUCTION
Reconstituted collagen-based hydrogels remain a widely studied class of scaffolds for engineering tissues.1,2 Although they offer clear advantages in biocompatibility, these materials are often too mechanically weak for direct use, and they must be strengthened by chemical or thermal crosslinking before implantation. Such treatments can increase the elastic moduli of collagen scaffolds by an order-of-magnitude or more in the hydrated state.3-6
It remains unclear to what extent such stiffening affects the behavior of cells grown inside the scaffold. Extensive studies with cultured cells have established that the stiffness of a substratum, whether in two or three-dimensional form, affects cell function.7-11 Given the requirement of stable vascular perfusion in the viability of nearly all engineered tissues, it is important to know whether scaffold crosslinking promotes or inhibits the stability of blood vessels. Although less is known about the role that lymphatic drainage plays in the function of engineered tissues, poor lymphatic stability is likely to result in tissue edema; thus, how scaffold crosslinking affects lymphatic vessels is also an important issue. Recent studies with cultured endothelial cells (ECs) have suggested that growth on stiff substrata sensitizes ECs to breakdown of barrier function and activation by cytokines.12-14In vivo, the effects of stiffness are less consistent, with recent studies claiming that stiffening of the vascular wall may be deleterious15 or indirectly beneficial16 (by limiting strain) to the function of overlying endothelium.
Here, we use microfluidic type I collagen gels to investigate how scaffold crosslinking affects vascular stability. This class of scaffolds is characterized by the capacity for immediate perfusion17,18; thus, we can generate and perfuse endothelial tubes without relying on self-organization and tubulogenesis of ECs into open structures. We used two small-molecular-weight organic crosslinkers, the natural product-derived genipin and the “zero-length” agent 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), both of which generate adducts of collagen that are considered non-cytotoxic.19-22 We first tested whether stiffened collagen destabilized vessels under conditions that we and others previously showed could maintain perfusion. Finding no harmful effect from crosslinking, we then analyzed whether these agents could affect vascular stability under perfusion conditions that induce rapid delamination of endothelium from collagen-based scaffolds. Since engineered tissues may benefit from stabilization of both blood and lymphatic vessels, we studied the effects of scaffold stiffness on tubes of blood and lymphatic vessel-derived ECs, under the sustained and intermittent perfusion that characterize these respective vessel types.
MATERIALS AND METHODS
Cell culture
We cultured human dermal blood and lymphatic microvascular endothelial cells (BECs and LECs, respectively; BEC lots 7F3585 and 6F4547 from Lonza, and LEC lots 6120704.1 and 0070602 from Promocell) on gelatin-coated dishes. Standard growth media consisted of MCDB 131 media (Caisson) with 10% heat-inactivated fetal bovine serum (Atlanta Biosciences), 1% glutamine-penicillin-streptomycin (Invitrogen), 1 μg/mL hydrocortisone (Sigma), 0.2 mM L-ascorbic acid 2-phosphate (Sigma), 2 U/mL heparin (Sigma), 25 μg/mL endothelial cell growth supplement (Biomedical Technologies) and 80 μM dibutyryl cyclic AMP (Sigma). Cells were passaged at a 1:3 or 1:4 ratio and discarded after passage seven.
Formation of open BEC tubes
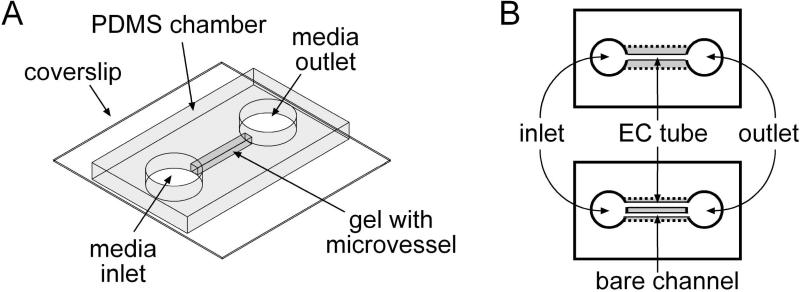
Gels of type I collagen (~8 mg/mL final concentration in PBS, from rat tail; BD Biosciences) were formed at 25°C around 120- or 140-μm diameter needles in 7.5- or 10-mm-long polydimethylsiloxane (PDMS) chambers, as described previously [Fig. 1(A)].23,24 After two hours of gelation, needles were removed to form bare channels. Some collagen scaffolds were stored overnight in phosphate-buffered saline (PBS) at 37°C before use.
Figure 1.
(A) Perspective and (B) top views of the vascular configurations used in this study. In (B), the widths of the gels are exaggerated to show the location of the endothelial tube and/or bare channel that each gel contains. Not shown are the polyethylene tubings that connect the inlet and outlet to external media reservoirs.
Bare collagen channels were either used as is or first crosslinked by perfusion with 1 or 20 mM genipin (Wako Biosciences) or with 0.5, 5, or 10 mg/mL EDC (Sigma) in PBS for two hours at 25°C. Treated collagen gels were flushed continuously for one hour with PBS to remove residual crosslinker before conditioning with growth media supplemented with 3% 70 kDa dextran (Sigma) for at least four hours. Conditioned gels were seeded with BECs by flowing concentrated cell suspension through the channels and allowing the cells to settle and adhere, as previously described23,25; BECs proliferated to form confluent tubes (i.e., vessels) [Fig. 1(B), top].
To test whether crosslinked scaffolds inhibited vascular function, we perfused a subset of microvessels (n = 61) with dextran-supplemented media at a pressure difference of 4.8 cm H2O. This pressure difference yielded a wall shear stress of ~10 dyn/cm2 and a positive transmural pressure, conditions that support stable vascular function in untreated gels.26,27 Shear stress was estimated by assuming flow obeyed Poiseuille's Law.
To test whether crosslinked scaffolds stabilized microvessels, we perfused some microvessels (n = 112) at a pressure difference of 2.2 cm H2O. At this lower pressure difference, the shear stress was ~5 dyn/cm2, a condition that induces rapid breakdown of vessels in untreated gels.26 We also formed microvessels (n = 34) that were paired with a parallel bare channel, as initially described by Price et al.26 Parallel channels in a single gel were made using two 120-μm needles that were placed ~0.3 mm apart in a ~9-mm-long PDMS chamber. We removed only one of the two needles and crosslinked some of the gels with 1 or 20 mM genipin as described above. Gels were then flushed and seeded, after which the remaining needle was removed to yield a BEC tube next to a parallel bare channel [Fig. 1(B), bottom]. Under perfusion with a pressure difference of 4.8 cm H2O, the tube and channel were exposed to the same axial pressure distribution, and the transmural pressure across the endothelium was nearly zero. This condition also leads to rapid vascular breakdown in untreated gels.26
Formation of open LEC tubes
LEC tubes were formed by an identical procedure as above, with the following modifications. The collagen channels were 120 μm in diameter and ~6.5 mm in length. Crosslinking conditions consisted of 20 mM genipin or 10 mg/mL EDC only. After seeding, samples (n = 37) were placed under intermittent, dropwise flow by adding ~80 μL of media to the well next to one channel end and ~50 μL to the opposite well. We replaced all media and regenerated the pressure difference every ~2 hrs on the day of seeding and twice daily thereafter.
Measurement of elastic moduli of collagen gels
We measured the elastic moduli of ~1-mm-thick, ~5-mm-diameter collagen gels by indentation. Gels were untreated or crosslinked with 1 or 20 mM genipin or with 0.5, 5, or 10 mg/mL EDC for two hours at ~25°C and then washed extensively with PBS. We gently placed stainless steel or aluminum spheres (Precision Balls) on top of the gels; metal spheres and collagen gels were completely submerged in PBS. The indentation depths of the spheres were measured after one hour from a side-view of the gels. We modeled the system as Hertz contact between a deformable material (gel) and an incompressible one (metal). In this case, the indentation modulus Eind is given by:
where R is the radius of the sphere (1.0 mm for steel, 0.8 mm for aluminum), ρ and ρPBS are the densities of metal (~8 g/cm3 for steel, 2.7 g/cm3 for aluminum) and PBS, g = 9.8 m/s2, and δ is the indentation depth.28
Measurement of hydraulic permeability of collagen hydrogels
We measured the hydraulic permeability of ~1×1×8 mm3 solid collagen hydrogels as described previously.29 Gels were untreated or crosslinked by interstitial flow of genipin or EDC solution for 2 hours at 25°C underã1.5 cm H2O pressure difference and flushed with PBS overnight at 37°C before measuring interstitial flow rates. Darcy permeability κ was calculated as:
where Q is the average flow rate of PBS over a ~5-hr period, L is the length of the gel (~8 mm), A is the cross-sectional area of the gel (~1 mm2), ΔP is the applied pressure difference, and ηPBS = 0.70 cP is the viscosity of PBS at 37°C.
Viability assay
To verify that cells were viable, we perfused microvessels three days after seeding with 2 μM calcein and 6 μM ethidium homodimer (Invitrogen) in media for fifteen minutes before imaging. All endothelial nuclei were visualized with 5 μg/mL Hoescht 33342 (Invitrogen).
Measurement of microvessel distension
Under standard flow conditions, the diameter of vessels increases over time in response to positive transmural pressure.23,26 We quantified distension as the ratio of the increase in microvessel diameter after three days of perfusion to the initial diameter of the unseeded channel (~120 or 140 μm). Phase-contrast images of microvessels were obtained with a Plan-Neo 10×/0.3 NA objective, and diameters were measured using a calibrated software ruler in Axiovision ver. 4.3 (Zeiss).
Focal leak assay
Permeability assays were performed on tubes three days after seeding using methods adapted from previous studies.25 Briefly, we supplemented perfusion media with 40 μg/mL Alexa Fluor 488-conjugated 10 kDa dextran (Invitrogen) without altering perfusion conditions. Fluorescence images were collected every minute at 10× magnification until the microvessel lumen was uniformly filled with dye and then for at least twelve more minutes.
“Focal” leaks were defined as regions of locally elevated fluorescence in the interstitial space near the vessel walls. The number of leaks were counted for each image and presented as the average number of leaks per millimeter of vessel length. To enhance detection of focal leaks in genipin-treated gels, which had high levels of background autofluorescence, we used MATLAB ver. 7.1 (Mathworks) to convert grayscale images into color topographical contour maps.
Lifespan assay
We defined microvessel “death” as the day when endothelial delamination or denuding from the gel was first observed and/or when the flow rate decreased below half of the peak flow rate. Delamination within 300 μm of vessel outlets was not counted towards statistical analysis of survival curves, in accordance with previous studies.26,30 Lifespans were monitored for two weeks and analyzed using Kaplan-Meier survival curves. Microvessels that experienced reduced flow due to outside causes, such as occlusion from debris in the perfusate, were censored from the analysis on the day the event occurred.
Delamination assay
Delamination heatmaps were obtained as previously described.27,31 Briefly, delamination was scored from stitched brightfield or phase-contrast images that were focused on the vessel midplane. The upper and lower edges of each microvessel yielded separate binary maps for stable (black) and delaminated (white) regions. For each vessel, we calculated the fraction of delaminated length by dividing the total length of delamination by the sum of the lengths of the two edges of the vessel. Binary delamination maps for a given scaffold treatment were stacked and averaged using a customized MATLAB code to generate a grayscale map of delamination frequency.
Statistical analysis
Numerical data are reported as mean ± standard deviation. We compared two sets of numerical data with two-tailed t-test with correction for unequal variances, multiple sets with one-way ANOVA, flow curves with two-way ANOVA (using time and treatment as the two variables), and survival curves with the log-rank test. We used Prism ver. 5 (GraphPad) to perform all tests, and considered p values less than 0.05 divided by the total number of possible comparisons to be statistically significant.
Numerical modeling
To estimate the decrease in the elastic strain energy of the collagen gel upon endothelial delamination, we constructed three-dimensional finite-element models of a 1-mm-long segment of a 120-μm-diameter vessel and the scaffold surrounding it, and solved them with COMSOL Multiphysics ver. 3.5a (Comsol). We modeled the collagen as a linear elastic solid under small strain, and treated the endothelium as a contractile thin film that exerts a uniform radial stress on the scaffold. We modeled a delaminated patch of endothelium by setting the radial stress at the scaffold to zero in a 20 μm × 20 μm region. The difference in total strain energies in the scaffold in the absence versus presence of the delaminated patch yielded the decrease in strain energy from delamination. Tripling the number of degrees of freedom from ~105 to ~3×105 by regular mesh refinement altered the computed energy decrease by <0.5%.
RESULTS
Characterization of genipin-treated gels
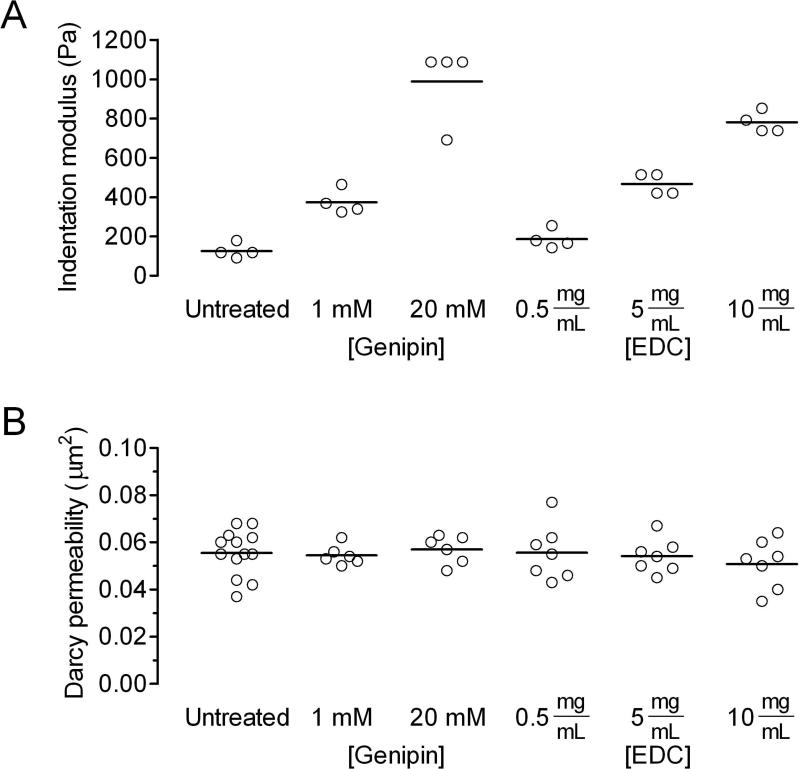
The indentation moduli of untreated collagen gels and those treated with 1 and 20 mM genipin were 130 ± 40 Pa, 370 ± 60 Pa, and 990 ± 200 Pa, respectively [Fig. 2(A)]. As expected, treatment with genipin led to an increase in elastic modulus (p = 0.0025 for untreated vs. 1 mM, and 0.0034 for untreated vs. 20 mM).
Figure 2.
Alteration of physical properties of collagen gels by genipin and EDC. (A) Elastic modulus, as measured by indentation. (B) Hydraulic permeability.
Since vascular stability in microfluidic scaffolds is affected by the hydraulic permeability of the scaffold27, we also measured the permeability of untreated and genipin-treated gels [Fig. 2(B)]. The Darcy permeabilities of untreated gels and those treated with 1 and 20 mM genipin were 0.056 ± 0.010 μm2, 0.054 ± 0.004 μm2, and 0.057 ± 0.006 μm2, respectively. The permeabilities were not significantly different (p > 0.5), which implies that treatment with genipin crosslinks the collagen gels without altering their pore structure.
Vascular function in genipin-treated gels under moderate perfusion conditions
Given recent studies that suggest stiffening of substrata can promote endothelial activation12,15, we hypothesized that crosslinking of collagen scaffolds with genipin would adversely affect the behavior of vessels formed from blood vessel-derived endothelial cells (BECs) within such scaffolds. To test this hypothesis, we formed BEC tubes under conditions that sustain long-term stability and barrier function in untreated collagen gels.26,30,31 These conditions consisted of perfusion with media that contained dibutyryl cyclic AMP and dextran, at a shear stress of at least 10 dyn/cm2.
In preliminary experiments, we found that vessels in untreated gels expanded under perfusion, whereas those in genipin-treated, stiff gels barely did (<5% radial distension). To ensure that the shear stress and perfusion pressures were similar across conditions, we used 120- and 140-μm-diameter channels when forming vessels in untreated and genipin-treated scaffolds, respectively. Untreated and genipin-treated scaffolds were made at lengths of ~7.5 and ~10 mm, respectively. In this way, we expected to keep the flow rates, vascular diameters, and shear stress the same across conditions after any expansion had occurred.
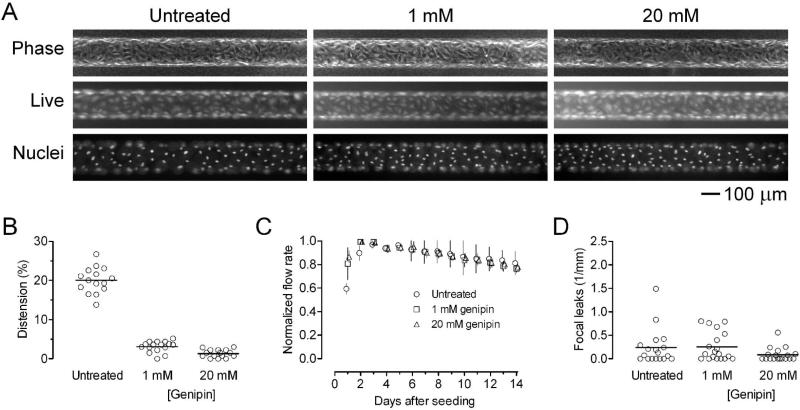
BECs that were seeded in genipin-treated gels adhered well and spread quickly. Whether made in untreated or crosslinked gels, vessels were viable on day 3 of perfusion and exhibited similar cell densities [Fig. 3(A)]. We did not observe any adherent dead cells under any condition, and vessels across the different gels looked similar by phase-contrast microscopy.
Figure 3.
BEC microvessels in genipin-treated collagen under moderate flow (shear stress of ~10 dyn/cm2). (A) Representative phase-contrast, Live/Dead, and Hoechst images on day 3 post-seeding. (B) Vascular distension on day 3. (C) Flow rates, normalized to the maximum flow rates for each sample (n = 10 for each condition). (D) Focal leak densities on day 3.
Vascular distension on day 3 was 20.0 ± 3.3%, 3.1 ± 1.4%, and 1.3 ± 1.0% for untreated gel and 1 mM and 20 mM genipin-treated gels, respectively [Fig. 3(B)]. Crosslinking of scaffolds with genipin decreased distension in a manner consistent with the observed changes in elastic moduli. Microvessels in untreated gels distended more than those in genipin-treated gels did (p < 0.0001 for untreated vs. 1 mM and for untreated vs. 20 mM). As designed, the shear stresses were not significantly different (p = 0.14): they were 11.5 ± 1.0 dyn/cm2 (n = 18), 11.1 ± 0.6 dyn/cm2 (n = 19), and 11.1 ± 0.6 dyn/cm2 (n = 18) for microvessels in untreated gels and in 1 and 20 mM genipin-treated gels, respectively.
In contrast to our hypothesis, vascular stability under moderate flow was not affected by crosslinking of gels. Whether made in untreated or genipin-treated gels, microvessels remained patent and sustained flow for two weeks [Fig. 3(C)]. Differences in the flow curves were not statistically significant (p > 0.5). A slight decrease in perfusion rate occurred over time in all three types of scaffolds (p < 0.0001), which we have noted before.27
Vascular barrier function was also unaffected by crosslinking of gels. We perfused vessels on day 3 with media that contained fluorescent dextran, and then monitored any leakage of solute. In particular, we measured the number of focal leaks, since substrate stiffening has been reported to promote formation of large endothelial gaps.12 The focal leak density was 0.24 ± 0.38 leaks/mm, 0.26 ± 0.30 leaks/mm, and 0.09 ± 0.14 leaks/mm in untreated gels and in 1 and 20 mM genipin-treated gels, respectively [Fig. 3(D)]. These differences were not statistically significant (p = 0.16).
Vascular function in genipin-treated gels under destabilizing perfusion conditions
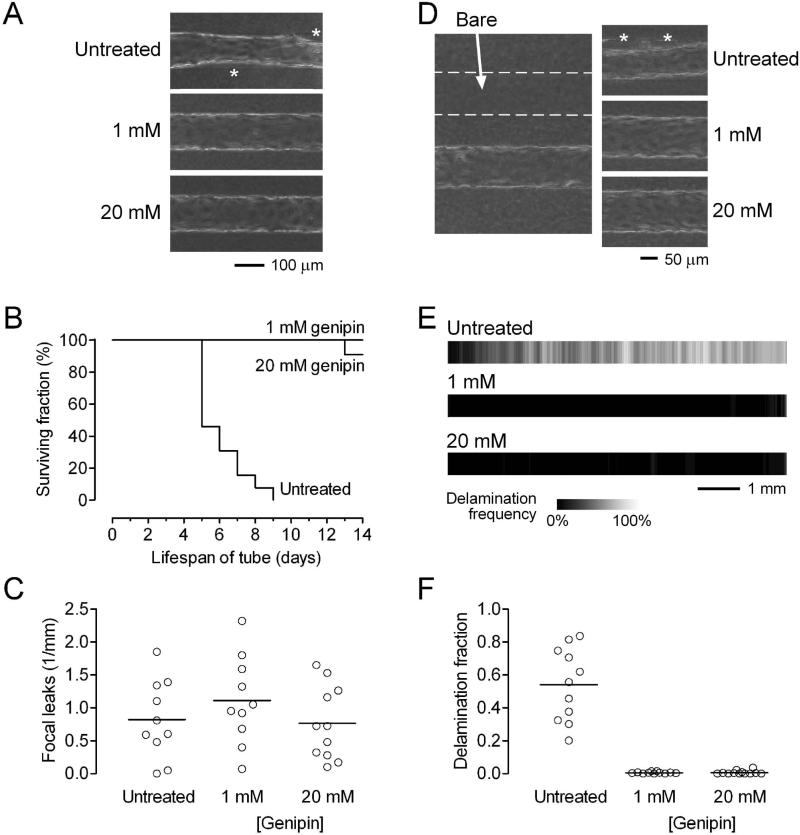
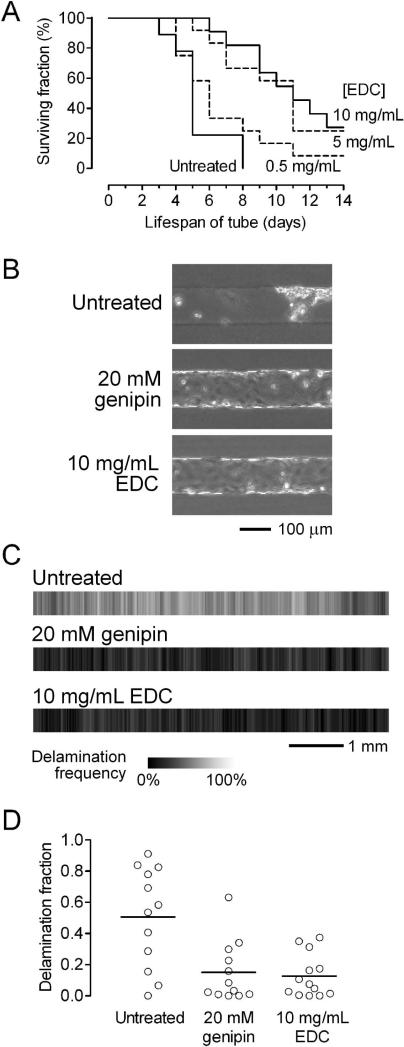
Previous studies suggested that the perturbing effects of substratum stiffness only become apparent during endothelial activation (e.g., with thrombin).12 Since our standard flow conditions are designed to keep the endothelium in a quiescent state, the endothelium in these vessels may not be primed to respond to scaffold crosslinking. Thus, we tested the hypothesis that scaffold crosslinking would accelerate the breakdown of vessels under destabilizing perfusion conditions. We have shown that perfusion under low shear stress (<10 dyn/cm2) or under near zero transmural pressure is sufficient to dramatically reduce vascular lifespan and barrier function.26,27 To test whether genipin-treated scaffolds could further destabilize vessels under low shear, we perfused vessels at a pressure difference of 2.2 cm H2O. With this lower pressure difference, the shear stresses were correspondingly much lower. Shear stress did not differ after three days of perfusion in microvessels made in untreated gels (5.9 ± 0.7 dyn/cm2, n = 20), gels treated with 1 mM genipin (6.1 ± 0.6 dyn/cm2, n = 19) and gels treated with 20 mM genipin (6.0 ± 0.7 dyn/cm2, n = 20) (p > 0.5). Consistent with past work, vessels in untreated scaffolds under low shear often delaminated from the scaffold, which led to progressive loss of perfusion [Fig. 4(A)]. Microvessels were considered “dead” when the flow rate decreased below half of the peak value; Kaplan-Meier survival analysis of vessels in untreated scaffolds showed an average lifespan of less than one week [Fig. 4(B)].
Figure 4.
BEC microvessels in genipin-treated collagen under destabilizing conditions (A-C: shear stress of ~5 dyn/cm2; D-F: transmural pressure of ~0 cm H2O). (A) Phase-contrast images on day 3. Asterisks indicate delaminated regions. (B) Vascular lifespan (n = 13, 12, and 12 for untreated, 1 mM genipin, and 20 mM genipin-treated gels, respectively). (C) Focal leak densities on day 3. (D) Left, phase-contrast image of a vessel adjacent to a bare channel (outlined by dotted lines). Right, images of vascular profile on day 3. Asterisks indicate delaminated regions. (E) Maps of delamination frequencies on day 3 (2n = 22, 22, and 24 for untreated, 1 mM genipin, and 20 mM genipin-treated gels, respectively). Flow is from left to right. (F) Fraction of vascular length that was delaminated by day 3.
To our surprise, nearly all microvessels in genipin-treated scaffolds survived for at least two weeks [Fig. 4(B)]. Their survival was significantly greater than that of vessels in untreated gels (p < 0.0001 for untreated vs. 1 mM and for untreated vs. 20 mM). Strangely, no significant difference was found in the density of focal leaks after three days of perfusion between vessels in untreated (0.8 ± 0.6/mm), 1 mM genipin-treated (1.1 ± 0.7/mm) and 20 mM genipin-treated (0.8 ± 0.6/mm) gels (p = 0.40) [Fig. 4(C)]. We note that the leak densities under low shear (~1/mm) were much greater than those under moderate shear (~0.2/mm) [Fig. 3(D)], irrespective of the scaffold. Thus, despite the lack of significant differences in hydraulic permeability, shear stress, and focal leak density, microvessels in genipin-treated gels had superior lifespan compared to those in untreated ones, when perfused at low shear. This result suggests that stiffening of scaffolds by genipin may itself act as an independent stabilizing factor.
A second destabilizing perfusion condition is the reduction of transmural pressure to nearly zero.27 To impose this condition, we perfused vessels in scaffolds that contained a parallel channel that was left unseeded [Fig. 4(D), left]. Previous numerical modeling indicated that the transmural pressure should be nearly zero along the length of the microvessel because the vessel and adjacent bare channel are driven by the same axial pressures.26 On day 3, the shear stresses of the vessels in these configurations were not significantly different: 9.4 ± 1.6 dyn/cm2 for untreated gels (n = 8); 10.2 ± 1.1 dyn/cm2 for 1 mM genipin-treated gels (n = 9); 10.0 ± 1.6 dyn/cm2 for 20 mM genipin-treated gels (n = 10) (p > 0.5). Vessels in untreated gels delaminated rapidly, often by day 3, whereas those in genipin-treated scaffolds rarely did [Fig. 4(D), right]. We used images of vessels to generate heatmaps of delamination frequency for the three types of scaffolds; these maps clearly showed that vessels in genipin-treated gels were resistant to delamination [Fig. 4(E)]. The fractional length of delamination of vessels was significantly higher in untreated gels (0.54 ± 0.22), compared to the 1 mM (0.005 ± 0.005) and 20 mM genipin-treated gels (0.007 ± 0.012) (p < 0.0001 for untreated vs. 1 mM and for untreated vs. 20 mM). These results indicate that crosslinking with genipin promotes vascular stability in collagen gels.
Vascular stability in EDC-treated gels under destabilizing perfusion conditions
To determine whether the stabilizing effects observed in crosslinked scaffolds were unique to genipin-induced crosslinking, we tested gels that were treated with the carbodiimide EDC. First, we selected EDC concentrations that yielded similar levels of mechanical stiffening as genipin did in flat collagen slabs; treatment with 5 and 10 mg/mL EDC resulted in gels of similar elastic moduli as that with 1 and 20 mM genipin did, respectively [Fig. 2(A)]. Treatment with 0.5 mg/mL EDC led to a slight, but not statistically significant, increase in stiffness (p = 0.11). As found with genipin, EDC did not alter the permeability of collagen gels (p > 0.5) [Fig. 2(B)].
We then formed BEC tubes in untreated and EDC-treated gels, and subjected them to destabilizing flow conditions (pressure difference of 2.2 cm H2O, shear stress of ~5 dyn/cm2). Consistent with a crosslinking-mediated mechanism, vascular stability improved in the scaffolds that were stiffened with 5 or 10 mg/mL EDC (p < 0.0001 for untreated vs. 5 mg/mL, and = 0.0006 for untreated vs. 10 mg/mL) [Fig. 5(A)]. Treatment of collagen with 0.5 mg/mL EDC, a condition that did not result in a statistically significant increase in gel stiffness, did not stabilize vessels under these flow conditions (p = 0.19). These results suggest that mechanical stiffening from crosslinking of scaffolds can promote vascular stability, regardless of the specific crosslinker used.
Figure 5.
Effect of EDC-induced crosslinking on stability of BEC microvessels, and effect of crosslinking on stability of LEC tubes. (A) Vascular lifespan of BEC vessels under low shear stress (n = 9, 12, 11, and 12 for untreated, 0.5 mg/mL EDC, 5 mg/mL EDC, and 10 mg/mL EDC-treated gels, respectively). (B) Phase-contrast images of LEC tubes two days after seeding. (C) Maps of delamination frequencies on day 2 (2n = 24, 24, and 26 for untreated, 20 mM genipin, and 10 mg/mL EDC-treated gels, respectively). Intermittent flow is from left to right. (D) Fraction of vascular length that was delaminated by day 2.
Stability of lymphatic tubes in crosslinked gels
To test whether crosslinking-induced vascular stability also applied to lymphatic endothelial cells (LECs), we used LECs to form tubes in untreated and crosslinked collagen gels. Like BECs, LECs adhered and spread well in untreated gels and in genipin- or EDC-treated gels. We then subjected the seeded channels to intermittent flow, since the flows present in lymphatics are unsteady.32 Pressure and shears in lymphatics in vivo are much smaller than in blood vessels, and lymphatic-like stresses should thus be extremely destabilizing in vitro. Indeed, LECs in untreated gels formed tubes that often delaminated catastrophically by day 2, leaving behind large swaths of barren collagen and clumps of LECs [Fig. 5(B)]. In crosslinked gels, LEC tubes were substantially more stable, as shown by phase-contrast images [Fig. 5(B)], delamination heatmaps [Fig. 5(C)], and comparison of delamination fractions [Fig. 5(D)]. Lymphatic tubes in genipin- or EDC-treated gels delaminated less than those in untreated ones did (p = 0.0041 for untreated vs. genipin, and 0.0019 for untreated vs. EDC). These results indicate that crosslinking of collagen gels helps to maintain LEC vascular stability.
DISCUSSION
This study demonstrates that crosslinking of type I collagen gels by genipin or EDC does not impair endothelial lifespan and barrier function in the context of perfused vessels in vitro. Instead, crosslinked gels promoted vascular stability under conditions that normally would result in rapid loss of perfusion. Since these effects occurred only at crosslinker doses that led to a significant change in elastic modulus (but not in hydraulic permeability or shear stress), our findings imply that scaffold stiffening itself can independently contribute to the functional stability of microvessels.
Scaffold crosslinking does not negatively affect vascular function
Recent studies using flat, functionalized polyacrylamide gels have found that the stiffer the substratum, the more activated the endothelium that is cultured atop it.12,14,15 Our original hypothesis was that the same stiffness-induced loss of function would occur in collagen gels, in the context of engineered vessels. Yet, we found that crosslinking (by genipin) did not reduce vascular stability or barrier function under moderate perfusion (shear stress of ~10 dyn/cm2).
We considered several explanations for the lack of stiffness-induced destabilization in these tubes. First, the crosslinker may “fix” the adherent cells and inhibit their ability to actively respond to the environment. Genipin and EDC, while less cytotoxic than a vigorous crosslinker like glutaraldehyde, are by no means benign.19,33 For this reason, we exhaustively flushed gels after crosslinking, and we did not observe differences in initial cell adhesion or spreading, or in the ability of seeded ECs to form an initially confluent tube. Cell viability stains indicated that culture on genipin- or EDC-crosslinked gels and potential exposure to residual soluble crosslinker did not adversely affect cell viability [Fig. 3(A), and data not shown].
Second, the studies with acrylamide gels noted that stiffness-induced EC activation occurred mainly in the presence of an additional activator; that is, substratum stiffness sensitized ECs, but did not activate them by itself.12,13 In our study, tubes that were grown under stabilizing conditions were constantly exposed to cyclic AMP, dextran, and moderate levels of shear stress. In previous work, we showed that these conditions promote barrier function and vascular stability.26,30,31,34 Thus, it is possible that the baseline perfusion conditions were not activating enough for scaffold stiffness to reveal a measurable deleterious effect. We think this explanation is insufficient, since destabilizing perfusion conditions also did not yield stiffness-induced instability (see below).
Third, the range of scaffold moduli analyzed in the current study is below those in previous studies. Reinhart-King and colleagues examined Young's moduli of 2.5-10 kPa, while van Niemo Amerongen and colleagues considered those in the range of 1.2-11 kPa;12,15 the values in these studies were chosen to reflect the mechanics of normal and atherosclerotic arterial walls, and were obtained in polyacrylamide gels. Although our gels were lower in modulus, the several-fold dynamic range matches that considered in previous studies; the 0.1-1 kPa range is routinely achievable with reconstituted collagen gels. We do not know whether a stiffness-induced instability would have occurred had we crosslinked the collagen even more. Practically, the degree of crosslinking we used was already sufficient to nearly eliminate deformation of the gel under perfusion, and many types of scaffolds that are being evaluated for tissue engineering applications have elastic moduli in the range we studied here.35
Scaffold crosslinking contributes to vascular stability
The current study supports the idea that scaffold crosslinking stabilizes, rather than destabilizes, vessels. Our previous work showed that in the presence of low shear stress (<10 dyn/cm2) or transmural pressure (~0 cm H2O), the endothelium quickly delaminates from microfluidic collagen gels.26,27 Surprisingly, scaffolds that were treated with genipin or EDC had superior survival and morphological stability when shear stress was ~5 dyn/cm2, a value that led to an average lifespan of <1 week in untreated gels. Measurement of focal leak density showed a greater leakiness under lower shear, but not specifically in any type of scaffold. Moreover, when transmural pressure was artificially held at ~0 cm H2O along the length of the vessel, the endothelium delaminated in untreated scaffolds but rarely in genipin-treated ones. Finally, under intermittent flow, LECs could form stable tubes in crosslinked gels but rarely in untreated ones. Altogether, these results with two different crosslinkers (genipin, EDC) and two endothelial cell types (BECs, LECs) indicate that crosslinking of scaffolds, and the subsequent stiffening, results in stabilization under perfusion conditions that normally would induce rapid breakdown.
Fracture mechanisms of stiffness-induced stabilization
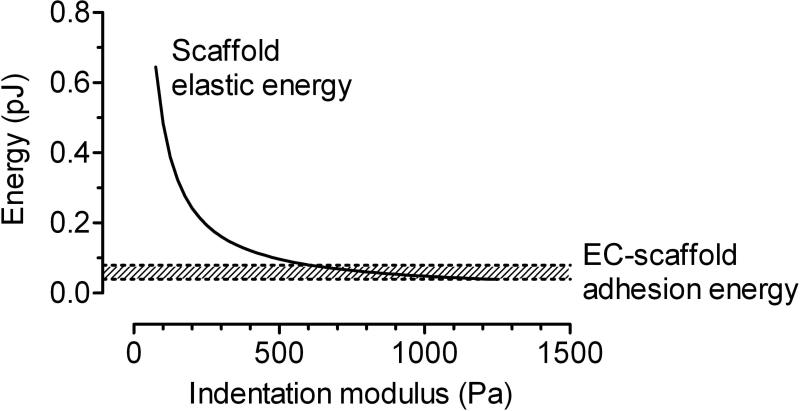
The most straightforward explanation for the enhanced vascular stability in stiff scaffolds is that the channels within a stiff scaffold maintain their shape and thus can support long-term perfusion. This effect may play a role in stabilization of flow rate, but it cannot explain why the endothelium delaminated less frequently in stiffer scaffolds. If we consider the endothelium to be a contractile thin film on the elastic scaffold, then delamination can be viewed as progressive fracture at the endothelial cell-scaffold interface. Similar coarse-grained approaches have been used to estimate the energies of cell-scaffold adhesion in “peel” tests.36 When the interface fractures, elastic and contractile energies are released as the scaffold and endothelium recoil, respectively. According to classical fracture theory, if the released energy exceeds the energy of interfacial adhesion, then fracture will proceed.37 We note that the elastic energy contained in the scaffold is inversely proportional to scaffold stiffness for a given applied stress.28 All other factors being equal, a stiffer scaffold will store less elastic energy, and hence endothelial delamination should be less energetically favorable.
To test whether this explanation is quantitatively plausible, we used finite-element modeling to calculate the scaffold elastic energy released as a function of scaffold stiffness (Fig. 6). For these models, we used parameter values that reflected experimental conditions in the current and previous studies30: EC contractility of 2.7 dyn/cm, and a minimum detectable delaminated area of 20×20 μm2. As predicted, the released elastic energy decreased with an increase in scaffold stiffness. Almost all of this energy resulted from strain of the scaffold, as EC contractile energy was negligible compared to the change in scaffold elastic energies. Reported values of interfacial adhesion energy for slow cell delamination from hydrogels fall in the range of 0.1-0.2 dyn/cm.38 Since the work of adhesion (shaded region in Fig. 6) is comparable to or exceeds the released elastic energy for stiff, but not compliant, scaffolds, the proposed fracture mechanism is quantitatively plausible, and suggests that stiffening scaffolds may be a generally applicable strategy to avoid cell delamination.
Figure 6.
Plot of elastic energy released from recoil of the scaffold upon endothelial delamination, as a function of scaffold elastic modulus. The larger the difference between this energy and the adhesion energy between the endothelium and scaffold (shaded region), the more likely delamination will be.
Other mechanisms, related to the effect of substratum stiffness on cell physiology, may also affect the stability of adhesion. On stiffer substrata, cell contractility tends to be larger, which would result in destabilization.39 On the other hand, the strength of integrin-extracellular matrix bonds appears to increase with stiffness.40,41 These effects are counteracting, and we have not assessed to what extent they contribute to the overall stabilizing effect of scaffold stiffness. Stiffness-independent mechanisms of stabilization may also be present, as chemical modification of collagen by genipin or EDC may affect the affinity with which endothelial cell integrins can bind it.
Implications for vascularization
Our results show that microvessels in crosslinked microfluidic collagen scaffolds exhibited increased long-term stability compared to those in untreated scaffolds. In past studies, we showed that perfusion conditions can be manipulated to promote vascular function.26,27,30 While such signals can be well-controlled in vitro, it is less clear how they could be maintained in vivo when the preformed vessels integrate with a host vascular supply. The current study provides a scaffold-dependent stabilizing factor (scaffold stiffness) that does not rely on constant control over perfusion conditions. In conjunction with recent work, this study suggests that the physical properties of the scaffold (elastic moduli and hydraulic permeability) play an important role in determining the effectiveness of vascularization. Stiffening, up to the ~1 kPa limit considered here, helps stabilize vascular adhesion and neither impairs nor improves barrier function. This result provides a simple strategy to promote blood and lymphatic vascularization in scaffolds suitable for engineering soft tissues.
CONCLUSIONS
This study demonstrates that crosslinking of type I collagen gels by genipin and EDC permits normal endothelial cell physiology and stabilizes blood and lymphatic endothelial microvessels subjected to destabilizing perfusion conditions in vitro. The stabilization correlated with mechanical stiffening, and can be rationalized by a decrease in stored elastic energy in stiff scaffolds. Although recent studies with flat polyacrylamide gels have found that substratum stiffening can weaken endothelial barrier function and increase contractility, the overall effect of stiffening collagen scaffolds on perfused vessels is stabilization. Both genipin and EDC are inexpensive and relatively non-cytotoxic, and routine treatment of collagen-based scaffolds with these or similar compounds may be warranted when long-term vascularization is desired. We note that these crosslinkers act via amine-based chemistry, so they may be applicable to other types of protein-based scaffolds such as fibrin or decellularized tissues.42 Moreover, the stiffness-induced protection against cell delamination may apply to other types of tubular structures, such as epithelial ducts and acini, that behave as contractile, adherent monolayers.
ACKNOWLEDGMENTS
We thank John Hutchinson, Dimitrije Stamenović, Victor Varner, and Billy Law for helpful discussions. Funding was provided by the National Heart, Lung, and Blood Institute under grant HL092335. A.H.K., R.L.T., and B.J.C. were supported through the Boston University Undergraduate Research Opportunities Program.
REFERENCES
- 1.Soller EC, Tzeranis DS, Miu K, So PTC, Yannas IV. Common features of optimal collagen scaffolds that disrupt wound contraction and enhance regeneration both in peripheral nerves and in skin. Biomaterials. 2012;33:4783–4791. doi: 10.1016/j.biomaterials.2012.03.068. [DOI] [PubMed] [Google Scholar]
- 2.Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 3.Yannas IV, Burke JF, Gordon PL, Huang C, Rubenstein RH. Design of an artificial skin. II. Control of chemical composition. J. Biomed. Mater. Res. 1980;14:107–132. doi: 10.1002/jbm.820140203. [DOI] [PubMed] [Google Scholar]
- 4.Duan X, Sheardown H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials. 2006;27:4608–4617. doi: 10.1016/j.biomaterials.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA. Crosslinking of collagen gels by transglutaminase. J. Biomed. Mater. Res. A. 2004;68:756–762. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, van der Werf KO, Fitié CFC, Bennink ML, Dijkstra PJ, Feijen J. Mechanical properties of native and cross-linked type I collagen fibrils. Biophys. J. 2008;94:2204–2211. doi: 10.1529/biophysj.107.111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 8.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown XQ, Bartolak-Suki E, Williams C, Walker ML, Weaver VM, Wong JY. Effect of substrate stiffness and PDGF on the behavior of vascular smooth muscle cells: implications for atherosclerosis. J. Cell. Physiol. 2010;225:115–122. doi: 10.1002/jcp.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VWM, Carman CV, Brain JD, Fredberg JJ, Butler JP, van Nieuw Amerongen GP. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am. J. Physiol. Cell Physiol. 2011;300:C146–C154. doi: 10.1152/ajpcell.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroka KM, Aranda-Espinoza H. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood. 2011;118:1632–1640. doi: 10.1182/blood-2010-11-321125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birukova AA, Tian X, Cokic I, Beckham Y, Gardel ML, Birukov KG. Endothelial barrier disruption and recovery is controlled by substrate stiffness. Microvasc. Res. 2013;87:50–57. doi: 10.1016/j.mvr.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JGN, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am. J. Pathol. 2006;168:1749–1761. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic scaffolds for tissue engineering. Nat. Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 18.Wong KHK, Chan JM, Kamm RD, Tien J. Microfluidic models of vascular functions. Annu. Rev. Biomed. Eng. 2012;14:205–230. doi: 10.1146/annurev-bioeng-071811-150052. [DOI] [PubMed] [Google Scholar]
- 19.Sundararaghavan HG, Monteiro GA, Lapin NA, Chabal YJ, Miksan JR, Shreiber DI. Genipin-induced changes in collagen gels: correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. A. 2008;87:308–320. doi: 10.1002/jbm.a.31715. [DOI] [PubMed] [Google Scholar]
- 20.Olde Damink LHH, Dijkstra PJ, van Luyn MJA, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–773. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Lau TT, Loh WL, Su K, Wang D-A. Cytocompatibility study of a natural biomaterial crosslinker--genipin with therapeutic model cells. J. Biomed. Mater. Res. B. 2011;97:58–65. doi: 10.1002/jbm.b.31786. [DOI] [PubMed] [Google Scholar]
- 22.Yu X, Liu F, Xu Y, Wan C. In vitro study in the endothelial cell compatibility and endothelialization of genipin-crosslinked biological tissues for tissue-engineered vascular scaffolds. J. Mater. Sci. Mater. Med. 2010;21:777–785. doi: 10.1007/s10856-009-3933-8. [DOI] [PubMed] [Google Scholar]
- 23.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006;71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Price GM, Tien J. Subtractive methods for forming microfluidic gels of extracellular matrix proteins. In: Bhatia SN, Nahmias Y, editors. Microdevices in Biology and Engineering. Artech House; Boston, MA: 2009. pp. 235–248. [Google Scholar]
- 25.Price GM, Tien J. Methods for forming human microvascular tubes in vitro and measuring their macromolecular permeability. In: Khademhosseini A, Suh K-Y, Zourob M, editors. Biological Microarrays (Methods in Molecular Biology. Vol. 671. Humana Press; Totowa, NJ: 2011. pp. 281–293. [DOI] [PubMed] [Google Scholar]
- 26.Price GM, Wong KHK, Truslow JG, Leung AD, Acharya C, Tien J. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials. 2010;31:6182–6189. doi: 10.1016/j.biomaterials.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong KHK, Truslow JG, Khankhel AH, Chan KLS, Tien J. Artificial lymphatic drainage systems for vascularized microfluidic scaffolds. J. Biomed. Mater. Res. A. 2013;101:2181–2190. doi: 10.1002/jbm.a.34524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber JR. Elasticity. Springer; New York, NY: 2010. p. 534. [Google Scholar]
- 29.Truslow JG, Price GM, Tien J. Computational design of drainage systems for vascularized scaffolds. Biomaterials. 2009;30:4435–4443. doi: 10.1016/j.biomaterials.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong KHK, Truslow JG, Tien J. The role of cyclic AMP in normalizing the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials. 2010;31:4706–4714. doi: 10.1016/j.biomaterials.2010.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung AD, Wong KHK, Tien J. Plasma expanders stabilize human microvessels in microfluidic scaffolds. J. Biomed. Mater. Res. A. 2012;100:1815–1822. doi: 10.1002/jbm.a.34137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zawieja DC. Contractile physiology of lymphatics. Lymphat. Res. Biol. 2009;7:87–96. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moshnikova AB, Afanasyev VN, Proussakova OV, Chernyshov S, Gogvadze V, Beletsky IP. Cytotoxic activity of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide is underlain by DNA interchain cross-linking. Cell. Mol. Life Sci. 2006;63:229–234. doi: 10.1007/s00018-005-5383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price GM, Chrobak KM, Tien J. Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc. Res. 2008;76:46–51. doi: 10.1016/j.mvr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD. Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromol. Biosci. 2009;9:20–28. doi: 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin MA, Engler AJ, Barber TA, Healy KE, Sweeney HL, Discher DE. Patterning, prestress, and peeling dynamics of myocytes. Biophys. J. 2004;86:1209–1222. doi: 10.1016/S0006-3495(04)74195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thouless MD. Fracture mechanics for thin-film adhesion. IBM J. Res. Develop. 1994;38:367–377. [Google Scholar]
- 38.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han SJ, Bielawski KS, Ting LH, Rodriguez ML, Sniadecki NJ. Decoupling substrate stiffness, spread area, and micropost density: a close spatial relationship between traction forces and focal adhesions. Biophys. J. 2012;103:640–648. doi: 10.1016/j.bpj.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 41.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 42.Haag J, Baiguera S, Jungebluth P, Barale D, Del Gaudio C, Castiglione F, Bianco A, Comin CE, Ribatti D, Macchiarini P. Biomechanical and angiogenic properties of tissue-engineered rat trachea using genipin cross-linked decellularized tissue. Biomaterials. 2012;33:780–789. doi: 10.1016/j.biomaterials.2011.10.008. [DOI] [PubMed] [Google Scholar]