Abstract
A new method for the experimental determination of the permeability of a small sample of a fluid-saturated hierarchically structured porous material is described and applied to the determination of the lacunar–canalicular permeability (KLC) in bone. The interest in the permeability of the lacunar–canalicular pore system (LCS) is due to the fact that the LCS is considered to be the site of bone mechanotransduction due to the loading-driven fluid flow over cellular structures. The permeability of this space has been estimated to be anywhere from 10−17 to 10−25 m2. However, the vascular pore system and LCS are intertwined, rendering the permeability of the much smaller-dimensioned LCS challenging to measure. In this study, we report a combined experimental and analytical approach that allowed the accurate determination of the KLC to be on the order of 10−22 m2 for human osteonal bone. It was found that the KLC has a linear dependence on loading frequency, decreasing at a rate of 2 × 10−24 m2/Hz from 1 to 100 Hz, and using the proposed model, the porosity alone was able to explain 86 % of the KLC variability.
Keywords: Bioengineering, Biomechanics, Human cortical bone, Interstitial fluid flow, Dynamic bone permeability
1 Introduction
Almost two decades ago, it was first proposed that the amplification of the mineralized tissue strain levels needed for osteocyte mechanosensation occurs when interstitial fluid flow is enhanced by the time-varying mechanical loads applied to bone (Weinbaum et al. 1994; You et al. 2001). Bone interstitial fluid flows through different discrete levels of porosity that coexist in cortical bone, which are nested hierarchically one inside another as a set of Russian dolls in microcirculatory pathways (Cowin et al. 2009). The largest pore size (~25 μm radius) is associated with the vascular porosity, which consists of the volume of all the tunnels in bone that contains blood vessels, nerves, and bone interstitial fluid and includes all the osteonal canals (primary and secondary) as well as transverse (Volkmann) canals (Malachanne et al. 2008; Li et al. 1987; Wen et al. 2010). The lacunar–canalicular porosity contains the osteocytes and bone fluid and consists of the volumes of all the osteocyte lacunae (~3–10 μm radius) and the canaliculi channels (~0.15–0.55 μm diameter) (Marotti 1990). The space between the osteocyte and the lacunar–canalicular wall is filled by the osteocyte’s glycocalyx and interstitial fluid. The smallest pore size in bone (<1 nm) is found in the collagen–apatite porosity, where most of the water is bound to ionic crystals in bone (Neuman et al. 1953; Wehrli and Fernandez-Seara 2005). A detailed review of interstitial fluid flow and mechanotransduction in bone was recently presented by Fritton and Weinbaum (2009).
The permeability (inverse of flow resistivity) of the lacunar–canalicular system (LCS) is a critical determinant of interstitial bone fluid flow, which is considered as the main mechanism to stimulate osteocyte cell processes in the LCS and initiate bone mechanotransduction. Accordingly, changes in the lacunar–canalicular permeability may play a key role in bone mechanotransduction as well as in the ability of bone to adapt its local tissue mass and architecture to its mechanical environment (Weinbaum et al. 1994; Cowin et al. 2009; Fritton and Weinbaum 2009). The permeability of the vascular system (KV) has been estimated from the vascular pore architecture (Johnson 1984; Dillaman 1984; Zhang et al. 1998; Swan et al. 2003) and has been experimentally measured using a traditional technique based on Darcy’s law (Rouhana et al. 1981; Li et al. 1987; Wen et al. 2010). This technique consists of measuring the volume of fluid flow per unit area and per unit time across a porous layer, which is then divided by the pore pressure gradient across the layer. Reported estimates and measurements of permeability associated with the vascular porosity span several orders of magnitude (10−11 to 10−17 m2). Unfortunately, Darcy permeability measurements are experimentally unfeasible for assessing the LCS permeability (KLC). The LCS permeability has been derived from theoretical analysis of the fluid flow in the pore architecture of the LCS and from combined experimental–numerical–analytical approaches using bone tissue from animal models. Theoretical estimates of KLC reported in the literature span several orders of magnitude, ranging from 10−17 to 10−20 m2, while experimental studies have reported a different range of LCS permeability, from 10−22 to 10−25m2. This wide range of values and the difference between theoretical and measured LCS permeability suggests that the permeability of the lacunar–canalicular system has not been determined with sufficient accuracy to date.
Measurements of the intrinsic lacunar–canalicular permeability of human cortical bone are scarce, and the effect of loading frequency on the dynamic permeability of the lacunar–canalicular system is practically unknown (Cardoso et al. 2013). In order to eliminate the influence of the vascular permeability in experimental measurements of the lacunar–canalicular permeability, Gailani and Cowin (2008) proposed to perform the permeability measurement in samples containing the lacunar–canalicular porosity only, such as a single osteon. An analytical solution of a saturated compressible poroelastic annular cylinder under an unconfined stress relaxation test was developed, and predictions from this model were compared to experimental stress relaxation measurements made on isolated osteons in vitro, i.e., considering zero blood pressure (Gailani et al. 2009; Gailani and Cowin 2008, 2011). Recently, a frequency-dependent analytical model of a fluid-saturated porous annular cylinder subjected to an axial cyclic strain loading was developed by Benalla et al. (2012). Formulas for the loss tangent, tan[δ(ω̄)], representing the frequency-dependent time-delay between the sinusoidal stress and strain in the LCS, were obtained as a function of the lacunar–canalicular permeability in the radial direction and loading frequency. In the present study, this frequency-dependent analytical model (Benalla et al. 2012) and measurements of loss tangent on isolated human osteons under harmonic loading are used (1) to determine the intrinsic lacunar–canalicular permeability in the radial direction of fresh osteonal samples, (2) to assess the effect of time post-isolation of osteons on lacunar–canalicular permeability measurements, (3) to evaluate the effect of loading frequency on the dynamic permeability of the lacunar–canalicular system, and (4) to investigate the relationship between porosity and permeability in the LCS.
2 Materials and methods
2.1 Formulation of the loss tangent with respect to frequency
The analytical model developed in Benalla et al. (2012) considers a transverse segment of an isolated osteon whose annular region (LCS) between the Haversian canal and cement line is subjected to an axial cyclic loading between two smooth, parallel, and impermeable plates. The contact between the specimen and the plates is assumed to be frictionless, and the applied load is considered to be uniformly distributed on the loaded surface. For boundary conditions, free flow is assumed between the LCS and the vascular pore system (VS) and zero flow is considered at the cement line boundary. The axial loading in the form of an applied strain is
| (1) |
where εo and ω are the amplitude and frequency, respectively. Under the same assumptions (Cowin et al. 2009) but for zero blood pressure, the governing equation of the pore pressure that leads to a permeability and frequency-dependent relationship between stress and strain was found to be (Benalla et al. 2012)
| (2) |
where ω̄ is the dimensionless frequency (Table 2 in Benalla et al. 2012) and the complex-valued modulus, C*(ω̄), is given by
| (3) |
The magnitude and phase of C*(ω̄) depend on the ci (i = 1 – 5), is the axial elastic normal stress constant, Krr is the lacunar–canalicular permeability in the radial direction (also simply referred to as KLC), and σo is the magnitude of the average resultant stress in the osteon, while δ(ω̄) is the phase angle between the applied strain and the resultant stress. Based on Euler’s formula, the dynamic modulus C*(ω̄) can be written as
| (4) |
where C′(ω̄) is known as the storage modulus and C″(ω̄) as the loss modulus of the cyclically loaded osteon. From Eq. (4), the expression of the loss tangent is
| (5) |
Formulas for the storage modulus, loss modulus and tan[δ(ω̄)] are presented in equations (29) and (30), respectively, in Benalla et al. (2012).
2.2 Formulation of the KLC with respect to loss tangent and frequency
In order to express the KLC with respect to tan[δ(ω̄)] and frequency, Eq. (3) is reformulated as
| (6) |
Separating Eq. (6) into real and imaginary parts gives
| (7) |
where βn and αn(n = 1, 3) are the coefficients of the two quadratic expressions in Eq. (7). Solutions of the quadratic expressions provide values of the KLC with respect to specified frequency values: C*(ω̄) is a component of the coefficients in the quadratic Eq. (6). Using Eqs. (4) and (5), C*(ω̄) is expressed with respect to tan[δ(ω̄)] as
| (8) |
Equation (8) permits the computation of complex modulus C*(ω̄) based on the experimental data of tan[δ(ω̄)] at different loading frequencies.
2.3 Sample preparation
Approximately one hundred osteons were extracted from the mid-diaphysis of fresh frozen femurs from three human female donors provided by the National Disease Resource Center. The ages of the donors were 53, 62, and 75 years old, and their medical history excluded any metabolic bone diseases or skeletal cancer. Femurs were kept at −80°C prior to cutting a 10-mm-length segment from the mid-diaphysis. Cortical segments were thawed, cleaned of soft tissue and bone marrow. Then 0.5-mm-thick cross-sections were machined from each segment using a low-speed diamond saw to obtain smooth and parallel surfaces. Each 0.5-mm-thick section was placed on the stage of an inverted biological microscope equipped with a small drilling machine and a diamond drill bit that was aligned with the center of the microscope field of view. An osteon with a circular or near circular shape was identified using the microscope and carefully isolated.
2.4 Micromechanical loading
A small uniaxial cyclic loading apparatus was developed to measure the stress produced in a single osteon undergoing harmonic strain. The experimental device works under displacement control using a digital controller connected in a closed loop to a piezoelectric linear actuator (P-841.10, Physik Instrumente, Auburn, MA). The linear actuator has a maximal push/pull force of 5 N and a minimal displacement increment of 3 nm. The piezoelectric actuator is vertically aligned to a 10 g load cell (Transducer Techniques, Temecula, CA). The osteon is placed over a stainless steel platen at the top of the load cell, immersed in PBS solution, preloaded, and cyclically strained with the piezoelectric actuator under frequencies ranging from 1 to 100 Hz. The cyclic strains applied by the piezoelectric actuator as well as the resultant stresses acquired from the load cell were collected with Lab-View and analyzed in MATLAB to determine the phase angle δ
Experiment 1. Effect of osteon’s storage time on the loss tangent measurements
The change in mechanical properties of post-mortem fresh frozen tissue due to the time elapsed after being thawed for experimental testing was investigated. For this purpose, two subsets of osteon specimens were cyclically loaded with a magnitude of 1,000 μ ε and 1 Hz frequency. The first set (9 osteons) was loaded five times every 60 min, hereafter designated as the hourly group. The second set (9 osteons) was loaded every 24 h for 5 days, and is henceforth named the daily group. Samples in the daily group were stored in PBS at −20°C and thawed before testing every day. Data from these tests determined the maximal time between the isolation and mechanical loading of the osteons in which changes in properties of the tissue are small, defining a time window to perform the experimental tests.
Experiment 2. Measurement of osteon’s loss tangent and KLC at different frequencies
A second experiment was performed to determine tan [δ(ω̄)] and Krr as a function of frequency in a larger set of samples. At twelve different frequencies (1, 2, 3, 5, 8, 10, 15, 20, 30, 50, 70, 100 Hz), cyclic strain was applied to all osteons, and the related tan[δ(ω̄)] was measured individually. The loss tangent was measured based on the slope between the applied strain and the resultant stress that determines the phase angle. Then, the KLC of each osteon was obtained based on curve fitting loss tangent experimental data to the analytical model over the range of loading frequencies. Prior to curve fitting of data into the model, the lacunar–canalicular porosity (φLC), Haversian canal radius, cement line radius, and mineralized tissue elastic modulus were obtained for each osteon based on high-resolution μCT images, light microscope images of the osteons, and a structural model of the LCS morphology, as described in the following sections.
2.5 μCT scanning
After micromechanical testing, osteons were scanned using a high-resolution μCT system (1172 SkyScan, Belgium). X-ray projections were generated from the sample every 0.3°, obtaining 680 consecutive views with 2.1-μm resolution (Nrecon, V.1.6.9, Skyscan, Belgium). Images were density calibrated in Hounsfield Units (HU) and mineral density, ρHA, using a calibration scan containing air, water, and two density phantoms (0.25 and 0.75 g/cm3 of hydroxyapatite). Cortical bone was segmented using a global thresholding procedure to exclude the volumes corresponding to the lacunae and the Haversian canal. Segmented cortical bone images were used to calculate the average tissue mineral density (TMD) in the tissue matrix of each osteon. In addition to the TMD, the total number of lacunae, the volume of the lacunae, and the dimensions of each osteon were determined. Also, μCT images were used to determine the alignment of the Haversian canal at the center of the sample and the absence of cracks or inhomogeneities (i.e., resorption areas) that might increase the sample’s permeability. Osteons that did not meet these requirements were discarded. A total of 18 osteons were retained for experiment 1 and 60 osteons for experiment 2.
2.6 Measurement of lacunar–canalicular porosity (φLC)
The lacunar–canalicular porosity was estimated for each tested osteon as the sum of the lacunar porosity, φLac (%), and the canalicular porosity, φCan (%). The lacunar porosity was assessed by automated 3D counting of closed porosities within the osteon specimen (CTAn, V.1.13, SkyScan, Belgium). The canalicular porosity was estimated using a structural model that used five parameters either taken from the literature or measured from the analyzed osteons. The volume of the osteonal specimen, Vs (mm3), the lacunar density, NLac/mm3, and the inner and outer diameter of the osteon, ri and ro (μm), were derived from μCT and light microscopy images, while the average distance between two neighboring lacunae was evaluated using a parallelepiped periodic unit cell (PPUC) that surrounded a lacuna. The side dimension, L (μm), of the PPUC represents the center-to-center distance between two neighboring lacunae (Beno et al. 2006).
| (9) |
Based on Eq. (9), the average canaliculi length is
| (10) |
where rLac is the average radius of lacunae in the radial direction, estimated to be approximately rLac = 2.5 μm (Beno et al. 2006). Finally, we use the diameter of the canaliculi reported in the literature as 259 ± 129 nm (You et al. 2004), and the number of canaliculi emanating from each lacuna as 62, based on the average of measurements reported in Beno et al. (2006) using the slicing method. The final formula for the canalicular porosity, φCan, is then
| (11) |
2.7 Tissue mineral density and elastic constants
In order to estimate the Young’s elastic modulus of each osteon, the ρHA was obtained from μCT density-calibrated VOIs. Using the relationship between ρHA and from Wagner et al. (2011)
| (12) |
where ρHA is the TMD obtained from the μCT, and the Young’s modulus of the matrix material in the radial direction, , was evaluated. The Young’s modulus in the axial direction, , was calculated based on the symmetry of the fourth-order transversely isotropic elasticity tensor,
| (13) |
where the Poisson’s ratios were and (Weinbaum et al. 1994).
2.8 Determination of the lacunar–canalicular permeability (KLC)
The lacunar–canalicular permeability was first determined from curve fitting of the analytical model (Benalla et al. 2012) against experimental data of tan[δ(ω̄)] measured in the 1–100 Hz frequency range. The curve fitting was performed in MATLAB using a least-squares algorithm with a single free parameter, KLC. Then, the lacunar–canalicular permeability was also obtained using Eq. (6), which represents the inverse solution for KLC from the analytical poroelasticity model of the osteon under cyclic loading.
2.9 Statistical analysis
Descriptive statistics were obtained for the lacunar density (NLac/mm3, #/mm3), lacunar porosity (φLac, %), canalicular porosity (φCan, %), lacunar–canalicular porosity (φLC, %), tissue mineral density (TMD, gHA/cm3), Young’s moduli in the radial, and axial directions ( and , GPa). Repeated measures ANOVA and Tukey’s multiple comparison tests were performed to identify differences in tan[δ(ω̄)] and Krr measured at different time points, as well as at different frequencies. Prior to performing statistical tests, normality was verified by the Kolmogorov–Smirnov test. All tests were performed using Prism statistics software (V.5.03, GraphPad, La Jolla, CA, USA) with a significance level of p < 0.05.
3 Results
3.1 Material properties and microstructure of isolated osteons
The mean ± SD values of the inner and outer radius for the sixty isolated human osteons were 41.5 ± 19.0 and 76.0 ± 32.5 μm, respectively. The volumetric lacunar density, NLac/mm3, was found to be (36.85 ± 9.99) × 103 lacunae per mm3. The average lacunar porosity, φLac, estimated from μCT scans was 1.69 ± 0.50 %, and using the structural model described by Eq. (9), the average canalicular porosity, φCan, was found to be 2.79 ± 0.91 % (Table 1). These two porosities lead to an estimated lacunar–canalicular porosity, φLC, of 4.51 ± 2.01 %. The average tissue mineral density, TMD, or volumetric hydroxyapatite density, ρHA/cm3, from μCT measurements was found to be 0.91 ± 0.06 gHA/cm3, which is used in Eqs. (12–13) to yield Young’s moduli (Wagner et al. 2011) estimates of GPa in the radial direction and GPa in the axial direction.
Table 1.
Measurements and estimates of micro/nanoarchitecture of the lacunar–canalicular network (updated from Cardoso et al. 2013)
| Reference | Method | Tissue | Lacunar porosity (%) |
|---|---|---|---|
| Schneider et al. (2007) | SR-μ CT | Mouse (B6) femoral mid-diaphysis | 1.3 |
| Tommasini et al. (2012) | SR-μ CT | Rat femoral diaphysis | 1.5 |
| Palacio-Mancheno et al. (2013) | μ CT | Rat tibial cortical metaphysis | 1.5 ± 0.44 |
| Present study | μ CT | Human femoral diaphysis | 1.69 ± 0.50 |
|
| |||
| Reference | Method | Tissue | Canalicular porosity (%) |
|
| |||
| Schneider et al. (2011) | FIB/SEM | Mouse (B6) femoral mid-diaphysis | 0.70 |
| Sharma et al. (2012) | CLSM | Rat tibial cortical metaphysis | 14 (overestimated due to partial volume effects) |
| Present study | μ CT | Human femoral diaphysis | 2.79 ± 0.91 |
|
| |||
| Reference | Method | Tissue | Lacuna per unit volume (#/mm3) |
|
| |||
| Hannah et al. (2010) | SR-μ CT | Human femur | 40–90 (×103) |
| Tommasini et al. (2012) | SR-μ CT | Rat femoral diaphysis | 56.5 (×103) |
| Sharma et al. (2012) | CLSM | Rat tibial cortical metaphysis | 67.3 ± 14.0 (×103) |
| Carter et al. (2013) | SR-μ CT | Human femur | 26–37 (×103) |
| Palacio-Mancheno et al. (2013) | μ CT | Rat tibial cortical metaphysis | 68.8 ± 13.2 (×103) |
| Present study | μ CT | Human femoral diaphysis | 36.85 ± 9.99 (×103) |
|
| |||
| Reference | Method | Tissue | Lacunar volume (μ m3) |
|
| |||
| McCreadie et al. (2004) | CLSM | Human hip | 455 ± 200 |
| Hannah et al. (2010) | SR-μ CT | Human femur | 290 ± 107 |
| Sharma et al. (2012) | CLSM | Rat tibial cortical metaphysis | 352 ± 30 |
| Carter et al. (2013) | SR-μ CT | Human femur | ~400 |
|
| |||
| Reference | Method | Tissue | Inter-lacuna separation (μ m) |
|
| |||
| Sugawara et al. (2005) | CLSM | Chick calvaria, E16 days old | 24.1 ± 2.8 |
| Hannah et al. (2010) | SR-μ CT | Human femur | 21.9 ± 6.3 |
| Sugawara et al. (2011) | CLSM | Chick calvaria, E16 days old | 23.5 ± 6.1 |
| Mouse calvaria (B6), E17 days old | 39.6 ± 11.6 | ||
| Sharma et al. (2012) | CLSM | Rat tibial cortical metaphysis | 24.8 ± 1.7 |
|
| |||
| Reference | Method | Tissue | Canaliculi diameter (nm) |
|
| |||
| Marotti (1990) | SEM | Human tibia | 150–550 |
| You et al. (2004) | TEM | Mouse female | 259 ± 129 |
| Sugawara et al. (2005) | CLSM | Chick calvaria, E16 days old | <500 |
| Lin and Xu (2011) | AFM | Bovine tibia, transverse direction | 426 ± 118 |
| Bovine tibia, radial direction | 459 ± 144 | ||
| Bovine tibia, longitudinal direction | 419 ± 113 | ||
| Schneider et al. (2011) | FIB/SEM | Mouse (B6) femoral mid-diaphysis | 95 |
| Sharma et al. (2012) | CLSM | Rat tibia, cortical metaphysis trab. remnants | 520 ± 42 |
| Rat tibia, cortical metaphysis lamellar region | 553 ± 33 | ||
| Rat tibia, cancellous metaphysis | 483 ± 24 | ||
| SEM | Rat tibia, cancellous metaphysis | 335 ± 32 | |
| TEM | Rat tibia, cancellous metaphysis | 228 ± 11 | |
|
| |||
| Reference | Method | Tissue | # canaliculi per lacuna |
|
| |||
| Sugawara et al. (2005) | CLSM | Chick calvaria, E16 days old | 52.7 ± 5.7 |
| Beno et al. (2006) | Estimated from (Remaggi et al. 1998) light microscopy data | Chick | 54 |
| Rabbit | 60 | ||
| Bovine | 85 | ||
| Horse | 115 | ||
| Dog | 81 | ||
| Human | 41 | ||
| Schneider et al. (2011) | FIB/SEM | Mouse (B6) femoral mid-diaphysis | 78 |
| Sugawara et al. (2011) | CLSM | Chick calvaria, E16 days old | 52.7 ± 6.4 |
| Mouse calvaria (B6), E17 days old | 49.7 ± 9.7 | ||
| Sharma et al. (2012) | CLSM | Rat tibia, primary canaliculi | 83.9 ± 14 |
| Rat tibia, secondary canaliculi | 387 ± 34 | ||
μCT micro-computed tomography, CLSM confocal laser scanning microscopy, SR-μCT synchrotron radiation-based micro-CT, TEM transmission electron microscopy, AFM atomic force microscopy, FIB/SEM focused ion beam/scanning electron microscopy
3.2 Effect of osteon freshness on loss tangent
Changes in loss tangent measurements due to the lack of tissue freshness during the time elapsed between the isolation and loading of the sample were analyzed hourly and daily. No significant differences were found in tan[δ(ω̄)] measurements within the first three hours after isolation of osteons (Fig. 1a). However, a small (~1%) but statistically significant (p < 0.05) change in tan[δ(ω̄)] was found when measurements were taken 4 and 5 h after the samples were extracted. In addition, a clear, statistically significant decrease of tan[δ(ω̄)] was found in measurements taken every 24 h, with tan[δ(ω̄)] found to be almost 5 % lower at the fifth day of testing (Fig. 1b).
Fig. 1.
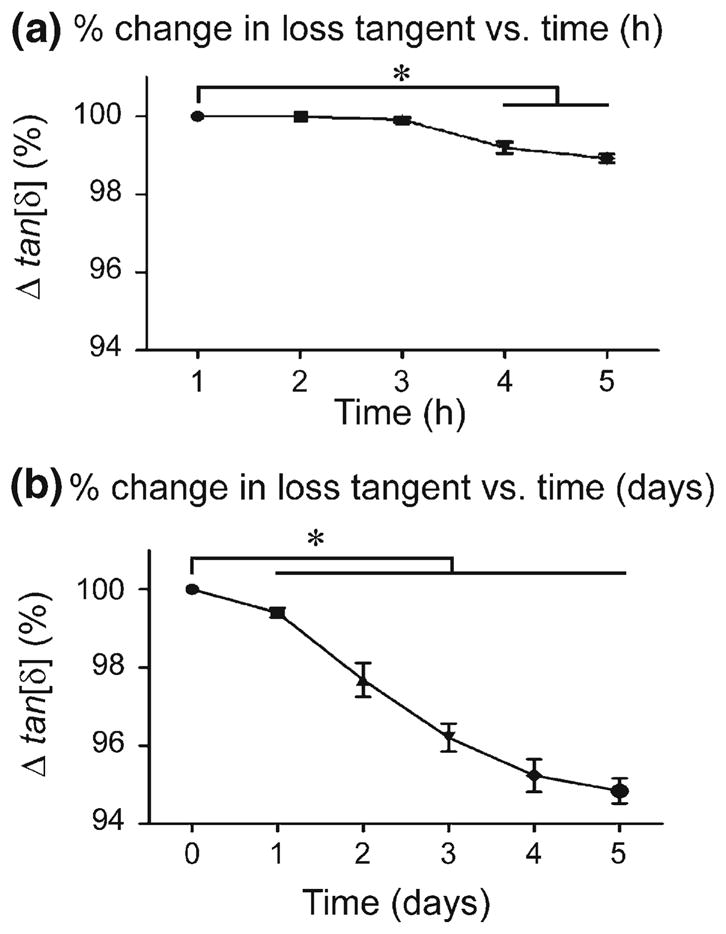
a The loss tangent (mean ± SD) versus time for a period of 5 h. b The loss tangent (mean ± SD) versus time for a duration of 5 days (* p<0.05, nine loaded osteons in each analysis)
The hourly and daily changes in tan[δ(ω̄)] (direct measurement from experiments) were also analyzed to determine whether such changes have a significant effect on KLC (indirect estimates using analytical model). A change of 1 % in tan[δ(ω̄)] observed in the osteon samples measured hourly resulted in a change in KLC of less than 0.1 %, while the 5 % change in tan[δ(ω̄)] from the osteon samples measured daily produced a change in KLC of approximately 10 %. Therefore, to maintain tissue freshness, testing of osteons in the second experiment of this study was performed within 5 h of specimen cutting.
3.3 Intrinsic and dynamic permeability of human LCS
In this study, KLC was obtained by two methods, namely the curve fitting of loss tangent data to the analytical model (Benalla et al. 2012) and by using the inverse solution of the poroelastic model (Eq. 6). In the first method, the experimental measurements of loss tangent at different loading frequencies were curve-fitted to the analytical model (Benalla et al. 2012), in which KLC is the fitting parameter. This approach resulted in an average lacunar–canalicular permeability in the osteonal radial direction, Krr = (2.34 ± 0.33) × 10−22m2 (Fig. 2). Changes in loss tangent due to frequency were not statistically significant different at frequencies below 3 Hz, but differences were significant at 5 Hz and higher frequencies when compared to the loss tangent measured at 1Hz. Also, the standard deviation of the tan[δ(ω̄)] decreased as the frequency increased. The KLC measurements have an 11.4% coefficient of variation (CV = 100 × standard deviation/mean). In the second method, using Eq. (6), the lacunar–canalicular permeability was found to vary between (3.27± 0.45) × 10−22m2 and (1.68± 0.11) × 10−22m2 when analyzed in the 1–100 Hz range, showing a linear decrease with respect to frequency at a rate of −0.02 × 10−22m2/Hz (Fig. 3). The ordinate at the origin (zero frequency) represents the intrinsic KLC, which was found to be 3.27 × 10−22m2. When compared to KLC measured at 1 Hz, the lacunar–canalicular permeability showed no significant differences below 20 Hz, but changes were significant at 30 Hz and higher frequencies.
Fig. 2.
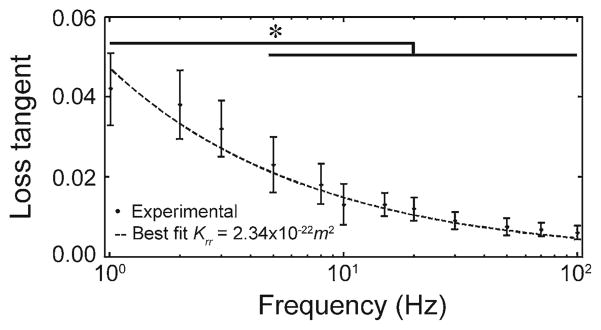
Curve fitting of the analytical model and experimental data of the loss tangent to evaluate the average lacunar–canalicular permeability, Krr = (2.34 ± 0.33) × 10−22m2(* p < 0.05, sixty loaded osteons)
Fig. 3.
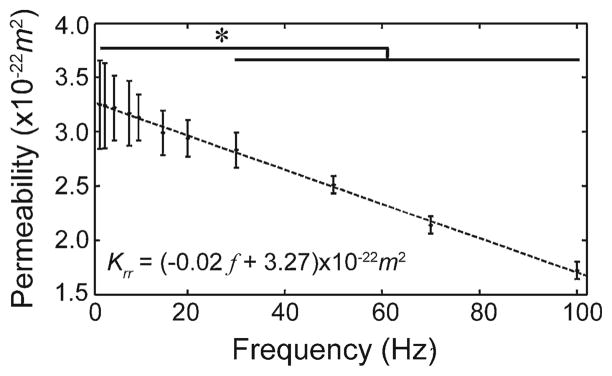
Lacunar–canalicular permeability versus frequency, Krr = (3.27 − 0.02 f ) × 10−22m2(* p < 0.05, sixty loaded osteons) from inverse poroelastic model of Krr
3.4 Loss tangent and KLC as a function of the lacunar–canalicular porosity
The loss tangent versus lacunar–canalicular porosity (φLC) displays a linear behavior within the narrow range of porosity investigated (Fig. 4). The correlation between these two variables is r2 = 0.54, and the equation of the linear fit model is tan[δ(ω̄)] = 0.003φLC + 0.027. Similarly, the analysis of KLC versus φLC exhibits a linear behavior, Krr = (0.27φLC + 1.14) × 10−22m2, and a strong correlation coefficient, r2 = 0.86 with p < 0.05 (Fig. 5).
Fig. 4.
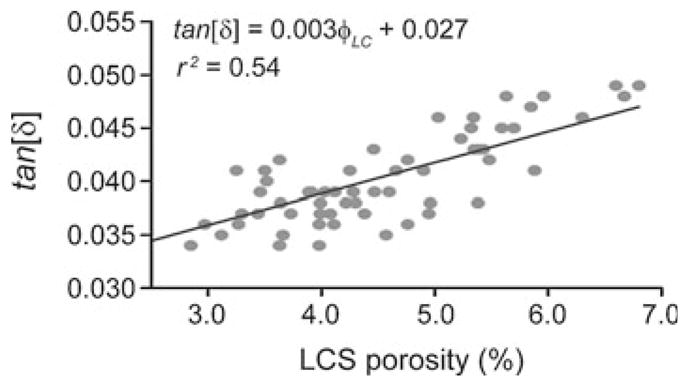
Loss tangent, tan [δ(ω = 2π)], measured at 1 Hz cyclic loading of isolated human osteons versus lacunar–canalicular porosity, φLC (sixty loaded osteons)
Fig. 5.
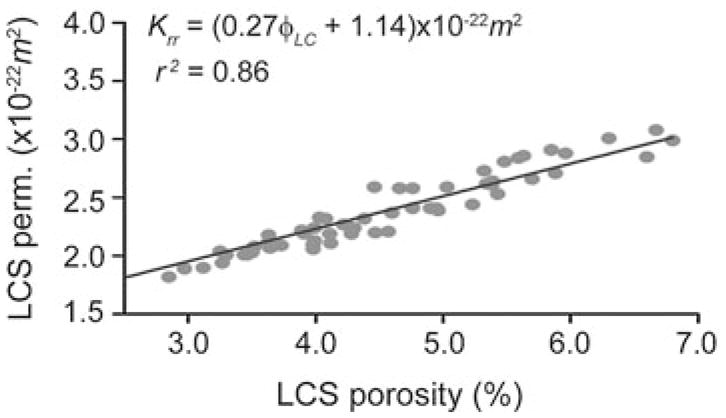
Lacunar–canalicular permeability in the radial direction of isolated human osteons, Krr, versus lacunar–canalicular porosity, φLC (sixty loaded osteons)
4 Discussion
4.1 Material properties and microstructure of isolated osteons
There are not many measurements of the lacunar–canalicular porosity (φLC) reported to date; however, recent advances in 3D imaging have lead to measurement of several microarchitectural parameters of the lacunar–canalicular system, from where φLC can be estimated. The lacunar–canalicular microarchitecture includes the volumetric lacunar density, which represents the number of osteocyte lacuna measured per unit volume (#/mm3), lacunar volume, inter-lacuna separation, canaliculi diameter, and number of primary and secondary (branching) canaliculi emanating from a lacuna (Table 1). The average lacunar porosity, φLac, estimated from μCT scans in this study, 1.69 ± 0.50 %, using 2-μm resolution is in close agreement with the 1.3 % from Schneider et al. (2007) using 0.7-μm resolution in mice, the 1.5 % from Tommasini et al. (2012) using 0.75-μm resolution in rats, and the 1.5 ± 0.44 % lacunar porosity values reported by Palacio-Mancheno et al. (2013) using 1-μm resolution. Based on the structural model described by Eq. (9), the average canalicular porosity, φCan, was found to be 2.79 ± 0.91 %, which is in between the range of values, 0.7 and 14 %, reported by Schneider et al. (2011) using focused ion beam/scanning electron microscopy (FIB/SEM) and Sharma et al. (2012) using confocal laser scanning microscopy (CLSM), respectively. However, measurements of the canalicular porosity using CLSM seem to be overestimated due to partial volume effects. In our study, the addition of the lacunar and canalicular porosities lead to an estimated lacunar–canalicular porosity, φLC, of 4.51±2.01 %. The volumetric lacunar density (NLac/mm3) of 36.85 ± 9.99(×103) lacunae per mm3 measured in this study also falls within the range of values, 26 – 90 × 103 lacunae/mm3, in other studies using synchrotron radiation-based micro-CT (SR-μCT) (Hannah et al. 2010; Tommasini et al. 2012; Carter et al. 2013), μCT (Palacio-Mancheno et al. 2013), and CLSM (Sharma et al. 2012). Interestingly, NLac/mm3 seems to be significantly larger in rodents when compared to human data. Studies using CLSM and SR-μCT have found the average lacunar volume to fall in the range of 290–455 μm3 (Sharma et al. 2012; McCreadie et al. 2004; Hannah et al. 2010; Carter et al. 2013). The inter-lacunar separation has been reported to vary between 22 and 40 μm in different species in CLSM studies (Sharma et al. 2012; Sugawara et al. 2011, 2005), and using SR-μCT (Hannah et al. 2010). Canaliculi morphology measurements were reported in the early work of Marotti (1990), Marotti et al. (1995, 1985) and in several more recent studies. The average canaliculi diameter spans a range of 100–700 nm using electron microscopy (Sharma et al. 2012; Marotti 1990; You et al. 2004; Schneider et al. 2011), CLSM (Sharma et al. 2012; Sugawara et al. 2005), and atomic force microscopy (AFM) (Lin and Xu 2011). Canalicular diameter measurements are considered to be overestimated in studies using CLSM systems because of partial volume effects. Finally, an average of 41–115 primary canaliculi per lacuna was reported using CLSM measurements and estimates from light microscopy measurements (Sharma et al. 2012; Sugawara et al. 2005; Beno et al. 2006), as well as using FIB/SEM (Schneider et al. 2011). Secondary canaliculi, which may branch from the primary canaliculi directly connected to a lacuna, were found to be ~ 390 canaliculi per lacuna (Sharma et al. 2012), which is four times higher than the number of primary canaliculi reported in the same study (Table 1). The tissue mineral density from μCT measurements found in this study using 2.1-μm resolution, 0.91 ± 0.06 gHA/cm3, is in general agreement with previous reports of TMD in human samples, 0.926 ± 0.035 and 1.047 ± 0.046gHA/cm3, measured at 13.5 and 3.4-μm scanning resolution (Souzanchi et al. 2012), respectively. These TMD values from human tissue are slightly lower than TMD values reported in rodents, 1.13–1.36 gHA/cm3 (Palacio-Mancheno et al. 2013; Matsumoto et al. 2006; Martin-Badosa et al. 2003; Windahl et al. 1999).
4.2 Effect of osteon freshness on loss tangent
A main objective of this study was to determine the KLC of fresh human bone after excluding the effect of the vascular system permeability. The freshness of the specimen is one of the most important factors for improving the accuracy in determining KLC. Bone tissue loses some of its freshness with time and storage conditions, despite precautions that can be taken. Keeping the bone specimens hydrated with physiological solution and performing the experiment immediately after the specimen isolation are critical for acquiring ex vivo measurements that are as close as possible to the in vivo condition. Plots of tan[δ(ω̄)] obtained from loaded osteons versus time show a decline of less than 1 % for a time duration of five hours and a decline of 5 % for a period of five days. The 1 % decrease of tan[δ(ω̄)] does not significantly affect the final result of KLC, while the 5 % decrease reduces KLC by 10 %. In this study, we have made every effort to preserve the freshness of the osteons by storing them immersed in PBS and loading them within five hours after isolation.
4.3 Intrinsic permeability of human LCS
The estimates of lacunar–canalicular permeability in Table 2 exhibit a broad range of values, from 10−17 to 10−25 m2, with a noticeable 2–3 orders of magnitude difference between the average permeability value obtained from theoretical approaches (10−17 to 10−20 m2) and experimental/theoretical approaches (10−22 to 10−25 m2). In this study, the values of KLC obtained by both curve fitting and inverse poroelastic model, 2.34 × 10−22m2 and 3.27 × 10−22m2, respectively, are much closer to the predicted values of KLC by several theoretical analyses. Overall, these KLC values lie in the middle range of values reported from theoretical and experimental studies (Table 2). Data also exhibit a small coefficient of variability (14.1 %) when compared to previous reports in the literature (Table 2), possibly due to the individual measurement of φLC and tissue elastic modulus for each osteon.
Table 2.
Measurements and estimates of intrinsic permeability in the vascular and lacunar–canalicular systems (updated from Cardoso et al. 2013)
| Reference | Method (theoretical estimate) | Tissue | VS permeability (m2) |
|---|---|---|---|
| Johnson (1984) | Theoretical study | – | 2.5×10−14 |
| Dillaman (1984) | Theoretical study | Rat | 1 × 10−13 to 1 × 10−11 |
| Zhang et al. (1998) | Theoretical study | – | 1 × 10−15 to 1 × 10−13 |
| Swan et al. (2003) | Theoretical study | – | 1.25 × 10−13 to 3 × 10−12 |
|
| |||
| Reference | Method (experimental measurement) | Tissue | VS permeability (m2) |
|
| |||
| Rouhana et al. (1981) | Perfusion | Bovine | 3 × 10−13 |
| Li et al. (1987) | Perfusion | Canine | 5 × 10−17 to 1 × 10−15 |
| Malachanne et al. (2008) | Compression and FEA | Bovine, ox femurs | 1.1 × 10−13 |
| Wen et al. (2010) | Perfusion | Canine | 4 × 10−17 |
|
| |||
| Reference | Method (theoretical estimate) | Tissue | LCS permeability (m2) |
|
| |||
| Weinbaum et al. (1994) | Ultrastructural model | – | 1.0 × 10−22 to 1.0 × 10−19 |
| Zhang et al. (1998) | Theoretical, adapted from Weinbaum et al. (1994) | – | 1.5 × 10−20 |
| Wang et al. (1999) | Theoretical, adapted from Weinbaum et al. (1994) | – | 1.0 × 10−21 to 1.3 × 10−19 |
| Smit et al. (2002) | Finite element analysis | – | 2.2 × 10−22 |
| Gururaja et al. (2005) | Theoretical, adapted from Weinbaum et al. (1994) | – | 7.5 × 10−20 |
| Beno et al. (2006) | Adapted from Weinbaum et al. (1994) | Chick, rabbit, bovine, horse, dog, human (data from Remaggi et al. 1998) | 1.0 × 10−22 to 1.0 × 10−19 |
| Lemaire et al. (2008) | Glycocalyx fiber permeability, adapted from Weinbaum et al. (1994) | – | 5.9 × 10−18 to 2.0 × 10−17 |
| Anderson et al. (2008) | Perfusion on scaled-up models and predictive virtual and stochastic computational models | Scaled-up, rapid prototyped models of human femoral neck | 2.7 × 10−18 to 8.7 × 10−18 |
| Cowin et al. (2009) | Poroelasticity theory | – | 1.5 × 10−20 |
| Goulet et al. (2009) | Carman–Kozeny permeability model | – | 1.0 × 10−18 to 1.0 × 10−20 |
| Kameo et al. (2010) | Morphology of LCP using CLSM | Swine tibia | 3.3 × 10−19 to 1.3 × 10−18 |
| Lemaire et al. (2012) | FEM, Carman–Kozeny permeability model | – | 1.0 × 10−19 to 1.0 × 10−18 |
|
| |||
| Reference | Method (experimental measurement) | Tissue | LCS permeability (m2) |
|
| |||
| Oyen (2008) | Nanoindentation poroelasticity | Human | 4.1 × 10−24 |
| Galli and Oyen (2009) | Nanoindentation poroelasticity | Human | 6.5 × 10−23 |
| Zhou et al. (2008) | FRAP poroelasticity | Mice | 1.0 × 10−21 |
| Gailani et al. (2009) | Stress relaxation single osteons poroelasticity | Bovine | 5.0 × 10−25 – 8.0 × 10−24 |
| Gardinier et al. (2010) | Step loading in vivo poroelasticity | Canine | 2.8 × 10−23 |
| Present study | Harmonic loading of isolated osteons | Human femoral diaphysis | 2.34 × 10−22 ± 0.33 × 10−22 |
There may be several reasons for the wide range of KLC in previous studies (Cardoso et al. 2013) The first reason is related to the specimen size. A limitation associated with data from experimental studies that have used millimeter size or larger bone samples is that the resulting measurements correspond to the combination of the VS and LCS permeability due to the intermingling of the vascular and lacunar–canalicular pores. Such permeability measurements are thus dominated by the vascular pores, which are much larger than the lacunar–canalicular pores. In order to measure the KLC only, the VS should not be included in the sample to be tested. The second reason is related to the material properties of the specimen. In most previous work, the material properties of the bone were adopted from the literature and generalized to all the samples. The adopted values may be close to the real ones; however, certain parameters, such as the φLC, may have a significant effect on the analytical model, which causes a significant variation in the determination of the KLC. The sensitivity of the model parameters (Benalla et al. 2012) indicated that the φLC and Young’s modulus were among the most influential parameters on the measurement of KLC. Therefore, instead of adopting a single value of φLC and Young’s modulus of the tissue matrix material from the literature, these parameters were determined separately for each osteon specimen. The average Young’s moduli of the matrix material in the radial (15.29 ± 1.31 GPa) and axial (18.72 ± 1.61 GPa) directions as well as the φLC (4.51 ± 2.01 %) were found to be in close agreement with values reported by others (Goulet et al. 2009; Orias et al. 2009; Cardoso et al. 2013). The individual determination of these parameters increased the accuracy in determining KLC. Third, this large variability in the lacunar–canalicular permeability is likely influenced by theoretical assumptions and experimental errors associated with the measurement of a very small quantity. Performing direct measurements on samples with very small permeability and very small size (i.e., samples 100–250 μm in diameter and 0.5 mm in height) is highly sensitive to measurement errors. Fourth, the freshness of the tissue seems very important to obtain appropriate values of permeability. When the tissue is no longer fresh, or has been treated (i.e., dehydrated, resin infiltrated, and embedded), its properties change, and the lacunar–canalicular pores may be not be as open as in their natural state and result in smaller permeability estimates. Fifth, several theoretical estimates analyze the lacunar–canalicular system considering open pores at both ends of the LCS. However, the knowledge of boundary conditions of the analytical model used to estimate the lacunar–canalicular permeability is generally imprecise. Sixth, there exist hydroelectrochemical effects associated with fluid flow in canaliculi (Lemaire et al. 2012) that could significantly influence the permeability of the LCS, which are not fully elucidated to date.
4.4 Dynamic permeability of human LCS
The second goal of this study was to investigate the dependence of KLC on loading frequency. It was found that as the frequency increases from 1 to 100 Hz, KLC decreases linearly at a rate of −0.02 × 10−22 m2/Hz, from 3.25 × 10−22m2 to 1.82 × 10−22m2 (Fig. 3). The inverse relationship between KLC and frequency is considered to be mainly related to the decrease in the loss modulus and the increase in the storage modulus in the matrix material (Figs. 3 and 4 in Benalla et al. (2012)). Because the relaxation time associated with the LCS is around 4.9 ms (Zhang et al. 1998), which corresponds to ~ 200 Hz, it is expected that the decreasing rate of KLC will increase after 200 Hz because the LCS pore pressure will not have enough time to relax entirely. The incomplete relaxation of the LCS will cause the interstitial fluid to lower its velocity between all the lacunae and through the canaliculi, which will amplify the decreasing rate of KLC at loading frequencies higher than 200 Hz. It was noted that the coefficients of variation of tan[δ(ω̄)] and KLC are larger at low frequency and keep decreasing as the frequency increases (Figs. 2 and 3). This observation is explained by the fact that at high frequencies, the mechanical behavior of the osteon becomes purely elastic (Figs. 3 and 4 in Benalla et al. 2012).
4.5 Effect of φLC on tan[δ(ω̄)] and KLC
The sensitivity study in Benalla et al. (2012) showed that φLC is one of the most influential parameters of the model inputs. Therefore, the variability of KLC and tan[δ(ω̄)] versus φLC was also studied. Strong relationships were found between tan[δ(ω̄)] and φLC (Fig. 4) as well as between KLC and φLC (Fig. 5) for the sixty loaded osteons. The high correlation, r2 = 0.86 (p < 0.05), of the permeability with respect to porosity (Fig. 5) shows that there is a strong linear relation-ship between the two parameters and suggests that the variability of KLC depends mainly on φLC. Thus, the equation of the linear fit model, Krr = (0.27φLCS + 1.14) × 10−22m2, represents an empirical formula to predict the KLC from φLC measurements. However, the correlation between loss tangent versus porosity, r2 = 0.54 (p < 0.05), illustrates that tan[δ(ω̄)] is not dependent on the φLC alone and that other parameters such as the elastic constants and inner and outer osteonal radius are also involved.
4.6 Study limitations
There are several limitations in this study. First, the idealization of the osteon and the Haversian canal as pure cylinders is not fully correct. The shape of the osteon and the Haversian canal is not purely circular, and their radii may slightly change from bottom to top. However, the small height of the osteon sections, 500 μm, minimizes the inaccuracy of this assumption. Second, in the evaluation of the lacunar–canalicular porosity, the canaliculi are considered to be straight tubes relating the lacunae with each other and with the Haversian canal. In reality, some of these canaliculi, especially the ones emanating from the apices of the lacunae, radiate along the vertical axis of the lacunae before they bend horizontally following the radial direction Marotti (1996). Nonetheless, only few canaliculi take that bent shape, which reduces their effect. The third limitation is related to the assumption of considering the outer boundary of the osteon to be impermeable. However, interstitial fluid flow across the cement line, if it exists, should be negligible compared to fluid flow at the surface of the Haversian canal. Finally, the φLac was estimated using μCT scans with a 2.1-μm resolution. A more accurate estimate of φLac could be obtained with a nanoCT system.
Acknowledgments
This work was supported by NSF (PHY-0848491, CMMI-1333560, MRI-0723027, and 1229449), NIH/NIA (AG34198), PSC-CUNY Research Award Program of the City University of New York and by a fellowship from the CUNY Graduate Center.
Footnotes
Conflict of interest All authors state that they have no conflicts of interest.
Contributor Information
L. Cardoso, Email: cardoso@engr.ccny.cuny.edu.
S. C. Cowin, Email: sccowin@gmail.com.
References
- Anderson EJ, Kreuzer SM, Small O, Tate MLK. Pairing computational and scaled physical models to determine permeability as a measure of cellular communication in micro- and nano-scale peri-cellular spaces. Microfluid Nanofluid. 2008;4(3):193–204. doi: 10.1007/s10404-007-0156-5. [DOI] [Google Scholar]
- Benalla M, Cardoso L, Cowin SC. Analytical basis for the determination of the lacunar-canalicular permeability of bone using cyclic loading. Biomech Model Mechanobiol. 2012;11(6):767–780. doi: 10.1007/s10237-011-0350-y. [DOI] [PubMed] [Google Scholar]
- Beno T, Yoon YJ, Cowin SC, Fritton SP. Estimation of bone permeability using accurate microstructural measurements. J Biomech. 2006;39(13):2378–2387. doi: 10.1016/j.jbiomech.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Cardoso L, Fritton SP, Gailani G, Benalla M, Cowin SC. Advances in assessment of bone porosity, permeability and interstitial fluid flow. J Biomech. 2013;46(2):253–265. doi: 10.1016/j.jbiomech.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter Y, Thomas CD, Clement JG, Peele AG, Hannah K, Cooper DM. Variation in osteocyte lacunar morphology and density in the human femur-a synchrotron radiation micro-CT study. Bone. 2013;52(1):126–132. doi: 10.1016/j.bone.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Cowin SC, Gailani G, Benalla M. Hierarchical poroelasticity: movement of interstitial fluid between porosity levels in bones. Philos Transact A Math Phys Eng Sci. 2009;367(1902):3401–3444. doi: 10.1098/rsta2009.0099. [DOI] [PubMed] [Google Scholar]
- Dillaman RM. Movement of ferritin in the 2-day-old chick femur. Anat Rec. 1984;209(4):445–453. doi: 10.1002/ar.1092090404. [DOI] [PubMed] [Google Scholar]
- Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech. 2009;41:347–374. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailani G, Benalla M, Mahamud R, Cowin SC, Cardoso L. Experimental determination of the permeability in the lacunar-canalicular porosity of bone. J Biomech Eng. 2009;131(10):101007. doi: 10.1115/1.3200908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailani G, Cowin SC. The unconfined compression of a porous annular cylindrical disk. J Mech Mater. 2008;40(6):507–523. [Google Scholar]
- Gailani G, Cowin SC. Ramp loading in Russian doll poroelasticity. J Mech Phys Solids. 2011;53:103–120. [Google Scholar]
- Galli M, Oyen ML. Fast identification of poroelastic parameters from indentation test. Comput Model Eng Sci. 2009;48:241–270. [Google Scholar]
- Gardinier JD, Townend CW, Jen KP, Wu Q, Duncan RL, Wang L. In situ permeability measurement of the mammalian lacunarcanalicular system. Bone. 2010;46(4):1075–1081. doi: 10.1016/j.bone.2010.01.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet GC, Coombe D, Martinuzzi RJ, Zernicke RF. Poroelastic evaluation of fluid movement through the lacunocanalicular system. Ann Biomed Eng. 2009;37(7):1390–1402. doi: 10.1007/s10439-009-9706-1. [DOI] [PubMed] [Google Scholar]
- Gururaja S, Kim HJ, Swan CC, Brand RA, Lakes RS. Modeling deformation-induced fluid flow in cortical bone’s canalicularlacunar system. Ann Biomed Eng. 2005;33(1):7–25. doi: 10.1007/s10439-005-8959-6. [DOI] [PubMed] [Google Scholar]
- Hannah KM, Thomas CDL, Clement JG, De Carlo F, Peele AG. Bimodal distribution of osteocyte lacunar size in the human femoral cortex as revealed by micro-CT. Bone. 2010;47(5):866–871. doi: 10.1016/j.bone.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Johnson MW. Behavior of fluid in stressed bone and cellular stimulation. Calcif Tissue Int. 1984;36(Suppl 1):S72–76. doi: 10.1007/BF02406137. [DOI] [PubMed] [Google Scholar]
- Kameo Y, Adachi T, Sato N, Hojo M. Estimation of bone permeability considering the morphology of lacunocanalicular porosity. J Mech Behav Biomed Mater. 2010;3(3):240–248. doi: 10.1016/j.jmbbm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Lemaire T, Lemonnier S, Naili S. On the paradoxical determinations of the lacunocanalicular permeability of bone. Biomech Model Mechanobiol. 2012 doi: 10.1007/s10237-011-0363-6. [DOI] [PubMed] [Google Scholar]
- Lemaire T, Naili S, Remond A. Study of the influence of fibrous pericellular matrix in the cortical interstitial fluid movement with hydroelectrochemical effects. J Biomech Eng. 2008;130(1):011001. doi: 10.1115/1.2838025. [DOI] [PubMed] [Google Scholar]
- Li GP, Bronk JT, An KN, Kelly PJ. Permeability of cortical bone of canine tibiae. Microvasc Res. 1987;34(3):302–310. doi: 10.1016/0026-2862(87)90063-x. [DOI] [PubMed] [Google Scholar]
- Lin Y, Xu S. AFM analysis of the lacunarcanalicular network in demineralized compact bone. J Microsc. 2011;241(3):291–302. doi: 10.1111/j.1365-2818.2010.03431.x. [DOI] [PubMed] [Google Scholar]
- Malachanne E, Dureisseix D, Canadas P, Jourdan F. Experimental and numerical identification of cortical bone permeability. J Biomech. 2008;41(3):721–725. doi: 10.1016/j.jbiomech.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Marotti G. The original contributions of the scanning electron microscope to the knowledge of bone structure. In: Bonucci E, Motta PM, editors. Ultrastructure of skeletal tissues. Boston: Kluwer Academic; 1990. pp. 19–39. [Google Scholar]
- Marotti G. The structure of bone tissues and the cellular control of their deposition. Ital J Anat Embryol. 1996;101(4):25–79. [PubMed] [Google Scholar]
- Marotti G, Ferretti M, Remaggi F, Palumbo C. Quantitative evaluation on osteocyte canalicular density in human secondary osteons. Bone. 1995;16(1):125–128. doi: 10.1016/s8756-3282(94)00019-0. [DOI] [PubMed] [Google Scholar]
- Marotti G, Muglia MA, Zaffe D. A SEM study of osteocyte orientation in alternately structured osteons. Bone. 1985;6(5):331–334. doi: 10.1016/8756-3282(85)90324-2. [DOI] [PubMed] [Google Scholar]
- Martin-Badosa E, Amblard D, Nuzzo S, Elmoutaouakkil A, Vico L, Peyrin F. Excised bone structures in mice: imaging at three-dimensional synchrotron radiation micro CT. Radiology. 2003;229(3):921–928. doi: 10.1148/radiol.2293020558. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yoshino M, Asano T, Uesugi K, Todoh M, Tanaka M. Monochromatic synchrotron radiation muCT reveals disuse-mediated canal network rarefaction in cortical bone of growing rat tibiae. J Appl Physiol. 2006;100(1):274–280. doi: 10.1152/japplphysiol.00495.2005. [DOI] [PubMed] [Google Scholar]
- McCreadie BR, Hollister SJ, Schaffler MB, Goldstein SA. Osteocyte lacuna size and shape in women with and without osteoporotic fracture. J Biomech. 2004;37(4):563–572. doi: 10.1016/S0021-9290(03)00287-2. [DOI] [PubMed] [Google Scholar]
- Neuman WF, Toribara TY, Mulryan BJ. The surface chemistry of bone 7. The hydration shell. J Am Chem Soc. 1953;75(17):4239–4242. [Google Scholar]
- Orias AAE, Deuerling JM, Landrigan MD, Renaud JE, Roeder RK. Anatomic variation in the elastic anisotropy of cortical bone tissue in the human femur. J Mech Behav Biomed. 2009;2(3):255–263. doi: 10.1016/j.jmbbm.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyen ML. Poroelastic nanoindentation responses of hydrated bone. J Mater Res. 2008;23(5):1307–1314. doi: 10.1557/Jmr2008.0156. [DOI] [Google Scholar]
- Palacio-Mancheno PE, Larriera AI, Doty SB, Cardoso L, Fritton SP. 3D assessment of cortical bone porosity and tissue mineral density using high-resolution micro-CT: effects of resolution and threshold method. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaggi F, Cane V, Palumbo C, Ferreti M. Histomorphometric study on the osteocyte lacunocanalicular network in animals of different species. I. Woven-fibered and parallel fibered bones. Ital J Anat Embryol. 1998;103:145–155. [PubMed] [Google Scholar]
- Rouhana SW, Johnson MW, Chakkalakal DA, Harper RA. Permeability of the osteocyte lacunocanalicular compact bone. Joint ASME ASCE Conf Biomech Symp AMD. 1981;43:169–172. [Google Scholar]
- Schneider P, Meier M, Wepf R, Muller R. Serial FIB/SEM imaging for quantitative 3D assessment of the osteocyte lacunocanalicular network. Bone. 2011;49(2):304–311. doi: 10.1016/j.bone.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Schneider P, Stauber M, Voide R, Stampanoni M, Donahue LR, Muller R. Ultrastructural properties in cortical bone vary greatly in two inbred strains of mice as assessed by synchrotron light based micro- and Nano-CT. J Bone Miner Res. 2007;22(10):1557–1570. doi: 10.1359/Jbmr.070703. [DOI] [PubMed] [Google Scholar]
- Sharma D, Ciani C, Marin PAR, Levy JD, Doty SB, Fritton SP. Alterations in the osteocyte lacunarcanalicular microenvironment due to estrogen deficiency. Bone. 2012;51(3):488–497. doi: 10.1016/j.bone.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit TH, Huyghe JM, Cowin SC. Estimation of the poroelastic parameters of cortical bone. J Biomech. 2002;35(6):829–835. doi: 10.1016/s0021-9290(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Souzanchi MF, Palacio-Mancheno P, Borisov YA, Cardoso L, Cowin SC. Microarchitecture and bone quality in the human calcaneus: local variations of fabric anisotropy. J Bone Miner Res. 2012;27(12):2562–2572. doi: 10.1002/jbmr.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara Y, Ando R, Kamioka H, Ishihara Y, Honjo T, Kawanabe N, Kurosaka H, Takano-Yamamoto T, Yamashiro T. The three-dimensional morphometry and cell-cell communication of the osteocyte network in chick and mouse embryonic calvaria. Calcif Tissue Int. 2011;88(5):416–424. doi: 10.1007/s00223-011-9471-7. [DOI] [PubMed] [Google Scholar]
- Sugawara Y, Kamioka H, Honjo T, Tezuka K, Takano-Yamamoto T. Three-dimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone. 2005;36(5):877–883. doi: 10.1016/j.bone.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Swan CC, Lakes RS, Brand RA, Stewart KJ. Micromechanically based poroelastic modeling of fluid flow in Haversian bone. J Biomech Eng. 2003;125(1):25–37. doi: 10.1115/1.1535191. [DOI] [PubMed] [Google Scholar]
- Tommasini SM, Trinward A, Acerbo AS, De Carlo F, Miller LM, Judex S. Changes in intracortical microporosities induced by pharmaceutical treatment of osteoporosis as detected by high resolution micro-CT. Bone. 2012;50(3):596–604. doi: 10.1016/j.bone.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DW, Lindsey DP, Beaupre GS. Deriving tissue density and elastic modulus from microCT bone scans. Bone. 2011;49(5):931–938. doi: 10.1016/j.bone.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Wang L, Fritton SP, Cowin SC, Weinbaum S. Fluid pressure relaxation depends upon osteonal microstructure: modeling an oscillatory bending experiment. J Biomech. 1999;32(7):663–672. doi: 10.1016/s0021-9290(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Wehrli FW, Fernandez-Seara MA. Nuclear magnetic resonance studies of bone water. Ann Biomed Eng. 2005;33(1):79–86. doi: 10.1007/s10439-005-8965-8. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27(3):339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Wen D, Androjna C, Vasanji A, Belovich J, Midura RJ. Lipids and collagen matrix restrict the hydraulic permeability within the porous compartment of adult cortical bone. Ann Biomed Eng. 2010;38(3):558–569. doi: 10.1007/s10439-009-9858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(−/−) mice. J Clin Invest. 1999;104(7):895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–1386. doi: 10.1016/s0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]
- You LD, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol. 2004;278(2):505–513. doi: 10.1002/ar.a.20050. [DOI] [PubMed] [Google Scholar]
- Zhang D, Weinbaum S, Cowin SC. Estimates of the peak pressures in bone pore water. J Biomech Eng. 1998;120(6):697–703. doi: 10.1115/1.2834881. [DOI] [PubMed] [Google Scholar]
- Zhou X, Novotny JE, Wang L. Modeling fluorescence recovery after photobleaching in loaded bone: potential applications in measuring fluid and solute transport in the osteocytic lacunarcanalicular system. Ann Biomed Eng. 2008;36(12):1961–1977. doi: 10.1007/s10439-008-9566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


