Abstract
Improving tendon repair using Functional Tissue Engineering (FTE) principles has been the focus of our laboratory over the last decade. Although our primary goals were initially focused only on mechanical outcomes, we are now carefully assessing the biological properties of our tissue-engineered tendon repairs so as to link biological influences with mechanics. However, given the complexities of tendon development and healing, it remains challenging to determine which aspects of tendon biology are the most important to focus on in the context of tissue engineering. To address this problem, we have formalized a strategy to identify, prioritize, and evaluate potential biological success criteria for tendon repair. We have defined numerous biological properties of normal tendon relative to cellular phenotype, extracellular matrix and tissue ultra-structure that we would like to reproduce in our tissue-engineered repairs and prioritized these biological criteria by examining their relative importance during both normal development and natural tendon healing. Here, we propose three specific biological criteria which we believe are essential for normal tendon function: 1) scleraxis-expressing cells; 2) well-organized and axially-aligned collagen fibrils having bimodal diameter distribution; and 3) a specialized tendon-to-bone insertion site. Moving forward, these biological success criteria will be used in conjunction with our already established mechanical success criteria to evaluate the effectiveness of our tissue-engineered tendon repairs.
1. Introduction
Tendon and ligament injuries continue to burden the U.S. population and economy, affecting over 110 million patients (United States Bone and Joint Initiative, 2008) and costing an estimated $30 billion annually (Praemer et al., 1999). Repairing these injuries remains a challenge, often resulting in long-term impairments, such as chronic tendinopathy and osteoarthritis (Rodrigues et al., 2013). Tissue engineering represents a novel approach to potentially improve tendon and ligament repair outcomes by combining cells, biomaterials, and in vitro pre-conditioning to prepare tissue-engineered constructs (TECs) for in vivo implantation. Taking this concept a step further, functional tissue engineering (FTE) establishes the importance of biomechanical aspects of the design process by focusing on how soft and/or hard tissues are normally loaded during activities of daily living (ADLs) (Butler et al., 2000; Guilak et al., 2003). By incorporating these concepts, the field is poised to more effectively design repairs that meet the demands of the in vivo setting. If we are to design truly successful repairs, we must understand both the mechanical and biological in vivo environments influencing the TEC following implantation. Building on the FTE paradigm to establish mechanical success criteria, we now seek to establish biological success criteria to further improve the evaluation of tissue-engineered repairs for clinical translation.
2. Using FTE principles to improve tendon repair
Since FTE was first described in 2000 (Butler et al., 2000), our laboratory has been designing tissue-engineered constructs to improve tendon healing following injury with the ultimate goal of clinical translation. Tendons contain compositionally and structurally distinct, but mechanically interconnected, regions regulated by the normal loading environment and the location within the body (Benjamin et al., 2008; Kjaer, 2004). When tendons are not repaired after injury, the natural tendon healing process often fails to restore normal mechanical properties, leading to increased rates of re-injury (Rettig et al., 2005) as well as tendinopathy and osteoarthritis in the long-term (Rodrigues et al., 2013). The standard of care for many acute tendon injuries is surgical repair, but post-operative clinical outcomes have been variable (Amin et al., 2013; Kato et al., 2002; Silfverskiold et al., 1993). As such, the concept of developing a tissue-engineered repair to augment the healing process remains an attractive alternative.
Guided by FTE principles, we established two primary mechanical success criteria to evaluate the effectiveness of our tissue-engineered constructs (composed of autologous, mesenchymal progenitor cells seeded in collagen scaffolds) to repair a rabbit central patellar tendon (PT) defect (Butler et al., 2008).
Exceeding Peak In Vivo Forces
One aspect of FTE is the importance of characterizing the normal mechanical properties of native tissue for benchmarking engineered constructs and in vivo repairs. We measured peak in vivo forces in the rabbit and goat, finding that tendons experience quite different levels of peak in vivo force (IVF). In the rabbit Achilles’, flexor digitorum profundus, and patellar tendons, in vivo loads range from 11-28% of a tissue’s failure force during moderate ADLs (Juncosa et al., 2003; Malaviya et al., 1998; West et al., 2004). However, in the goat model, the percentage approaches 40% of tensile failure load (Korvick et al., 1996). These results suggested that TECs must be designed to accommodate the differences between species and tissue sites.
Matching Normal Tangent Stiffness up to Peak IVFs with a Safety Factor
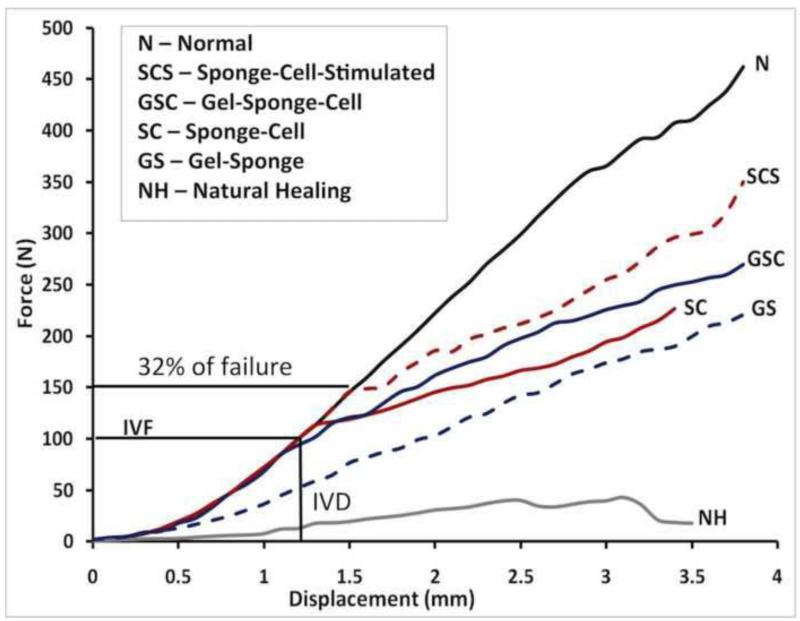
Using the rabbit central PT defect model, we concluded that any TEC repair must match the tangent stiffness of normal PT up to the peak IVFs but also incorporate a safety factor to account for potential overloading during more strenuous activities. Working towards this goal, we strategically improved our repairs by optimizing the cell density, scaffold material, and mechanical preconditioning of the TEC in culture before implantation (Butler et al., 2008). Not only did we generate 12-week repairs of the central PT that exceeded peak IVFs recorded in this model, but we also matched the tangent stiffness of the normal tendon up to 50% beyond peak IVFs (Fig. 1).
Fig. 1. Our laboratory’s iterative efforts to improve tendon repair in a rabbit central patellar tendon defect model using a functional tissue engineering approach.
Natural healing (NH) resulted in significantly inferior biomechanical outcomes when compared to the normal uninjured tendon (N). Over the next decade, we developed tissue-engineered constructs (TECs) for augmenting tendon healing by incorporating cells, collagen scaffolds and in vitro tensile stimulation. Our best TEC repair (denoted as SCS) consisted of a collagen sponge, a mesenchymal stem cell population, and in vitro tensile stimulation, achieving 32% of normal patellar tendon failure force and exceeding tangent stiffness for activities of daily living. Adapted with permission from (Butler et al., 2008).
3. Need for developing biological success criteria
While previous studies have shown our TECs produce an improved mechanical repair outcome compared to natural healing, we still have not fully restored normal tendon structure and function in the following two ways:
Repair tissue does not match tangent stiffness up to at least 40% of failure force, which corresponds to the highest measured in vivo loads we have recorded (Korvick et al., 1996). While matching tangent stiffness up to 32% of failure force is 50% above the peak IVFs recorded in the rabbit PT, there is still room for improvement and focusing on mechanics alone may be insufficient to reach this goal.
We do not adequately understand the biological mechanisms which lead to the successful repairs seen previously. There are several questions that still remain which may help explain these results. Specifically, what was the cell phenotype at the time of harvest, after preconditioning in culture, and following repair? How was the matrix assembled during the repair process and how does its composition change over time? How do interactions between cells, matrix and the local mechanical environment lead to regional differences in tissue properties? These are all questions that would not only help explain these results but also provide potential predictive success criteria for future experiments.
Ultimately, tendon repairs must be able to withstand in vivo mechanical forces to be truly considered a clinical success. Successful mechanical outcomes are, in turn, a function of an ordered sequence of appropriate biological processes. Therefore, we believe it is critical for the field to begin establishing biological parameters which lead to mechanical and clinical benchmarks of successful repair. To address these shortcomings, we propose adapting the FTE paradigm to establish biological success criteria.
4. Strategy to develop biological success criteria
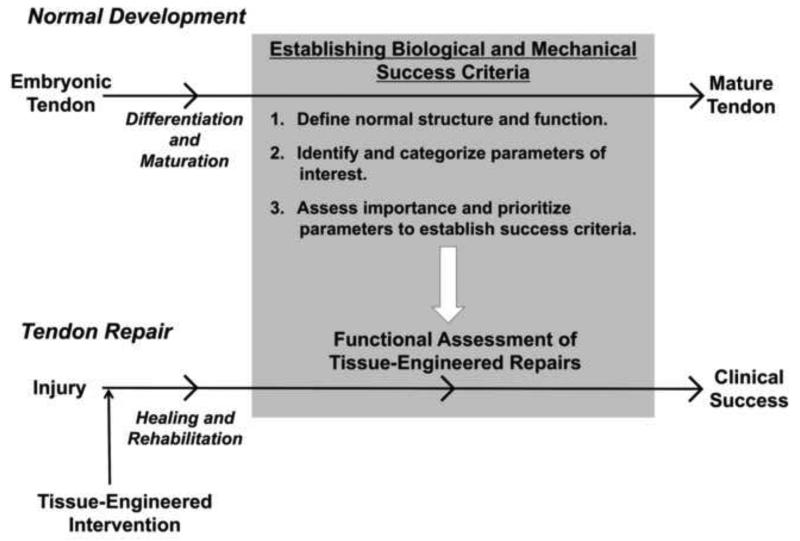
We have previously discussed how our lab has used FTE principles to create mechanical success criteria by 1) measuring normal in vivo tendon forces, 2) selecting mechanical parameters based on sub-failure tendon mechanics, and 3) prioritizing a subset of these parameters to establish mechanical success criteria for functional assessment of our tissue-engineered repairs. We can now use this same approach to generate biological success criteria for tendon repair. First, we must define what constitutes the normal biological properties of tendon. Second, we need to identify biological parameters from the numerous available candidates that are critical to tendon function. Finally, we should assess how deviations in these biological parameters affect mechanical outcomes in order to prioritize which biological properties are crucial for successful tendon repair (Fig. 2).
Fig. 2. Our strategy to establish mechanical and biological success criteria for functional assessment of tissue-engineered tendon repairs.
Building on the FTE paradigm we used to develop mechanical design criteria, we now seek to establish biological design criteria to more fully characterize these repairs. Our strategy is to investigate the normal biological processes responsible for tendon development and maturation, identify and categorize the biological parameters that may be important, and finally, assess how these chosen biological parameters affect the mechanical outcomes of tissue repair. This approach can be applied to other tissue systems and injury models to begin developing connections between the biological processes and the mechanical outcomes.
Mature tendon cells (tenocytes) express necessary transcription factors and signaling ligands to maintain their phenotype and synthesize extracellular matrix proteins which form the scaffolding for the living tendon tissue. The cellular phenotype and extracellular matrix composition varies along the tendon length, generally dividing the tissue into a myotendinous junction, tendon midsubstance and enthesis (Benjamin and Ralphs, 1998; Thomopoulos et al., 2003), thereby producing regional variations in mechanical properties (Arruda et al., 2006; Butler et al., 1990; Rigozzi et al., 2009). Such compositional variation necessitates that we define normal tendon biological parameters based upon the 1) cellular phenotype, 2) extracellular matrix, and 3) regionalization of these two parameters within the tissue ultra-structure.
Understanding tendon development is critical to defining what makes a “tendon a tendon” and allows for the identification of markers which are required for proper tendon formation and potentially necessary for successful repair following injury. While many potential biological properties could be used to establish success criteria for tendon repair, prioritizing these criteria is essential to improving the efficiency and effectiveness of the process. Our lab has been using a strategy of comparing normal tendon development to natural healing to identify potential targets to modulate in future repair studies. Here we introduce three biological success criteria relative to the repair’s cellular phenotype, extracellular matrix and tendon ultra-structure, which we believe are essential for normal tendon function (Fig. 3).
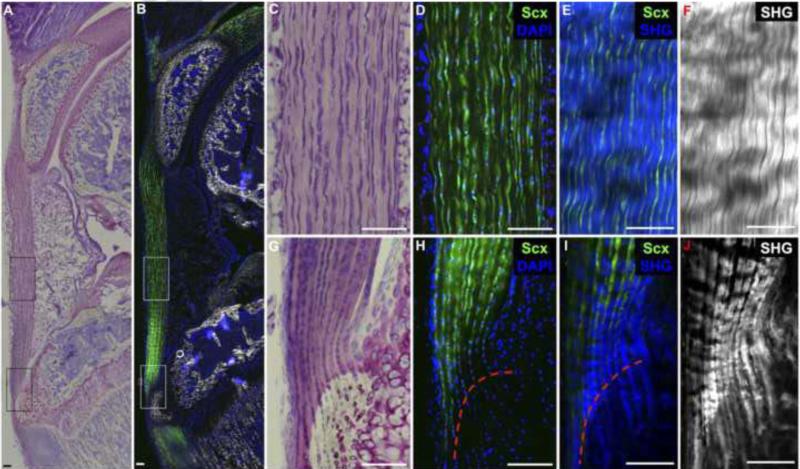
Fig. 3. Biological success criteria include 1) Scleraxis-expressing cells situated between 2) densely-packed collagen fibers aligned along the axis of tension and 3) a zonal enthesis with unmineralized and mineralized fibrocartilage.
Toluidine blue staining (A,C,G) and ScxGFP fluorescence (B,D,H) in a 4-week old murine patellar tendon depict highly aligned tendon fibroblasts within the tendon midsubstance and stacked, rounded cells within the zonal insertion site that are predominately ScxGFP+. Two photon images of tendon midsubstance (E,F) and enthesis (I,J) depict highly-aligned, densely-packed collagen fibers (second harmonic generation signal in blue and grey) within the tendon midsubstance that extend through the enthesis into the underlying bone. The tidemark (red dotted line) indicates the junction between the unmineralized and mineralized fibrocartilage. Scale bars = 100μm.
5. Comparing normal development and natural healing to select biological design criteria
5.1. Cellular phenotype: Scleraxis-expressing cells
The developmental biology field has begun to identify mechanisms leading to normal tendon development, namely transcription factors which aid in characterizing cell phenotype and function to regulate the expression of other important tendon genes, such as extracellular matrix proteins. Scleraxis (Brent et al., 2003; Murchison et al., 2007; Pryce et al., 2009; Schweitzer et al., 2001), mohawk homeobox (Ito et al., 2010; Liu et al., 2010) and early growth response 1 (Guerquin et al., 2013; Léjard et al., 2011) are three of the more extensively studied tenogenic transcription factors. All have been shown to be important regulators of normal tendon development and maturation, as loss of expression of these markers results in phenotypes ranging from severely impaired tendon function in load-bearing tendons (Murchison et al., 2007) to abnormalities in collagen fibrillogenesis and impaired biomechanical properties (Guerquin et al., 2013; Ito et al., 2010; Léjard et al., 2011; Liu et al., 2010). Moreover, spatial variations in the expression of these transcription factors can alter the development of the tendon enthesis. For example, a chondrogenic-tenogenic progenitor pool of cells resides at the enthesis during embryonic development. These cells dually express scleraxis (Scx) and the chondrogenic transcription factor SRY- box containing gene 9 (Sox9), which give rise to the fibrocartilage in the enthesis (Blitz et al., 2013; Sugimoto et al., 2013). Scx-expressing progenitors are required for enthesis formation, as Scx knockout mice exhibit impaired bone tuberosity formation (Blitz et al., 2009). Furthermore, conditionally knocking out Sox9 or disrupting chondrogenic signaling pathways in Scx-expressing cells also results in defective enthesis formation in limb tendons (Liu et al., 2013; Sugimoto et al., 2013).
Of the aforementioned markers, Scx appears to be the most critical regulator of tendon formation known to date. Scx expression defines tendon progenitors during limb condesation (Brent et al., 2003; Schweitzer et al., 2001), and it continues to be expressed in mature tenocytes (Fig. 3). It regulates expression of other tenogenic markers including type I collagen (Léjard et al., 2007), tenascin-C (Edom-Vovard et al., 2002; Schweitzer et al., 2001), and tenomodulin (Shukunami et al., 2006). Its expression during development is so critical that knocking it out results in disorganized and reduced collagen content and a loss of function in many axial and limb tendons (Murchison et al., 2007). However, its role in tendon healing and repair are only beginning to be clarified.
Scleraxis expression during tendon healing exhibits distinct spatiotemporal patterns. Although Scx remains slightly down-regulated during early stages of tendon natural healing (compared to normal tendon) (Dyment et al., 2013; Eliasson et al., 2009; Scott et al., 2011), its expression increases in the tendon callus during later stages of healing (Scott et al., 2011). Furthermore, mechanical loading of the healing tendon during late-stage remodeling can further increase Scx expression (Eliasson et al., 2009). Interestingly, Scx expression during healing appears to originate from a paratenon source of progenitors (Dyment et al., 2013). These cells migrate to the wound site then express Scx as the tenascin-rich provisional matrix transitions to a collagenous matrix. While direct correlations with biomechanical properties have yet to be made, increased Scx expression during remodeling appears to be commensurate with increased biomechanical properties (Dyment et al., 2011; Scott et al., 2011).
Scx expression is necessary, but certainly not sufficient, for the formation of mechanically functional tendon. Although Scx has a role in tendon development and healing, it is also up-regulated in the fibrotic response of many other tissues (Bagchi and Czubryt, 2012; Espira et al., 2009; Mendias et al., 2012). As fibrotic scar is generally characterized by disorganized collagen assembly, biological success criteria should include an organized and competent extracellular matrix to give rise to improved tendon mechanical properties.
5.2. Extracellular matrix: Collagen organization and alignment
Tendons are collagenous tissues composed predominantly of collagen type I (von der Mark, 1981), along with lesser amounts of collagen types III (Lapiere et al., 1977), V (Dressler et al., 2002; Wenstrup et al., 2011), VI (Izu et al., 2011; Ritty et al., 2003) and X (Fujioka et al., 1997), and additional matrix proteins including proteoglycans, such as decorin (Zhang et al., 2006) and lumican (Ezura et al., 2000). Hierarchical in nature, the tendon matrix is composed of collagen microfibrils and fibrils, which aggregate to form fibers. Given their highly organized structure, tendons exhibit high tensile strength, resisting loading in the axial direction, vital to normal tendon function in the body (Galloway et al., 2013; Wren et al., 2001).
The complex process through which collagen fibrils are processed, assembled, and organized is known as collagen fibrillogenesis (Blissett et al., 2009; Zhang et al., 2005). Collagen fibrillogenesis is highly regulated during tendon development, involving the interaction of integrins, collagens, and collagen binding proteins (Kadler et al., 2008). Early in development, small diameter fibrils predominate. As the tendon grows and begins to experience loading, collagen fibrils become longer and larger in diameter, resulting in a matrix that assumes a bimodal distribution of both large (~100-150 nm) and small (~40-75 nm) collagen fibrils to form the mature tendon (Goh et al., 2012). The larger diameter fibrils are thought to resist tensile loading and the smaller diameter fibrils to negate creep and improve fibril binding strength (Parry et al., 1978). Proteoglycans, such as decorin (Zhang et al., 2006), biglycan (Lechner et al., 2006), fibromodulin (Hedlund et al., 1994), and lumican (Ezura et al., 2000), also play a key role in collagen fibrillogenesis by regulating collagen fibril interactions and ultimately tendon mechanics.
Researchers disagree about why bimodal fibril distributions are not restored after injury. Some attribute the inferior mechanical properties to an altered collagen fibrillogenesis process, with a predominance of small diameter fibrils that never form large diameter fibrils (Oryan et al., 2012; Rigozzi et al., 2010). Others contend it may not be the size of the fibrils, but their total number and/or packing density that ultimately affects the mechanical outcome (Battaglia et al., 2003; Frank et al., 1992). Regardless, natural tendon healing typically results in scar tissue formation, consisting of small collagen fibrils that are not oriented along the direction of loading. Further complicating the healing process and rehabilitation is the formation of adhesions between tendons and other structures. Tendon adhesions generally affect intrasynovial flexor tendons, such as in the finger, and can reduce joint range of motion, often leading to pain and discomfort (Khanna et al., 2009; Wong et al., 2009).
Given that tendons are highly loaded structures vital to skeletal and joint movement, understanding their matrix composition is vital to developing effective tissue-engineered repairs. While an aligned collagen matrix is necessary for normal mechanical function, the composition of the matrix varies along the length of the tendon, producing regional variations in mechanical properties. Therefore, as we develop biological success criteria for tendon repair, we must also consider regional differences in the tissue ultra-structure.
5.3. Tissue ultra-structure: Zonal fibrocartilaginous enthesis
Tendons provide the physical linkages between muscle and bone and serve to transmit muscular forces to the skeleton. Thus, a functional tendon actually consists of three specialized tissue regions: the myotendinous junction, the tendon midsubstance, and the tendon-to-bone insertion site (enthesis).
The enthesis is particularly critical for proper mechanical function because it facilitates force transmission between the compliant tendon and the much stiffer bone while also ameliorating potentially damaging stress concentrations that would otherwise accumulate at this interface (Liu et al., 2011; Thomopoulos et al., 2006). Uninjured entheses exhibit gradations in cell phenotype, biochemical composition, matrix organization, and mineral distribution along their length (Genin et al., 2009; Thomopoulos et al., 2003). The enthesis contains rounded fibrochondrocytes (Ralphs et al., 1998) and large amounts of type II and X collagen as well as proteoglycans and glycoproteins such as aggrecan, biglycan, and tenascin C (de Palma et al., 2004; Waggett et al., 1998; Wang et al., 2006). Due to differences in collagen fiber organization and crimp pattern (Lake et al., 2010; Miller et al., 2012; Stouffer et al., 1985), local strains near the insertion are often 2-3 times greater than in the tendon midsubstance under sub-failure tensile loads (Butler et al., 1990; Gilday et al., in review).
As discussed previously, the transcription factors Scx and Sox9 appear to be critical for enthesis formation (Blitz et al., 2013; Sugimoto et al., 2013). Recent evidence also indicates that Indian hedgehog (IHH) signaling is involved in enthesis differentiation, and knocking out this pathway in Scx-expressing cells during development results in morphologic and biomechanical deficits that persist into adulthood (Liu et al., 2012; Liu et al., 2013). However, even if the correct biological cues are in place, experiments using botox to inhibit muscular contraction have shown that muscle loading is also required for normal enthesis formation (Schwartz et al., 2013). In fact, the presence of mature fibrocartilage at the enthesis is correlated with increased compressive loads in this region (Benjamin and Ralphs, 1998), indicating that both biologic and mechanical factors are important players in enthesis development and maturation.
Unfortunately, once damaged, the complex structure of the enthesis is not regenerated during natural healing. Deposition of disorganized scar tissue at the healing tendon-bone interface and the absence of a morphologically normal fibrocartilage transition region results in altered biomechanical properties and premature failures at the enthesis (Galatz et al., 2006; Kinneberg et al., 2011; Newsham-West et al., 2007; Rodeo et al., 1993). Thus, restoring the fibrocartilaginous interface between tendon and bone represents our third biological success criterion for tissue-engineered tendon repair.
6. Discussion
Establishing a set of biological success criteria to accompany the mechanical design goals of tendon repair would greatly benefit both the clinical and tissue engineering communities. The idea of creating biological design standards for engineered tissues is not new (Functional Tissue Engineering Conference Group, 2008; Nawroth and Parker, 2013), but linking structure to function in biological systems remains a significant challenge. This is certainly true in the case of tendon healing and repair as the complexity of these processes makes it difficult to parse out which biological criteria make the largest contributions to tendon mechanics and long-term repair outcomes. To address this problem, we have defined aspects of normal tendon biology relative to cellular phenotype, extracellular matrix and tissue ultra-structure that we would like to reproduce in our tissue-engineered repairs. We are prioritizing these biological criteria by examining their role and relative importance during natural tendon formation and tendon healing. In future tissue engineering studies, we will assess whether achieving these chosen biological criteria improves repair biomechanics.
Although straightforward in principle, this strategy does have challenges. 1) Many aspects of normal tendon biology are not well understood with other biological markers of normal tendon function still waiting to be discovered. Even very fundamental questions such as “what defines a tenocyte?” are still being debated. 2) Tendons are dynamic tissues whose biologic properties depend on anatomical and mechanical cues, which adds a layer of complexity to our strategy (Birch, 2007; Mackey et al., 2008). 3) From a clinical perspective, patient factors such as age (Klatte-Schulz et al., 2012; Yu et al., 2013), gender (Magnusson et al., 2007; Miller et al., 2007), disease (Abate et al., 2013; de Oliveira et al., 2011), and activity level (Obst et al., 2013; Reeves, 2006) affect normal tendon properties, making it even more difficult to establish a set of biological success criteria that would be broadly applicable. 4) Prioritizing biological criteria is currently a rather subjective process since few published studies have linked a particular biological criterion with a functional mechanical outcome. We chose to compare and contrast normal tendon development (a model of successful tendon formation) with inadequate natural healing. We reasoned that biological criteria present during normal development but absent in natural healing might be effective initial targets to modulate during repair. However, this approach to prioritizing biological success criteria is predicated on the assumption that successful tendon healing should spatially and temporally resemble normal tendon development, which may not be the case.
While our approach represents one possible strategy for establishing a set of biological success criteria for tendon repair, other approaches should be investigated. 1) Recent evidence indicates that some animal models, such as the Murphy Roth’s Large (MRL) mouse, display “super healing” capabilities (Clark et al., 1998). Uncovering biological differences between super healers and normal healers may reveal new biological criteria that significantly affect tendon repair. 2) Studies investigating the underlying genetic and/or structural differences between patients with good and poor surgical outcomes could identify biological criteria most highly correlated with clinical success.
Establishing clear linkages between biology and mechanics in tissue-engineered tendon repairs will require well-designed and appropriately controlled studies that isolate specific biological criteria and quantify their effects on mechanical repair outcomes. However, such studies are inherently difficult because biological criteria are often qualitative in nature and may be difficult to measure. Biological criteria also vary widely across both spatial and temporal scales. Small, early changes in one biological criterion (for example, expression of a certain transcription factor) may result in drastic changes in mechanics later on, but these correlations are hard to detect. Furthermore, biological processes are often interrelated and many compensatory mechanisms are activated if normal biology is altered in any way, making it difficult to isolate the effects of a single factor. Finally, establishing biological and mechanical homology across species is challenging but will be required if novel tendon repair strategies are to be translated towards the clinic. This is a particularly difficult task for functional tissue engineers, since investigators use different injury models and assess different mechanical repair outcomes at different time points.
7. Conclusion
The tissue engineering field needs to develop better strategies and adopt more unified approaches for the identification, prioritization, and evaluation of biological success criteria for tissue repair. Our laboratory has developed a general strategy in which we: 1) identify and categorize biological parameters of normal tendon based on cellular phenotype, extracellular matrix and tissue ultra-structure; 2) select a subset of biological parameters by examining their relative importance during both normal development and natural healing; and 3) prioritize these parameters by experimentally assessing whether they affect mechanical outcomes in a tissue engineering scenario. This paradigm, although presented here only in the context of tendon repair, could be applied to any load-bearing tissue in the body. We have begun using this strategy to select specific biological success criteria critical to normal tendon function. In our view, a successful tendon repair must not only meet the stated mechanical design limits but also exhibit 1) scleraxis-expressing cells embedded within 2) a well-organized and axially-aligned collagen matrix that is 3) securely attached to bone via a fibrocartilaginous enthesis as a protection against long term failure. Future tissue engineering studies linking these biological success criteria with improved in vivo mechanical outcomes will be critical for validating the utility of this approach. Ultimately, understanding which biological criteria are most predictive of successful mechanical outcomes will expedite and streamline the tissue engineering process and result in improved treatments for tendon injuries.
Acknowledgements
The authors would like to acknowledge funding from NIH grants R01-AR056943-05, T32-GM063483, R01-AR54713, R01-AR052374, and T90-DE021989 and the NSF IGERT training program 333377. The authors would like to thank Drs. Cy Frank (U. of Calgary), Richard Brand (OREF) and Jack Lewis (U. of Minnesota) for stimulating discussion regarding the strategy proposed in this paper. The authors also want to recognize the many other collaborators over the past 36 years who have contributed to aspects of this work. These individuals include bioengineering colleagues at the University of Cincinnati (Drs. Hani Awad, Aditya Chaubey, Kumar Chokalingam, John Cummings, Matthew Dressler, Edward Grood, Bala Haridas, John Holden, Shawn Hunter, Natalia Juncosa-Melvin, Sanjit Nirmalanandhan, Frank Noyes, and Donald Stouffer, as well as David Glos, Matthew Harris, and John West); veterinary and human surgery colleagues in Cincinnati (Drs. Greg Boivin, Chris Casstevens, Marc Galloway, Brian Grawe, Michael Greiwe, Samer Hasan, Namdar Kazemi, Keith Kenter, and Donna Korvick); researchers at Cincinnati Children’s Hospital (Lindsey Aschbacher-Smith, Jane Florer, Chris Frede, and Drs. Chia-Feng Liu, Richard Wenstrup and Christopher Wylie); bioengineering, biological, and design collaborators and co-authors in the musculoskeletal field (Ms. Mary Beth Privitera and Drs. Al Banes, Arnold Caplan, David Fink, Steve Goldstein, Steve Gordon, Farsh Guilak, Peter Maye, Van Mow, David Mooney, Heather Powell, David Rowe, Jeff Ruberti, Ronen Schweitzer, Randall Young, and Savio Woo).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abate M, Schiavone C, Salini V, Andia I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford, England) 2013;52:599–608. doi: 10.1093/rheumatology/kes395. [DOI] [PubMed] [Google Scholar]
- Amin NH, Old AB, Tabb LP, Garg R, Toossi N, Cerynik DL. Performance outcomes after repair of complete achilles tendon ruptures in national basketball association players. The American Journal of Sports Medicine. 2013;41:1864–1868. doi: 10.1177/0363546513490659. [DOI] [PubMed] [Google Scholar]
- Arruda EM, Calve S, Dennis RG, Mundy K, Baar K. Regional variation of tibialis anterior tendon mechanics is lost following denervation. Journal of Applied Physiology. 2006;101:1113–1117. doi: 10.1152/japplphysiol.00612.2005. [DOI] [PubMed] [Google Scholar]
- Bagchi RA, Czubryt MP. Synergistic roles of scleraxis and Smads in the regulation of collagen 1α2 gene expression. Biochimica Et Biophysica Acta. 2012;1823:1936–1944. doi: 10.1016/j.bbamcr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Battaglia TC, Clark RT, Chhabra A, Gaschen V, Hunziker EB, Mikic B. Ultrastructural determinants of murine achilles tendon strength during healing. Connective Tissue Research. 2003;44:218–224. [PubMed] [Google Scholar]
- Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. Journal of Anatomy. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. Journal of Anatomy. 1998;193(Pt 4):481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch HL. Tendon matrix composition and turnover in relation to functional requirements. International Journal of Experimental Pathology. 2007;88:241–248. doi: 10.1111/j.1365-2613.2007.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissett AR, Garbellini D, Calomeni EP, Mihai C, Elton TS, Agarwal G. Regulation of collagen fibrillogenesis by cell-surface expression of kinase dead DDR2. Journal of Molecular Biology. 2009;385:902–911. doi: 10.1016/j.jmb.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development. 2013;140:2680–2690. doi: 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, , Johnson RL, Tabin CJ, Schweitzer R, Zelzer E. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Developmental Cell. 2009;17:861–873. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Butler DLD, Sheh MYM, Stouffer DCD, Samaranayake VAV, Levy MSM. Surface strain variation in human patellar tendon and knee cruciate ligaments. Journal of Biomechanical Engineering. 1990;112:38–45. doi: 10.1115/1.2891124. [DOI] [PubMed] [Google Scholar]
- Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, Awad H. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. Journal of Orthopaedic Research. 2008;26:1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- Butler DL, Goldstein SA, Guilak F. Functional Tissue Engineering: The Role of Biomechanics. Transactions of the ASME. 2000;122:1–6. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clinical Immunology and Immunopathology. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- de Oliveira RR, Lemos A, de Castro Silveira PV, da Silva RJ, de Moraes SR. Alterations of tendons in patients with diabetes mellitus: a systematic review. Diabetic Medicine : A Journal of the British Diabetic Association. 2011;28:886–895. doi: 10.1111/j.1464-5491.2010.03197.x. [DOI] [PubMed] [Google Scholar]
- de Palma L, Marinelli M, Meme L, Pavan M. Immunohistochemistry of the enthesis organ of the human Achilles tendon. Foot & Ankle International. 2004;25:414–418. doi: 10.1177/107110070402500609. [DOI] [PubMed] [Google Scholar]
- Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. Journal of Orthopaedic Research. 2002;20:1315–1322. doi: 10.1016/S0736-0266(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Dyment NA, Kazemi N, Aschbacher-Smith L, Barthelery NJ, Kenter K, Gooch C, Shearn JT, Wylie C, Butler DL. The relationships among spatiotemporal collagen gene expression, histology, and biomechanics following full-length injury in the murine patellar tendon. Journal of Orthopaedic Research. 2011;30:28–36. doi: 10.1002/jor.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyment NA, Liu C, Kazemi N, Aschbacher-Smith L, Kenter K, Breidenbach AP, Shearn JT, Wylie C, Rowe DW, Butler DL. The Paratenon Contributes to Scleraxis-Expressing Cells during Patellar Tendon Healing. PLoS ONE. 2013;8:e59944. doi: 10.1371/journal.pone.0059944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edom-Vovard F, Schuler B, Bonnin M, Teillet M, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Developmental Biology. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- Eliasson P, Andersson T, Aspenberg P. Rat Achilles tendon healing: Mechanical loading and gene expression. Journal of Applied Physiology. 2009;107:399–407. doi: 10.1152/japplphysiol.91563.2008. [DOI] [PubMed] [Google Scholar]
- Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IMC, Czubryt MP. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. Journal of Molecular and Cellular Cardiology. 2009;47:188–195. doi: 10.1016/j.yjmcc.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. Journal of Cell Biology. 2000;151:779–787. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C, McDonald D, Bray D, Bray R, Rangayyan R, Chimich D, Shrive N. Collagen fibril diameters in the healing adult rabbit medial collateral ligament. Connective Tissue Research. 1992;27:251–263. doi: 10.3109/03008209209007000. [DOI] [PubMed] [Google Scholar]
- Fujioka H, Wang GJ, Mizuno K, Balian G, Hurwitz SR. Changes in the expression of type- X collagen in the fibrocartilage of rat Achilles tendon attachment during development. Journal of Orthopaedic Research. 1997;15:675–681. doi: 10.1002/jor.1100150508. [DOI] [PubMed] [Google Scholar]
- Functional Tissue Engineering Conference Group Evaluation criteria for musculoskeletal and craniofacial tissue engineering constructs: a conference report. Tissue Engineering Part A. 2008;14:2089–2104. doi: 10.1089/ten.tea.2007.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. Journal of Orthopaedic Research. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- Galloway MT, Lalley AL, Shearn JT. The role of mechanical loading in tendon development, maintenance, injury, and repair. The Journal of Bone and Joint Surgery. 2013;95:1620–1628. doi: 10.2106/JBJS.L.01004. American Volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, Thomopoulos S. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophysical Journal. 2009;97:976–985. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilday SD, Casstevens EC, Kenter K, Shearn JT, Butler DL. Murine Patellar Tendon Bioimechanical Properties and Regional Strain Patterns During Natural Tendon-to-Bone Healing After Acute Injury. Journal of Biomechanics. doi: 10.1016/j.jbiomech.2013.10.029. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh KL, Holmes D, Lu Y, Purslow PP, Bechet D, Kadler K, Wess T. Bimodal collagen fibril diameter distributions direct age-related variations in tendon resilience and resistance to rupture. Journal of Applied Physiology. 2012;113:878–888. doi: 10.1152/japplphysiol.00258.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerquin M, Charvet B, Nourissat G, Havis E, Ronsin O, Bonnin M, Ruggiu M, Olivera-Martinez I, Robert N, Lu Y, Kadler KE, Baumberger T, Doursounian L, Berenbaum F, Duprez D. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. The Journal of Clinical Investigation. 2013;123:3564–3576. doi: 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Butler DL, Goldstein SA, Mooney D. Functional Tissue Engineering. Springer; New York: 2003. p. 426. [Google Scholar]
- Hedlund H, Mengarelli-Widholm S, Heinegard D, Reinholt FP, Svensson O. Fibromodulin distribution and association with collagen. Matrix Biology. 1994;14:227–232. doi: 10.1016/0945-053x(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Ito Y, Toriuchi N, Yoshitaka T, Ueno-Kudoh H, Sato T, Yokoyama S, Nishida K, Akimoto T, Takahashi M, Miyaki S, Asahara H. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10538–10542. doi: 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izu Y, Ansorge HL, Zhang G, Soslowsky LJ, Bonaldo P, Chu M, Birk DE. Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biology. 2011;30:53–61. doi: 10.1016/j.matbio.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncosa N, West JR, Galloway MT, Boivin GP, Butler DL. In vivo forces used to develop design parameters for tissue engineered implants for rabbit patellar tendon repair. Journal of Biomechanics. 2003;36:483–488. doi: 10.1016/s0021-9290(02)00459-1. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Hill A, Canty-Laird E. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Current Opinion in Cell Biology. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Minami A, Suenaga N, Iwasaki N, Kimura T. Long-term results after primary repairs of zone 2 flexor tendon lacerations in children younger than age 6 years. Journal of Pediatric Orthopedics. 2002;22:732–735. [PubMed] [Google Scholar]
- Khanna A, Friel M, Gougoulias N, Longo UG, Maffulli N. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? British Medical Bulletin. 2009;90:85–109. doi: 10.1093/bmb/ldp013. [DOI] [PubMed] [Google Scholar]
- Kinneberg KRC, Galloway MT, Butler DL, Shearn JT. Effect of implanting a soft tissue autograft in a central-third patellar tendon defect: biomechanical and histological comparisons. Journal of Biomechanical Engineering. 2011;133:091002. doi: 10.1115/1.4004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiological Reviews. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Klatte-Schulz F, Pauly S, Scheibel M, Greiner S, Gerhardt C, Schmidmaier G, Wildemann B. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. European Cells & Materials. 2012;24:74–89. doi: 10.22203/ecm.v024a06. [DOI] [PubMed] [Google Scholar]
- Korvick DL, Cummings JF, Grood ES, Holden JP, Feder SM, Butler DL. The use of an implantable force transducer to measure patellar tendon forces in goats. Journal of Biomechanics. 1996;29:557–561. doi: 10.1016/0021-9290(95)00036-4. [DOI] [PubMed] [Google Scholar]
- Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Tensile properties and fiber alignment of human supraspinatus tendon in the transverse direction demonstrate inhomogeneity, nonlinearity, and regional isotropy. Journal of Biomechanics. 2010;43:727–732. doi: 10.1016/j.jbiomech.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiere CM, Nusgens B, Pierard GE. Interaction between collagen type I and type III in conditioning bundles organization. Connective Tissue Research. 1977;5:21–29. doi: 10.3109/03008207709152608. [DOI] [PubMed] [Google Scholar]
- Lechner BE, Lim JH, Mercado ML, Fallon JR. Developmental regulation of biglycan expression in muscle and tendon. Muscle & Nerve. 2006;34:347–355. doi: 10.1002/mus.20596. [DOI] [PubMed] [Google Scholar]
- Léjard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MHA, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-Œ±1(I) collagen gene in tendon fibroblasts. Journal of Biological Chemistry. 2007;282:17665–17675. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- Léjard V, Blais F, Guerquin M, Bonnet A, Bonnin M, Havis E, Malbouyres M, Bidaud CB, Maro G, Gilardi-Hebenstreit P, Rossert J, Ruggiero F, Duprez D. EGR1 and EGR2 involvement in vertebrate tendon differentiation. Journal of Biological Chemistry. 2011;286:5855–5867. doi: 10.1074/jbc.M110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Aschbacher-Smith L, Bathelery NJ, Dyment N, Butler DL, Wylie C. Spatial and temporal expression of molecular markers and cell signals during normal development of the mouse patellar tendon. Tissue Engineering - Part A. 2012;18:598–608. doi: 10.1089/ten.tea.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Breidenbach A, Aschbacher-Smith L, Butler D, Wylie C. A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PLoS ONE. 2013;8:e65411. doi: 10.1371/journal.pone.0065411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Watson SS, Lan Y, Keene DR, Ovitt CE, Liu H, Schweitzer R, Jiang R. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Molecular and Cellular Biology. 2010;30:4797–4807. doi: 10.1128/MCB.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Birman V, Chen C, Thomopoulos S, Genin GM. Mechanisms of Bimaterial Attachment at the Interface of Tendon to Bone. Journal of Engineering Materials and Technology. 2011;133:011006. doi: 10.1115/1.4002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Heinemeier KM, Koskinen SO, Kjaer M. Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connective Tissue Research. 2008;49:165–168. doi: 10.1080/03008200802151672. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen M, Langberg H, Miller B, Haraldsson B, Westh EK, Koskinen S, Aagaard P, Kjaer M. The adaptability of tendon to loading differs in men and women. International Journal of Experimental Pathology. 2007;88:237–240. doi: 10.1111/j.1365-2613.2007.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya P, Butler DL, Korvick DL, Proch FS. In vivo tendon forces correlate with activity level and remain bounded: evidence in a rabbit flexor tendon model. Journal of Biomechanics. 1998;31:1043–1049. doi: 10.1016/s0021-9290(98)00123-7. [DOI] [PubMed] [Google Scholar]
- Mendias CL, Gumucio JP, Davis ME, Bromley CW, Davis CS, Brooks SV. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle & Nerve. 2012;45:55–59. doi: 10.1002/mus.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Hansen M, Olesen JL, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. Tendon collagen synthesis at rest and after exercise in women. Journal of Applied Physiology. 2007;102:541–546. doi: 10.1152/japplphysiol.00797.2006. [DOI] [PubMed] [Google Scholar]
- Miller KS, Connizzo BK, Feeney E, Soslowsky LJ. Characterizing local collagen fiber realignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. Journal of Biomechanics. 2012;45:2061–2065. doi: 10.1016/j.jbiomech.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Nawroth JC, Parker KK. Design standards for engineered tissues. Biotechnology Advances. 2013;31:632–637. doi: 10.1016/j.biotechadv.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsham-West R, Nicholson H, Walton M, Milburn P. Long-term morphology of a healing bone-tendon interface: a histological observation in the sheep model. Journal of Anatomy. 2007;210:318–327. doi: 10.1111/j.1469-7580.2007.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obst SJ, Barrett RS, Newsham-West R. Immediate effect of exercise on achilles tendon properties: systematic review. Medicine and Science in Sports and Exercise. 2013;45:1534–1544. doi: 10.1249/MSS.0b013e318289d821. [DOI] [PubMed] [Google Scholar]
- Oryan A, Moshiri A, Meimandi-Parizi AH. Short and long terms healing of the experimentally transverse sectioned tendon in rabbits. Sports Medicine, Arthroscopy, Rehabilitation, Therapy & Technology. 2012;4:14. doi: 10.1186/1758-2555-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proceedings of the Royal Society of London, Series B. 1978;203:305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. Rosemont, IL. American Academy of Orthopaedic Surgeons; 1999. p. 182. [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralphs JR, Benjamin M, Waggett AD, Russell DC, Messner K, Gao J. Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. Journal of Anatomy. 1998;193(Pt 2):215–222. doi: 10.1046/j.1469-7580.1998.19320215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves ND. Adaptation of the tendon to mechanical usage. Journal of Musculoskeletal & Neuronal Interactions. 2006;6:174–180. [PubMed] [Google Scholar]
- Rettig AC, Liotta FJ, Klootwyk TE, Porter DA, Mieling P. Potential risk of rerupture in primary achilles tendon repair in athletes younger than 30 years of age. The American Journal of Sports Medicine. 2005;33:119–123. doi: 10.1177/0363546504268720. [DOI] [PubMed] [Google Scholar]
- Rigozzi S, Muller R, Snedeker JG. Collagen fibril morphology and mechanical properties of the Achilles tendon in two inbred mouse strains. Journal of Anatomy. 2010;216:724–731. doi: 10.1111/j.1469-7580.2010.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigozzi S, Muller R, Snedeker JG. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. Journal of Biomechanics. 2009;42:1547–1552. doi: 10.1016/j.jbiomech.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Roth R, Heuser JE. Tendon cell array isolation reveals a previously unknown fibrillin-2-containing macromolecular assembly. Structure. 2003;11:1179–1188. doi: 10.1016/s0969-2126(03)00181-3. [DOI] [PubMed] [Google Scholar]
- Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. The Journal of Bone and Joint Surgery. 1993;75:1795–1803. doi: 10.2106/00004623-199312000-00009. American Volume. [DOI] [PubMed] [Google Scholar]
- Rodrigues MT, Reis RL, Gomes ME. Engineering tendon and ligament tissues: present developments towards successful clinical products. Journal of Tissue Engineering and Regenerative Medicine. 2013;7:673–686. doi: 10.1002/term.1459. [DOI] [PubMed] [Google Scholar]
- Schwartz AG, Lipner JH, Pasteris JD, Genin GM, Thomopoulos S. Muscle loading is necessary for the formation of a functional tendon enthesis. Bone. 2013;55:44–51. doi: 10.1016/j.bone.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Scott A, Sampaio A, Abraham T, Duronio C, Underhill TM. Scleraxis expression is coordinately regulated in a murine model of patellar tendon injury. Journal of Orthopaedic Research. 2011;29:289–296. doi: 10.1002/jor.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Developmental Biology. 2006;298:234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Silfverskiold KL, May EJ, Oden A. Factors affecting results after flexor tendon repair in zone II: a multivariate prospective analysis. The Journal of Hand Surgery. 1993;18:654–662. doi: 10.1016/0363-5023(93)90312-Q. [DOI] [PubMed] [Google Scholar]
- Stouffer DC, Butler DL, Hosny D. The relationship between crimp pattern and mechanical response of human patellar tendon-bone units. Journal of Biomechanical Engineering. 1985;107:158–165. doi: 10.1115/1.3138536. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, Hiraki Y, Shukunami C. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013;140:2280–2288. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Marquez JP, Weinberger B, Birman V, Genin GM. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. Journal of Biomechanics. 2006;39:1842–1851. doi: 10.1016/j.jbiomech.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. Journal of Orthopaedic Research. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- United States Bone and Joint Initiative . The Burden of Musculoskeletal Diseases in the United States. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2008. [Google Scholar]
- von der Mark K. Localization of collagen types in tissues. International Review of Connective Tissue Research. 1981;9:265–324. doi: 10.1016/b978-0-12-363709-3.50012-7. [DOI] [PubMed] [Google Scholar]
- Waggett AD, Ralphs JR, Kwan AP, Woodnutt D, Benjamin M. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biology. 1998;16:457–470. doi: 10.1016/s0945-053x(98)90017-8. [DOI] [PubMed] [Google Scholar]
- Wang INE, Mitroo S, Chen FH, Lu HH, Doty SB. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. Journal of Orthopaedic Research. 2006;24:1745–1755. doi: 10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, Soslowsky LJ, Birk DE. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. The Journal of Biological Chemistry. 2011;286:20455–20465. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Juncosa N, Galloway MT, Boivin GP, Butler DL. Characterization of in vivo Achilles tendon forces in rabbits during treadmill locomotion at varying speeds and inclinations. Journal of Biomechanics. 2004;37:1647–1653. doi: 10.1016/j.jbiomech.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Wong JKF, Lui YH, Kapacee Z, Kadler KE, Ferguson MWJ, McGrouther DA. The cellular biology of flexor tendon adhesion formation: an old problem in a new paradigm. The American Journal of Pathology. 2009;175:1938–1951. doi: 10.2353/ajpath.2009.090380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren TA, Yerby SA, Beaupre GS, Carter DR. Mechanical properties of the human achilles tendon. Clinical Biomechanics. 2001;16:245–251. doi: 10.1016/s0268-0033(00)00089-9. [DOI] [PubMed] [Google Scholar]
- Yu TY, Pang JH, Wu KP, Chen MJ, Chen CH, Tsai WC. Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes. BMC Musculoskeletal Disorders. 2013;14:2. doi: 10.1186/1471-2474-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. Journal of Musculoskeletal Neuronal Interactions. 2005;5:5–21. [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. Journal of Cellular Biochemistry. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]