Abstract
Background
Despite increased identification of spotted fever group rickettsioses (SFGR) in animals and arthropods, human SFGR are poorly characterized in Taiwan.
Methods
Patients with suspected Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever from April 2004 to December 2009 were retrospectively investigated for SFGR antibodies (Abs). Sera were screened for Rickettsia rickettsii Abs by indirect immunofluorescence antibody assay (IFA), and those with positive results were further examined for Abs against R. rickettsii, R. typhi, R. felis, R. conorii, and R. japonica using micro-immunofluorescence (MIF) tests. Polymerase chain reaction (PCR) for detection of SFGR DNA was applied in those indicated acute infections. Case geographic distribution was made by the geographic information system software.
Results
A total of 413 cases with paired serum, including 90 cases of Q fever, 47 cases of scrub typhus, 12 cases of murine typhus, 6 cases of leptospirosis, 3 cases of dengue fever, and 255 cases of unknown febrile diseases were investigated. Using IFA tests, a total of 49 cases with 47 (11.4%) and 4 (1.0%) cases had sera potentially positive for R. rickettsii IgG and IgM, respectively. In the 49 cases screened from IFA, MIF tests revealed that there were 5 cases of acute infections (3 possible R. felis and 2 undetermined SFGR) and 13 cases of past infections (3 possible R. felis and 10 undetermined SFGR). None of the 5 cases of acute infection had detectable SFGR DNA in the blood specimen by PCR. Possible acute infection of R. felis was identified in both one case of Q fever and scrub typhus. The geographic distribution of SFGR cases is similar with that of scrub typhus.
Conclusions
Human SFGR exist and are neglected diseases in southern Taiwan, particularly for the species closely-related to R. felis.
Introduction
Rickettsioses are a group of diseases that historically include rickettsial diseases, ehrlichiosis, anaplasmosis, scrub typhus (caused by Orientia tsutsugamushi), and Q fever (caused by Coxiella burnetii), but Ehrlichia and Anaplasma were removed from the family Rickettsiaceae, Orientia from the genus, and Coxiella from alpha-proteobacteria [1]. These diseases are common zoonoses in humans that may present as a fever of unknown origin in clinical settings. Current classification of rickettsial diseases includes 3 biogroups: spotted fever group rickettsioses (SFGR), typhus group rickettsioses (TGR), and scrub typhus [1], [2]. SFGR are found worldwide, the geographic distribution varying for different Rickettsia spp., and the causative rickettsiae are often named according to the location or the arthropod vectors where they are first described [1], [2]. Most SFGR are tick-borne, except Rickettsia akari and R. felis, which are mite-borne and flea-borne, respectively [1], [3].
SFGR are emerging infections, and many of the causative rickettsiae have been identified as human pathogens, but the human pathogenicity of some SFGR are unknown [1], [3], [4]. They are neglected emerging diseases, particularly in developing countries [5]–[7]. In East Asia, human SFGR infections of R. japonica in Japan [8]–[10], R. conorii, R. japonica, and R. felis in South Korea [11], [12], R. sibirica, R. conorii, R. akari, and R. heilongjiangensis in China [13], [14], and R. sibirica in Mongolia [15] have been identified. In Taiwan, there have been increased reports of isolation and identification of SFGR, including novel strains, from arthropods in recent years [16]–[22]. However, serological investigation of human SFGR was poorly characterized [23], [24]. Although Q fever, scrub typhus, and murine typhus are widely reported by clinicians in Taiwan [25]–[31], human SFGR infections have rarely been identified, except one imported human case of an African tick bite fever in 2009 (caused by R. africae) [32] and one indigenous R. felis infection in 2008 [33]. In addition, many suspected cases of Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever reported to Centers for Disease Control, Taiwan (Taiwan CDC) are excluded by confirmatory tests for each suspected disease. It is reasonable to speculate that SFGR might account for some of these cases that have either similar clinical manifestations or share exposure to arthropod vectors as a risk factor but are treated with agents effective against rickettsioses without appropriate diagnosis.
The aim of this study is to investigate the seroepidemiology of SFGR in patients who had suspected cases of Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever in southern Taiwan.
Methods
Ethics Statement
This study was approved by the Ethics Committee of the E-Da Hospital (EMRP-097-117). The committee waived the need for written informed consent because the demographic information and clinical data were retrospectively recorded, and all of the data were collected anonymously.
Study Setting and Selection of Study Cases
E-Da hospital, a regional and referral hospital comprising 1200 beds, locates at southern Taiwan (Figure 1A). Patients who were clinically suspected Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever were enrolled because these diseases are common zoonoses or arthropod-vectored diseases in Taiwan and are clinically difficult to differentiate them from SFGR. The confirmation or exclusion of each disease was determined according to the final reports of notifiable diseases of Taiwan CDC.
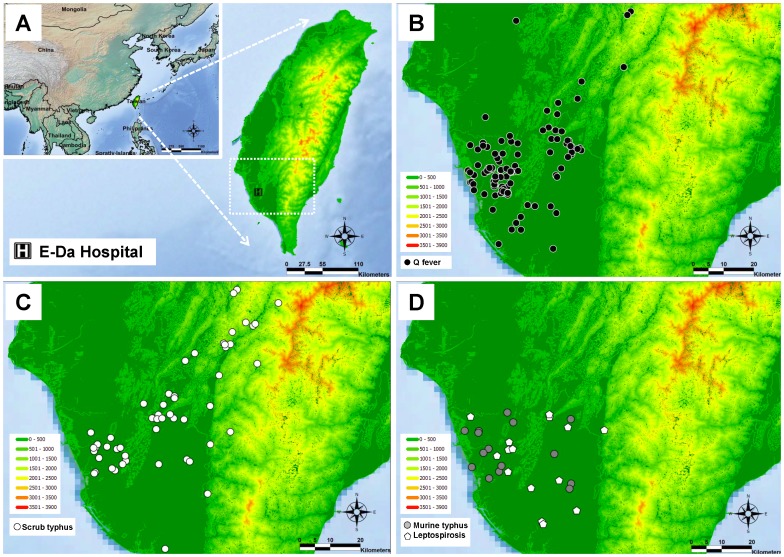
Figure 1. The case distribution of confirmed Q fever, scrub typhus, murine typhus, and leptospirosis.
A: The geographic location of Taiwan and E-Da hospital. B: The case distribution of acute Q fever (black circles). C: The case distribution of scrub typhus (white circles). D: The case distribution of murine typhus (gray circles) and leptospirosis (white pentagons).
Diagnosis of Q Fever, Scrub Typhus, Murine Typhus, Leptospirosis, and Dengue Fever
Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever are notifiable diseases in Taiwan, and clinicians are requested to report patients who are clinically suspected of having these diseases to Taiwan CDC. Paired blood specimens (acute and convalescent phase) are collected and sent to the contract laboratories of Taiwan CDC for confirmatory or exclusive diagnosis. Q fever was diagnosed by an anti-phase II antigen IgM titer of ≥1∶80 and a 4-fold or greater increase of anti-phase II antigen IgG titer in paired sera using indirect immunofluorescence antibody assay (IFA), or positive detection of C. burnetii DNA in the blood by polymerase chain reaction (PCR). Scrub typhus was diagnosed by an antibody titer of IgM ≥1∶80 and 4-fold or greater rise of total antibody (IgG+IgA+IgM) titer in paired sera for Karp, Kato, or Gilliam strain of O. tsutsugamushi using IFA, or positive detection of O. tsutsugamushi DNA in the blood by PCR. Murine typhus was diagnosed by an antibody titer of IgM ≥1∶80 and a 4-fold or greater rise of titer of IgG against to R. typhi in paired sera using IFA, or positive detection of R. typhi DNA in the blood by PCR. Dengue fever was diagnosed by a blood specimen positive for NS1 antigen, a dengue virus gene detected by PCR, or specific IgM against the dengue virus detected by enzyme-linked immunosorbent assay (ELISA). Leptospirosis was diagnosed by a 4-fold or greater increase of specific antibody in paired serum by microscopic agglutination test (MAT) or Leptospira spp. isolated from urine. Patients who were excluded from the initial suspected notifiable diseases by Taiwan CDC were classified as cases of unknown febrile diseases in this study.
Screening Test for SFGR Antibodies by IFA Kits
Blood samples were comprised of the residual specimens obtained for the purpose of diagnosing Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever for the Taiwan CDC. Specimens were stored at −80°C until analysis. Serum was first screened for SFGR (R. rickettsii) and TGR (R. typhi) antibodies by a commercially available IFA kits (Rickettsia IFA IgG [IF0100G] and IgM [IF0100M], Focus Diagnostic, USA). The positive and negative controls of human serum used in the procedures were contained in the IFA kits. Assays were performed according to the manufacturer’s instructions and the test titer started at 1∶40. An antibody titer of ≥1∶80 is considered as positive reaction. Serum with an antibody titer of ≥1∶40 was considered to be potentially positive for SFGR antibodies and was included further micro-immunofluorescence (MIF) test.
Detection of Antibodies against Selected Species of SFGR by MIF Kits
To detect antibodies to selected antigens of SGFR simultaneously, a commercially available customized MIF kits (Rickettsia screen IFA IgG [R50G-120] and IgM [R50M-120] antibody kits) (Fuller Laboratories, USA) were used. Antigens of R. felis, R. japonica, and R. conorii were selected because they were SFGR commonly reported in Asia and Taiwan. Antigens of R. rickettsii and R. typhi used in IFA kits were selected as well for comparison. The positive and negative controls of human serum used in the procedures were contained in the MIF kits. The procedures were performed according to the manufacturer’s instructions. The test titer started at 1∶32 and an antibody titer of ≥1∶64 is considered as positive reaction.
Interpretation of Serological Cross-reaction and SFGR Infections
Acute infection of SFGR: either a 4-fold or greater increase of IgG titer in paired sera or a positive detection of IgM specific for Rickettsia species; Past infection of SFGR: either an IgG titer ≥1∶80 by IFA or ≥1∶64 by MIF without a 4-fold or greater increase of titer, and IgM negative; Cross-reaction interpretation: a rickettsial antigen was considered to represent the agent of infection when IgG or IgM antibody titers against this antigen were at least 2 serial dilutions higher than titers of IgG or IgM antibody against other rickettsial antigens [3], [34], [35]; Undetermined SFGR infection: acute or past infection of SFGR without a representative species of SFGR could be determined by the above criteria.
Detection of SFGR DNA in Blood Specimens
The blood specimens of acute infection of SFGR were included for SFGR DNA detection. PCR tests with primers targeting 17-kDa protein gene, groEL, and ompB of Rickettsia spp. were performed as previously described [33]. A nested PCR was also applied to detect gltA and rompB, as described previously [11]. The DNA extracted from R. felis was used as the positive control for PCR assays [17].
Clinical Characteristics and Geographic Distribution of SFGR Infection Cases
The demographic data, clinical manifestations, findings of image examinations, results of laboratory examinations, and antimicrobial treatments were recorded retrospectively by medical chart review. The map of case geographic distribution was made by marking the resident address of each case using SuperGIS Desktop software (Supergeo Technologies Inc.) and free vector and raster map data obtained from Natural Earth (a public domain map dataset, http://www.naturalearthdata.com).
Results
Studied Cases
From April 2004 to December 2009, a total of 413 cases clinically suspected of Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever with paired blood specimens (acute and convalescent phase) available at E-Da hospital were included in this study. According to the final reports of Taiwan CDC, 90 cases of Q fever, 47 cases of scrub typhus, 12 cases of murine typhus, 6 cases of leptospirosis, and 3 cases of dengue fever were determined. The other 255 cases were unknown febrile diseases.
Serological Results of Antibodies against R. rickettsii by IFA Tests
A total of 49 (11.9%) cases, including 47 (11.4%) and 4 (1.0%) cases for IgG and IgM, respectively, had R. rickettsii antibody titer of ≥1∶40 in either acute or convalescent phase sera (Table 1).
Table 1. Titers of antibodies against R. rickettsii a in 413 cases of clinically suspected Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever.
| Clinical final diagnosisb | R. rickettsii antibody titer, n (%) | |||||||
| > = 1∶40 | 1∶40 | 1∶80 | 1∶160 | 1∶320 | 1∶640 | 1∶1280 | 1∶2560 | |
| R. rickettsii IgG (n = 413) | 47 (11.4) | 28 (6.8) | 12 (2.9) | 2 (0.5) | 2 (0.5) | 3 (0.7) | 0 (0) | 0 (0) |
| Q fever (n = 90) | 7 (7.8) | 5 (5.6) | 0 (0) | 1 (1.1) | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) |
| Scrub typhus (n = 47) | 8 (17.0) | 4 (8.5) | 3 (6.4) | 0 (0) | 0 (0) | 1 (2.1) | 0 (0) | 0 (0) |
| Murine typhus (n = 12) | 3 (25.0) | 2 (16.7) | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) |
| Leptospirosis (n = 6) | 1 (16.7) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dengue fever (n = 3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| UFD (n = 255) | 28 (11.0) | 17 (6.7) | 9 (3.5) | 0 (0) | 1 (0.4) | 1 (0.4) | 0 (0) | 0 (0) |
| R. rickettsii IgM (n = 413) | 4 (1.0) | 3 (0.7) | 0 (0) | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Q fever (n = 90) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Scrub typhus (n = 47) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Murine typhus (n = 12) | 3 (25) | 3 (25.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Leptospirosis (n = 6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dengue fever (n = 3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| UFD (n = 255) | 1 (0.4) | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Indirect immunofluorescence antibody assay (Rickettsia IFA IgG and IgM, Focus Diagnostic, USA).
According to the final reports of Taiwan CDC.
UFD = Unknown febrile disease.
Serological Results of Antibodies against Selected Species of SFGR and Detection of SFGR DNA
Of the 49 cases with R. rickettsii antibody titer of ≥1∶40 by IFA tests, MIF tests revealed that there were 5 cases of acute infection of SFGR (3 R. felis and 2 undetermined SFGR) and 13 cases of past infection of SFGR (3 R. felis and 10 undetermined SFGR) (Table 2). There were 9 cases of cross-reaction caused by infection of R. typhi (the titer of R. typhi was at least 4-fold greater than other tested Rickettsia spp.), and 22 cases were excluded as not infected by the tested Rickettsia spp. (titer was 1∶40 by IFA, but non-reactive by MIF tests). Among the 5 cases of acute infections, all had acute phase sera and only one (case 1) had acute phase peripheral blood monocytes (PBMCs) available for PCR test. However, none had detectable SFGR DNA.
Table 2. Resultsa of micro-immunofluorescence (MIF) testsb of patients who are positive for R. rickettsii IgG or IgM by indirect immunofluorescence antibody assay (IFA) testsc.
| IFA IgG IFA IgM | MIF IgG | MIF IgM | |||||||||||
| SFGR | SFGR | TGR | SFGR | TGR | |||||||||
| Clinical final diagnosisd | R. rickettsia | R. rickettsii | R. conorii | R. japonica | R. felis | R. typhi | R. rickettsii | R. conorii | R. japonica | R. felis | R. typhi | ||
| Acute infection of SFGR | |||||||||||||
| Case 1 | UFD | 0/80 | 0/0 | 0/32 | 0/128 | 0/128 | 0/256 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 2 | UFD | 40/320 | 0/160 | 0/1024 | 0/1024 | 0/2048 | 0/4096 * | 0/0 | 0/128 | 0/32 | 0/0 | 0/256 * | 0/0 |
| Case 3 | Q fever | 0/160 | 0/0 | 0/64 | 0/64 | 0/64 | 0/256 * | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 4 | Scrub typhus | 80/640 | 0/0 | 128/512 | 512/512 | 256/256 | 1024/4096 * | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 5 | UFD | 0/640 | 0/0 | 0/512 | 0/2048 | 0/2048 | 0/4096 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Past infection of SFGR | |||||||||||||
| Case 6 | UFD | 80/80 | 0/0 | 0/0 | 32/32 | 32/32 | 32/32 | 0/32 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 7 | UFD | 0/80 | 0/0 | 32/0 | 0/0 | 0/0 | 0/32 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 8 | UFD | 0/80 | 0/0 | 0/0 | 0/0 | 0/0 | 0/32 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 9 | UFD | 40/80 | 0/0 | 32/32 | 32/32 | 32/64 | 128/64 * | 32/64 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 10 | Q fever | 160/640 | 0/0 | 32/32 | 0/32 | 0/32 | 128/128 * | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 11 | Scrub typhus | 0/40 | 0/0 | 0/32 | 0/64 | 64/64 | 128/256 * | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 12 | Q fever | 0/40 | 0/0 | 32/64 | 0/0 | 0/0 | 32/64 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 13 | Q fever | 40/40 | 0/0 | 32/32 | 64/64 | 64/64 | 128/128 | 32/32 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 14 | Leptospirosis | 0/160 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 15 | UFD | 40/80 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 16 | UFD | 80/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 17 | UFD | 80/80 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Case 18 | Scrub typhus | 40/80 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
Presentation of antibody titers: acute phase titer/convalescent phase titer.
Customized micro-immunofluorescence assay (Rickettsia screen IFA IgG and IgM antibody kit, Fuller Laboratories, USA).
Indirect immunofluorescence antibody assay (Rickettsia IFA IgG and IgM, Focus Diagnostic, USA).
*Considered as the representative pathogen of infection.
SFGR = Spotted fever group rickettsiae; TGR = Typhus group rickettsiae; UFD = Unknown febrile disease.
Clinical Characteristics of Acute Infection of SFGR
The clinical characteristics of the 5 cases of acute infection of SFGR and one previously reported case are summarized in Table 3. The most common manifestations were fever, chills, headache, elevated liver enzymes, and thrombocytopenia. Only one (case 2) had itching skin rash during the course of presentation. However, it was suspected to be an allergic reaction to moxifloxacin by the clinical assessment. Two had history of animal contact, but none had reported contact with cats or bitten by insects or arthropods. Case 3 and case 4 were Q fever and scrub typhus, respectively, confirmed by Taiwan CDC.
Table 3. Clinical characteristics of cases of acute infection of spotted fever group rickettsioses (SFGR) in Taiwan.
| Case No. | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Other case [33] a |
| Clinical finaldiagnosis | UFD | UFD | Q fever | Scrub typhus | UFD | NA |
| Identified SFGR | UndeterminedSFGR | Possible R. felis | Possible R. felis | Possible R. felis | UndeterminedSFGR | R. felis |
| Age/Sex | 48/F | 50/M | 31/M | 67/M | 40/M | 27/F |
| Underlying diseases | Nil | Alcoholism | Alcoholism, HCV | HBV, HCV, cirrhosis,diabetes mellitus | Nil | Nil |
| Clinical symptomsand signs | Fever, chills, | Fever, chills,headache, skinrash, relativebradycardia | Fever, chills,headache, abdominalpain, myalgia,relative bradycardia | Fever, chills, cough,abdominal pain,myalgia, relativebradycardia | Fever, chills,headache | Fever, chills,headache, fatigue,acute polyneuropathy |
| Resident in mountainarea/rural | Nil | Yes | Yes | Yes | Nil | Nil |
| Recent travel in mountain/rural area | Yes | Nil | Nil | Nil | Nil | Nil |
| Animal contact history | Dogs, chicken | Nil | Cattle | Nil | Nil | Nil |
| Insects/arthropods bites | Nil | Nil | Nil | Nil | Nil | Nil |
| Chest x-ray | Normal | Normal | Normal | Increased infiltrationover right lower lung | Normal | Normal |
| Abdominal ultrasonography | Fatty liver | Fatty liver | Fatty liver,hepatosplenomegaly | Cirrhosis, splenomegaly,fatty liver, gallstonesand polyps | Fatty liver | NA |
| Laboratory examination | Normal bloodcell count,elevatedliver enzymes(ALT/AST:122/188 U/L) | Thrombocytopenia(platelet count:113000/uL),elevated liverenzymes (ALT/AST:155/191 U/L) | Leukocytosis(WBC:14210/uL),elevated liverenzymes(ALT/AST:150/214 U/L) | Thrombocytopenia(platelet count:149000/uL), elevatedliver enzymes (ALT/AST:108/113 U/L) | Thrombocytopenia(69000/uL), elevatedliver enzymes(ALT/AST:208/46 U/L) | Normal blood cellcount, liver enzymes |
| Antimicrobial treatment | Doxycycline | Moxifloxacin,doxycycline | Doxycycline | Doxycycline,levofloxacin | Doxycycline | Doxycycline |
Case 6 was reported by Tsai et al. [33].
Normal range of blood examinations: WBC (3600∼10600/uL), platelet count (150000∼400000 uL), ALT (0∼40 U/L), AST (0∼38 U/L).
UFD = Unknown febrile disease; HBV = Hepatitis B virus; HCV = Hepatitis C virus; ALT = Alanine transaminase; AST = Aspartate transaminase; WBC = White blood cell count; NA = Not available.
The Geographic Distribution of Q Fever, Scrub Typhus, Murine Typhus, Leptospirosis, and SFGR
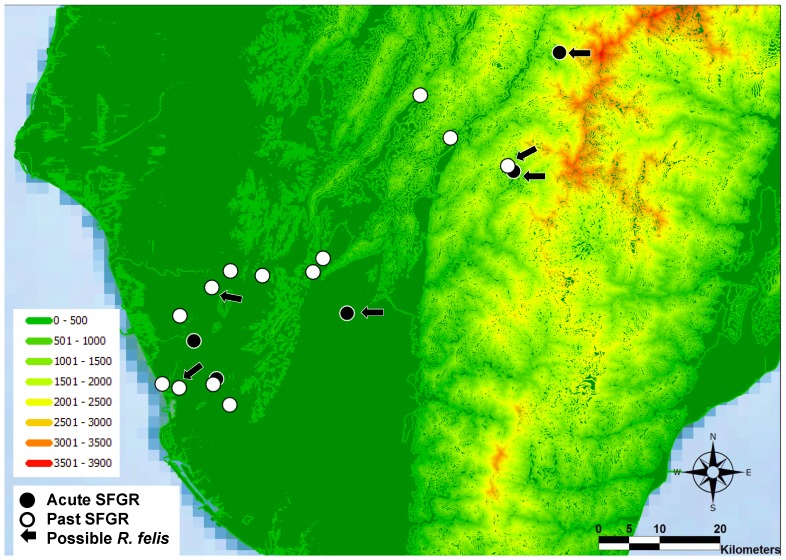
The geographic distribution of cases of Q fever, scrub typhus, murine typhus, and leptospirosis are illustrated in Figure 1 (B∼D). Except nearly half of scrub typhus cases were distributed in mountainous areas, most cases of Q fever, murine typhus, and leptospirosis were distributed in plains areas. Figure 2 illustrates the distribution of the 18 cases of SFGR infections listed in Table 2. The cases of SFGR infections were distributed in both mountainous and plains areas, similar to scrub typhus.
Figure 2. The case distribution of spotted fever group rickettsioses (SFGR) infections.
Black circles: The 5 cases of acute infection SFGR. White circles: The 10 cases of past infection of SFGR. Black arrows: The 6 cases of possible Rickettsia felis infection.
Discussion
SFGR is poorly characterized in Taiwan, particularly regarding human infections. Only two human serologic studies of SFGR conducted approximately 2 decades ago [23], [24]. During 1983 to 1989, 3.5 to 4.4% of sera samples from 113 residents in Tainan City, located in southern Taiwan, were positive for the R. sibirica antibody [23]. In 1995, none of 548 cases suspected as scrub typhus and murine typhus had serum that was positive for R. rickettsii and R. conorii antibodies [24]. Recent studies identified several species of SFGR closely related to R. rhipicephali [16], [21], R. australis [16], R. japonica [20], R. conorii [20], and R. felis [16]–[18], [20], [21], or novel strain of SFGR [22] in arthropods trapped from cats, dogs, or rodents. In additional, serological study in small animals had revealed infections of R. felis in cats [17] and R. conorii in rodents [19]. However, despite increased identification of SFGR in animals and arthropods, no further indigenous human SFGR was reported after the first case of human R. felis infection identified in 2008 [33]. The results of this retrospective serological investigation illustrate that human SFGR infections do exist in Taiwan, but may be underappreciated.
Rickettsia felis, a rickettsiae belonging to SFGR, is the causative pathogen of so-called flea-borne spotted fever (or cat-flea typhus), which is regarded as an emerging global rickettsiosis [34], [36]–[38]. Although isolation or molecular evidence of R. felis has been reported in fleas, ticks, mites, and mosquito [39], the cat flea (Ctenocephalides felis) is the only known biological vector of R. felis [40]. However, direct evidence of transmission from C. felis to animals has not been determined and no viable R. felis has been isolated from a vertebrate host yet [36], [40]. The clinical manifestation of human infection with R. felis is similar to murine typhus (caused by R. typhi) or other rickettsioses, which makes the clinical diagnosis difficult [38]. In Asia, emerging human R. felis infections have been reported in the Thai-Myanmar border [35], South Korea [11], Laos [41], and Taiwan [33]. In our study, most cases of SFGR infections have higher antibody titers against R. felis than other tested Rickettsia spp., including 3 acute and 3 past infections of R. felis by serological interpretation (Table 2). This is consistent with the recent findings derived from investigations of R. felis in animals and arthropods in Taiwan [16]–[18], [20], [21]. However, no SFGR DNA was detected in this study. This might due to only one case had acute phase PBMC obtained after administration of doxycycline and the others had sera available for PCR examination. In a critical view, however, we just have identified human infection of SFGR close-related to R. felis in Taiwan for lack of direct molecular evidence or isolation of R. felis.
Serological diagnosis of SFGR is challenge because cross-reaction is notoriously found between the species of SFGR. Western blot assays and cross-absorption studies are the recommended methods to distinguish species, but they demand large amounts of antigens derived from different Rickettsia specie [1]. Even in national reference centers, most antigens of SFGR may not be available for testing and Taiwan as well [3]. Comparing with IFA, MIF can simultaneously detect antibodies to a number of rickettsial antigens with the same drop of serum in a single well containing multiple rickettsial antigen dots [42]. For consideration of cost and availability of reagents, we used commercial available IFA and MIF kits for serological screening and identification of potentially representative SFGR, respectively. However, regarding to R. rickettsii antibodies, some cases of SFGR infections might be missed if either IFA or MIF were used only (Table 2). Although difference in titers between antigens was applied for interpreting serological cross-reaction as previous literatures [3], [34], [35], the higher titers of R. felis and non-reaction by MIF in our cases could be related to other species close-related to R. felis (such as R. australis and R. akari) and those species not used in this study or still unknown in Taiwan, respectively. This indicates the complexity of serological cross-reaction between species of SFGR, and variant sensitivity and specificity of serological reagents for different SFGR species.
The most common manifestations of the 5 cases of acute SFGR infections and one other previously reported case were fever, chills, headache, elevated liver enzymes, and thrombocytopenia (Table 3), which are similar to the symptoms of other rickettsioses in Taiwan [28], [29], [31]. Among the 5 cases, 3 were possible R. felis (case 2, 3, and 4) and the other 2 (case 1 and 5) were undetermined SFGR infections. All of them had received antimicrobial agents effective against rickettsioses and none had been suspected SFGR and reported to Taiwan CDC for confirmatory test. According, cases of human SFGR might be well treated in clinical without appropriate and definitive diagnosis in Taiwan.
Notably, 2 of the 5 cases of acute SFGR infections were also confirmed with Q fever (case 3) and scrub typhus (case 4) by Taiwan CDC. Among the 13 case of past SFGR infections, 3 cases were Q fever, 2 were scrub typhus, and one was leptospirosis (Table 3). Although Q fever had been reported to serologically cross-reactive with R. conorii and R. typhi [43], most of the our cases had higher antibody titer of R. felis than R. conorii, and were non-reactive to R. typhi (Table 3). Concomitant infection with Q fever and tick-borne rickettsioses has been reported, including R. conorii [44], [45], R. slovaca [44], and R. africae [44]. In southern Taiwan, concomitant infections with Q fever and scrub typhus [46], murine typhus and Q fever, and murine and scrub typhus [31] were identified. This indicates that patients suspected or confirmed with zoonosis or arthropod-vectored diseases might also have been exposed to the environment, animal, or arthropod vectors containing various SFGR [16]–[21], and are at risk of infection by SFGR in southern Taiwan. The clinicians have neglected this possibility and not conducted diagnostic approaches for SFGR. The overlapping distribution of zoonosis and SFGR cases illustrated by Figures 1 and 2, particularly of scrub typhus, could partially support this speculation. The present study not only proves human SFGR existing in Taiwan, but also provides the first evidence of possible concomitant infection of SFGR (possible R. felis) with Q fever and scrub typhus in Taiwan.
This retrospective serology and clinical study has limitations. Only antigens of 5 Rickettsia spp. were tested, and other potential SFGR might be unrecognized. Due to the lack of isolation and antigens of tested Rickettsia spp., Western blot assays and cross absorption tests were not available for further identification of the responsible SFGR. Because this was a retrospective study on residual blood specimens, not all acute infection cases had appropriate specimens for the molecular detection of SFGR, and the antibody reactivity might have decayed with time from collection to test.
This work illuminates that human SFGR infection does exist and is unappreciated in southern Taiwan where Q fever, scrub typhus, murine typhus, leptospirosis, and dengue fever are endemic. This observation, accompanied by increasing SFGR detected in animals and arthropods, indicates that human SFGR infection should not be overlooked, particularly for R. felis. Announcement and education regarding SFGRs, especially for clinicians and populations at high risk of acquisition, could be helpful for uncovering potential human cases. Meanwhile, establishment of standardized diagnostic methods and the inclusion of SFGR as notifiable diseases would be helpful for better characterizing these emerging diseases in Taiwan.
Funding Statement
This work was supported by research grants from the National Science Council [NSC 98-2745-B-650-001 and NSC100-2314-B-037-001-MY3] and E-Da Hospital [EDAHP98008]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Raoult D, Roux V (1997) Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev 10: 694–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jensenius M, Fournier PE, Raoult D (2004) Rickettsioses and the international traveler. Clin Infect Dis 39: 1493–1499. [DOI] [PubMed] [Google Scholar]
- 3. Parola P, Davoust B, Raoult D (2005) Tick- and flea-borne rickettsial emerging zoonoses. Vet Res 36: 469–492. [DOI] [PubMed] [Google Scholar]
- 4. Parola P, Paddock CD, Raoult D (2005) Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev 18: 719–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mediannikov O, Diatta G, Fenollar F, Sokhna C, Trape JF, et al.. (2010) Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl Trop Dis 4. [DOI] [PMC free article] [PubMed]
- 6. Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, et al. (2013) Etiology of severe non-malaria febrile illness in Northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis 7: e2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maina AN, Knobel DL, Jiang J, Halliday J, Feikin DR, et al. (2012) Rickettsia felis infection in febrile patients, western Kenya, 2007–2010. Emerg Infect Dis 18: 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahara F (1997) Japanese spotted fever: report of 31 cases and review of the literature. Emerg Infect Dis 3: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahara F (2006) Rickettsioses in Japan and the far East. Ann N Y Acad Sci 1078: 60–73. [DOI] [PubMed] [Google Scholar]
- 10. Uchida T, Uchiyama T, Kumano K, Walker DH (1992) Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int J Syst Bacteriol 42: 303–305. [DOI] [PubMed] [Google Scholar]
- 11. Choi YJ, Jang WJ, Ryu JS, Lee SH, Park KH, et al. (2005) Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg Infect Dis 11: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung MH, Lee SH, Kim MJ, Lee JH, Kim ES, et al. (2006) Japanese spotted fever, South Korea. Emerg Infect Dis 12: 1122–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu XB, Na RH, Wei SS, Zhu JS, Peng HJ (2013) Distribution of tick-borne diseases in China. Parasit Vectors 6: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qi Y, Xiong X, Wang X, Duan C, Jia Y, et al. (2013) Proteome analysis and serological characterization of surface-exposed proteins of Rickettsia heilongjiangensis . PLoS One 8: e70440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu QH, Chen GY, Jin Y, Te M, Niu LC, et al. (1995) Evidence for a high prevalence of spotted fever group rickettsial infections in diverse ecologic zones of Inner Mongolia. Epidemiol Infect 115: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsui PY, Tsai KH, Weng MH, Hung YW, Liu YT, et al. (2007) Molecular detection and characterization of spotted fever group rickettsiae in Taiwan. Am J Trop Med Hyg 77: 883–890. [PubMed] [Google Scholar]
- 17. Tsai KH, Lu HY, Huang JH, Wang PJ, Wang HC, et al. (2009) Rickettsia felis in cat fleas in Taiwan Vector Borne Zoonotic Dis. 9: 561–563. [DOI] [PubMed] [Google Scholar]
- 18. Tsai KH, Huang CG, Fang CT, Shu PY, Huang JH, et al. (2011) Prevalence of Rickettsia felis and the first identification of Bartonella henselae Fizz/CAL-1 in cat fleas (Siphonaptera: Pulicidae) from Taiwan. J Med Entomol 48: 445–452. [DOI] [PubMed] [Google Scholar]
- 19. Kuo CC, Huang CL, Wang HC (2011) Identification of potential hosts and vectors of scrub typhus and tick-borne spotted fever group rickettsiae in eastern Taiwan. Med Vet Entomol 25: 169–177. [DOI] [PubMed] [Google Scholar]
- 20. Kuo CC, Huang JL, Lin TE, Wang HC (2012) Detection of Rickettsia spp. and host and habitat associations of fleas (Siphonaptera) in eastern Taiwan. Med Vet Entomol 26: 341–350. [DOI] [PubMed] [Google Scholar]
- 21. Hsu YM, Lin CC, Chomel BB, Tsai KH, Wu WJ, et al. (2011) Identification of Rickettsia felis in fleas but not ticks on stray cats and dogs and the evidence of Rickettsia rhipicephali only in adult stage of Rhipicephalus sanguineus and Rhipicephalus haemaphysaloides. Comp Immunol Microbiol Infect Dis 34: 513–518. [DOI] [PubMed] [Google Scholar]
- 22. Tsai KH, Wang HC, Chen CH, Huang JH, Lu HY, et al. (2008) Isolation and identification of a novel spotted fever group rickettsia, strain IG-1, from Ixodes granulatus ticks collected on Orchid Island (Lanyu), Taiwan. Am J Trop Med Hyg 79: 256–261. [PubMed] [Google Scholar]
- 23. Takada N, Fujita H, Yano Y, Huang WH, Khamboonruang C (1993) Serosurveys of spotted fever and murine typhus in local residents of Taiwan and Thailand compared with Japan. Southeast Asian J Trop Med Public Health 24: 354–356. [PubMed] [Google Scholar]
- 24. Chen HL, Chen HY, Chung CL, Lin TH, Wang GR, et al. (1997) [Primary surveillance of spotted fever group antibodies on rats in the Kinmen area] [In Chinese]. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 30: 115–121. [PubMed] [Google Scholar]
- 25. Lee HC, Ko WC, Lee HL, Chen HY (2002) Clinical manifestations and complications of rickettsiosis in southern Taiwan. J Formos Med Assoc 101: 385–392. [PubMed] [Google Scholar]
- 26. Lai CH, Huang CK, Chin C, Chung HC, Huang WS, et al. (2008) Acute Q fever: an emerging and endemic disease in southern Taiwan. Scand J Infect Dis 40: 105–110. [DOI] [PubMed] [Google Scholar]
- 27. Chen NY, Huang PY, Leu HS, Chiang PC, Huang CT (2008) Clinical prediction of endemic rickettsioses in northern Taiwan–relevance of peripheral blood atypical lymphocytes. J Microbiol Immunol Infect 41: 362–368. [PubMed] [Google Scholar]
- 28. Lai CH, Huang CK, Chen YH, Chang LL, Weng HC, et al. (2009) Epidemiology of acute Q Fever, scrub typhus, and murine typhus, and identification of their clinical characteristics compared to patients with acute febrile illness in southern Taiwan. J Formos Med Assoc 108: 367–376. [DOI] [PubMed] [Google Scholar]
- 29. Lai CH, Huang CK, Weng HC, Chung HC, Liang SH, et al. (2008) Clinical characteristics of acute Q fever, scrub typhus, and murine typhus with delayed defervescence despite doxycycline treatment. Am J Trop Med Hyg 79: 441–446. [PubMed] [Google Scholar]
- 30. Lai CH, Huang CK, Weng HC, Chung HC, Liang SH, et al. (2009) The difference in clinical characteristics between acute Q fever and scrub typhus in southern Taiwan. Int J Infect Dis 13: 387–393. [DOI] [PubMed] [Google Scholar]
- 31. Chang K, Chen YH, Lee NY, Lee HC, Lin CY, et al. (2012) Murine typhus in southern Taiwan during 1992–2009. Am J Trop Med Hyg 87: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsai KH, Lu HY, Huang JH, Fournier PE, Mediannikov O, et al. (2009) African tick bite Fever in a Taiwanese traveler returning from South Africa: molecular and serologic studies. Am J Trop Med Hyg 81: 735–739. [DOI] [PubMed] [Google Scholar]
- 33. Tsai KH, Lu HY, Tsai JJ, Yu SK, Huang JH, et al. (2008) Human case of Rickettsia felis infection, Taiwan. Emerg Infect Dis 14: 1970–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perez-Arellano JL, Fenollar F, Angel-Moreno A, Bolanos M, Hernandez M, et al. (2005) Human Rickettsia felis infection, Canary Islands, Spain. Emerg Infect Dis 11: 1961–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parola P, Miller RS, McDaniel P, Telford SR 3rd, Rolain JM, et al. (2003) Emerging rickettsioses of the Thai-Myanmar border. Emerg Infect Dis 9: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perez-Osorio CE, Zavala-Velazquez JE, Arias Leon JJ, Zavala-Castro JE (2008) Rickettsia felis as emergent global threat for humans. Emerg Infect Dis 14: 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Renvoise A, Joliot AY, Raoult D (2009) Rickettsia felis infection in man, France. Emerg Infect Dis 15: 1126–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parola P (2011) Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect 17: 996–1000. [DOI] [PubMed] [Google Scholar]
- 39. Socolovschi C, Pages F, Ndiath MO, Ratmanov P, Raoult D (2012) Rickettsia species in African Anopheles mosquitoes. PLoS One 7: e48254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reif KE, Macaluso KR (2009) Ecology of Rickettsia felis: a review. J Med Entomol 46: 723–736. [DOI] [PubMed] [Google Scholar]
- 41. Phongmany S, Rolain JM, Phetsouvanh R, Blacksell SD, Soukkhaseum V, et al. (2006) Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis 12: 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. La Scola B, Raoult D (1997) Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol 35: 2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vardi M, Petersil N, Keysary A, Rzotkiewicz S, Laor A, et al. (2011) Immunological arousal during acute Q fever infection. Eur J Clin Microbiol Infect Dis 30: 1527–1530. [DOI] [PubMed] [Google Scholar]
- 44. Rolain JM, Gouriet F, Brouqui P, Larrey D, Janbon F, et al. (2005) Concomitant or consecutive infection with Coxiella burnetii and tickborne diseases. Clin Infect Dis 40: 82–88. [DOI] [PubMed] [Google Scholar]
- 45. Janbon F, Raoult D, Reynes J, Bertrand A (1989) Concomitant human infection due to Rickettsia conorii and Coxiella burnetii . J Infect Dis 160: 354–355. [DOI] [PubMed] [Google Scholar]
- 46. Lai CH, Chen YH, Lin JN, Chang LL, Chen WF, et al. (2009) Acute Q fever and scrub typhus, southern Taiwan. Emerg Infect Dis 15: 1659–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]