Abstract
Background
The aims of this study were to investigate the cancer incidence and risk in HIV/AIDS patients relative to the general population in Taiwan.
Methods
Using Taiwan’s National Health Insurance Research Database, 15,269 HIV/AIDS patients were identified between 1998 and 2009. Gender-specific incidence densities (IDs) of both AIDS-defining cancers (ADC) and non-AIDS-defining cancers (NADC) after HIV infection were calculated. Age-, sex- and period-adjusted standardized incidence rates (SIRs) were obtained using 1.8 million people from the general population as controls.
Results
A total of 1,117 male and 165 female HIV/AIDS patients were diagnosed with cancer. Non-Hodgkin lymphoma (n=196; ID=328.79/100,000 person-years) and cervical cancer (n = 50; ID = 712.08/100,000 person-years) were the most common ADCs, while liver cancer (n=125; ID=184.52/100,000 person-years) and colon cancer (n=11; ID=156.66/100,000 person-years) were the most common NADCs in males and females, respectively. Period-adjusted gender-specific ADC and NADC rates decreased from more than 1,500 cases/100,000 person-years to less than 500 cases/100,000 person-years (p <0.001 for trend). SIRs of ADCs and NADCs also decreased. However, relative to the general population, increased SIRs were still seen for most cancers, many of which had an infectious etiology. The highest SIRs in ADCs and NADCs were seen in Kaposi's sarcoma (SIR=298.0, 95%CI=258.16, 343.85) and anal cancer (SIR=19.10, 95%CI=12.80, 27.50).
Conclusion
This study showed that although the cancer incidence rates have significantly decreased in the HAART era, HIV/AIDS patients were still at increased risk of ADCs and most NADCs. Cancer screening, especially for infection-related NADCs, should therefore be promoted.
Keywords: HIV, AIDS, standardized incidence rates, cancer
Introduction
After the introduction of highly active antiretroviral therapy (HAART), improvement in survival was seen in people living with HIV/AIDS (PLWHA).1–6 This has led to growing interest in the epidemiology of chronic illnesses in these patients. Cancer has become one of the chronic health conditions extensively studied in settings where HAART is delivered regularly to patients. In Taiwan, HAART has been offered to people with HIV free of charge since 1997. Since the diagnosis of the first HIV case in 1984, the HIV prevalence in Taiwan has reached 0.09% (representing a cumulative total of 20,801 diagnoses) by the end of 2011.
While many studies on cancer among PLWHA have been conducted in Europe and the United States, very few have been reported in Asian countries.7–20 These studies in Western countries reported increased standardized incidence rates (SIRs) of infection-related cancers in HIV/AIDS patients. However, because some infection-related cancers are relatively uncommon in Western countries, their SIR could not be calculated. Some of these cancers, such as those of the oral cavity, nasopharynx and liver, are more common in Asia. Therefore, we conducted such a study in Taiwan to confirm the impact of HIV infection on cancers, particularly those linked to infectious etiology, to determine whether the SIR was increased in other cancers previously not thought to be associated with infection, and to postulate the possibility of an infectious etiology for these cancers. In this study we determined the cancer incidence among PLWHA in Taiwan and compared their incidence to that of the general population by using Taiwan’s National Health Insurance Research Database (NHIRD).
Materials and methods
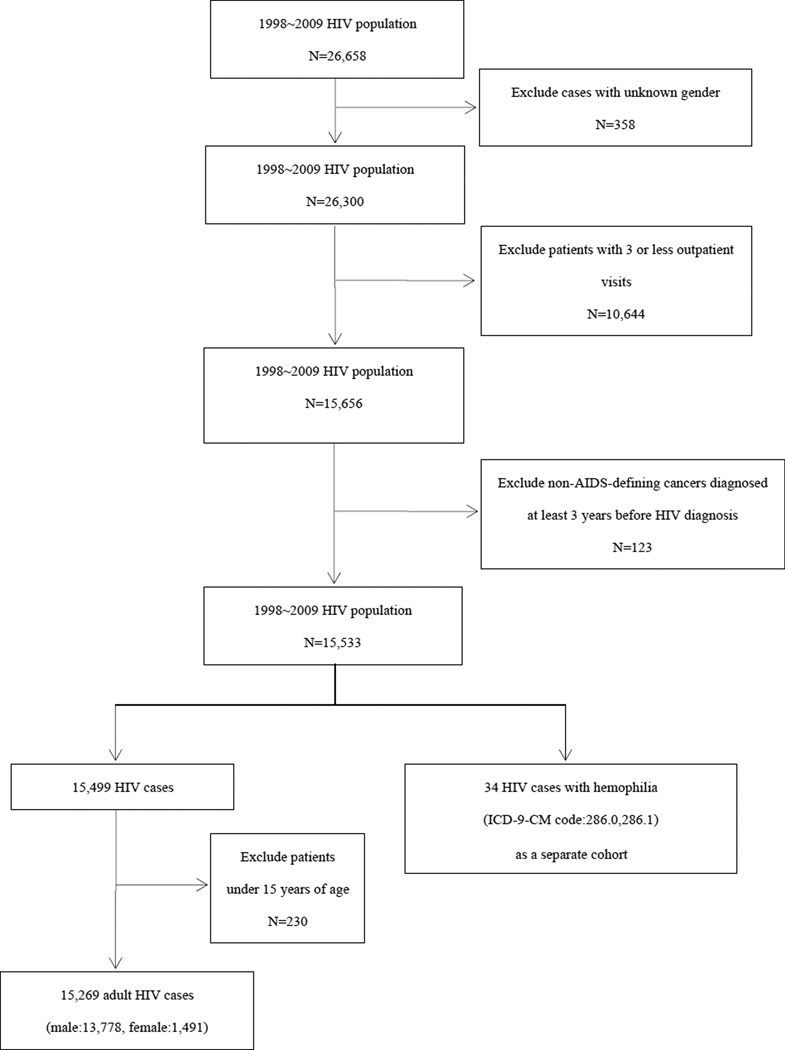
Study population (Figure 1)
Figure 1.
HIV/AIDS population selection flowchart
In 1995, Taiwan launched a single-payer National Health Insurance program, and as of 2007, 22.60 million of Taiwan's 22.96 million population were enrolled in this program. Each year, the Bureau of National Health Insurance, Taiwan, collects data, including registration files and original claim data for reimbursement, from the National Health Insurance, and sorts it into data files. These data files are de-identified by scrambling the identification codes of patients, medical institutions and physicians and sent to the National Health Research Institutes, Taiwan, to form the original files of the NHIRD. Therefore, these files contain all the records of individuals enrolled in the National Health Insurance program, and they are available for research purposes only.
With approval from the National Health Research Institutes, Taiwan, the NHIRD was accessed at The Collaboration Center of Health Information Application (CCHIA), Department of Health, Executive Yuan, Taiwan. The NHIRD was searched for individuals with HIV/AIDS in the 1998–2009 databases, and 26,658 PLWHA were identified.21 PLWHA were defined as patients diagnosed with ICD-9-CM codes 042–044, 7958, or V08 who were tested for viral load or CD4 count (order codes: 26017A1, 14074B, 12071A, 12071B, 12073A, 12073B, 12074A, 12074B). Patients of unknown gender (358 patients) and those with less than three outpatient visits (10,644 patients) were excluded from this study. In suspected HIV cases who were ELISA-positive, physicians sometimes used the above-mentioned ICD-9-CM codes in their medical records and insurance claims to order confirmatory tests. In cases where the tests results came back negative, the coding was revised. Therefore, by excluding cases with these ICD-9-CM codes with less than three office visits, we avoided the inclusion of false-positive cases in our study.
PLWHA subsequently diagnosed with cancer were identified based on the ICD-9-CM diagnosis codes. Review of subjects at the first HIV clinic visit showed that many of these patients were in the advanced stages of AIDS. Therefore, it is possible that these patients developed non-AIDS-defining cancers many years ago. Previous studies backdated cases up to 5 years for the distant pre-AIDS period.9 In our study we included non-AIDS defining cancers diagnosed at the earliest 3 years before the first HIV clinic visit. Individuals already diagnosed with cancer at least 3 years before HIV diagnosis (123 patients) were excluded from this study.
In the remaining 15,533 patients, 34 HIV cases with hemophilia were identified and analyzed as a separate cohort. The number of patients in the resultant cohort was 15,449. Subsequently, 230 patients aged under 15 years were excluded from the cohort, and the final number of patients was 15,269. The follow-up period was from the first HIV/AIDS clinic visit until the dates of any cancer diagnosis, death, or December 31, 2009. An ELISA test is usually performed prior to or during the first HIV/AIDS clinic visit, and sometimes patients do not return for follow-up until 6 to 12 months later. By excluding patients who did not return for follow-up twice we were able to remove cases with uncertain diagnosis. However, if in subjects with confirmed HIV infection during the first clinic visit the follow-up period does not include the time between the first and third visits, a significant period of follow-up is omitted in subjects with confirmed HIV diagnosis. Therefore, we used the first HIV/AIDS clinic visit as the starting date for the calculation of the follow-up period.
In order to calculate expected rates of cancer, data were obtained from a database linked by the Office of Statistics of the Department of Health using the NHIRD and death certificate database. This dataset consisted of 1.8 million individuals randomly sampled from the Registry for Beneficiaries of the NHIRD, which maintains the registration data and all the original claim data of every person who was a beneficiary of the National Health Insurance program during the period 1998–2009. There are approximately 23.72 million individuals in this registry. Random selection was performed with SAS or SPSS software. Two million random numbers were obtained in the 1 to 2,147,483,646 range. Numbers appearing more than once and those not matching an existing serial number in the National Health insurance registry were deleted (approximately 200,000–300,000). The remaining 1.8 million numbers were included in the dataset. There was no significant difference in the gender distribution between the patients in the randomly selected subset and the original NHIRD.
The demographic variables collected on each subject included sex, age at first clinic visit, and year of first clinic visit. New cancer cases among PLWHA were identified by selecting those with insurance claims whose recorded data contained ICD-9-CM codes and A- codes of cancers. Date of cancer diagnosis was identified as the date when a patient was first diagnosed with cancer. Kaposi’s sarcoma, non-Hodgkin lymphoma, and cervical cancer were categorized as AIDS-defining cancers (ADCs), while all other cancer types were categorized as non-AIDS defining cancers (NADCs). All cancer subtypes, including in situ and invasive cancers, were included in this study.
The number of PLWHA cases in the NHIRD was compared to that provided by the Centers for Disease Control (CDC).22 (Supplementary Figure 1) There was an IDU-related HIV outbreak in 2005 and this dramatic increase in cases was seen in the CDC registry; however, this increase was seen in 2006 in the NHIRD. Cases were reported to the CDC within 24 hours of diagnosis. Many of the newly-diagnosed cases were in prison at that time and were not covered by the National Health Insurance. Therefore, they were added to the NHIRD after release from prison the following year resulting in this time lag. While we did not have direct measures of HIV-positive status nor access to CDC data, the health insurance definitions used in this study yielded very similar results in terms of HIV-positive patient numbers to those direct measures used by the CDC, therefore supporting the validity of our determination of HIV case numbers.
Statistical analysis
The incidence density (ID) and SIR for each cancer type were calculated in this cohort of PLWHA. Person-years analysis was performed in strata of age, sex, calendar period and cancer type to estimate the ID and SIR in the PLWHA cohort. The start date for the calculation of person-years was the date of first HIV/AIDS clinic visit and the end date was December 31, 2009 or the date of death for each person who died during the follow-up period. The ID of each type of cancer after HIV infection was calculated by dividing the number of observed cancer cases by the total person years at risk for that cancer.
The SIR for each cancer type was calculated by dividing the observed number of cases by the number that would be expected if age-, sex-, and calendar-period-specific rates of the comparison population applied.23 In this study, the 1.8 million controls were used for calculation of the SIRs. The 95% confidence interval (CI) was calculated by Poisson distribution.
All analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC). A two tailed p value of less than 0.05 was considered statistically significant.
Results
Cancer incidence among PLWHA
The ID (per 100,000 person-years) of different types of cancer, both ADCs and NADCs, were calculated. (Table 1) A total of 13,778 male patients contributed 59,613.34 person-years, while 1,491 female patients contributed 7,021.65 person-years to the cohort. A total of 1,117 male and 165 female HIV/AIDS patients were diagnosed with cancer. Non-Hodgkin lymphoma (n=196; ID=328.79/100,000 person-years) was the most common ADC in males, while cervical cancer was the most common ADC in females (n = 29; ID = 413.01/100,000 person-years). Kaposi's sarcoma was also common in both male and female HIV/AIDS patients (ID=315.37/100,000 person years in males and ID=85.45/100,000 person years in females). The most common NADC in males was liver and biliary system cancer (n = 125; ID = 184.52/100,000 person-years), followed by oral cavity cancer (n = 78; ID = 130.84/100,000 person-years) and bronchus and lung cancer (n =73; ID = 122.46/100,000 person-years); while the most common NADC in females was colon cancer (n = 11; ID = 156.66/100,000 person-years), followed by breast cancer (n =10; ID = 142.42/100,000 person-years) and bronchus and lung cancer (n=9, ID = 128.18/100,000 person-years).
Table 1.
Incidence density and standardized incidence ratio of AIDS-related and non-AIDS-related cancers in male and female Taiwanese HIV-1/AIDS adult patients enrolled in the National Health Insurance between 1998–2009 in Taiwan
| Male (age≧15; N=13,778) | Female (age≧15; N=1,491) | Total (age≧15; N=15,269) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CANCER | Person-years: 59,613.34 | Person-years: 7,021.65 | Person-years: 66,634.99 | |||||||||
| Cases | ID φ | SIR | 95% CI | Cases | ID φ | SIR | 95% CI | Cases | ID φ | SIR | 95% CI | |
| Total | 1,117 | 144 | 1,189 | |||||||||
| AIDS-related | 384 | 53 | 408 | |||||||||
| Kaposi's sarcoma | 188 | 315.37 | 158.46 | (136.97,183.24)† | 6 | 85.45 | 1233.22 | (452.59,2688.42)† | 194 | 291.14 | 298.04 | (258.16,343.85)† |
| Non-Hodgkin Lymphoma | 196 | 328.79 | 23.66 | (20.51,27.28)† | 18 | 256.35 | 22.38 | (13.27,35.36)† | 214 | 321.15 | 26.12 | (22.78,29.90)† |
| Cervix | 29 | 413.01 | 13.95 | (9.35,20.09)† | ||||||||
| Non AIDS-related | 733 | 91 | 781 | |||||||||
| Lip | 6 | 10.06 | 5.58 | (2.05,12.16)† | 0 | - | - | 6 | 9 | 8.54 | (3.13,18.62)† | |
| Oral cavity | 78 | 130.84 | 5.65 | (4.49,7.09)† | 4 | 56.97 | 12.4 | (3.37,31.74)† | 82 | 123.06 | 9.23 | (7.39,11.52)† |
| Oropharynx and hypopharynx | 19 | 31.87 | 4.8 | (2.89,7.49)† | 0 | - | - | 19 | 28.51 | 5.4 | (3.25,8.42)† | |
| Nasopharynx | 72 | 120.78 | 7.35 | (5.79,9.31)† | 4 | 56.97 | 7.04 | (1.91,18.02)† | 76 | 114.05 | 9.62 | (7.63,12.10)† |
| Esophagus | 17 | 28.52 | 6 | (3.50,9.60)† | 0 | - | - | 17 | 25.51 | 9.26 | (5.40,14.82)† | |
| Stomach | 22 | 36.9 | 4.95 | (3.10,7.47)† | 1 | 14.24 | 1.63 | (0.04,9.08) | 23 | 34.52 | 4.93 | (3.13,7.40)† |
| Small Intestine | 2 | 3.35 | 3.51 | (0.42,12.67) | 0 | - | - | 2 | 3 | 3.73 | (0.45,13.47)† | |
| Colon | 67 | 112.39 | 7.02 | (5.47,8.97)† | 11 | 156.66 | 7.53 | (3.76,13.48)† | 78 | 117.06 | 7.53 | (5.99,9.44)† |
| Rectum or rectosigmoid junction | 32 | 53.68 | 5.93 | (4.05,8.39)† | 2 | 28.48 | 2.57 | (0.31,9.28) | 34 | 51.02 | 5.99 | (4.15,8.37)† |
| Anus or anal canal | 27 | 45.29 | 18.45 | (12.16,26.94)† | 2 | 28.48 | 10.02 | (1.21,36.17)† | 29 | 43.52 | 19.1 | (12.80,27.50)† |
| Liver and intrahepatic duct | 125 | 184.52 | 4.2 | (3.46,5.06)† | 8 | 85.45 | 2.6 | (0.84,6.08) | 133 | 174.08 | 5.5 | (4.54,6.59)† |
| Pancreas | 10 | 16.77 | 5.38 | (2.58,9.90)† | 4 | 56.97 | 17.03 | (4.63,43.60)† | 14 | 21.01 | 7.56 | (4.13,12.70)† |
| Larynx | 9 | 15.1 | 5.21 | (2.39,9.90)† | 0 | - | - | 9 | 13.51 | 7.82 | (3.58,14.86)† | |
| Bronchus and lung | 73 | 122.46 | 7.73 | (6.10,9.77)† | 9 | 128.18 | 7.3 | (3.34,13.87)† | 82 | 123.06 | 8.52 | (6.82,10.63)† |
| Melanoma | 4 | 6.71 | 10.46 | (2.85,26.78)† | 0 | - | - | 4 | 6 | 7.96 | (2.17,20.38)† | |
| Non-melanoma skin | 38 | 63.74 | 15.59 | (11.01,21.45)† | 1 | 14.24 | 2.83 | (0.07,15.76) | 39 | 58.53 | 14.92 | (10.58,20.46)† |
| Breast | 2 | 3.35 | 11.84 | (1.43,42.74)† | 10 | 142.42 | 2.09 | (1.00,3.85)† | 12 | 18.01 | 0.59 | (0.31,1.03) |
| Uterus | 5 | 71.21 | 6.91 | (2.24,16.10)† | ||||||||
| Ovary | 4 | 56.97 | 5.06 | (1.38,12.95)† | ||||||||
| Prostate | 17 | 28.52 | 3.48 | (2.03,5.57)† | ||||||||
| Testis | 17 | 28.52 | 7.48 | (4.36,11.97)† | ||||||||
| Kidney and renal pelvis | 11 | 18.45 | 3.13 | (1.56,5.60)† | 4 | 56.97 | 5.84 | (1.59,14.95)† | 15 | 22.51 | 3.56 | (1.99,5.87)† |
| Brain | 22 | 36.9 | 7.27 | (4.56,10.98)† | 8 | 113.93 | 18.15 | (7.82,35.76)† | 30 | 45.02 | 8.28 | (5.59,11.84)† |
| Thyroid | 2 | 3.35 | 0.89 | (0.11,3.21) | 3 | 42.73 | 2.34 | (0.48,6.83) | 5 | 7.5 | 0.67 | (0.22,1.56) |
| Hodgkin's lymphoma | 11 | 18.45 | 9.42 | (4.70,16.86)† | 1 | 14.24 | 7.82 | (0.20,43.56) | 12 | 18.01 | 9.35 | (4.83,16.36)† |
| Multiple myeloma | 7 | 11.74 | 5.99 | (2.40,12.34)† | 2 | 28.48 | 16.03 | (1.94,57.87)† | 9 | 13.51 | 8.19 | (3.75,15.56)† |
| Leukemia | 26 | 43.61 | 2.44 | (1.59,3.59)† | 4 | 56.97 | 4.39 | (1.19,11.24)† | 30 | 45.02 | 2.93 | (1.98,4.19)† |
| Bladder | 17 | 28.52 | 3.27 | (1.91,5.23)† | 4 | 56.97 | 4.19 | (1.14,10.73)† | 21 | 31.51 | 3.53 | (2.19,5.40)† |
Incidence density per 100,000 person years
The SIR is significant
As expected, the IDs of cancers more common in Asia were also found to be quite high in this study. The IDs in males and females for liver cancer were 184.52 and 85.45/100,000 person-years, for oral cavity cancer were 130.84 and 56.97/100,000 person-years, and for cancer of the nasopharynx were 120.78 and 56.97/100,000, respectively.
SIRs of different cancer types
The SIR of different cancer types was calculated using the cancer IDs of PLWHA and the general population. (Table 1) The SIR of Kaposi’s sarcoma was markedly increased in males (SIR=158.46, 95%CI=139.67, 183.24) and in females (SIR=1233.2, 95%CI=452.59, 2688.42) when compared to the general population. Increased SIRs were also seen in the other ADCs, including Non-Hodgkin lymphoma and cervical cancer. Among NADCs, the highest SIR in males was seen in cancer of the anus or anal canal (SIR=18.45, 95%CI=12.16, 26.94), while the highest SIR in females was seen in brain cancer (SIR=18.15, 95%CI=7.82, 35.76). (Table 1)
Table 2 compares our results to those of previous studies in Western countries. 9,15,18,20 For the most part, patterns of increased cancer risk in our study were similar to those of the other studies. The insignificant decreased risks of breast and thyroid cancers seen in our study were also seen in studies in the United States and the United Kingdom. While studies in the United States showed decreased prostate and bladder cancer risks, our study showed increased risks.
Table 2.
Comparison of SIRs of our study with those of studies in the United States and United Kingdom
| Population | Taiwan HIV/AIDS population | US Frisch et al. 2001 | US Engels et al. 2008 | UK Newnham et al. 2005 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up period, Cohort entry |
1998–2009 HIV entry | 1980–1998 AIDS entry | 1991–2002 HIV entry | 1985–2001 HIV entry | ||||||||
| ID φ | SIR | 95% CI | ID φ | SIR | 95% CI | ID φ | SIR | 95% CI | ID φ | SIR | 95% CI | |
| AIDS-related | ||||||||||||
| Kaposi's sarcoma | 291.14 | 298.04 | (258.16,343.85)† | 1960.2 | 177.7 | (173.2,182.3)† | 93 | 1300 | (1100,1500)† | 586.79 | 247.4 | (231.8,263.8)† |
| Non-Hodgkin Lymphoma | 321.15 | 26.12 | (22.78,29.90)† | 1104.2 | 72.81 | (70.36,75.32)† | 109 | 7.3 | (6.4,8.4)† | 407.16 | 42.61 | (39.29,45.91)† |
| Cervix | 413.01 | 13.95 | (9.35,20.09)† | 15.19 | 5.2 | (3.81,6.93)† | 44 | 2.9 | (1.9,4.2)† | 1.89 | 1 | (0.21,2.92) |
| Non AIDS-related | ||||||||||||
| Leukemia | 45.02 | 2.93 | (1.98,4.19)† | 57.79 | 3.6 | (3.09,4.17)† | 2 | 0.5 | (0.1,1.5) | 11.98 | 2.5 | (1.51,3.90)† |
| Hodgkin's lymphoma | 18.01 | 9.35 | (4.83,16.36)† | 26.75 | 11.5 | (10.61,12.45)† | 19 | 5.6 | (3.9,7.8)† | 23.95 | 5.6 | (3.95,7.67)† |
| Multiple myeloma | 13.51 | 8.19 | (3.75,15.56)† | 16.18 | 2.6 | (1.92,3.44)† | 4 | 1.4 | (0.6,2.7) | 3.78 | 2.7 | (1.00,5.94) |
| Lip | 9 | 8.54 | (3.13,18.62)† | 2.31 | 3.1 | (1.89,4.79)† | ||||||
| Oral cavity | 123.06 | 9.23 | (7.39,11.52)† | 24.44 | 3 | (2.4,3.9)† | 14 | 1.7 | (1.1,2.5)† | 3.78 | ||
| Oropharynx and hypopharynx | 28.51 | 5.4 | (3.25,8.42)† | 5.61 | 6 | (3.5,9.7)† | ||||||
| Nasopharynx | 114.05 | 9.62 | (7.63,12.10)† | 8.92 | 2.6 | (1.7,3.7)† | 2.52 | 5 | (1.4,12.8)† | |||
| Esophagus | 25.51 | 9.26 | (5.40,14.82)† | 10.9 | 1.6 | (1.10,2.25)† | 3 | 1.1 | (0.4,2.4) | 1.26 | 0.5 | (0.06,1.81) |
| Stomach | 34.52 | 4.93 | (3.13,7.40)† | 19.81 | 2 | (1.53,2.57)† | 4 | 0.9 | (0.4,1.9) | 1.26 | 0.4 | (0.05,1.44) |
| Small Intestine | 3 | 3.73 | (0.45,13.47)† | 3.3 | 1.3 | (0.62,2.39) | 2 | 1.7 | (0.4,5.0) | 1.26 | 3.39 | (0.4,12.04) |
| Colon | 117.06 | 7.53 | (5.99,9.44)† | 52.17 | 0.9 | (0.8,1.04)* | 15 | 0.8 | (0.5,1.1)* | 8.82 | 0.9 | (0.5,1.5)* |
| Recturn or rectosigmold junction | 51.02 | 5.99 | (4.15,8.37)† | |||||||||
| Anus or anal canal | 43.52 | 19.1 | (12.80,27.50)† | 78.26 | 33.79 | (29.48,38.55)† | 10 | 9.2 | (5.5,15)† | 11.35 | 23.08 | (13.33,35.56)† |
| Liver and intrahepatic duct | 174.08 | 5.5 | (4.54,6.59)† | 28.73 | 7.7 | (6.17,9.50)† | 8 | 2.7 | (1.5,4.6)† | 8.19 | 5.6 | (3.01,9.67)† |
| Pancreas | 21.01 | 7.56 | (4.13,12.70)† | 14.86 | 2.39 | (1.75,3.20)† | 8 | 2.2 | (1.2,3.6)† | 1.89 | 0.8 | (0.16,2.31) |
| Larynx | 13.51 | 7.82 | (3.58,14.86)† | 38.97 | 2.8 | (2.32,3.35)† | 8 | 2.6 | (1.4,4.2)† | 3.15 | 2 | (0.65,4.67) |
| Bronchus and lung | 123.06 | 8.52 | (6.82,10.63)† | 79.58 | 4.5 | (4.19,4.82)† | 59 | 2.6 | (2.1,3.1)† | 24.58 | 2.2 | (1.57,3.01)† |
| Melanoma | 6 | 7.96 | (2.17,20.38)† | 47.88 | 1.3 | (1.10,1.53)† | 4 | 0.6 | (0.2,1.2) | 1.26 | 0.2 | (0.02,0.72)† |
| Non-melanoma skin | 58.53 | 14.92 | (10.58,20.46)† | 44.12 | 19.61 | (15.16,24.57)† | ||||||
| Breast | 18.01 | 0.59 | (0.31,1.03) | 47.22 | 1.1 | (0.93,1.30) | 18 | 0.8 | (0.5,1.1) | 7.56 | 0.8 | (0.41,1.40) |
| Uterus | 71.21 | 6.91 | (2.24,16.10)† | 3.96 | 0.9 | (0.47,1.58) | 2 | 0.2 | (0.0,1.0) | |||
| Ovary | 56.97 | 5.06 | (1.38,12.95)† | 7.59 | 1.5 | (0.95,2.26) | 13 | 2.1 | (0.9,4.1) | 1.26 | 1 | (0.12,3.61) |
| Prostate | 28.52 | 3.48 | (2.03,5.57)† | 47.88 | 0.7 | (0.59,0.82) | 17 | 0.3 | (0.2,0.5)† | 3.15 | 0.9 | (0.29,2.08) |
| Testis | 28.52 | 7.48 | (4.36,11.97)† | 55.15 | 1.76 | (1.51,2.05)† | 5 | 0.7 | (0.3,1.6) | 11.98 | 1.1 | (0.66,1.72) |
| Kidney and renal pelvis | 22.51 | 3.56 | (1.99,5.87)† | 26.09 | 1.5 | (1.19,1.87)† | 6 | 1 | (0.5,1.8) | 3.78 | 1.1 | (0.40,2.37) |
| Bladder | 31.51 | 3.53 | (2.19,5.40)† | 13.54 | 0.6 | (0.43,0.81)† | 1.89 | 0.5 | (0.10,1.46) | |||
| Brain | 45.02 | 8.28 | (5.59,11.84)† | 51.51 | 3.5 | (2.97,4.09)† | 1 | 0.3 | (0.0,1.1) | 5.67 | 1 | (0.46,1.90) |
| Thyroid | 7.5 | 0.67 | (0.22,1.56) | 11.23 | 0.8 | (0.55,1.12) | 2 | 0.4 | (0.1,1.1) | 0.63 | 0.4 | (0.01,2.23) |
The SIR is significant
SIRs for cancers of colon, rectum and rectosigmoid junction
Trend analysis
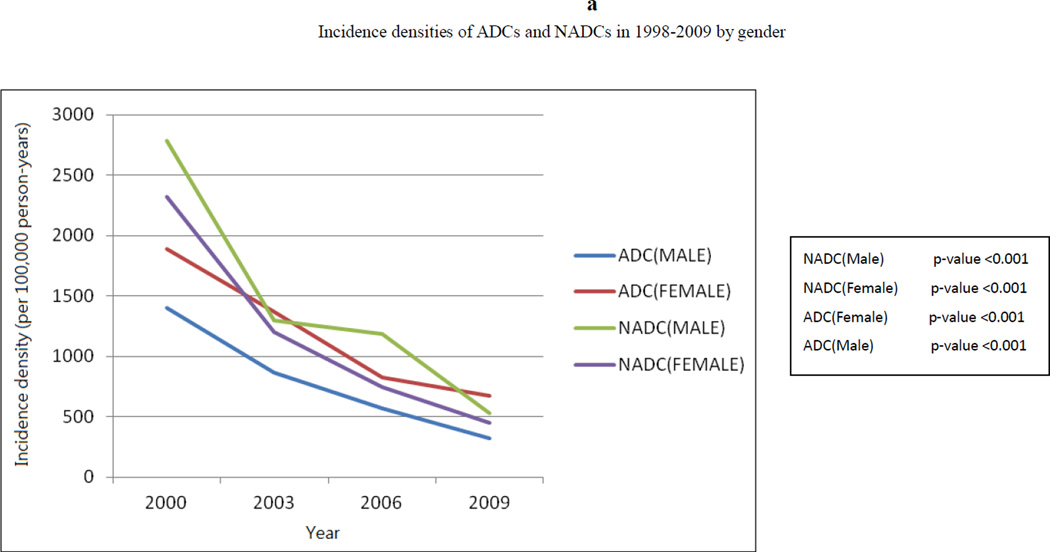
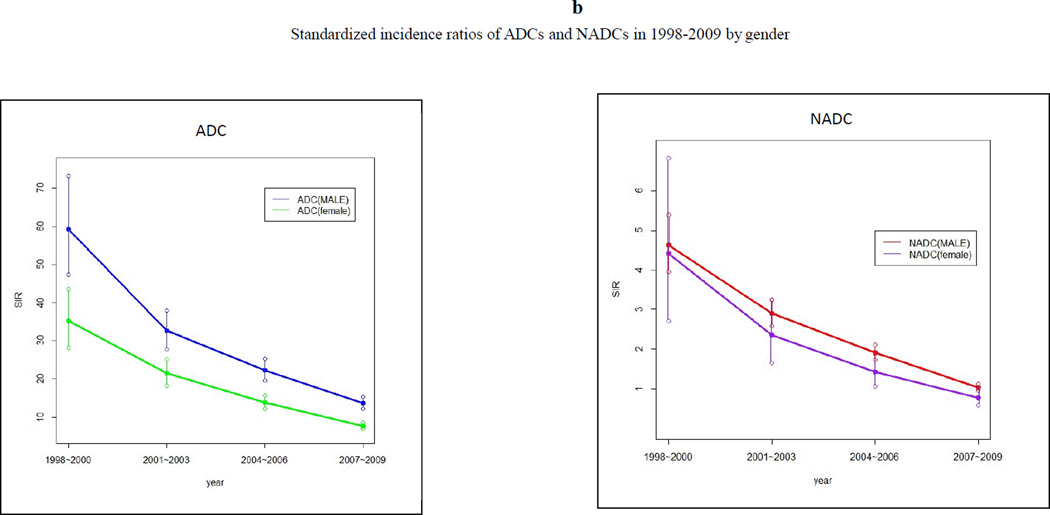
Trend analysis showed that from 1998 to 2009, period-adjusted gender-specific ADC and NADC rates decreased from more than 1,500 cases/100,000 person-years to less than 500 cases/100,000 person-years (p <0.001 for trend). (Figure 2a) A similar decrease in SIRs of ADCs and NADCs was seen in both males and females. (Figure 2b) Nevertheless, the SIRs remained increased for both ADCs and NADCs in males and females relative to the general population, except for a statistically insignificant decreased SIR for NADCs in females in 2007–2009. Overall, the SIRs were higher in males than in females.
Figure 2.
(a) Trend of incidence densities of ADCs and NADCs in 1998–2009 by gender, and (b) trend of standardized incidence ratios of ADCs and NADCs in 1998–2009 by gender.
Comparison with Catastrophic Illness Registry
Taiwan possesses another nationwide registry called the Catastrophic Illness Registry (CIR), which records patients with major illnesses, including all cancers. Comparison of data between the NHIRD and the CIR showed that in the general population, the case numbers were similar for most cancers. (Supplementary Table 1) Discrepancies were found for cancers of the anus, rectum or rectosigmoid junction, kidney and renal pelvis, ovary, and multiple myeloma. However, the SIRs remained increased for most of these cancers if the CIR was used instead. Our calculations showed that based on the CIR, the SIRs were 37.92 (CI=23.16, 58.58) for anal cancer, 4.65 (CI=2.95, 6.97) for cancer of the rectum and rectosigmoid junction, 2.50 (CI=1.20, 4.60) for cancer of the kidney and renal pelvis, 1.38 (CI=0.02, 7.66) for cancer of the ovary, and 6.31 (CI=2.53, 13.0) for multiple myeloma.
Discussion
While epidemiological studies on the incidence of cancer in PLWHA have been conducted, this is the first such study performed in Asia. In this study, the incidence of malignancies in PLWHA and relative to the general population was determined in a 12-year cohort in the post-HAART era. For the most part, our results are in line with those previously reported in Western studies.9,15,18–20 Kaposi's sarcoma was mostly thought to be rare in Asia;24 however, our study showed that Kaposi's sarcoma was a relatively common ADC in HIV/AIDS patients in Taiwan. The high prevalence of Kaposi's sarcoma may be explained by risk behavior as about half of male HIV/AIDS patients in Taiwan are men having sex with men (MSM) and one third are injection drug users (IDUs).25
Similarly increased SIRs were seen for men (SIR=18.45, ID=45.29/100,000 person years) and women (SIR=10.02, ID=28.48/100,000 person-years) for anal cancer. Anal cancer may be due to anal sex and HPV infection. All female HIV/AIDS patients in Taiwan were heterosexual, but it is unclear whether they engaged in anal sex. However, it is known that anal sex may not be necessary for anal HPV infection.26 Increased SIRs for colon cancer were seen in both males and females, and a possible infectious etiology, while not yet proven, has been proposed. Studies by Bodaghi et al.27 and Chen et al.28 suggested that colorectal cancer could be related to HPV 16 infection. In the past, Taiwan was a civilization whose diet was based on rice, seafood and pork. In the last few decades, Taiwan underwent a gradual westernization of diet, with an increased consumption of raw or partially cooked beef. This change in diet may have resulted in infectious pathogens being unknowingly ingested and could be associated with carcinogenesis.
Cancers of the oral cavity and nasopharynx, known to be associated with betel nut chewing and HPV infection, are common in the general population in Taiwan, with a cumulative incidence of 25.09/100,000 population in 2008.29 Liver cancer, which is related to HBV and HCV infection, is also common in Taiwan, with an incidence of 45.86/100,000 population.25 In this study, HIV infection was shown to further contribute to the risks of these common cancers as seen by the increased SIRs. However, it is hard to determine whether HIV plays a direct role, or if people who are more likely to contract HIV through risky sexual behavior or injection drug use, are more likely to contract other risk factors for the cancers such as HCV and HPV infection.
Lung and bronchus cancer was one of the more common NADCs. A statistically increased risk of lung cancer was seen in females in this cohort when compared to the general population. In the past 10 years, many adolescents and adults in Taiwan have picked up smoking habits. In the general population, about 46% of adults are non-smokers and 54% are past or current smokers.30 Similarly, about 40% of HIV patients in Taiwan never smoked, 33.5% are current smokers, and 26.7% are past smokers.31 While these smoking rates may appear similar, they are not identical, and smoking intensity may be different in HIV patients. Taiwanese women spend considerable amounts of time in the kitchen, and Taiwanese cuisine is heavy on fried and sautéed dishes. Previously, Cheng et al. reported an association between HPV infection and lung cancer in Taiwan.32 Therefore, tobacco use, second-hand smoke, cooking fumes and an infectious etiology may all play a role in lung carcinogenesis.
In this PLWHA cohort, increased susceptibility to most cancers was seen, which is in agreement with the theory that long-term immunosuppression in PLWHA caused by HAART may lead to increased risk of most types of cancer when compared to the general population. This study confirmed that PLWHA were at increased risk of cancers related to infection, and this may be related to deficiencies in their immune system. Overall, increased SIRs were seen in cancers related to HHV-8 (Kaposi’s sarcoma), EBV (non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and cancer of the nasal pharynx), HBV/HCV (cancer of the liver), H. pylori (cancer of the stomach), HPV (cancers of the cervix, uterus, anus or anal canal, oral cavity, oropharynx and hypopharynx, and possibly non-melanoma skin cancer and cancers of the lip, esophagus and larynx), and HTLV-1 (leukemia).
While a causal role has not yet been established for some cancers, a link has been proposed between HPV type 18 and prostate cancer,33 HPV, EBV and the mouse mammary tumor virus (MMTV) and breast cancer,34 HPV semen infection and testicular cancer,35 and polyomaviruses and bladder cancer.36 In our study, increased risks of prostate cancer, testicular cancer and bladder cancer were seen. However, SIRs for these cancers were significantly higher than those reported in Western countries. HPV and EBV infections are relatively common in the United States and Europe, and we would have expected similar SIRs in all studies. While we do not have an explanation for this discrepancy, it suggests that while HIV-infected patients may be at a higher risk of infectious comorbidities that may be related to some cancers, other factors may also play a role in carcinogenesis in HIV-infected patients.
The greatest strength of this study is that it was based on a nationwide registry rather than hospital-based registries, which made the calculation of cancer incidences more accurate. Some previous studies followed subjects after the onset of AIDS, and included person-years in the 5 years before AIDS in the study of PLWHA,15,20 while other studies included only the post-AIDS period.9,18 Because many of these studies covered both the pre-HAART and post-HAART eras, the degree of immune deficiency of subjects varied widely depending on the type of AIDS treatment received and the natural history of HIV infection. Our study included all adults residing in Taiwan diagnosed with HIV/AIDS during a 12-year post-HAART period, which made the immune status of the subjects more homogeneous.
The SIR of ADCs and some NADCs is associated with the degree of immune dysfunction. A limitation of this registry-based study was that we could not obtain clinical data such as CD4 count and analyze cancer incidence by degree of immune dysfunction.
This study showed increased risk of ADCs and most NADCs in PLWHA in Taiwan. With an increasing number of PLWHA and increased survival due to the advent of HAART, cancer has gradually become a very important comorbidity in these patients with deficient immune systems. Results from this study confirm that PLWHA are at increased risk of most cancers with respect to the general population, and support the theory that PLWHA may be at higher risk of infectious comorbidities that may play an important role in carcinogenesis. Therefore, preventive measures must be undertaken to decrease the risk of cancers, especially infection-related cancers, in PLWHA.
Supplementary Material
Supplementary Figure 1. Number of HIV/AIDS cases in 1998–2009 according to the Centers for Disease Control and NHIRD
Acknowledgements
We would like to thank Drs. Ann Shih and Ken N. Kuo from the Institute of Population Health Sciences of the National Health Research Institutes for their help in data collection and Andrew E. Grulich for his help in study design. This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, Taiwan, and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes. Funding was provided in part through the Asia Pacific HIV Research Collaboration, an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institute of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), and from the Dutch Ministry of Foreign Affairs through a partnership with Stichting Aids Fonds. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
References
- 1.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998 Feb 11;279(6):450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Phillips AN, Friis-Moller N, et al. Response to antiretroviral therapy among patients exposed to three classes of antiretrovirals: results from the EuroSIDA study. Antivir. Ther. 2002 Mar;7(1):21–30. [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. 2006 Sep;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.d'Arminio Monforte A, Sabin CA, Phillips A, et al. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Arch. Intern. Med. 2005 Feb 28;165(4):416–423. doi: 10.1001/archinte.165.4.416. [DOI] [PubMed] [Google Scholar]
- 6.Ruxrungtham K, Brown T, Phanuphak P. HIV/AIDS in Asia. Lancet. 2004 Jul 3–9;364(9428):69–82. doi: 10.1016/S0140-6736(04)16593-8. [DOI] [PubMed] [Google Scholar]
- 7.Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA. Immune deficiency and risk for malignancy among persons with AIDS. J. Acquir. Immune Defic. Syndr. 2003 Apr 15;32(5):527–533. doi: 10.1097/00126334-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 8.Goedert JJ, Cote TR, Virgo P, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998 Jun 20;351(9119):1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 9.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001 Apr 4;285(13):1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 10.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007 Jul 7;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg MJ, Neuhaus J, Bower M, et al. Risk of cancers during interrupted antiretroviral therapy in the SMART study. AIDS. 2007 Sep 12;21(14):1957–1963. doi: 10.1097/QAD.0b013e3282ed6338. [DOI] [PubMed] [Google Scholar]
- 12.Cancer ICoHa. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J. Natl. Cancer Inst. 2000 Nov 15;92(22):1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 13.Grulich AE, Li Y, McDonald A, Correll PK, Law MG, Kaldor JM. Rates of non-AIDS-defining cancers in people with HIV infection before and after AIDS diagnosis. AIDS. 2002 May 24;16(8):1155–1161. doi: 10.1097/00002030-200205240-00009. [DOI] [PubMed] [Google Scholar]
- 14.Grulich AE, Li Y, McDonald AM, Correll PK, Law MG, Kaldor JM. Decreasing rates of Kaposi's sarcoma and non-Hodgkin's lymphoma in the era of potent combination anti-retroviral therapy. AIDS. 2001 Mar 30;15(5):629–633. doi: 10.1097/00002030-200103300-00013. [DOI] [PubMed] [Google Scholar]
- 15.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006 Aug 1;20(12):1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 16.Petoumenos K. The role of observational data in monitoring trends in antiretroviral treatment and HIV disease stage: results from the Australian HIV observational database. J. Clin. Virol. 2003 Feb;26(2):209–222. doi: 10.1016/s1386-6532(02)00119-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, Dore GJ, Zhang F, Lim PL, Chen YM. Hepatitis B and C virus coinfection in The TREAT Asia HIV Observational Database. J. Gastroenterol. Hepatol. 2007 Sep;22(9):1510–1518. doi: 10.1111/j.1440-1746.2007.05062.x. [DOI] [PubMed] [Google Scholar]
- 18.Dal Maso L, Franceschi S, Polesel J, et al. Risk of cancer in persons with AIDS in Italy, 1985–1998. Br. J. Cancer. 2003 Jul 7;89(1):94–100. doi: 10.1038/sj.bjc.6601017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dal Maso L, Polesel J, Serraino D, et al. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br. J Cancer. 2009 Mar 10;100(5):840–847. doi: 10.1038/sj.bjc.6604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newnham A, Harris J, Evans HS, Evans BG, Moller H. The risk of cancer in HIV-infected people in southeast England: a cohort study. Br. J. Cancer. 2005 Jan 17;92(1):194–200. doi: 10.1038/sj.bjc.6602273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. http://www.nhri.org.tw/ NHIRD/en/index.htm.
- 22.HIV/AIDS statistics and drug substitution treatment statistics. [Accessed May 22, 2011]; http://www.cdc.gov.tw/mp.asp?mp=1.
- 23.Breslow NE, Day NE. Statistical methods in cancer research. Volume II--The design and analysis of cohort studies. IARC Sci. Publ. 1987;(82):1–406. [PubMed] [Google Scholar]
- 24.Petoumenos K, Hui E, Kumarasamy N, et al. Cancers in the TREAT Asia HIV Observational Database (TAHOD): a retrospective analysis of risk factors. Journal of the International AIDS Society. 2010;13:51. doi: 10.1186/1758-2652-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YJ, Lee CM, Chen M, et al. Molecular epidemiology of HIV-1 infection in Taiwan from 2005 to 2008: further spread of CRF07_BC and emergence of CRF07_BC/subtype B dual infection. J. Acquir. Immune Defic. Syndr. 2012 Apr 15;59(5):438–446. doi: 10.1097/QAI.0b013e3182454ea3. [DOI] [PubMed] [Google Scholar]
- 26.Kojic EM, Cu-Uvin S, Conley L, et al. Human papillomavirus infection and cytologic abnormalities of the anus and cervix among HIV-infected women in the study to understand the natural history of HIV/AIDS in the era of effective therapy (the SUN study) Sex. Transm. Dis. 2011 Apr;38(4):253–259. doi: 10.1097/OLQ.0b013e3181f70253. [DOI] [PubMed] [Google Scholar]
- 27.Bodaghi S, Yamanegi K, Xiao SY, Da Costa M, Palefsky JM, Zheng ZM. Colorectal papillomavirus infection in patients with colorectal cancer. Clin. Cancer Res. 2005 Apr 15;11(8):2862–2867. doi: 10.1158/1078-0432.CCR-04-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen TH, Huang CC, Yeh KT, et al. Human papilloma virus 16 E6 oncoprotein associated with p53 inactivation in colorectal cancer. World J Gastroenterol. 2012 Aug 14;18(30):4051–4058. doi: 10.3748/wjg.v18.i30.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summary of statistics on causes of death in Taiwan. [Accessed January 30, 2012];2008 http://www.doh.gov.tw.
- 30.Wu IHEE, Sansgiry SS, Peters RJ, Yang M, Abughosh S. Cigarette smoking among Taiwanese adults. Epidemiology. 2011;1(3):107. [Google Scholar]
- 31.Wu PY, Hung CC, Liu WC, et al. Metabolic syndrome among HIV-infected Taiwanese patients in the era of highly active antiretroviral therapy: prevalence and associated factors. J. Antimicrob. Chemother. 2012 Apr;67(4):1001–1009. doi: 10.1093/jac/dkr558. [DOI] [PubMed] [Google Scholar]
- 32.Cheng YW, Chiou HL, Sheu GT, et al. The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res. 2001 Apr 1;61(7):2799–2803. [PubMed] [Google Scholar]
- 33.Whitaker NJ, Glenn WK, Sahrudin A, Orde MM, Delprado W, Lawson JS. Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate. 2013 Feb 15;73(3):236–241. doi: 10.1002/pros.22562. [DOI] [PubMed] [Google Scholar]
- 34.Amarante MK, Watanabe MA. The possible involvement of virus in breast cancer. J. Cancer Res. Clin. Oncol. 2009 Mar;135(3):329–337. doi: 10.1007/s00432-008-0511-2. [DOI] [PubMed] [Google Scholar]
- 35.Garolla A, Pizzol D, Bertoldo A, et al. Testicular cancer and HPV semen infection. Front. Endocrinol. (Lausanne) 2012;3:172. doi: 10.3389/fendo.2012.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robles C, Viscidi R, Malats N, et al. Bladder cancer and seroreactivity to BK, JC and Merkel cell polyomaviruses: The Spanish bladder cancer study. Int. J Cancer. 2013 Aug 1;133(3):597–603. doi: 10.1002/ijc.28053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Number of HIV/AIDS cases in 1998–2009 according to the Centers for Disease Control and NHIRD