Abstract
Neonatal monosodium glutamate (MSG) administration increases adiposity, decreases energy expenditure and is associated with arcuate nucleus (Arc) destruction. Disrupted brown adipose tissue (BAT) thermogenesis underlies some of these effects, although, interscapular BAT temperature (TIBAT) has not been measured. Therefore, we tested the effects of neonatal MSG or vehicle administration in Siberian hamsters and, when they were adults, measured TIBAT during acute cold exposure. The Arc and its projection to the hypothalamic paraventricular nucleus (PVH) are both components of the CNS outflow circuits to IBAT, with the latter implicated in BAT thermogenesis that could be compromised by MSG treatment. Using a viral transneuronal tract tracer, pseudorabies virus (PRV), we also tested whether the components of these circuits were intact. As adults, MSG-treated hamsters had significantly increased body mass and some white fat pad masses, markedly reduced Arc Nissl and neuropeptide staining, and PVH neuropeptide fiber staining. Cold-exposed (18 h at 5 °C) MSG- and vehicle-treated hamsters initially maintained TIBAT, but the ability of the former waned after 2 h being significantly decreased by 18 h. PRV immunoreactive fibers/cells were not altered by neonatal MSG treatment despite substantial Arc and PVH destruction. MSG- and vehicle-treated hamsters given an exogenous norepinephrine challenge showed identical increases in the duration and peak of TIBAT. Thus, the inability of MSG-treated animals to sustain TIBAT in the cold is not due to any obvious MSG-induced deletions of central sympathetic outflow circuits to IBAT, but appears to be extrinsic to the tissue nevertheless.
Keywords: Obesity, Thermoregulation, Arcuate nucleus, Pseudorabies virus, Neuropeptide Y, Agouti-related protein, Lipolysis, Monosodium glutamate
1. Introduction
A greater appreciation of the CNS control of peripheral metabolism, including the link between brain and adipose tissues, has developed recently, especially the role of the sympathetic nervous system (SNS) in the regulation of lipid storage/mobilization and thermogenesis by white and brown adipose tissue (WAT and BAT), respectively (for reviews see: Bartness and Bamshad, 1998; Bartness and Song, 2007b; Himms-Hagen, 1991). The exact mechanisms underlying the ability of the SNS to control lipid mobilization/thermogenesis remain to be precisely defined, however.
The preponderance of research on the central control of energy intake and expenditure has focused on the hypothalamus (for review see: Cone et al., 2001), with an even narrower focus on the arcuate nucleus (Arc). The Arc initially received attention because lesions of this nucleus, produced by neonatal administration of monosodium glutamate (MSG), result in obesity (e.g., Olney, 1969). The Arc has been the focus of further research because it has receptors for leptin (e.g., Hakansson et al., 1996; Mercer et al., 1996), the adipokine (Zhang et al., 1994) that is thought to be important in energy balance (for review see: Arch, 2005). More specifically, the Arc contains two major cell types — neurons synthesizing orexigenic neuropeptides [neuropeptide Y (NPY) and agouti-related protein (AgRP)] and anorexigenic peptides [proopiome-lanocortin (POMC) and cocaine-amphetamine related transcript (CART); for review see: (Cone et al., 2001)]. Both sets of neurons are targets for the actions of leptin on energy balance (for review see: Berthoud, 2002). Of the efferents from the Arc, there are prominent NPY/AgRP and CART/POMC projections innervating the hypothalamic paraventricular nucleus (PVH; Bai et al., 1985; Cowley et al., 1999). Although the roles of these Arc neural populations in altering energy intake are well-accepted, appreciation of their involvement in energy expenditure is increasing (e.g., Bing et al., 1997; Kong et al., 2003; Morris et al., 1998; Schoelch et al., 2002).
A classic approach to understanding the role of any brain site for a given physiological response, such as energy balance, is to destroy it. Complete Arc electrolytic lesions, although difficult, have been accomplished (Choi et al., 1999), but carry with them a high probability of destroying the median eminence/pituitary gland which greatly complicates the interpretation of this type of Arc lesion. An alternative approach to Arc destruction is offered by neonatal administration of monosodium glutamate (MSG). MSG is able to penetrate the brain (e.g., Olney, 1969) via the underdeveloped neonatal blood brain barrier (BBB) in several brain areas, including the area postrema (AP; e.g., Leitner and Bartness, 2008a; Phelix and Hartle, 1990; Takasaki, 1978), but most notably in the Arc where up to 80–90% of neurons can be ablated (Olney, 1969). This neonatal MSG treatment produces a distinct adult phenotype of decreases in linear growth (Tamura et al., 2002), increases in body fat (Leitner and Bartness, 2008a) and sympathetic nervous system (SNS) dysfunction, most notably for the latter resulting in decreased BAT thermogenesis (e.g., Moss et al., 1985b; Yoshida et al., 1984).
BAT is the major organ responsible for non-shivering thermogenesis (e.g., Foster and Frydman, 1978; for review see: Cannon and Nedergaard, 2004). The SNS innervation of BAT (Bamshad et al., 1999; Foster et al., 1982a,b) is critical for heat production during cold acclimation (Heldmaier et al., 1989; Himms-Hagen, 1986), overeating (Rothwell and Stock, 1982) and other conditions (for review see: Bartness and Song, 2005; Himms-Hagen, 1991). Of the BAT depots, interscapular brown adipose tissue (IBAT) receives the most attention because of its size, accessibility and clear innervation (for review see: Bartness and Song, 2005).
It appears that the impaired BAT thermogenesis in neonatally-treated MSG animals contributes to the genesis of their obesity in adulthood, most likely through reduction in the sympathetic drive to BAT (Morris et al., 1998), rather than an intrinsic defect in the underlying mechanisms controlling BAT thermogenesis [e.g., the functioning of uncoupling protein-1 (UCP-1)]. That is, MSG-induced obesity is associated with decreases in BAT norepinephrine (NE) turnover (Nishioka et al., 1988; Rehorek et al., 1987; Yoshida et al., 1985), a neurochemical measure of sympathetic drive. These decreases in BAT NE turnover may be due to several factors including inappropriately low synthesis/release of NE from the SNS nerve terminals that innervate the tissue and/or MSG-induced disruption in the central SNS outflow circuits to BAT resulting in decreased NE release compared with animals that are neurally intact centrally.
Using the viral transneuronal retrograde tract tracer, pseudorabies virus (PRV), the CNS origins of SNS outflow circuits to IBAT have been determined in Siberian hamsters (Bamshad et al., 1999; Song et al., 2008), laboratory rats (Bamshad et al., 1999; Cano et al., 2003; Oldfield et al., 2002) and mice (Voss-Andreae et al., 2007). Among the many SNS outflow sites across the neuroaxis ultimately innervating IBAT, and of interest to the present study, are the Arc and one of its primary projection sites, the PVH (Bamshad et al., 1999; Bamshad et al., 1999; Song et al., 2008). Both the PVH (Freeman and Wellman, 1987; Yoshimatsu et al., 1993) and the Arc have been strongly implicated in the control of thermogenesis (Bing et al., 1997; Kong et al., 2003; Morris et al., 1998; Schoelch et al., 2002). Thus, the possibility exists that one of the extrinsic factors contributing to the ineffective IBAT thermogenic response to cold exposure of MSG-treated animals is the disruption of the brain–SNS–IBAT circuitry caused by the destruction of the Arc and/or its PVH projections.
Therefore, the present experiment tests IBAT function in adult hamsters that were treated with MSG- or its vehicle neonatally and that had temperature transponders implanted under IBAT for telemetric recording of IBAT temperature (TIBAT) during cold exposure (18 h at 5 °C). To avoid the cold-induced increase in food intake that occurs in this species (Brito et al., 2008) that could dampen the cold-triggered stimulation of BAT thermogenesis, all animals also were simultaneously food deprived. Several days later we tested the intrinsic function of BAT thermogenesis by injecting NE systemically and measuring TIBAT. It should be noted that, surprisingly, despite dozens of studies testing the response of BAT to several different stimuli in MSG-treated animals, direct measures of TIBAT have not been tested and indeed, such measures are infrequently performed for any thermogenic challenge in any species. Instead, apparent surrogates of BAT thermogenesis are most frequently measured especially UCP-1 gene expression. MSG damage was quantified by standard histology for cells (Nissl staining) and more detailed analysis of the phenotype of the destroyed neurons using immunohistochemistry for a variety of neurochemicals. In a second experiment using PRV, we tested whether the SNS outflow circuitry from brain to IBAT in MSG-treated was compromised due to Arc destruction and/or its projections to the PVH, two sites that are part of the SNS outflow from brain to IBAT noted above (Bamshad et al., 1999; Song et al., 2008).
2. Results
2.1. Verification of MSG-induced Arc, PVH and AP neuroanatomical damage
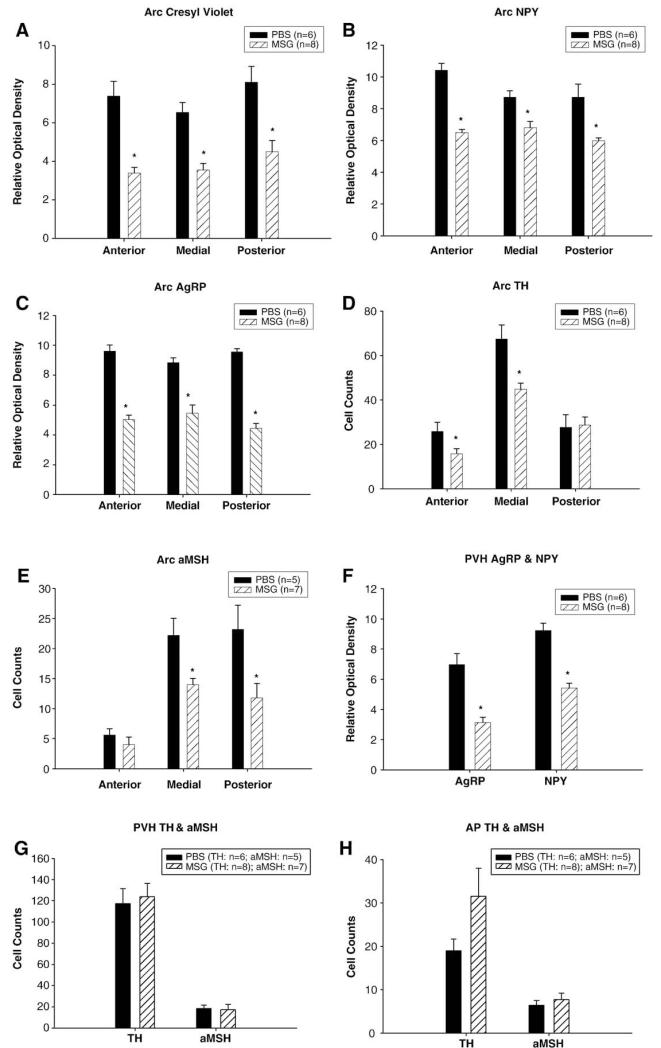
MSG treatment significantly decreased cresyl echt violet Nissl staining, NPY- and AgRP-immunoreactive (ir) fibers throughout the anterior, medial and posterior regions of the Arc (Figs. 1A–C and 2A–C). By contrast, MSG treatment only significantly decreased TH-ir cells at the anterior and medial Arc level and α-melanocyte-stimulating hormone (α-MSH)-ir cells at the medial and posterior Arc levels (Figs. 1D, E and 2D, E). In the PVH (Figs. 1F, G), MSG treatment decreased NPY- and AgRP fiber-ir (Figs. 2F, G), but did not affect TH-ir (Fig. 2H) and α-MSH-ir in the PVH. Cresyl echt violet Nissl staining (PBS: 6.8±0.9; MSG: 7.0±0.3), TH- and α-MSH-ir cell bodies in the AP (Fig. 1H) were not affected by MSG treatment.
Fig. 1.
Mean±SEM relative optical density of Nissl staining (A), neuropeptide Y (NPY)- (B) and agouti-related protein (AgRP)- (C) immunoreactive (ir) fibers, and Mean±SEM cells of tyrosine hydroxylase (TH)- (D) and α-melanocyte-stimulating hormone (αMSH)- (E) immunoreactive (ir) cells at three level of the arcuate (ARC) and Mean±SEM relative optical density of NPY- and AgRP-ir fibers (F) in the hypothalamic paraventricular nucleus (PVH) and Mean±SEM TH- and αMSH-ir cells in the PVH (G) and area postrema (AP; H) of adult male Siberian hamsters treated neonatally with monosodium glutamate (MSG) or phosphate buffered saline (PBS; *Ps<0.05 MSG vs. PBS).
Fig. 2.
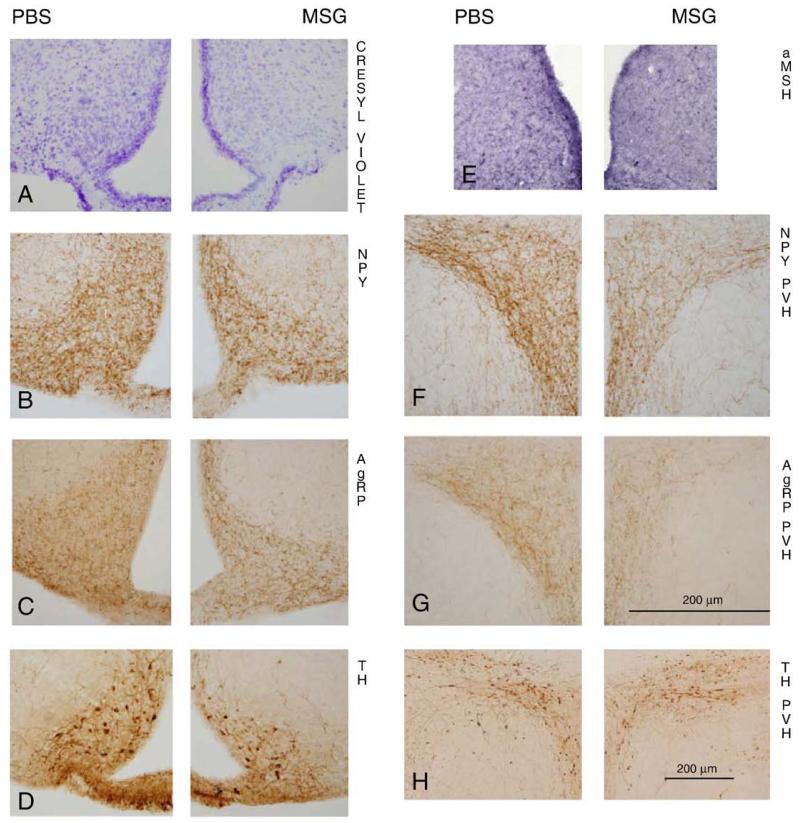
Representative photomicrographs of staining/immunohistochemistry of cresyl echt violet (A), neuropeptide Y (NPY; B) and agouti-related protein (AgRP; C) of the arcuate nucleus (Arc); and tyrosine hydroxylase (TH; D) and α-melanocyte-stimulating hormone (α-MSH; E)-immunoreactive (ir) cells in more posterior sections of the Arc; and NPY (F)-ir fibers, AgRP(G) -ir fibers and TH-ir cells (H) of the hypothalamic paraventricular nucleus (PVH) of adult male Siberian hamsters treated neonatally with monosodium glutamate (MSG; right panels) or phosphate buffered saline (PBS; left panels). Photomicrographs A–G were taken with the same magnification (20×), bar shown in G, while photomicrograph H was taken with 10× magnification.
2.2. Verification of MSG-induced obesity on body and fat pad masses
Neonatal MSG treatment resulted in significantly increased body mass of ~3 month old hamsters compared with their phosphate buffered saline (PBS) vehicle controls (Fig. 3A). In addition, MSG significantly increased retroperitoneal WAT (RWAT) mass (Fig. 3B). Across the various WAT pads, only epididymal WAT (EWAT) mass was significantly decreased for both MSG- and PBS-treated groups with cold exposure/^food deprivation evident even several days later (Fig. 3B).
Fig. 3.
Mean±SEM g body (A) and individual fat pad mass (B) of adult male Siberian hamsters treated neonatally with monosodium glutamate (MSG) or phosphate buffered saline (*Ps<0.05 MSG vs PBS, #Ps<0.05 cold/food deprived vs group control) after body mass recovered to baseline levels after cold exposure and NE injections.
2.3. Effects of cold exposure/food deprivation on TIBAT, body mass and serum glycerol and free fatty acid (FFA) concentrations in MSG- and PBS-treated hamsters
At the initiation of the cold exposure/food deprivation, MSG-treated hamsters had significantly decreased basal TIBAT compared with their PBS controls (Figs. 4A, B). This difference was not significant during the recovery phase (36 h later) or during NE injections (Fig. 6).
Fig. 4.
Mean±SEM TIBAT during room temperature/food deprivation (A) and 18 h cold exposure/food deprivation (B) of adult male Siberian hamsters treated neonatally with monosodium glutamate (MSG) or phosphate buffered saline (PBS; *Ps<0.05 MSG vs PBS; #Ps<0.05 MSG cold/food deprived vs PBS cold/food deprived).
Fig. 6.
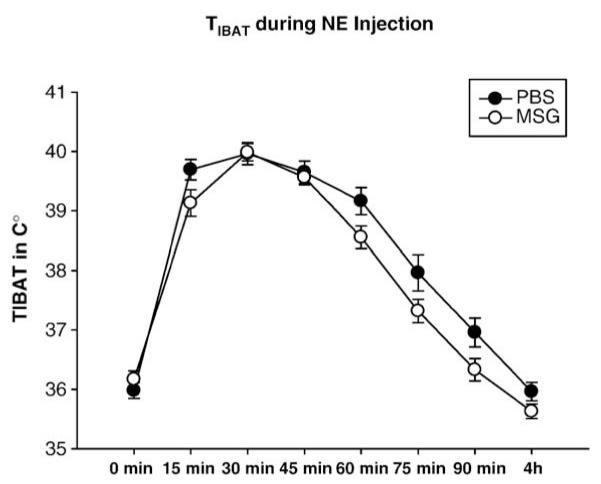
Mean±SEM TIBAT of adult male Siberian hamsters treated neonatally with monosodium glutamate (MSG) or phosphate buffered saline (PBS) after systemic (ip) injection of norepinephrine (NE; 0.4 mg/kg free base).
With cold exposure/food deprivation, TIBAT of food-deprived, cold-exposed PBS hamsters remained steady across the 18 h test, whereas TIBAT of MSG cold-exposed, food-deprived hamsters progressively decreased beginning about 2 h after cold initiation and becoming significantly less than PBS-treated cold-exposed controls by 18 h (Fig. 4B). Two cold-exposed, food-deprived MSG animals were torpid [stereotypic curled body posture, head tucked between legs, slow breathing, cold to touch; (Bartness et al., 1989), TIBAT<27°C], a finding not seen in any animal in the other groups.
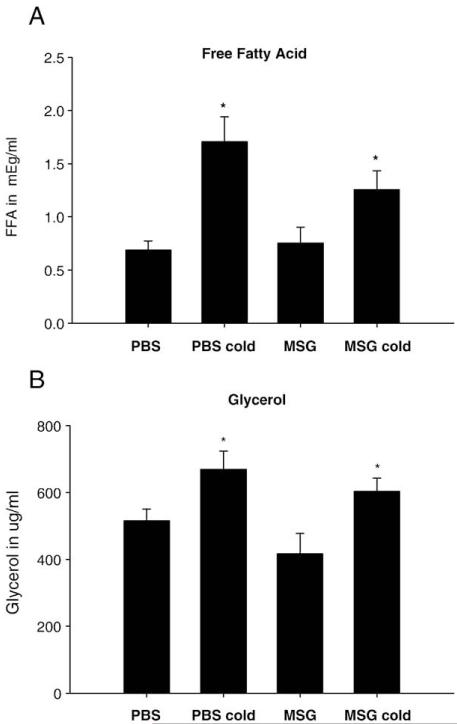
18 h of cold exposure/food deprivation significantly decreased body mass in both MSG and PBS-treated Siberian hamsters approximately to the same extent compared with their respective room temperature food-deprived controls (data not shown). To test for circulating indicators of WAT lipid mobilization, serum FFAs and glycerol, the products of lipolysis, were increased in both cold-exposed, food-deprived groups compared with their respective food-deprived controls at room temperature (Figs. 5A, B), but were not different from each other.
Fig. 5.
Mean±SEM serum free fatty acid (FFA) concentrations (mEg/ml) (A) and glycerol concentrations (μg/ml) (B) of adult male Siberian hamsters treated neonatally with monosodium glutamate (MSG) or phosphate buffered saline [PBS; *Ps<0.05 control (room temperature/food deprivation) vs. cold/food deprivation].
2.4. Effects of a NE challenge on TIBAT
Despite the declining TIBAT of MSG-treated hamsters compared with their PBS counterparts during cold exposure/food deprivation (Fig. 4), systemically applied exogenous NE resulted in a similar NE-induced increase in TIBAT (Fig. 6). Specifically, TIBAT was increased compared with baseline values at 15, 30, 45, 60 and 75 min post injection similarly for both groups (Fig. 6). Moreover, the NE-triggered peak in TIBAT occurred at the same temperature (~40 °C) and time (30 min post injection) for both groups.
2.5. Effects of MSG on SNS outflow circuitry to IBAT
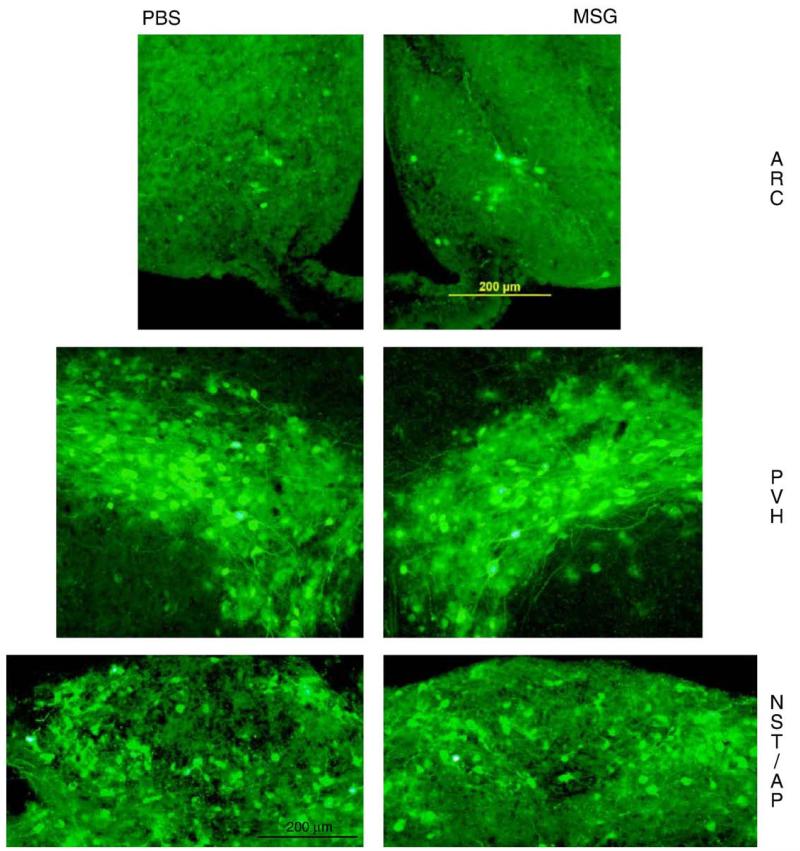
We quantified several forebrain and brainstem areas previously shown to be principal components of the SNS outflow circuitry from brain to IBAT (Bamshad et al., 1999; Song et al., 2008; e.g., Table 1). The number of PRV-infected cells did not differ between MSG- and PBS-treated animals in any nucleus/area (Table 1), including, surprisingly, the Arc. PRV-labeled cells in both groups were prevalent in the medial preoptic area, dorsomedial hypothalamic nucleus, as well as the nucleus of the solitary tract of the brainstem (Table 1). Infections also occurred in neurons of the Arc and PVH (especially the PVH parvocellular part and to a lesser extent PVH magnocellular divisions) of the forebrain and AP of the brainstem (Fig. 7). This labeling confirms previously identified areas associated with the CNS–SNS–IBAT circuitry (Bamshad et al., 1999; Song et al., 2008).
Table 1. Number of PRV-labeled cells (mean ± SEM) per nucleus I from neonatallymonosodiumglutamate (MSG)- and phosphate I buffered saline (PBS)-treated male Siberian hamsters quantified when they were adults after inoculation of pseudorabies virus (PRV) into interscapular brown adipose tissue (IBAT).
| Area | PBS | MSG |
|---|---|---|
| Forebrain (hypothalamus) | ||
| Medial preoptic area | 49±12 | 54±38 |
| Lateral hypothalamus | 48±12 | 45±20 |
| Suprachiasmatic nucleus | 31±20 | 13±4 |
| Anterior hypothalamus | 23±4 | 20±11 |
| Zona incerta | 22±5 | 28±8 |
| Dorsomedial nucleus | 68±21 | 39±13 |
| Posterior hypothalamus | 23±2 | 21±5 |
| Ventromedial hypothalamus | 13±4 | 14±6 |
| Arcuate nucleus | ||
| Anterior arcuate nucleus | 6±2 | 9±3 |
| Medial arcuate nucleus | 8±3 | 19±12 |
| Posterior arcuate nucleus | 19±5 | 17±7 |
| Hypothalamic paraventricular nucleus | ||
| Hypothalamic magnocellular | 36±7 | 48±13 |
| paraventricular nucleus | ||
| Hypothalamic parvocellular | 64±22 | 53±7 |
| paraventricular nucleus | ||
| Other forebrain areas | ||
| Nucleus reuniens | 5±2 | 5±2.0 |
| Midbrain | ||
| Periaqueductal gray area | ||
| Dorsomedial periaqueductal gray | 17+4 | 10±5 |
| Dorsolateral periaqueductal gray | 13±4 | 4±6 |
| Lateral periaqueductal gray | 23±3 | 20±8 |
| Ventrolateral periaqueductal gray | 43±6 | 34±9 |
| Brainstem | ||
| Cranial nerve V | 45±7 | 35±5 |
| Noradrenergic cell field | 16±4 | 17±5 |
| Ventral spinocerebellar tract | 11±2 | 12±3 |
| Cranial nerve VII | 21±7 | 7±5 |
| Lateral paragigantocellular nucleus | 29±9 | 35±5 |
| Raphe magnus nucleus | 5±1 | 9±2 |
| Raphe pallidus | 13+1 | 10±1 |
| Gigantocellular complex | ||
| Gigantocellular reticular nucleus | 9±2 | 9±2 |
| Anterior gigantocellular reticular nucleus | 23±8 | 31±7 |
| Ventral gigantocellular reticular nucleus | 25 ±7 | 33±9 |
| Adrenergic cells/rostroventrolateral reticular nucleus |
14±4 | 30±5 |
| Intermediate reticular nucleus | 12±3 | 15±5 |
| Raphe obscurus | 11±5 | 9±2 |
| Solitary tract nucleus | 46±10 | 66±17 |
| Area postrema | 46±18 | 15±5 |
Fig. 7.
Photomicrographs of PRV-labeled cells for the arcuate nucleus (Arc), hypothalamic paraventricular nucleus (PVH) and nucleus of the solitary tract with the area postrema (NST/AP) of monosodium glutamate (MSG; right panels) — and phosphate buffered saline (PBS; left panels)-treated hamsters. Photomicrographs were taken with the same magnification (20×).
3. Discussion
The purpose of this study was to test the effects of neonatal MSG treatment on a direct measure of BAT function, TIBAT, for the first time in any species. More specifically, we tested the response of TIBAT to cold exposure, with the additional challenge of food deprivation to obviate the cold-induced increase in food intake seen in Siberian hamsters (Brito et al., 2008). This also was the first test of BAT function in MSG-treated Siberian hamsters, except for an indirect test of neonatal MSG administration on adult torpor responses (Pelz et al., 2008). TIBAT was initially increased in MSG-treated hamsters exposed to the cold similarly to their vehicle controls; however, with increased cold exposure, the ability to maintain this increased TIBAT diminished beginning at 2 h and by 18 h, it significantly decreased compared with controls. This inability to maintain increased TIBAT by MSG-treated hamsters was not due to an intrinsic defect in BAT because when given an exogenous NE challenge, the peak and duration of the increased TIBAT were identical between MSG- and PBS-treated hamsters.
We conducted an extensive immunohistological analysis of the Arc, PVH and AP to document the MSG destruction in the present experiment. In previous studies by others where MSG lesions were induced neonatally, sometimes there was no histology (e.g., Bueno et al., 2005; Dolnikoff et al., 2001) or at best in the vast majority of the studies, only Nissl staining (e.g., Maletinska et al., 2006; Olney, 1969; Schoelch et al., 2002), as well as ignoring other brain sites potentially affected by MSG such as the AP (Ebling et al., 1998). We found that neonatal MSG injections produced profound Arc neuronal destruction when verified in adult hamsters including decreased overall Arc cell density [cresyl violet (Nissl) staining], as well as decreased Arc TH-, AgRP-, α-MSH- and NPY-ir cells and decreased NPY and AgRP-ir fibers in the PVH, a primary Arc projection site. By contrast, TH- and α-MSH-ir cell bodies in the AP were not reduced in MSG-treated animals. It is not possible, however, to ascribe the defect in the TIBAT response to cold/food deprivation to the loss of any of the phenotypically-defined neurons. Instead, the purpose of this analysis was to indicate the degree of MSG-induced neuroanatomical destruction including the phenotype of some of the cells that were destroyed.
It has been reported previously (Ebling et al., 1998) that neonatal MSG treatment in this species spares a small population of TH-ir cells in the dorsal medial aspects of the Arc (dmpArc), an area that contains approximately 15% of Arc PRV infection after IBAT injection (Barrett et al., 2009). In the present experiment, we also found no destruction of TH-ir cells in the posterior section, which includes the dmpArc. Thus, the dmpArc could possibly account for the lack of decreased PRV-labeling in MSG-treated Siberian hamsters.
Because the AP was not affected here, the ablation of only the Arc allowed us also to test the role of the Arc and its efferent connections in TIBAT responses to acute cold exposure using indwelling IBAT temperature transponders — a relatively novel direct measure of IBAT thermogenesis in MSG-treated animals that we have used previously in this species (Brito et al., 2007; Leitner and Bartness, 2008b; Song et al., 2008). Indeed, to our knowledge, this is the only study directly measuring TIBAT, rather than a surrogate of thermogenesis (e.g., UCP-1 gene expression, NETO, guanosine-5′-diphosphate binding) in thermally challenged, MSG-treated animals of any species. Moreover, this is an infrequent direct in vivo measure of TIBAT in rodents for any treatment (e.g., Flaim et al., 1976; Morimoto et al., 1986).
We found that MSG-treated hamsters had significantly decreased TIBAT before cold exposure, at 0 h (Fig. 4). This difference, however, was found inconsistently throughout the study. The exact reason for this discrepancy is unknown, however, we believe that the occasional decreased TIBAT by MSG-treated animals compared with their controls is due to the increased baseline levels of the stress hormone corticosterone (Dolnikoff et al., 1988), and increased sensitivity of our animals to stress, as seen by increased corticosterone secretion to even relatively mild stressors frequently observed in MSG-treated rodents (e.g., Dolnikoff et al., 1988; Larsen et al., 1994). Corticosterone, in turn, is well known for its inhibitory effects on UCP-1 and BAT thermogenesis (e.g., Moriscot et al., 1993; Soumano et al., 2000; Strack et al., 1995), especially in MSG-treated animals (Tokuyama and Himms-Hagen, 1989). Therefore, heightened corticosterone secretion may underlie the decreased TIBAT when observed during baseline conditions.
Although precisely how this decrease in TIBAT might affect body temperature (Tb) cannot be discerned from the present study, but the decreased TIBAT potentially could have contributed to the genesis of obesity in MSG-treated Siberian hamsters. In a recent study of torpor responses in MSG-treated Siberian hamsters (Pelz et al., 2008), no differences were reported for Tb between these animals and their controls. This also is consistent with studies reporting indistinguishable rectal temperature (Tb) between MSG- and vehicle-treated mice (Moss et al., 1985a). In addition, resting metabolic rate in MSG-treated laboratory mice (Poon and Cameron, 1978), and colonic temperature and total energy expenditure in MSG-treated rats (Schoelch et al., 2002; Yehuda et al., 1991) are significantly decreased compared with controls suggesting possible contribution of decreased thermogenesis to the development of the MSG-triggered obesity in our study. Because Tb and TIBAT can be dissociated (Halvorson et al., 1990; Kobayashi et al., 1999; Madden and Morrison, 2009; Zaretskaia et al., 2002), we can only speculate that decreased Tb contributed to the production of MSG-induced obesity in our animals because we did not measure Tb here.
With cold induction/food deprivation, MSG-treated hamsters were unable to maintain TIBAT across this 18 h challenge compared with their PBS counterparts, but were able to maintain short-lived TIBAT responses (e.g. 2 h). The similar early (~1–2 h) TIBAT response to cold between the two groups is consistent with acute cold- (1 h at 5 °C) induced increases in NE turnover and guanosine-5′-diphosphate binding in both MSG- and vehicle-treated mice (Yoshioka et al., 1989), suggesting that some aspect of MSG treatment impairs the ability to maintain the short-lived increased BAT thermogenesis. The apparent extrinsic deficit in BAT functioning appears response-specific, rather than being a global SNS deficit, consistent with the notion of differential control of sympathetic drive to peripheral tissues (for review see: Bartness and Song, 2007a; Morrison, 2001). Indeed, diet-induced thermogenesis is normal in MSG-treated laboratory mice (Moss et al., 1985a). Moreover, MSG did not produce an overall deficit in brain–SNS functioning because another sympathetic neurally-mediated response, lipolysis (for review see: Bartness and Bamshad, 1998; Bartness and Song, 2007b), was unimpaired in MSG-treated hamsters compared to their PBS counterparts. That is, both groups showed significantly increased cold-induced plasma concentrations of FFAs and glycerol, the products of NE-triggered lipolysis, as we found previously with MSG-treated Siberian hamsters that were only food deprived (Leitner and Bartness, 2008a).
Because the impact of TIBAT is not only dependent on sympathetic drive to BAT, but also on blood flow in response to the cold (Foster and Frydman, 1979), one might postulate that decreases in blood flow to IBAT of MSG-treated hamsters may have contributed to reduced TIBAT. Adult rats, however, treated with MSG neonatally have normal blood flow to BAT in response to β3-adrenoceptor stimulation (Iwase et al., 2000), a manipulation that is interchangeable with acute cold exposure in terms of its ability to stimulate BAT thermogenesis, at least in Siberian hamsters (Bocker et al., 1982). Therefore, a dysfunction in blood flow to IBAT of MSG-treated animals in the present study does not seem likely.
The ability of exogenous systemic NE injection to increase TIBAT virtually identically in MSG- and PBS-treated hamsters in the present study indicates that the inability of IBAT to respond in a normal thermogenic manner to acute cold exposure is not due to an intrinsic malfunction in brown adipocyte adrenoceptor activation or intracellular signaling/processes. Instead, the defect appears extrinsic in origin, but not due to any apparent structural alteration in brain–SNS–IBAT circuitry, as tested here. The notion that the malfunction in BAT of MSG-treated animals is extrinsic to the tissue has been suggested previously based on experiments in neonatally MSG-treated laboratory mice (Moss et al., 1985b), as also was found here in Siberian hamsters. More mechanistically, rather than structurally, this IBAT thermogenesis deficit of MSG-treated, cold-exposed animals may be due to insufficient SNS drive to IBAT, as measured by NE turnover. Indeed, IBAT NE turnover is significantly decreased in cold-exposed, MSG-treated mice compared with their vehicle-treated counterparts (Dulloo and Young, 1991; Yoshida et al., 1984, 1985). It has been suggested that this decrease in sympathetic drive is due to initial defects in NE synthesis, rather than release (Dulloo and Young, 1991). Such reductions in NE synthesis ultimately resulting in decreases in release, could result in decreases in mobilization of BAT triacylglycerol (Moss et al., 1985b), uncoupling protein-1 gene expression (Tsukahara et al., 1998) and/or the local generation of triiodothyronine by type II T4 5′-deiodinase, all factors necessary for normal BAT uncoupling and hence increases in BAT temperature (Tsukahara et al., 1997). Any or all of these possible defects would help explain the inability to sustain an increase in TIBAT with cold exposure once the terminal pools of NE were depleted by their release in MSG-treated animals. Regardless of whether the defect in BAT sympathetic nerves is due to decreased NE synthesis or release, it seems specific to IBAT NE terminals, as lipolysis occurs normally in MSG-treated Siberian hamsters as shown here, discussed above and demonstrated previously (Leitner and Bartness, 2008a).
To assess possible structural damage to the central SNS outflow circuits to IBAT, we injected PRV into IBAT and found, somewhat surprisingly given the extensive MSG-induced Arc damage, that Arc PRV-infected neurons of MSG hamsters were not reduced compared to PBS controls, nor were there any obvious significant differences in the number of PRV-infected neurons across several forebrain, midbrain and brainstem nuclei — sites previously shown to be components of the SNS outflow circuitry from brain to IBAT using the PRV tract-tracing methodology in this species (Bamshad et al., 1999; Song et al., 2008) and in laboratory rats (Berthoud et al., 2005; Cano et al., 2003; Oldfield et al., 2002). This lack of an effect on PRV-labeling in SNS outflow circuitry to IBAT also was evident in the PVH, a major Arc projection destination that exhibited significant and marked decreases in NPY- and AgRP-ir fibers. Although we quantified PRV-infected cells in 10 hypothalamic sites (as well as subregions of the Arc and PVH), one non-hypothalamic forebrain site, one midbrain site and its divisions (PAG) and 13 brainstem areas (including subregions of the GI), we did not assay all infected areas of the brain and potentially could have missed areas that had compromised SNS outflow cells as a result of neonatal MSG treatment. We did, however, assay what are acknowledged as brain sites most likely to be affected by MSG (Arc, PVH and AP) as well as the major components of the SNS outflow circuitry from brain to IBAT.
Neonatal MSG treatment produced increases in body mass including an increase in one WAT pad mass (RWAT), responses expected as this treatment is obesity promoting in laboratory rats and mice (Dulloo and Young, 1991; Moss et al., 1985b; Yoshida et al., 1985) as well as in identically treated female Siberian hamsters seen by us previously (Leitner and Bartness, 2008a). Although increased food consumption in MSG-administered female Siberian hamsters (Leitner and Bartness, 2008a) has been suggested, the genesis of the MSG-induced increased body and lipid mass in the present study is unknown because we did not measure food intake, or energy expenditure as noted above. Here, only RWAT mass was increased in MSG-injected neonatal male Siberian hamsters compared with PBS-treated controls. Others, however, have reported no change in adiposity of MSG-treated male Siberian hamsters (Ebling et al., 1998), which serves to reinforce the notion of lesser effects of neonatal MSG administration in males compared with females. More specifically, in identically treated females, MSG treatment induced a more broadly represented adult-expressed obesity showing increases in all major WAT pad masses (Leitner and Bartness, 2008a). In male neonatally-treated MSG laboratory rats, both the RWAT and EWAT masses are typically increased (Nascimento Curi et al., 1991; Racek et al., 2001). The mechanism behind the lack of MSG-induced increased IWAT and EWAT mass in the present study is unknown, but the MSG-induced fat pad-specific effect on RWAT mass seen here would have been missed if only one fat pad was assayed (usually EWAT) as is typically the case in previous MSG studies as well as other conditions that promote obesity (for review see: Bartness and Song, 2007b).
Collectively, the present data demonstrate that an intact Arc and/or its projections to the PVH and perhaps elsewhere (e.g., perifornical area; Bai et al., 1985; Cowley et al., 1999) are required for a full, normal thermogenic response of TIBAT to cold exposure/food deprivation and that the progressive inability to maintain TIBAT is not due to an inability to respond thermogenically to NE, as evidenced by the virtually identical TIBAT increases between MSG- and PBS-treated hamsters. Finally, the major purpose of the study was to test if the underlying extrinsic basis for the inability of MSG-treated animals to show appropriate cold-induced thermogenic BAT responses was due to disruption of the CNS–SNS–IBAT circuitry. There was, however, no obvious structural difference in the central SNS outflow circuitry to IBAT, as suggested by the lack of a decrease in PRV-labeled cells in several areas across the neural access previously shown to be components of this circuitry. Thus, the factor extrinsic to BAT that is altered by neonatal MSG treatment such that adult animals are unable to exhibit normal BAT thermogenic responses to an acute cold challenge remains unexplained.
4. Experimental procedures
4.1. Animals and housing
All experiments were approved by the Georgia State University Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health and United States Department of Agriculture guidelines. Siberian hamsters (Phodopus sungorus) were housed in a vivarium where the temperature, relative humidity and photocycle were 20±1.5 °C, 50±5% and 16L:8D hours (lights on at 0300), respectively throughout the experiment. Animals were given Purina lab chow 5001 (TestDiet, Brentwood, MO) and tap water ad libitum unless otherwise noted. Hamsters were weaned at 21 days post-partum and singly housed in polypropylene cages (27.8 × 17.5 × 1 3.0 cm) containing corn cob bedding (The Andersons, Maumee, OH) and cotton nestlets (Ancare, Belmore, NY) until used in the present experiment.
4.2. MSG treatment
Forty-three male Siberian hamsters from our breeding colony were injected subcutaneously into the dorsal dermis area of the interscapular region from postnatal Day 1 to 5 (postnatal Day 0=day of birth) with 25 μl of MSG (Sigma-Aldrich, St. Louis, MO) to deliver 4 mg/g body mass or vehicle [0.1 M phosphate buffer (PBS), pH 7.4] as previously described (Leitner and Bartness, 2008a). This dose and injection schedule was chosen based on the successful production of the MSG-syndrome in this species (Ebling et al., 1998). All animals within a litter were injected with MSG or PBS to minimize pup cannibalization that can occur with differential treatment of members of the same litter in this species (unpublished observations). Five animals (3 MSG, 2 PBS), however, had to be excluded from the analysis due to unusually low body masses as ~2.5 month old adults (<32 g), resulting in 22 MSG and 16 PBS animals.
4.3. Transponder implants
At ~2.5 month of age, each individual had a temperature transponder implanted under the left IBAT pad to directly measure TIBAT telemetrically, as we have done previously (Brito et al., 2007; Song et al., 2008). In brief, under isoflurane anesthesia, a midline incision along the cephalad dorsal surface of the body was made and the IBAT pads were exposed. A temperature transponder (Implantable Program-mable Temperature Transponder [IPTT] 300, BioMedic Data Systems, Seaford, DE) was tied to sterile suture silk and then attached to the left IBAT pad with the temperature sensitive portion of the IPTT-300 (copper end) directed at the medial border of the left IBAT pad. The IBAT pad was moistened with sterile saline, the incision was then closed with sterile wound clips and nitrofurozone powder was applied to minimize the risk of infection. At the end of the experiment, hamsters were overdosed with pentobarbital sodium (300 mg/kg; Nembutal, Ovation Pharmaceuticals, Inc. Lake Forest, IL) and the transponder location was verified by visually examining the position of the copper end of the transponder relative to the medial border of the left IBAT pad. Two animals had to be excluded from the analysis due to movement of the transponder to other dorsal subcutaneous tissues (e.g., WAT) resulting in 20 MSG and 16 PBS animals to be included for analysis.
4.4. Acute cold exposure/food deprivation procedures and blood serum analysis
At ~12 weeks of age, animals were weighed, and food and bedding was removed from their cheek pouches. They were then placed in clean, empty cages with access to only water. Food deprivation was added to the cold exposure to eliminate the cold-induced increases in food intake seen in this species (Brito et al., 2008) that likely would affect TIBAT and consequently the room temperature animals also were food deprived to permit comparisons between both housing temperatures. Half the animals remained at room temperature (22 °C) and the other half were transferred to the cold (5 °C), the latter provided by a lighted, modified chromatography refrigerator equipped with a muffin fan to assure air exchanges equivalent to that of the vivarium. Temperature treatments lasted for 18 h and began at 0800 h. TIBAT was measured 2 h before (data not shown) and at 0, 2, 6, 10, 14 and 18 h after cold exposure. In addition, one recovery TIBAT measure (36 h) was taken the next day. At 18 h, all animals were anesthetized with isoflurane and orbital blood was taken for future assay of serum glycerol and FFA concentrations as a general measure of the products of lipolysis. Blood (~500 μl) was collected with heparinized glass capillary tubes, stored in culture tubes overnight, centrifuged at 4 °C for 20 min at 2000 RPM. Serum was removed and stored at −80 °C until analysis. Commercially available glycerol (Sigma-Aldrich, St. Louis, MO) and FFA (WAKO Chemicals, Richmond, VA) kits were used according to the manufacturers’ instructions.
4.5. NE challenge
Ten days after the acute cold test, we tested the response of TIBAT to a NE challenge to determine if any defect in IBAT thermogenesis was of extrinsic origin, in which case the MSG- and PSB-treated animals would respond similarly, or if intrinsic, then the MSG-treated hamsters would have an impaired response compared with the PSB-injected controls. In addition, this test also served as a functional assay of the temperature transponders (Song et al., 2008). NE (+) bitartrate salt hydrate (Sigma-Aldrich, St. Louis, MO) was injected subcutaneously (0.4 mg/kg free base; Bocker et al., 1982) 10 days after the cold experiment, when animals had recovered to their pre-experimental body mass. Basal TIBAT was established with three baseline measures 30 min apart before the beginning of the NE injections, with the final reading serving as the baseline used for data analysis. At 0730 h, all animals were injected with NE and TIBAT was measured every 15 min for 90 min with a final reading 4 h post injection.
4.6. PRV injection
To test if MSG treatment produced a disruption in the brain–SNS–IBAT neurocircuitry that may underline the impairment of IBAT function in these animals, we used PRV methodology to trace these circuits. Therefore, a subset of animals (5 MSG-treated, 5 PBS-treated) was anesthetized with isoflurane and the scapular area was shaved and wiped with ethanol (50%). To expose IBAT, an interscapular incision was made with care taken to avoid damaging the underlying vasculature and musculature. A series of injections of PRV 152 (GFP-expressing PRV strain, a generous gift of Dr. Lynn Enquist, Princeton University, Princeton, NJ) was made using a 1.0 μl micro-syringe at five loci (7.5×107 pfu/ml; 150 nl/loci) within the right IBAT pad (the left IBAT contained transponder implant) to evenly distribute the virus, as previously described (Song et al., 2008). The animals were terminated 5.5 days post injection, in accordance to our previously determined time course for IBAT PRV infections in this species (Bamshad et al., 1998; Song et al., 2008).
4.7. Tissue harvesting and perfusions
PRV-injected animals were excluded from the tissue harvesting. Animals were anesthetized with an overdose (300 mg/kg) of pentobarbital sodium once animals had recovered from the NE challenge and the right IWAT, and bilateral RWAT and EWAT depots were removed and weighed for an integrated measure of lipolysis. The animals were then perfused transcardially between 1200 h and 1600 h with ~125 ml isotonic saline, followed by ~125 ml of 4% paraformaldehyde in 0.1 M PBS solution (pH 7.4). A subset of animals (7 MSG, 5 PBS) was perfused transcardially with 4% paraformaldehyde and 2.5% acrolein in phosphate buffer (pH ~6.8), to enhance α-MSH-ir. All brains were stored in 4% paraformaldehyde and transferred to sucrose (30% and 0.1% sodium azide) after 24 h until they were processed for immunohistochemistry (IHC). Brains were sectioned in the coronal plane on a freezing microtome at a thickness of 35 μm. Brain sections were stored in 0.1 M PBS with 0.1% sodium azide at 4 °C and processed within 30 days.
4.8. MSG lesion verification: cresyl echt violet staining and IHC for NPY, AgRP and TH
Methods for IHC have been described previously (Leitner and Bartness, 2008a). In brief, to reduce background staining, sections were incubated in 0.6% H2O2 in 0.1 M PBS with 0.4% Triton X-100 (PBTx) for 30 min, rinsed with 0.1 M PBS (×3) and a blocking step was introduced using 10% normal goat serum (Biomeda, Foster City, CA) in PBTx. Sections were then incubated overnight at room temperature in primary antibodies for TH, NPY and AgRP in 2% normal goat serum in PBTx. Antibodies (mouse anti-TH 1: 10,000; rabbit anti-NPY 1:10,000; rabbit anti-AgRP 1:10,000) were obtained commercially (Immunostar, Hudson, WI). All sections for each antibody were processed simultaneously to eliminate differences in staining that would compromise accurate quantification by the imaging system. After rinsing with 0.1 M PBS (×3), sections were incubated in the secondary antibodies of biotinylated goat anti-mouse (TH) and biotinylated goat-anti-rabbit (NPY, AgRP; Vector Laboratories, Burlingame, CA) for 2 h at room temperature in 2% normal goat serum in PBTx. Next, the sections were bathed in avidin biotin solution (Vector Laboratories, Burlingame, CA) at 1: 500 for 1 h at room temperature. Slides were then rinsed in 0.1 M PBS (×3) and 3.3′-diaminobenzidine tetrahydrochloride (DAB) was used as a peroxidase substrate in the presence of 0.0025% hydrogen peroxide in 0.1 M PBS to produce a chromogen for visualization. Finally, the sections were rinsed with 0.1 M PBS (×3), mounted onto microscope slides (Superfrost, Fisher Scientific, Suwannee, GA) and dried overnight. The slides were then dehydrated and delipidated through a series of alcohol concentrations and xylene and finally coverslipped using Permount (Fisher Scientific, Suwannee, GA). An additional set of sections were stained with cresyl echt violet for Nissl body visualization as a general indicator of cell destruction.
4.9. IHC for α-MSH
Acrolein perfused brains were used for α-MSH staining to increase sensitivity. Sections for α-MSH were rinsed in 0.1 M PBS (4×) and incubated in 1% Na borohydride for 20 min. Sections were then rinsed in 0.1 M PBS (10×) and placed in primary antibody (rabbit anti-α-MSH, Immunostar Hudson, WI) diluted in PBTx for 48 h at 4 °C. After removal from the antiserum and rinsing in PBS (10×), sections were placed in biotinylated secondary antibody (biotinylated goat-anti-rabbit; Vector Laboratories, Burlingame, CA) used at 1:600 for 1 h. Sections were cleared from the secondary antibody through rinsing with 0.1 M PBS (4×) before placing sections in avidin biotin solution (Vector Laboratories, Burlingame, CA) at 4.5 μl/ml for 1 h at room temperature. Sections were then rinsed in sodium acetate (0.5 M) (3×) and placed in nickel sulfate–DAB chromogen solution for 10–15 min. The diluted nickel sulfate–DAB chromogen solution was prepared using 0.002 g DAB, 0.25 g nickel sulfate and 8.3 μl 3% H2O2 in 40 ml sodium acetate. After final rinsing in sodium acetate (3×) and then in 0.1 M PBS (3×) to stop the reaction, sections were then processed as detailed above.
4.10. PRV visualization
Brains of PRV-injected animals were stored, sliced and mounted using Vectashield mounting medium for fluorescence (Vector Laboratories, Inc. Burlingame, CA) in darkness because of the use of the GFP-expressing PRV strain. PRV staining was visualized and quantified (cell counts) within 7 days under green fluorescence (Olympus BX41 microscope) for labeling from the level of the POA, PVH, LH and the brainstem focusing on the RMg, RPa, Rob and NST, all areas shown to be highly involved in the BAT SNS outflow circuitry (Bamshad et al., 1999; Berthoud et al., 2005; Cano et al., 2003; Oldfield et al., 2002; Song et al., 2008).
4.11. Density and cell counts
TH- and α-MSH-ir cells were visually quantified microscopically in the Arc, PVH and AP (Nikon eclipse E800, 34357, Japan; Camera: Retiga EXi Qimaging, 32-0062A-143, Canada) by an observer blind to the experimental condition. Optical density analysis of NPY- and AgRP-ir fiber staining, as well as Nissl staining in the Arc, PVH and AP was accomplished using a commercial computer-assisted imaging system (IP lab, Scientific Image Processing; Version 3.9.1, Mac OS 10.4.7, Scanalytic, Inc., VA). The program, as previously described (Leitner and Bartness, 2008a), quantifies intensity of staining after background subtraction within the area of interest. Each brain site/section was scored bilaterally three times, averaged and an averaged background (three measures) was subtracted. Because the Arc has a long rostral–caudal shape and because of the uneven distribution of neuropeptides within the Arc (for review see: Chronwall, 1985; Sawchenko, 1998), it often is divided into thirds rostro-caudally to identify the location of labeled cells/fibers (Legradi and Lechan, 1998) and we followed this convention here and previously (Leitner and Bartness, 2008a). Three levels of the Arc were analyzed using a mouse brain atlas to guide in the identification of brain regions (Paxinos and Franklin, 2007), as there is no Siberian hamster brain atlas commercially available and the size and shape of most mouse brain areas are similar to that of Siberian hamsters. The sections quantified approximately corresponded to the stereotaxic atlas Arc coordinates of: anterior region (bregma: −1.46 mm), medial region (bregma: −1.70 mm) and posterior region (bregma: −2.18 mm). In addition, the PVH (bregma: −1.06 mm) was analyzed for NPY- and AgRP-ir fibers, and for TH and α-MSH-ir cells and the AP (bregma: −7.48), and the AP (bregma: −7.48) was analyzed for Nissl staining, and for TH and α-MSH-ir cells.
4.12. Statistical analysis
Data for body mass, fat pads, FFA and glycerol were analyzed by a two-way analysis of variance (ANOVA) [2×2; treatment (MSG/PBS)×Cold and Food Deprived/Non-Cold and Food Deprivated group] with Bonferroni post hoc tests (GraphPad Prism version 4.00; GraphPad Software, San Diego, CA) when appropriate. Differences in histology were quantified using a one-way ANOVA (MSG vs PBS). Temperature during cold exposure, NE injections and PRV-labeled cells were analyzed with an ANOVA for repeated measures. Differences between means were considered significant if P<0.05. Exact probabilities and test values were omitted for simplicity and clarity of presentation.
Acknowledgments
The authors thank the animal care facility for support during the study, Dr. Yogendra Shrestha for help with temperature recordings and Bruce Smith for technical advice during PRV injections. We thank Dr. Ann Murphy for use of the microscope/software for computer-assisted quantification of the immunohistochemistry and Dr. Jamie LaPrairie Beale for assistance with this equipment. In addition, we thank Dr. Brett Teubner for helpful comments on an earlier version of this manuscript.
This work was supported in part by NIDDK grant R37-DK-078358 to T.J.B. and the Center for Behavioral Neuroscience Viral Tract Tracing Core at Georgia State University through the STC Program of the National Science Foundation under agreement no. IBN-9876754.
REFERENCES
- Arch JR. Central regulation of energy balance: inputs, outputs and leptin resistance. Proc. Nutr. Soc. 2005;64:39–46. doi: 10.1079/pns2004407. [DOI] [PubMed] [Google Scholar]
- Bai FL, Yamano M, Shiotani Y, Emson PC, Smith AD, Powell JF, Tohyama M. An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing neurons in the rat hypothalamus. Brain Res. 1985;331:172–175. doi: 10.1016/0006-8993(85)90730-9. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Barrett P, van den Top M, Wilson D, Mercer JG, Song CK, Bartness TJ, Morgan PJ, Spanswick D. Short photoperiod induced decrease of histamine H3 receptors facilitates activation of hypothalamic neurons in the Siberian hamster. Endocrinology. 2009 doi: 10.1210/en.2008-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am. J. Physiol. 1998;275:R1399–R1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Song CK. Innervation of brown adipose tissue and its role in thermogenesis. Can. J. Diabetes. 2005;29:420–428. [Google Scholar]
- Bartness TJ, Song CK. Brain-adipose tissue neural crosstalk. Physiol. Behav. 2007a;91:343–351. doi: 10.1016/j.physbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Song CK. Sympathetic and sensory innervation of white adipose tissue. J. Lipid Res. 2007b;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Elliott JA, Goldman BD. Control of torpor and body weight patterns by a seasonal timer in Siberian hamsters. Am. J. Physiol. 1989;257:R142–R149. doi: 10.1152/ajpregu.1989.257.1.R142. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem. Cell Biol. 2005;123:147–156. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- Bing C, Pickavance L, Wang Q, Frankish H, Trayhurn P, Williams G. Role of hypothalamic neuropeptide Y neurons in the defective thermogenic response to acute cold exposure in fatty Zucker rats. Neuroscience. 1997;80:277–284. doi: 10.1016/s0306-4522(97)00121-8. [DOI] [PubMed] [Google Scholar]
- Bocker H, Steinlechner S, Heldmaier G. Complete cold substitution of noradrenaline-induced thermogenesis in the Djungarian hamster, Phodopus sungorus. Experientia. 1982;38:262. doi: 10.1007/BF01945102. [DOI] [PubMed] [Google Scholar]
- Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–53347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2008;294:R1445–R1452. doi: 10.1152/ajpregu.00068.2008. [DOI] [PubMed] [Google Scholar]
- Bueno AA, Oyama LM, Estadella D, Habitante CA, Bernardes BS, Ribeiro EB, Oller do Nascimento CM. Lipid metabolism of monosodium glutamate obese rats after partial removal of adipose tissue. Physiol. Res. 2005;54:57–65. doi: 10.33549/physiolres.930527. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Choi S, Sparks R, Clay M, Dallman MF. Rats with hypothalamic obesity are insensitive to central leptin injections. Endocrinology. 1999;140:4426–4433. doi: 10.1210/endo.140.10.7064. [DOI] [PubMed] [Google Scholar]
- Chronwall BM. Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides. 1985;6:1–11. doi: 10.1016/0196-9781(85)90128-7. [DOI] [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 2001;25(Suppl. 5):S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Dolnikoff M, Martin-Hidalgo A, Machado UF, Lima FB, Herrera E. Decreased lipolysis and enhanced glycerol and glucose utilization by adipose tissue prior to development of obesity in monosodium glutamate (MSG) treated-rats. Int. J. Obes. Relat. Metab. Disord. 2001;25:426–433. doi: 10.1038/sj.ijo.0801517. [DOI] [PubMed] [Google Scholar]
- Dolnikoff MS, Kater CE, Egami M, de Andrade IS, Marmo MR. Neonatal treatment with monosodium glutamate increases plasma corticosterone in the rat. Neuroendocrinology. 1988;48:645–649. doi: 10.1159/000125076. [DOI] [PubMed] [Google Scholar]
- Dulloo AG, Young JB. Effects of monosodium glutamate and gold thioglucose on dietary regulation of sympathetic nervous system activity in rodents. Metabolism. 1991;40:113–121. doi: 10.1016/0026-0495(91)90160-x. [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Arthurs OJ, Turney BW, Cronin AS. Seasonal neuroendocrine rhythms in the male Siberian hamster persist after monosodium glutamate-induced lesions of the arcuate nucleus in the neonatal period. J. Neuroendocrinol. 1998;10:701–712. doi: 10.1046/j.1365-2826.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- Flaim KE, Horowitz JM, Horwitz BA. Functional and anatomical characteristics of the nerve brown adipose tissue interaction in the rat. Pflugers Arch. 1976;365:9–14. doi: 10.1007/BF00583622. [DOI] [PubMed] [Google Scholar]
- Foster DO, Frydman ML. Brown adipose tissue: the dominant site of nonshivering thermogenesis in the rat. Experientia. 1978;(Suppl. 32):147–151. doi: 10.1007/978-3-0348-5559-4_16. [DOI] [PubMed] [Google Scholar]
- Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can. J. Physiol. Pharm. 1979;57:257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- Foster DO, Depocas F, Zaror-Behrens G. Unilaterality of the sympathetic innervation of each pad of interscapular brown adipose tissue. Can. J. Physiol. 1982a;60:107–113. doi: 10.1139/y82-018. [DOI] [PubMed] [Google Scholar]
- Foster DO, Depocas F, Zuker M. Heterogeneity of the sympathetic innervation of rat interscapular brown adipose tissue via intercostal nerves. Can. J. Physiol. Pharm. 1982b;60:747–754. doi: 10.1139/y82-104. [DOI] [PubMed] [Google Scholar]
- Freeman PH, Wellman PJ. Brown adipose tissue thermogenesis induced by low level electrical stimulation of hypothalamus in rats. Brain Res. Bull. 1987;18:7–11. doi: 10.1016/0361-9230(87)90026-8. [DOI] [PubMed] [Google Scholar]
- Hakansson ML, Hulting AL, Meister B. Expression of leptin receptor mRNA in the hypothalamic arcuate nucleus — relationship with NPY neurones. NeuroReport. 1996;7:3087–3092. doi: 10.1097/00001756-199611250-00059. [DOI] [PubMed] [Google Scholar]
- Halvorson I, Gregor L, Thornhill JA. Brown adipose tissue thermogenesis is activated by electrical and chemical (l-glutamate) stimulation of the ventromedial hypothalamic nucleus in cold-acclimated rats. Brain Res. 1990;522:76–82. doi: 10.1016/0006-8993(90)91579-6. [DOI] [PubMed] [Google Scholar]
- Heldmaier G, Klaus S, Wiesinger H, Friedrichs U, Wenzel M. Cold acclimation and thermogenesis. In: Malan A, Canguilhem B, editors. Living in the Cold II. Montrouge, France; John Libbey Eurotext: 1989. pp. 347–358. [Google Scholar]
- Himms-Hagen J. Brown adipose tissue and cold-acclimation. In: Trayhurn P, Nicholls DG, editors. Brown Adipose Tissue. Arnold; London: 1986. pp. 214–268. [Google Scholar]
- Himms-Hagen J. Neural control of brown adipose tissue thermogenesis, hypertrophy, and atrophy. Front. Neuroendocinol. 1991;12:38–93. [Google Scholar]
- Iwase M, Ichikawa K, Tashiro K, Iino K, Shinohara N, Ibayashi S, Yoshinari M, Fujishima M. Effects of monosodium glutamate-induced obesity in spontaneously hypertensive rats vs. Wistar Kyoto rats: serum leptin and blood flow to brown adipose tissue. Hypertens. Res. 2000;23:503–510. doi: 10.1291/hypres.23.503. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Osaka T, Namba Y, Inoue S, Kimura S. Involvement of sympathetic activation and brown adipose tissue in calcitonin gene-related peptide-induced heat production in the rat. Brain Res. 1999;849:196–202. doi: 10.1016/s0006-8993(99)02154-x. [DOI] [PubMed] [Google Scholar]
- Kong WM, Stanley S, Gardiner J, Abbott C, Murphy K, Seth A, Connoley I, Ghatei M, Stephens D, Bloom S. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J. 2003;17:1688–1690. doi: 10.1096/fj.02-0805fje. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Mikkelsen JD, Jessop D, Lightman SL, Chowdrey HS. Neonatal monosodium glutamate treatment alters both the activity and the sensitivity of the rat hypothalamo-pituitary-adrenocortical axis. J. Endocrinol. 1994;141:497–503. doi: 10.1677/joe.0.1410497. [DOI] [PubMed] [Google Scholar]
- Legradi G, Lechan RM. The arcuate nucleus is the major source for neuropeptide Y-innervation of thyrotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology. 1998;139:3262–3270. doi: 10.1210/endo.139.7.6113. [DOI] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. Food deprivation-induced changes in body fat mobilization after neonatal monosodium glutamate treatment. Am. J. Physiol. 2008a;294:R775–R783. doi: 10.1152/ajpregu.00369.2007. [DOI] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. Sympathetic circuitry to BAT and the response to acute cold in monosodium-glutamate treated Siberian hamsters. Am. J. Physiol. 2008b doi: 10.1016/j.brainres.2009.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2009;296:R831–R843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletinska L, Toma RS, Pirnik Z, Kiss A, Slaninova J, Haluzik M, Zelezna B. Effect of cholecystokinin on feeding is attenuated in monosodium glutamate obese mice. Regul. Pept. 2006;136:58–63. doi: 10.1016/j.regpep.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J. Neuroendocrinol. 1996;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- Morimoto A, Murakami N, Nakamori T, Watanabe T. Suppression of non-shivering thermogenesis in the rat by heat-seeking behaviour during cold exposure. J. Physiol. 1986;380:541–549. doi: 10.1113/jphysiol.1986.sp016301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriscot A, Rabelo R, Bianco AC. Corticosterone inhibits uncoupling protein gene expression in brown adipose tissue. Am. J. Physiol. 1993;265:E81–E87. doi: 10.1152/ajpendo.1993.265.1.E81. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Tortelli CF, Filippis A, Proietto J. Reduced BAT function as a mechanism for obesity in the hypophagic, neuropeptide Y deficient monosodium glutamate-treated rat. Regul. Pept. 1998:75–76. 441–447. doi: 10.1016/s0167-0115(98)00100-1. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Differential control of sympathetic outflow. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2001;281:R683–R698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- Moss D, Ma A, Cameron DP. Cafeteria feeding promotes diet-induced thermogenesis in monosodium glutamate-treated mice. Metabolism. 1985a;34:1094–1099. doi: 10.1016/0026-0495(85)90152-0. [DOI] [PubMed] [Google Scholar]
- Moss D, Ma A, Cameron DP. Defective thermoregulatory thermogenesis in monosodium glutamate-induced obesity in mice. Metabolism. 1985b;34:626–630. doi: 10.1016/0026-0495(85)90089-7. [DOI] [PubMed] [Google Scholar]
- Nascimento Curi CM, Marmo MR, Egami M, Ribeiro EB, Andrade IS, Dolnikoff MS. Effect of monosodium glutamate treatment during neonatal development on lipogenesis rate and lipoprotein lipase activity in adult rats. Biochem. Int. 1991;24:927–935. [PubMed] [Google Scholar]
- Nishioka H, Yoshida T, Yoshioka K, Kondo M. Studies on the regulation mechanism for sympathetic nervous system activity—using hypothalamic obese mice. Nippon Naibunpi Gakkai Zasshi. 1988;64:554–563. doi: 10.1507/endocrine1927.64.7_554. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2007. [Google Scholar]
- Pelz KM, Routman D, Driscoll JR, Kriegsfeld LJ, Dark J. Monosodium glutamate-induced arcuate nucleus damage affects both natural torpor and 2DG-induced torpor-like hypothermia in Siberian hamsters. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2008;294:R255–R265. doi: 10.1152/ajpregu.00387.2007. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Hartle DK. Systemic glutamate induces degeneration of a subpopulation of tyrosine hydroxylase-immunoreactive neurons in the rat area postrema. Brain Res. 1990;516:335–340. doi: 10.1016/0006-8993(90)90938-8. [DOI] [PubMed] [Google Scholar]
- Poon TK, Cameron DP. Measurement of oxygen consumption and locomotor activity in monosodium glutamate-induced obesity. Am. J. Physiol. 1978;234:E532–E534. doi: 10.1152/ajpendo.1978.234.5.E532. [DOI] [PubMed] [Google Scholar]
- Racek L, Lenhardt L, Mozes S. Effect of fasting and refeeding on duodenal alkaline phosphatase activity in monosodium glutamate obese rats. Physiol. Res. 2001;50:365–372. [PubMed] [Google Scholar]
- Rehorek A, Kerecsen L, Muller F. Measurement of tissue catecholamines of obese rats by liquid chromatography and electrochemical detection. Biomed. Biochim. Acta. 1987;46:823–827. [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Neural regulation of thermogenesis. Trends Neurosci. 1982;5:124–126. [Google Scholar]
- Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J. Comp. Neurol. 1998;402:435–441. [PubMed] [Google Scholar]
- Schoelch C, Hubschle T, Schmidt I, Nuesslein-Hildesheim B. MSG lesions decrease body mass of suckling-age rats by attenuating circadian decreases of energy expenditure. Am. J. Physiol: Endocrinol. Metab. 2002;283:E604–E611. doi: 10.1152/ajpendo.00439.2001. [DOI] [PubMed] [Google Scholar]
- Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am. J. Physiol. 2008;295:R417–R428. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumano K, Desbiens S, Rabelo R, Bakopanos E, Camirand A, Silva JE. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol. Cell. Endocrinol. 2000;165:7–15. doi: 10.1016/s0303-7207(00)00276-8. [DOI] [PubMed] [Google Scholar]
- Strack AM, Bradbury MJ, Dallman MF. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am. J. Physiol. 1995;268:R183–R191. doi: 10.1152/ajpregu.1995.268.1.R183. [DOI] [PubMed] [Google Scholar]
- Takasaki Y. Studies on brain lesion by administration of monosodium l-glutamate to mice. I. Brain lesions in infant mice caused by administration of monosodium l-glutamate. Toxicology. 1978;9:293–305. doi: 10.1016/0300-483x(78)90013-6. [DOI] [PubMed] [Google Scholar]
- Tamura H, Kamegai J, Shimizu T, Ishii S, Sugihara H, Oikawa S. Ghrelin stimulates GH but not food intake in arcuate nucleus ablated rats. Endocrinology. 2002;143:3268–3275. doi: 10.1210/en.2002-220268. [DOI] [PubMed] [Google Scholar]
- Tokuyama K, Himms-Hagen J. Adrenalectomy prevents obesity in glutamate-treated mice. Am. J. Physiol. 1989;257:E139–E144. doi: 10.1152/ajpendo.1989.257.2.E139. [DOI] [PubMed] [Google Scholar]
- Tsukahara F, Uchida Y, Ohba K, Nomoto T, Muraki T. Defective stimulation of thyroxine 5′-deiodinase activity by cold exposure and norepinephrine in brown adipose tissue of monosodium glutamate-obese mice. Horm. Metab. Res. 1997;29:496–500. doi: 10.1055/s-2007-979087. [DOI] [PubMed] [Google Scholar]
- Tsukahara F, Uchida Y, Ohba K, Ogawa A, Yoshioka T, Muraki T. The effect of acute cold exposure and norepinephrine on uncoupling protein gene expression in brown adipose tissue of monosodium glutamate-obese mice. Jpn. J. Pharmacol. 1998;77:247–249. doi: 10.1254/jjp.77.247. [DOI] [PubMed] [Google Scholar]
- Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Carasso RL, Mostofsky DI. The facilitative effects of alpha-MSH and melanin on learning, thermoregulation, and pain in neonatal MSG-treated rats. Peptides. 1991;12:465–469. doi: 10.1016/0196-9781(91)90085-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nishioka H, Nakamura Y, Kondo M. Reduced norepinephrine turnover in mice with monosodium glutamate-induced obesity. Metabolism. 1984;33:1060–1063. doi: 10.1016/0026-0495(84)90238-5. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nishioka H, Nakamura Y, Kanatsuna T, Kondo M. Reduced norepinephrine turnover in brown adipose tissue of pre-obese mice treated with monosodium-l-glutamate. Life Sci. 1985;36:931–938. doi: 10.1016/0024-3205(85)90388-1. [DOI] [PubMed] [Google Scholar]
- Yoshimatsu H, Egawa M, Bray GA. Sympathetic nerve activity after discrete hypothalamic injections of l-glutamate. Brain Res. 1993;601:121–128. doi: 10.1016/0006-8993(93)91702-t. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Yoshida T, Kondo M. Effect of acute cold-exposure on norepinephrine turnover and thermogenesis in brown adipose tissue and metabolic rate in MSG-induced obese mice. Jpn. J. Physiol. 1989;39:957–962. doi: 10.2170/jjphysiol.39.957. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]