Abstract
Myofibroblasts, the primary cells associated with corneal stromal haze (opacity), can be derived from both cornea-derived and bone marrow-derived precursor cells. In the present study, the role of TGFβ or PDGF blockage on bone marrow-derived myofibroblast development was investigated using a green fluorescent protein (GFP) chimeric bone marrow mouse model and plasmid vectors that blocked TGFβ or PDGF signaling. At the peak of corneal haze one month after irregular phototherapeutic keratectomy the central stroma had significantly less alpha-smooth muscle actin (α-SMA)-positive cells derived from GFP+ bone marrow-derived cells or GFP- keratocyte/corneal fibroblast-derived cells when corneas were treated with the TGFβ blocking vector pGFPC1.TGFRBKDEL or the PDGF blocking vector pCMV.PDGFRB.23KDEL compared with the corresponding empty vector treated or untreated control groups. In individual animals, 30 to 60% of myofibroblasts were derived from bone marrow-derived precursor cells and 40 to 70% of myofibroblasts were derived from keratocyte-derived precursor cells. TGFβ and PDGF regulate corneal myofibroblast development from bone marrow-derived precursor cells and keratocyte/corneal fibroblast-derived precursor cells.
1. Introduction
Stromal scarring or opacity can be a serious complication following corneal injury or surgery. During the corneal wound healing process, a complex stromal response is initiated that can lead to the formation of α-smooth muscle actin (α-SMA)-expressing myofibroblasts and their deposition of large quantities of abnormal extracellular matrix components, including collagen types not found in the normal cornea (Wilson, 2012). Myofibroblasts in the cornea can be derived from a variety of precursor cells, including keratocyte/corneal fibroblast-derived cells(Jester, Rodrigues and Herman, 1987; Masur et al., 1996) and bone marrow-derived cells (Barbosa et al., 2010; Singh et al., 2013). Myofibroblasts also originate from bone marrow-derived cells in other tissues such as skin and liver, including specialized precursor cells called fibrocytes (Scholten et al., 2011; Mori et al., 2005).
TGFβ and PDGF have been found to be important modulators of myofibroblast differentiation in the cornea and other tissues (Desmoulière et al., 1993; Diegelmann et al., 2004; Masur et al., 1996; Jester, et al., 2002; Stramer et al., 2003; Stramer et al., 2005; Koesters et al., 2010; LeBleu and Kalluri, 2011; Singh et al., 2011; Singh et al., in press). In one of these studies (Singh et al, 2011), vectors that interfered with TGFβ and PDGF signaling were shown to block myofibroblast generation in the cornea after haze-inducing injury. In the present study, these same vectors were used in chimeric mice with bone marrow-derived cells that expressed green fluorescent protein (GFP) to determine if TGFβ and PDGF modulate development of myofibroblasts from bone marrow-derived cells and keratocyte/corneal fibroblast-derived cells in mouse corneas.
2. Method and material
2.1. Animals
Six to eight week old C57BL/6 (Stock Number: 664) or C57/BL/6-Tg(UBC-GFP)30 Scha/J (Stock Number: 4353) female mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). All animals were treated in accordance with the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and these experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the Cleveland Clinic.
2.2. Harvesting of bone marrow cells from GFP+ mice (C57/BL/6-Tg(UBC-GFP)30 Scha/J)
The C57/BL/6-Tg(UBC-GFP)30 Scha/Jtransgenic mice expressed enhanced green fluorescent protein (GFP) in all nucleated cells under the transcriptional control of a human ubiqutin C promoter. Mice homozygous for the transgene are viable, fertile, normal in size and do not display any gross physical or behavioral abnormalities other than green fluorescence of all organs, including cornea and skin. GFP+ mice were euthanised by CO2 inhalation. Bone marrow were harvest as previously described (Singh et al., 2013). Briefly, the long bones of both legs were removed, maintained in chilled PBS, and flushed with DMEM medium using 26-gauge needles. Bone marrow cells/stem cells that were found concentrated at the joints were harvested by scratching the bone gently with a scalpel and needle, and flushing with DMEM medium. Clumps of bone marrow cells in the medium were gently dissociated using a 1 ml pipette to form a single-cell suspension. Suspensions obtained were centrifuged at 1500 rpm for 10 minutes at 4°C to obtain a cell pellet. Red blood c ells were lysed by adding chilled sterile water. Then, 10X PBS (1:10) was added and the mixture gently vortexed. Cells counts were obtained and viability checked using trypan blue. Cells were suspended in PBS at a final concentration of 20x106 cells/ml.
2.3. Irradiation of recipient mice
C57BL/6 wild type mice were irradiated two hours prior to cell injection using a 137Cs Mark 1 irradiator (J.L. Sheperdand Associates, Glendale, CA). All recipient mice received a single dose of 600 rads of whole-body irradiation. Irradiated animals rested for two hours with unlimited food and water before bone marrow injection.
2.4. GFP+ bone marrow transplantation in recipient mice
Irradiated recipient WT C57BL/6 mice were placed under a ultraviolet lamp briefly to dilate the tail veins. The tails were sterilized using 70% ethanol and 0.5 ml of bone marrow cell suspension (20 X 106 cells/ml) was injected intravenously using a 30-guage needle. Animal mortality, if any, was observed within one to two weeks and was usually attributable to insufficient delivery of bone marrow cells and resulting systemic infection.
2.5. Fluorescence-activated cell sorting (FACS)
FACS was performed as described earlier (Singh et al., 2013) at one month after transfer of GFP+ cells in all chimeric mice. Blood was collected from the tail vein using heparin-coated capillary tubes. Blood collected in capillary tubes was diluted in 1 ml of phosphate buffered saline (PBS) in FACS tubes and maintained at 4°C. Red blood cells were lysed with chilled water and the solution was reconstituted with one-tenth volume of 10X PBS, followed by centrifugation. The white blood cell pellet was washed in staining buffer (filtered PBS with 1% BSA and 0.1% sodium azide), pelleted at 500 rpm and resuspended in 200 μl of staining buffer. Samples were analyzed with a Becton–Dickinson FAC Scanner (Franklin Lakes, New Jersey). All peripheral blood lymphocyte preparations were stained with propidium iodide for detection of procedure-induced cell death. Dead cells were gated on SSC and FL2 plots and excluded for further analysis. SSC vs. FL1 plots were analyzed to assess the percentage of GFP-positive cells in the bone marrow-derived cells of each chimeric mouse. Only mice having more than 90% GFP chimerization were used in subsequent experiments (Singh et al., 2013).
2.6. Irregular phototherapeutic keratectomy (PTK) in mice and plasmid vectors
Anterior stromal haze was induced in the mouse corneas with irregular PTK (Mohan, et al, 2008). Briefly, mice were anesthetized with an intraperitoneal injection of 130 μg of ketamine and 8.8 μg of xylazine per gram of body weight. One drop of 1% proparacaine HCl (Alcon, Ft. Worth, TX, USA) was applied to the eye. Each GFP+ chimeric mouse had epithelial scrape with a #64 Beaver blade (Beaver-Visitec International, Inc., Waltham, MA) in one eye. This was followed by application of forty-five 2.0 mm diameter PTK laser pulses through a fine metallic screen with the VISX S4 IR laser (AMO, Irvine, CA).
Animals were divided into five treatment groups. The experiment was repeated and yielded consistent results in the two experiments. In each group, the eye was treated with 30 μl of pGFPC1.TGFRBKDEL, pCMV.PDGFRB.23KDEL, control empty pGFPC1 vector or control empty pCMV vector at a concentration of 1000 ng/ml after PTK. The vector solution was applied to the exposed stroma immediately after PTK and every 24 hours thereafter for four days. The plasmid vectors are directly taken up by corneal stromal cells until the epithelium heals and epithelial barrier function is restored (Hao et al., 2010). The epithelium was healed in all eyes at four days after PTK.
The design of the vectors used to block TGFβ or PDGF signaling were previously described (Kaur et al., 2009b, Singh et al., 2011). Briefly, the pCMV.PDGFRB.23KDEL vector expresses domains 2 and 3 of human platelet-derived growth factor receptor beta (PDGFR-β) that are responsible for binding platelet-derived growth factor (PDGF-B), with endoplasmic reticulum retention signal KDEL tag sequence on 3′ end. The pGFPC1.TGFRBKDEL vector expresses domains 2 and 3 of human TGFβ receptor II that are responsible for binding TGFβ, with the endoplasmic reticulum retention signal KDEL tag sequence on 3′ end. Both expressed recombinant peptides have been shown to bind the corresponding mouse growth factors (N. Singh and B. Ambati, unpublished data, 2008). The expressed recombinant proteins trigger anomalous intracellular sorting of PDGF-B and TGFβ to the endoplasmic reticulum once they are bound to the receptor domains and sequester the cytokines within cells and, thus, interfere with proper signaling by the cytokines (Singh et al. 2005; Singh et al., 2006; Kaur et al., 2009b; Singh et al., 2011; Kaur and Wilson, unpublished data, 2008). The recombinant proteins expressed by the KDEL-expressing vectors do not work to interrupt receptor-mediated signaling directly but lead to intracellular sequestration of their respective growth factors so that intracellular levels of the growth factors available to bind the native receptors are markedly reduced (Singh, et al, 2005). These studies showed that when rabbit corneal fibroblasts were transfected with the PDGF-blocking vector or mouse corneal fibroblasts were transfected with the TGFβ-blocking vector (the TGFβ vector expressed protein does not bind rabbit TGFβ, Kaur and Wilson, unpublished data, 2008) the proliferation of the cells in response to the corresponding growth factors was inhibited (Singh et al., 2011; Kaur and Wilson, unpublished data, 2008) and that when corneal fibroblasts were transfected with the TGFβ-blocking vector, but not control empty vector, myofibroblast differentiation of mouse corneal fibroblasts in response to exogenous TGFβ-1 was inhibited (Singh et al, 2011). In addition, prior in vitro studies showed that PDGF receptor and TGFβ receptor accumulated in membrane bound organelles in corneal fibroblasts transfected with the PDGF-blocking vector and TGFβ-blocking vector, respectively (Kaur et al., 2009b and Kaur and Wilson, unpublished data, 2008). This approach was previously used for a VEGF-1 receptor fragment and cells transfected with the Flt24K recombinant construct-expressing vector that combined the VEGF receptor-1 fragment with KDEL reduced intracellular levels of free VEGF (Singh, et al, 2005).
The following experimental groups were included: 1) control group with no treatment (n=12); 2) empty pGFPC1 vector (n=10); 3) empty pCMV vector (n=11); 4) pGFPC1.TGFRBKDEL vector (n=11); and 5) pCMV.PDGFRB.23KDEL vector (n=11). All treated eyes received one drop of 0.3% ciprofloxacin antibiotic twice a day at 30 minutes after an application of vector solution until the epithelium was healed.
2.7. Immunohistochemistry
Immunohistochemical staining was performed on experimental and control tissue sections as previously described (Singh et al., 2012 and 2013). Briefly, sections from the central cornea were washed with PBS twice for five minutes each and permeabilized with 1% triton X-100 for one hour at room temperature. Sections were incubated with 4% normal goat serum with 1% BSA in PBS at room temperature for one hour to block non-specific binding. Sections were stained for α-smooth muscle actin with rabbit polyclonal antibody (ab5694, Abcam, San Francisco, CA) diluted 1:100 in 1% normal goat serum in PBS and/or for GFP with chicken polyclonal antibody (ab13970, Abcam) diluted 1:750 in 1% normal goat serum in PBS for 90 minutes. Sections were washed with PBS and then incubated with respective secondary antibodies (A11037 Invitrogen, Grand Island, NY) Alexa Fluor 594 goat anti-rabbit IgG, 1:200 dilution or A11039 (Invitrogen) Alexa Fluor 488 goat anti-chicken IgG, 1:1000 dilution, for 60 minutes before washing with PBS four times. Immunohistochemical controls were performed with omitted primary antibody. Coverslips were mounted with Vectashield containing DAPI (Vector Laboratories Inc., Burlingame, CA) to allow visualization of all nuclei in the tissue sections.
2.8 Quantification of cells
α-SMA+ and GFP+ cells were counted real time at the microscope. In each case, counts of α-SMA+ or GFP+ cells were performed in central cornea by counting the number of cells per 400X full thickness column in the central cornea and averaging the counts for three adjacent fields, as previously described (Singh et al., 2011). The sections were viewed and photographed with a Leica DM5000 microscope equipped with Q-Imaging Retiga 4000RV (Surrey, BC, Canada) camera and ImagePro software (Leica). All IHC staining was performed at least three times to insure the results were consistent.
2.9. Statistical Analysis
All statistical analyses were performed using the R software (version 2.12). A Poisson regression was used with the counts of positive cells followed by a multiple comparison of means by Tukey methods with Bonferroni correction to assess the pairwise comparisons between the groups. Variations were reported as standard errors. A two-tailed P value of less than 0.05 was considered statistically significant.
3. Result
Effect of cytokine blockade on α-SMA expressing cells
The results of this study confirm that in this model after irregular PTK in the mouse similar percentages of stromal myofibroblasts were derived from keratocyte/corneal fibroblast-derived precursor cells and bone marrow-derived precursor cells. In individual animals, 30 to 60% of myofibroblasts were derived from GFP+ bone marrow-derived precursor cells and 40 to 70% of myofibroblasts were derived from GFP- keratocyte-derived precursor cells, although 5 to 10% of the latter cells could have been bone-marrow derived cells since chimerization was 90 to 95% based on fluorescent cell sorting evaluation of blood samples from each animal.
At one month after irregular PTK the central corneal stroma had significantly fewer α-SMA-positive cells when treated with the TGFβ signal-blocking vector pGFPC1.TGFRBKDEL or the PDGF signal-blocking vector pCMV.PDGFRB.23KDEL (Fig. 1) compared with the corresponding empty vector group or the untreated control group (Table 1 and Table 2). The central stroma had significantly fewer α-SMA-positive/GFP-positive cells (myofibroblasts derived from bone marrow-derived cells) when treated with the TGFβ signal-blocking vector or the PDGF signal-blocking vector compared to the corresponding empty vector group or the untreated control group (Fig. 1, Table 1 and Table 2). The central stroma also had significantly fewer α-SMA-positive/GFP-negative cells (myofibroblasts derived from keratocyte/corneal fibroblast cells) when treated with the TGFβ signal-blocking vector or the PDGF signal-blocking vector compared to the corresponding empty vector group or the untreated control group (Fig. 1, Table 1 and Table 2).
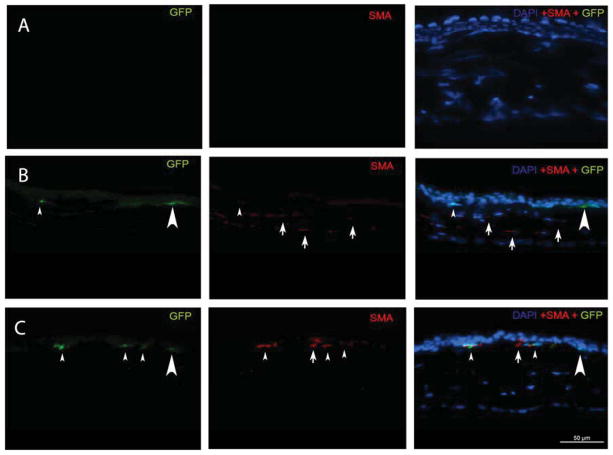
Fig. 1.
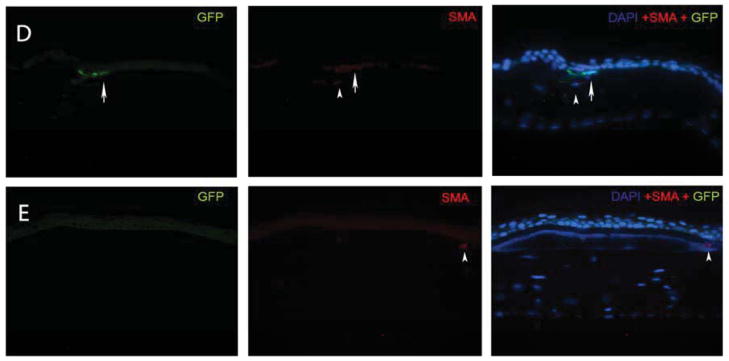
Representative α-SMA and GFP immunocytochemical staining of cornea sections obtained from chimeric mice at one month after irregular PTK. The left lane shows GFP stained green; the middle lane shows α-SMA stained red and the right lane is an overlay of the DAPI, GFP and α-SMA staining. Arrows indicate representative α-SMA+GFP+ myofibroblasts and arrowheads indicate representative α-SMA+GFP-myofibroblasts. A: A cornea at one month after irregular PTK that had immunocytochemistry with omitted primary antibodies as a control. B: A cornea that had irregular PTK but no vector treatment. C: A control cornea that had PTK and was treated with empty pCMV vector. D. A control cornea that had PTK and was treated with PDGF-B blocking vector pCMV.PDGFRB.23KDEL. E. A cornea that had PTK and was treated with the TGFβ blocking vector pGFPC1.TGFRBKDEL. GFP+ cells that are SMA- in these panels (examples large arrowheads in B and C), especially those adjacent to the epithelium, could represent myofibroblast precursor cells that have not yet matured to the point where they express α-SMA (Chaurasia et al., 2009). Quantitation was performed real-time at the microscope and not from captured images such as those shown in this figure.
Table 1.
Myofibroblast cells per 400X central corneal column
| SMA+ cells ± S.E. | SMA+GFP+cells ± S.E. | SMA+GFP-cells± S.E. | |
|---|---|---|---|
| Control untreated | 6.8 ± 0.9 | 2.8 ± 0.5 | 4.0 ± 1.0 |
| Empty pGFPC1 vector | 7.3 ± 1.5 | 2.8 ± 0.4 | 4.5 ± 0.7 |
| Empty pCMV vector | 8.1 ± 1.3 | 3.4 ± 0.6 | 4.6 ± 0.8 |
| pGFPC1.TGFRBKDEL vector | 2.6 ± 0.3 | 1.4 ± 0.2 | 1.2 ± 0.3 |
| pCMV.PDGFRB.23KDEL vector | 4.4 ± 0.7 | 2.2 ± 0.3 | 2.3± 0.9 |
Note that all quantitation was performed real-time at the microscope and not from captured images.
Table 2.
Anova p-value comparisons of myofibroblasts in different treatment groups
| SMA+ cells | SMA+GFP+cells | SMA+GFP-cells | |
|---|---|---|---|
| Empty pGFPC1 vector vs. untreated control | 1.00 | 1.00 | 1.00 |
| Empty pCMV vector vs. untreated control | 0.04 | 1.0 | 0.90 |
| pGFPC1.TGFRBKDEL vector vs. untreated control | <0.001 | <0.01 | <0.01 |
| pCMV.PDGFRB.23KDEL vector vs. untreated control | <0.01 | <0.01 | <0.01 |
| Empty pCMV vector vs. empty pGFPC1 vector | 1.00 | 0.92 | 1.00 |
| pGFPC1.TGFRBKDEL vector vs. empty pGFPC1 vector | <0.001 | <0.01 | <0.01 |
| pCMV.PDGFRB.23KDEL vector vs. empty pGFPC1 vector | <0.01 | 0.03 | <0.01 |
| pGFPC1.TGFRBKDEL vector vs. empty pCMV vector | <0.001 | <0.01 | <0.01 |
| pCMV.PDGFRB.23KDEL vector vs. empty pCMV vector | <0.001 | <0.01 | <0.01 |
| pGFPC1.TGFRBKDEL vector vs. pCMV.PDGFRB.23KDEL vector | <0.01 | 0.24 | 0.28 |
4. Discussion
Corneal wound healing is a complex process involving interplay between epithelial cells, resident stromal cells, bone marrow-derived cells (inflammatory cells, progenitor cells and possibly stem cells), and nerves, as well as soluble and cell-associated mediators (such as TGF-β, PDGF, IL-1, matrix metalloproteinases, etc.), and extracellular matrix components such as the basement membrane (Cotsarelis G., 2006; Diegelmann et al., 2004; Kaur et al., 2009a and b; Martins et al., 2013; Wilson SE, 2012).
Loss of transparency (haze) during corneal wound healing is associated with the generation and persistence of α-SMA-positive myofibroblasts, which are less transparent than quiescent keratocytes due to decreased synthesis of crystallins, and production of disorganized extracellular matrix by these cells and corneal fibroblasts (Jester et al., 1999 and 2012a and 2012b; Wilson et al., 1996). The progenitor cells for corneal myofibroblasts can be derived from keratocyte/corneal fibroblast-derived resident cells (Mazur, et al., 1996; Jester, et al., 1999) or bone marrow-derived cells that migrate into the cornea in large numbers after injury (Barbosa et al., 2010, Singh, et al., 2013). In prior studies (Kaur et al., 2009b; Singh et al., 2011), blockade of TGFβ and PDGF signaling was shown to reduce myofibroblast generation after corneal injury irrespective of the progenitors from which the myofibroblasts develop. The results of the present study extend those results and demonstrate that blockade of TGFβ and PDGF signaling using the pGFPC1.TGFRBKDEL and pCMV.PDGFRB.23KDEL vectors reduced generation of corneal myofibroblasts from both keratocyte/stromal fibroblast-derived progenitors (α-SMA+,GFP− stromal cells) and from bone marrow-derived progenitors (α-SMA+,GFP+ stromal cells). TGFβ blockade appears to have a more critical role on myofibroblast generation compared to PDGF blockade in this model. In a prior study where the cellular origin of myofibroblasts could not be determined (Singh et al., 2011) there was little added effect of combining TGFβ blockade and PDGF blockade and, therefore, the combination of both vectors was not studied in the current study.
The results in this study support development of corneal myofibroblasts from both bone marrow-derived cells and keratocyte/corneal fibroblast-derived cells. All chimeric mice used in this study were greater than 90% chimerized (Singh et al., 2013) but it is not currently possible to generate mice that are 100% chimerized. This means that even if all myofibroblasts in mouse corneas originated from bone marrow-derived cells, it would be expected that a maximum of approximately 10% of the corneal myofibroblasts that developed would have been GFP-. However, 40 to 70% of the myofibroblasts that developed either in control vector, pGFPC1.TGFRBKDEL vector, or pCMV.PDGFRB.23KDEL vector treated mice were GFP-. Thus, many of these myofibroblasts likely did not develop from bone marrow-derived cells but probably from progenitor cells derived from keratocytes/corneal fibroblasts (Mazur, et al., 1996; Jester, et al., 1999; Singh et al., 2012). An earlier study found a higher proportion of myofibroblasts derived from bone marrow-derived precursors (greater than 90%) than keratocyte/cornea fibroblast precursors (less than 10%) in a mouse PTK model (Barbosa et al., 2010). The investigators are uncertain if this relates to mouse strain differences, small differences in the PTK method, or other factors. In any case, it is clear that myofibroblasts are derived from both precursor types in most individual mouse corneas at one month after irregular PTK.
In vitro co-culture studies of corneal fibroblasts and bone marrow-derived cells confirmed that TGFβ is a critical cytokine modulating corneal myofibroblast generation and demonstrated that the presence of bone marrow-derived cells augments myofibroblast generation from corneal fibroblasts and, conversely, the presence of corneal fibroblasts augments myofibroblast generation from bone marrow-derived cells (Singh et al., 2012). Thus, in vivo, it is likely that communications between resident keratocyte/corneal fibroblast-derived cells and bone marrow-derived cells, as well as epithelium-derived TGFβ and PDGF penetration into the anterior stroma regulated by the epithelial basement membrane (Torricelli et al. 2013a and 2013b), are critical factors in the development and persistence of myofibroblasts during corneal wound healing. The bone marrow-derived cells that migrate into the cornea and differentiate into myofibroblasts may be “fibrocytes” (Bucala, et al., 1994; Mori et al., 2005) but more investigation is needed for confirmation of this origin. The importance of the basement membrane in the mouse model is supported by marked reduction in myofibroblast generation in mouse eyes when PTK without irregularity (no screen) is performed compared to PTK with irregularity (with screen) that retards basement membrane regeneration (Mohan et al., 2008). Irregular PTK in the mouse eye interferes with normal regeneration of the epithelial basement membrane and results in increased penetration of epithelium-derived TGFβ and PDGF in the anterior stroma, similar to the difference between photorefractive keratectomy (PRK) for low myopia correction compared with PRK for high myopia correction in rabbits and humans (Torricelli et al., 2013a and 2013b). These results are consistent with in vitro studies that have demonstrated an important regulatory role for TGFβ in the regulation of myofibroblast development from either keratocyte-derived corneal fibroblasts (Jester, et al., 1999; Mazur, et al., 1996; Stramer et al., 2003; Stramer et al., 2005, Singh, et al., in press) or bone marrow-derived cells (Singh et al., 2012).
In summary, the results of this study support the importance of both TGFβ and PDGF in modulating the development of myofibroblasts from both resident keratocyte/stromal fibroblast-derived precursor cells and bone marrow-derived precursor cells. Future studies may be directed at more specific identification of the bone marrow-derived precursor cells and clinically useful methods to block development of corneal myofibroblasts after corneal injury.
Highlights Wilson.
Corneal myofibroblasts are derived from bone marrow-derived cells
Corneal myofibroblasts are derived from keratocyte-corneal fibroblast-derived cells
TGF beta and PDGF modulate myofibroblast development from both precursors
Acknowledgments
Supported in part by US Public Health Service grants EY10056 and EY015638 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY. We thank Vandana Agrawal for her technical assistance in the study. We acknowledge statistician Mr. Hasnat Ali for the statistical analysis. We thank the flow core facility at the Lerner Research Institute for analysis of flow data.
Footnotes
Proprietary interest statement: None of the authors have any proprietary or financial interests in the topics discussed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbosa FL, Chaurasia SS, Cutler A, Asosingh K, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp Eye Res. 2010;91:92–96. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res. 2009;89:133–9. doi: 10.1016/j.exer.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–68. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC. Wound Healing: An overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- Hao J, Li SK, Kao WW, Liu CY. Gene delivery to cornea. Brain Res Bull. 2010;81:256–61. doi: 10.1016/j.brainresbull.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Brown D, Pappa A, Vasiliou V. Myofibroblast differentiation modulates keratocyte crystallin protein expression, concentration, and cellular light scattering. Invest Ophthalmol Vis Sci. 2012a;53:770–778. doi: 10.1167/iovs.11-9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGF beta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGF beta, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–57. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavanagh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for “corneal” crystallins. J Cell Sci. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Jester JV, Nien CJ, Vasiliou V, Brown DJ. Quiescent keratocytes fail to repair MMC induced DNA damage leading to the long-term inhibition of myofibroblast differentiation and wound healing. Molecular Vision. 2012b;18:1828–1839. [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Rodrigues MM, Herman IM. Characterization of avascular corneal wound healing fibroblasts. New insights into the myofibroblast. Am J Pathol. 1987;127:140–8. [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Wilson SE. Corneal myofibroblast viability: opposing effects of IL-1 and TGF beta-1. Exp Eye Res. 2009a;89:152–158. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Medeiros FW, Agrawal V, Salomao MQ, Singh N, Ambati BK, Wilson SE. Corneal stroma PDGF blockade and myofibroblast development. Exp Eye Res. 2009c;88:960–5. doi: 10.1016/j.exer.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koesters R, Kaissling B, Lehir M, Picard N, Theilig F, Gebhardt R, Glick AB, Hähnel B, Hosser H, Gröne HJ, Kriz W. Tubular overexpression of transforming growth factor-beta1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632–43. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu VS, Kalluri R. Blockade of PDGF receptor signaling reduces myofibroblast number and attenuates renal fibrosis. Kidney Int. 2011;80:1119–21. doi: 10.1038/ki.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins VL, Caley M, O’Toole EA. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013;351:255–68. doi: 10.1007/s00441-012-1410-z. [DOI] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996;93:4219–23. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Stapleton WM, Sinha S, Netto MV, Wilson SE. A novel method for generating corneal haze in anterior stroma of the mouse eye with the excimer laser, Exp. Eye Res. 2008;86:235–40. doi: 10.1016/j.exer.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori L, Bellini A, Stacey MA, Schmidt M, Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Scholten D, Reichart D, Paik YH, Lindert J, Bhattacharya J, Glass CK, Brenner DA, Kisseleva T. Migration of fibrocytes in fibrogenic liver injury. Am J Pathol. 2011;179:189–98. doi: 10.1016/j.ajpath.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Amin S, Richter E, Rashid S, Scoglietti V, Jani PD, Wang J, Kaur R, Ambati J, Dong Z, Ambati BK. Flt-1 intraceptors inhibit hypoxia-induced VEGF expression in vitro and corneal neovascularization in vivo. Invest Ophthalmol Vis Sci. 2005;46:1647–52. doi: 10.1167/iovs.04-1172. [DOI] [PubMed] [Google Scholar]
- Singh N, Jani PD, Suthar T, Amin S, Ambati BK. Flt-1 intraceptor induces the unfolded protein response, apoptotic factors, and regression of murine injury-induced corneal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:4787–93. doi: 10.1167/iovs.06-0419. [DOI] [PubMed] [Google Scholar]
- Singh V, Agrawal V, Santhiago MR, Wilson SE. Stromal fibroblast bone marrow-derived cell interactions: implications for myofibroblast development in the cornea. Exp Eye Res. 2012;98:1–8. doi: 10.1016/j.exer.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Barbosa FL, Torricelli AAM, Santhiago MR, Wilson SE. Transforming growth factor β and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp Eye Res. 2014 doi: 10.1016/j.exer.2014.01.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Jaini R, Torricelli AA, Tuohy VK, Wilson SE. A method to generate enhanced GFP+ chimeric mice to study the role of bone marrow-derived cells in the eye. Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.10.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Santhiago MR, Barbosa FL, Agrawal V, Singh N, Ambati BK, Wilson SE. Effect of TGFβ and PDGF-B blockade on corneal myofibroblast development in mice. Exp Eye Res. 2011;93:810–817. doi: 10.1016/j.exer.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer BM, Austin JS, Roberts AB, Fini ME. Selective reduction of fibrotic markers in repairing corneas of mice deficient in Smad3. J Cell Physiol. 2005;203:226–32. doi: 10.1002/jcp.20215. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–46. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Agrawal V, Santhiago MR, Wilson SE. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophth Vis Sci. 2013a;54:4026–33. doi: 10.1167/iovs.13-12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AAM, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: Structure, function and disease. Invest Ophth Vis Sci. 2013b;54:6390–400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, Chwang EL. Epithelial injury induces keratocyte apoptosis: hypothesized role for the Interleukin-1 system in modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–338. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]