Abstract
A study of epilepsy patients with a reproducible range of photoparoxysmal responses (PPR) (epileptiform discharges evoked by flashing lights) has been used as a “proof-of-concept” trial to determine if novel potential antiepileptic drugs (AEDs) should proceed in development. The standard design for this trial requires a 3-day inpatient stay and is single-blind. We evaluated two marketed and effective AEDs—one narrow-spectrum [carbamazepine (CBZ)], and one broad-spectrum [levetiracetam (LEV)]—using a novel double-blinded, cross-over outpatient version of the trial to detect acute drug effects of the two marketed AEDs on photosensitivity. We tested 6 patients with a known stable photosensitivity response, using single oral doses of CBZ 400 mg and LEV 1000 mg, compared to 2 test days with single placebo doses. Patients who received LEV had the lowest mean PPR (compared with placebo and CBZ). The mixed effect model showed a significant effect of LEV in all eye closure conditions (p < 0.001). There was no evidence of a significant change in PPR after CBZ or placebo treatment. In conclusion, LEV 1000 mg, but not CBZ 400 mg, was effective in suppressing photosensitivity within a 6-h period compared with placebo showing the ability of our novel photosensitivity trial design to demonstrate effects of broad-spectrum AEDs. We cannot confirm the ability of the photosensitivity trial to detect the narrow-spectrum AED CBZ in our design. The novel outpatient study design is feasible and is expected to reduce costs compared with previous methodology.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0243-0) contains supplementary material, which is available to authorized users.
Keywords: Photosensitive epilepsy, clinical trial, antiepileptic drug, carbamazepine, levetiracetam
Introduction
Patients with epilepsy often have intermittent seizures that occur at variable times. Consequently, trials evaluating new antiseizure medications require that large numbers of patients be evaluated over several months of treatment to accurately assess treatment effects. In such circumstances, it is extremely useful to conduct a “proof-of-concept” (POC) study, which would screen potential antiseizure agents for possible efficacy before proceeding to lengthy and expensive studies that expose many patients to a new compound. The photosensitivity POC trial design has been used successfully to evaluate potential antiseizure effects of new agents in early stage development in small groups of patients with photically-induced generalized epileptiform responses on their electroencephalogram (EEG), called photoparoxysmal responses (PPR). The number of flash frequencies at which PPR can be elicited [delineated by upper and lower thresholds elicited during intermittent photic stimulation (IPS)] can be used as a quantitative measure of photosensitivity and therefore epileptogenic threshold. When patients with PPR receive single test doses of possible antiseizure drugs, changes in the number of frequencies at which a PPR response is identified on EEG can be used to screen for antiepileptic effects without triggering seizures. This trial design has been used frequently, as there is a lack of relatively straightforward trial designs to assess POC for potential antiepileptic drug (AED) effectiveness in humans.
In photosensitivity studies, the patient is exposed to IPS at 14 predetermined unequally separated frequencies in order to detect changes in response around typical upper and lower frequency thresholds (e.g., 2 Hz, 5 Hz, 8Hz, 10 Hz, etc.). Each flash frequency that elicits a photosensitive response is considered one “step”. The ranges in Hz between the upper bound and the lower bound for each patient are transformed into a metric, called the standardized photosensitive range (SPR). The maximum SPR is 14, based on the total number of flash frequencies tested. As an example, if a patient had a lower photosensitivity bound of 10 and an upper bound of 30, this corresponds to an SPR of 8 (including the frequencies of 10, 13, 15, 18, 20, 23, 25, 30). The photosensitivity “range” for a given person between their lowest and highest IPS frequencies at which they have PPR elicited can then be defined. The number of steps at which a patient is sensitive can be measured repeatedly (e.g., 5–8 times) over the course of a single day, providing a stable baseline to compare with periods during which patients receive test medications. Reduction in photosensitivity range following administration of a single dose of a potential antiseizure drug can be easily quantified by comparing changes in the number of response “steps”. If patients with a relatively stable IPS response are used as patients, studies can be performed using a study design that requires only 5–6 patients per dose tested. This protocol has been used to identify the antiepileptic effect of a number of AEDs (some now approved, some still in development), most notably levetiracetam (LEV), lamotrigine (LTG), brivaracetam, carisbamate, JZP4, YKP3089, and valproate (VPA) [1–6]. Drugs also have been tested that are sedatives, but not anticonvulsants, and these have failed to suppress photosensitivity [7], indicating that the assessment of PPR is both sensitive and specific.
Typically, the photosensitivity study is conducted as an inpatient non-randomized treatment protocol. Patients are admitted, and the PPR is repeatedly tested over 3 days after administration of different drugs or placebo. However, to avoid carry-over treatment effects sometimes seen with even a single dose of some drugs (e.g., VPA) [8], placebo is always given on the first day and drug on the second day in this study protocol. As the principal investigator is aware of the drug order, the study design is single-blind.
In contrast, our study was performed in an outpatient setting with a randomized, double-blind cross-over design, bringing patients back at spaced intervals (to avoid carry-over effects) to perform repeated PPR testing limited to 6 h after dose. In addition, we included 2 placebo testing sessions, both to screen for placebo effects on PPR and to determine the variability in PPR across test days and the precision of measuring PPR ranges.
In order to test our new trial design, we utilized two AEDs that are already approved for use. The first drug selected was carbamazepine (CBZ), which is considered to be “narrow-spectrum”. As many patients with photosensitivity have generalized epilepsy and generalized spike-wave, there is controversy as to whether CBZ is effective in the photosensitivity model. The second drug was LEV, which is considered to be effective in both focal and generalized epilepsy (“broad-spectrum”), and has already been proven to be effective in standard inpatient photosensitivity trial design [2]. We used this drug to determine “assay sensitivity” for our new design.
CBZ is a typical sodium channel blocker that has been shown in humans and animals to sometimes increase generalized spike wave activity when given chronically [9]. In this study, we tested response to IPS after a single dose of CBZ and compared it to response to IPS after a single dose of LEV. We hypothesized that if CBZ was able to block or reduce the PPR in response to IPS, it would be a very strong sign that the photosensitivity trial design is able to identify a wide variety of drugs, including “narrow spectrum” ones that are effective for treating focal epilepsy. However, if CBZ either did not affect, or possibly aggravated, the PPR in a sufficient number of patients this would be an indication that the photosensitivity POC protocol may be most sensitive for screening effectiveness of relatively broad-spectrum AEDs such as LEV.
Methods
The design was a double-blind, randomized, placebo-controlled, 2-center outpatient trial in 6 patients with a known stable PPR on EEG (established with a screening EEG and a baseline day of PPR evaluation). The study was approved by the clinical sites’ institutional review boards, and all patients gave written consent to participate.
Study doses were tested on 1 day with a minimum of 2 weeks of treatment washout between test days. Patients received single doses of placebo on 2 study days and of active medication (CBZ, LEV) on 2 separate study days. Single oral doses of CBZ 400 mg and LEV 1000 mg were each compared with doses of placebo. During the study days, several procedures and IPS assessments were performed at 5 predetermined times over the course of the day, 1 pre-dose and 4 post-dose. Patients who received LEV as a background AED were only randomized to CBZ versus placebo.
Drugs and matching placebo were prepared by the research pharmacy at the University of Pennsylvania using over-encapsulation of all medications. They also prepared the randomization list using random permutations (www.randomization.com).
Eligible patients were men or women aged 16–60 years with a diagnosis and history of epilepsy for which they were treated with at most 2 concomitant AEDs, and had a reproducible PPR on EEG of at least 3 points on the SPR scale in at least 1 eye condition. Patients were excluded if they had serious medical or psychiatric conditions, a history of non-epileptic seizures, were receiving AEDs that were hepatic enzyme inducers, had a history of seizure aggravation or allergy when taking CBZ or LEV, or did not agree to use effective birth control.
Patients identified as having a generalized PPR on a prior EEG underwent screening no more than 21 days prior to the first dosing visit. At this visit, patients had IPS assessments at 09:30, 11:00, 12:00, 14:00, and 16:00 hours. At least 3 of the EEGs performed during the screen visit had to demonstrate a PPR of at least 3 points on the SPR scale in at least 1 eye condition. Patients underwent a full medical history, and neurological and physical examination. Routine blood work was obtained for liver function tests, electrolytes, complete blood count, AED levels, and serum pregnancy test (in women).
Patients who changed background AEDs after screening, were required to return for an additional screening day. If this day differed from visit 1 by more than 3 points at 3 times in any single eye condition, they were removed from the study.
Visits 2–5 were planned to occur at intervals of at least 2 weeks. Patients were instructed to refrain from strenuous exercise within 48 h and from alcoholic beverages within 24 h of each visit. During each visit, caffeine intake remained stable. Patients were instructed to get similar amounts of sleep on each visit day.
On treatment days, patients presented to the epilepsy center at 08:00 hours. At 09:30 hours they underwent a pre-dose IPS session. At 10:00 hours they received a dose of placebo, CBZ 400 mg, or LEV 1000 mg, in a blinded fashion. IPS sessions were repeated at 1, 2, 4, and 6 hours after dosing. A brief neurological examination and vital signs were performed 4 h after dosing. Blood was collected for AED levels at 1 and 6 h after dosing, and was sent to the laboratory by an unblinded nurse who selected the correct AED. All other study personnel remained blinded to AED results until study completion.
All IPS assessments were performed using the systematic protocol previously described by Kasteleijn et al. [2]. A standardized EEG photic stimulation procedure was used: 5 s of intermittent white flashes by a Grass PS 33 photic stimulator (1 Joule) with a 7-s pause.
Flashes were administered at standard frequencies of 2, 5, 8, 10, 13, 15, 18, 20, 23, 25, 30, 40, 50, and 60 Hz in 3 eye conditions (eye closure, eyes closed, and eyes open). To determine the upper and the lower IPS thresholds, each frequency was initially assessed in all eye conditions, starting at 2 Hz. As soon as generalized EEG epileptiform activity appeared, the stimulation for that particular frequency in a particular eye condition was terminated. The frequency at which the first PPR occurred was considered the lower bound of the range of frequencies at which the patient was sensitive in that eye condition. A lower bound was determined in each eye condition. Similar assessments were carried out starting at 60 Hz and descending through the standard frequencies. Again, the stimulator was turned off if a generalized response was seen, to avoid occurrence of a seizure, and the sequence was stopped at that frequency for that specific eye condition. This was considered the upper bound of the range to which the patient was photosensitive. The patient is presumed to be sensitive to all the frequencies between the upper and lower bound (these frequencies are not tested because of a high seizure risk at the most sensitive frequencies). The ranges in Hz between the upper bound and the lower bound for each patient were transformed into a metric, the SPR. Reduction of the range was defined as reduction of at least 3 frequency levels (SPR reduction of 3) out of a maximum of 14 on the SPR scale or complete abolition of the PPR.
One of the authors (D.K.) acted as an independent reviewer and determined all sensitivity ranges in a blinded fashion.
Statistical Analysis
The data were analyzed using mixed effect models to account for the correlation among measurements on the same patients [10]. Random effect models were used to account for the correlation of measurements taken within the same patient. Random effect models are more flexible and more efficient that repeated measures model, and can handle missing observations within a patient without removing the entire patient from the analysis. Data were first reduced by calculating the average of the photosensitivity range measurements taken 1, 2, 4, and 6 h after dosing for each visit. This average was used as the dependent variable, while drug (placebo, CBZ 400 mg, LEV 1000 mg) was used as the independent variable in all models. Patients were entered as random effects. Means and ranges of PPR for each patient, across each day for each eye condition (eye closure, eyes closed and eyes opened) for each treatment were also evaluated descriptively. Graphical displays of the data for each patient allowed exploration of inter- and intrapatient variability.
Results
Nine patients were screened for the study. Of these, 3 did not display sufficient photosensitivity and therefore were not enrolled. Six patients (3 men and 3 women) were enrolled and completed the study. Two of the patients were monozygotic twins. By history, 5 had idiopathic generalized epilepsy and 1 (patient #3) had partial onset seizures (with a family history of generalized epilepsy). One patient had an increase of VPA comedication during the study. This patient was rescreened, and as he still met inclusion criteria, he completed the remaining visits. Patients were all receiving 1 or 2 background drugs for the duration of the study. These were LTG/VPA (patients #4 and #5), LTG/zonisamide (patient #6), LEV (patient #2), VPA (patient #1), and zonisamide (patient #3). The patient who was on LEV did not complete the LEV arm, as per protocol.
Table 1 presents serum concentrations of CBZ and LEV evaluated at 1 and 6 h after dosing. Table 2 includes information about background drugs and serum concentrations. Background drug levels that were available were consistent with patient adherence with these doses.
Table 1.
Levels of study medication at hour 1 and hour 6 after blinded study administration
| Patient | Study drug | |||
|---|---|---|---|---|
| CBZ (mcg/mL) 1 h | CBZ (mcg/mL) 6 h | LEV (ug/mL) 1 h | LEV (ug/mL) 6 h | |
| 1 | < 3 | < 3 | 28 | 9 |
| 2 | < 3 | < 3 | NA | NA |
| 3 | 1.5 | 6.1 | 1.9 | 19.2 |
| 4 | <0.5 | 5 | 15.2 | 7.9 |
| 5 | 2.5 | 4.7 | 12.3 | 7.9 |
| 6 | <0.5 | 2.6 | 15.2 | 9.2 |
CBZ = carbamazepine; LEV = levetiracetam; NA = not applicable
Table 2.
Demographics of enrolled patients
| Patient | Duration (days in study) | Min./max. intervisit interval (days) | Syndrome | Seizure types by history | Concomitant AED doses | Range of AED levels |
|---|---|---|---|---|---|---|
| 1 | 307 | 1/169 | Idiopathic generalized | PGTC, absence | Visit 1–4: VPA ER 500 mg QPM Visit 5: VPA ER 1000 mg QPM | VPA ER 500 mg: 18.5–37.1 VPA ER 1000 mg: 79.9–85.1 |
| 2 | 35 | 7/21 | Juvenile myoclonic epilepsy | Absence, PGTC, myoclonic | LEV 375 mg AM; 1250 mg PM | LEV: 10–24 mcg/mL |
| 3 | 57 | 6/19 | Partial epilepsy | CPS, SGTC | ZNS 100 mg AM; 200 mg PM | ZNS: 16.8–26.9 mcg/mL |
| 4 | 72 | 14/21 | Juvenile myoclonic epilepsy | PGTC, myoclonic | VPA 500 mg BID LTG 75 mg BID | VPA: 88–97 mcg/mL LTG: 9.7–12.8 mcg/mL |
| 5 | 93 | 16/35 | Idiopathic generalized | PGTC, absence | VPA 500 mg BID LTG 75 mg BID | VPA: 83–121 mcg/mL LTG: 9.2 - 11 |
| 6 | 56 | 6/18 | Idiopathic generalized | PGTC, absence | ZNS 75 mg BID LTG 75 mg BID | ZNS: 10.9–16.9 mcg/mL LTG: 2.4–3.8 mcg/mL |
AED = antiepileptic drug; PGTC = primary generalized tonic–clonic; CPS = complex partial seizure; SGTC = secondarily generalized tonic–clonic; VPA = valproate; ER = extended release; QPM = once in the evening; LEV = levetiracetam; ZNS = zonisamide; LTG = lamotrigine
All CBZ levels were < 3 mg/l at hour 1, and 3 were > 4 mg/l at hour 6 (patients #3 #,4, and #5). LEV levels were always higher at hour 1 than hour 6, except in patient #3. Peak LEV levels were between 10 and 20 mg/l, except for patient #1, who achieved a level of 28 mg/l.
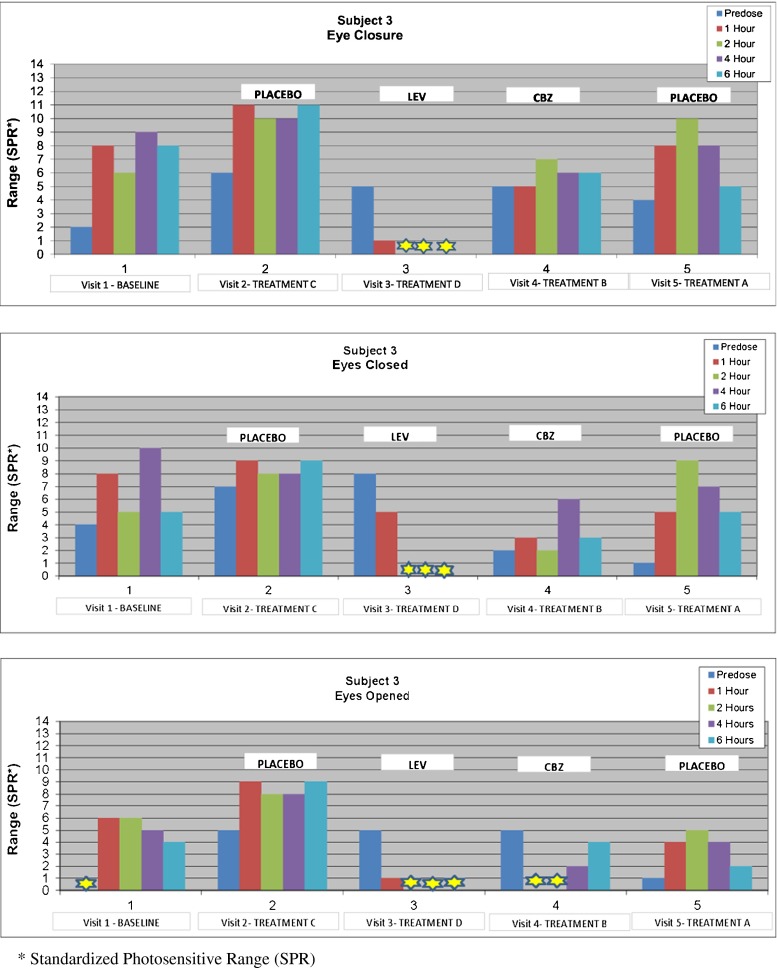
PPR treatment results are shown in Fig. 1, and an example of PPR response in a single patient is shown in Fig. 2. Patients who received LEV had the lowest mean PPR compared with the other treatments (placebo, CBZ). The mixed effect model showed a significant effect of LEV in all eye conditions (p < 0.001) (Table 3). There was no evidence of a significant decrease for CBZ or the placebo treatments on PPR. Moreover, complete suppression of PPR in the eyes closed condition at 2 time points occurred only with LEV, and was present in 3/5 patients who completed the LEV arm. In most patients, CBZ neither raised nor lowered the mean PPR, and did not produce complete suppression with eye closure in the patients. Changes in PPR did not appear to be influenced by CBZ serum concentrations. In fact, at hour 6, when the CBZ level was higher than at hour 1, the PPR was lower in only 1 patient than at hour 1, was higher in 4 patients, and the same in 1 patient. There did not seem to be a direct concentration relationship for LEV and PPR. There was no consistent effect of placebo treatments or the time of testing in the day (e.g., morning or afternoon) on changes in PPR.
Fig. 1.
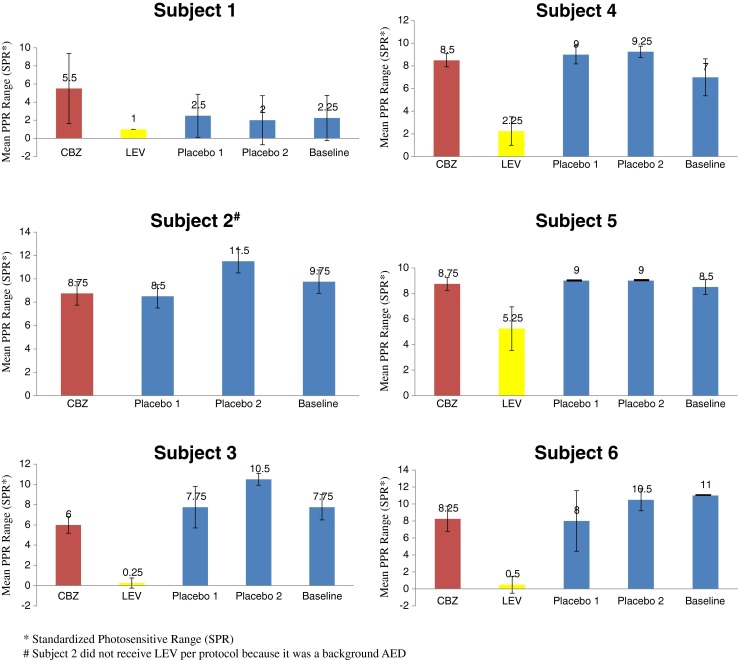
Mean photoparoxysmal responses (PPR) range across 4 (1–6 h) treatment and baseline test periods, each on different days, for 6 patients with eye closure condition. Mean PPR was not reduced by carbamazepine (CBZ), but was reduced by levetiracetam (LEV) in all patients compared to placebo. *SPR = standardized photosensitive range; #patient 2 did not receive LEV per protocol because it was a background antiepileptic drug
Fig. 2.
Example of photoparoxysmal response changes for patient 3 during the 3 eye conditions. The visits occurred on different days. *SPR = standardized photosensitive range. LEV = levetiracetam; CBZ = carbamazepine
Table 3.
Results of fixed effects model comparing effects of carbamazepine (CBZ), levetiracetam (LEV) and placebo treatments on photoparoxysmal responses. Statistically significant results are in bold
| Eyes closed | Eye closure | Eyes open | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Error | p | Estimate | Error | p | Estimate | Error | p | |
| CBZ | −0.54 | 0.94 | 0.5700 | −0.08 | 0.97 | 0.9300 | −1.10 | 0.98 | 0.2800 |
| LEV | −5.22 | 1.00 | < 0.0001 | −5.56 | 1.03 | < 0.0001 | −4.68 | 1.05 | 0.0005 |
| Placebo 1 | −0.38 | 0.94 | 0.6900 | −0.25 | 0.97 | 0.8000 | 0.65 | 0.99 | 0.5200 |
| Placebo 2 | 0.63 | 0.94 | 0.5100 | 1.08 | 0.97 | 0.2800 | −0.30 | 0.99 | 0.7700 |
| Intercept | 6.63 | 1.11 | 7.71 | 1.06 | 5.55 | 0.93 | |||
Discussion
This study is the first double-blind, placebo-controlled photosensitivity study, and showed the feasibility of an efficient outpatient protocol to screen compounds for antiseizure effects. Prior studies have been single-blind, inpatient studies, where the treatment day would immediately follow the placebo day. An outpatient photosensitivity study allows treatment washout periods between study treatments and also permits double-blinding of study treatments. Outpatient photosensitivity testing is also much less expensive than several day inpatient studies—an important consideration in early screening of potential therapies. We found PPR were relatively stable across and within study days, including baseline and placebo treatment periods—this permitted drug effects to be easily identified. We demonstrated strong effects of LEV on reducing or abolishing PPR, and minimal or no effects of CBZ on PPR (no exacerbations). This may be owing to relatively low serum concentrations of CBZ; however, 400-mg doses are typically the highest doses well tolerated by drug-naïve patients, and CBZ concentrations of 4.6–6.1 mg/l in 3 patients did not reduce PPR. Moreover, patients had reduced PPR during periods of low serum concentrations of LEV. It is therefore reasonable to conclude that not all AEDs will produce an effect on photosensitivity using single, low doses.
Previous studies have shown a reduction in photosensitivity with almost all effective AEDs tested. However, these single-blind studies were performed in patients kept in the hospital at rest for 24-h placebo baseline periods followed by study treatments. The effect of nonrandom order of treatment is currently unknown and the outpatient protocol with double-blind, placebo-controlled treatment that incorporates treatment washout periods is a more objective design. The criteria to assess changes in PPR also vary across previous inpatient single-blind studies. These range from reductions in PPR of 3 steps at 1–3 separate time points in 1 eye condition or all eye conditions. In 1 trial, a significant change was determined as a reduction of PPR by 3 steps at 3 time points on a given day, and no increase on that day compared with the placebo day [11]. Only 4 post-drug PPR’s were assessed per day in our study, meaning a reduction in 3/4 time points would be needed to occur to achieve this outcome criterion. Nonetheless, this number of reductions occurred in 1 patient in the current study when comparing one placebo day with another, raising the question of whether this is always a reliable measure of drug effect. Most previous studies assess PPR 8 times per day, and only 3/8 time points with improvement would be required to show a reduction to be considered a “positive” result. The fixed effects model we used here could be an effective alternative strategy for identifying treatment effects.
Our results show that a double-blind outpatient study is feasible in terms of both cost and accuracy. However, substantial time and effort are required to complete all visits, and we included baseline and 2 placebo treatment days in order to examine variability of PPR during sequential outpatient assessments. Our study results suggest that a slightly condensed design with 1 placebo-treatment day and a limited baseline “screening” evaluation may be warranted for an efficient outpatient screening protocol.
Our findings also suggest that the photosensitivity trial design is a screening study and may identify compounds that are effective in reducing photosensitive spiking in single test doses. The POC protocol has been successful in identifying multiple drugs in early phase drug development that were subsequently effective in large clinical trials. Our findings show possible limitations of the study design, as it did not demonstrate significant anticonvulsant effects of CBZ tables within a 6-h period. This finding shows that absence of a PPR effect of a drug tested in only 1 dose should not be interpreted as evidence that the drug has no effect at all. CBZ is clearly a highly efficacious drug in patients with focal epilepsy, yet was either ineffective or poorly effective in this model at the standard dosage of 400 mg. A previous study by Binnie et al. [4] reported that of 4 patients (3 with generalized seizures; 1 with partial onset seizures) treated with CBZ 400 mg doses (given as a liquid for rapid absorption), 2 had PPR abolishment. The study did not report serum levels [5] and it is unclear whether other factors, such as effects of concomitant AEDs, influenced CBZ responses.
Nonetheless, the photosensitivity trial design appears to be useful as a POC screening protocol for identifying possible antiseizure drugs. When a clear change from placebo is confirmed (as it was for LEV) it confirms delivery of drug to the brain and can be used as initial evidence of drug efficacy. Changes in PPR correlated with plasma concentrations of compounds can also be helpful bridges from preclinical testing and can potentially help identify optimal doses and duration of treatment effects. The outpatient photosensitivity protocol provides efficient screening for drugs that are effective in the photosensitivity trial design.
Electronic supplementary material
(PDF 4839 kb)
Acknowledgments
Funding was provided by The Epilepsy Research Foundation with supplemental funding by GlaxoSmithKline for support of the database, statistician, and EEG/IPS assessments. The Epilepsy Study Consortium provided oversight for the study, including site coordination, recruitment, EEG training, case report form development, regulatory document development, and data handling. We would like to thank Christina Ma from the Clinical Research Unit at The University of Calgary for providing database assistance.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Kasteleijn-Nolst Trenite DG, Genton P, Parain D, et al. Evaluation of brivaracetam, a novel SV2A ligand, in the photosensitivity model. Neurology. 2007;69:1027–1034. doi: 10.1212/01.wnl.0000271385.85302.55. [DOI] [PubMed] [Google Scholar]
- 2.Kasteleijn- Nolst Trenité DGA, Marescaux C, Stodieck S, Edelbroek PM, Oosting J. Photosensitive epilepsy: a model to study the effects of antiepileptic drugs. Evaluation of the piracetam analogue levetiracetam. Epilepsy Res 1996;25:225–230. [DOI] [PubMed]
- 3.Binnie CD. Preliminary evaluation of potential anti-epileptic drugs by single dose electrophysiological and pharmacological studies in patients. J Neural Transm. 1988;72:259–266. doi: 10.1007/BF01243424. [DOI] [PubMed] [Google Scholar]
- 4.Binnie CD, van Emde Boas W, Kasteleijn-Nolste-Trenite DGA, et al. Acute effects of lamotrigine (BW430C) in persons with epilepsy. Epilepsia 1986;27:248–254. [DOI] [PubMed]
- 5.Binnie CD, Kasteleijn-Nolst Trenite DGA, de Korte R. Photosensitivity as a model for acute antiepileptic drug studies. Electroencephalogr Clin Neurophysiol. 1986;63:35–41. doi: 10.1016/0013-4694(86)90060-X. [DOI] [PubMed] [Google Scholar]
- 6.Trenite DG, French JA, Hirsch E, et al. Evaluation of carisbamate, a novel antiepileptic drug, in photosensitive patients: an exploratory, placebo-controlled study. Epilepsy Res. 2007;74:193–200. doi: 10.1016/j.eplepsyres.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Kasteleijn-Nolst Trenite DG, van Emde Boas W, Groenhout CM, Meinardi H. Preliminary assessment of the efficacy of Org 6370 in photosensitive epileptic patients: paradoxical enhancement of photosensitivity and provocation of myoclonic seizures. Epilepsia 1992;33:135–141. [DOI] [PubMed]
- 8.Rowan AJ, Binnie CD, Warfield CA, Meinardi H, Meijer JW. The delayed effect of sodium valproate on the photoconvulsive response in man. Epilepsia. 1979;20:61–68. doi: 10.1111/j.1528-1157.1979.tb04776.x. [DOI] [PubMed] [Google Scholar]
- 9.Berkovic SF. Aggravation of generalized epilepsies. Epilepsia. 1998;39:S11–S14. doi: 10.1111/j.1528-1157.1998.tb05115.x. [DOI] [PubMed] [Google Scholar]
- 10.Molenberghs G, Verbeke G. Models for discrete longitudinal data. New York, Springer, 2005.
- 11.Biton V, Abou-Khalil B, Kasteleijn-Nolst Trenité D, et al. A clinical study of the effect of ICA-105665 on photic-induced paroxysmal EEG responses. American Epilepsy Society Abstract 2010;1:271.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 4839 kb)