Abstract
The mechanisms of epileptogenesis in pediatric epileptic syndromes are diverse, and may involve disturbances of neurodevelopmental trajectories, synaptic homeostasis, and cortical connectivity, which may occur during brain development, early infancy, or childhood. Although genetic or structural/metabolic factors are frequently associated with age-specific epileptic syndromes, such as infantile spasms and West syndrome, other syndromes may be determined by the effect of immunopathogenic mechanisms or energy-dependent processes in response to environmental challenges, such as infections or fever in normally-developed children during early or late childhood. Immune-mediated mechanisms have been suggested in selected pediatric epileptic syndromes in which acute and rapidly progressive encephalopathies preceded by fever and/or infections, such as febrile infection-related epilepsy syndrome, or in chronic progressive encephalopathies, such as Rasmussen encephalitis. A definite involvement of adaptive and innate immune mechanisms driven by cytotoxic CD8+ T lymphocytes and neuroglial responses has been demonstrated in Rasmussen encephalitis, although the triggering factor of these responses remains unknown. Although the beneficial response to steroids and adrenocorticotropic hormone of infantile spasms, or preceding fever or infection in FIRES, may support a potential role of neuroinflammation as pathogenic factor, no definite demonstration of such involvement has been achieved, and genetic or metabolic factors are suspected. A major challenge for the future is discovering pathogenic mechanisms and etiological factors that facilitate the introduction of novel targets for drug intervention aimed at interfering with the disease mechanisms, therefore providing putative disease-modifying treatments in these pediatric epileptic syndromes.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0265-2) contains supplementary material, which is available to authorized users.
Keywords: West syndrome, infantile spasms, FIRES, epileptogenesis, Rasmussen encephalitis.
Introduction
The pathogenic mechanisms of epileptogenesis in pediatric epileptic syndromes are quite heterogeneous, and may involve overlapping biological processes that include neuronal excitability, synaptic, and neuronal connectivity, adaptive and genetic mechanisms, and immunological responses. Although neurodevelopmental abnormalities and genetic factors determine mechanisms of epileptogenesis in several pediatric epileptic syndromes, such as those manifesting with infantile spasms (IS) [1–3], other factors, such as immune-mediated responses against the central nervous system (CNS), or environmental challenges, such as fevers and/or infections, may influence the development of aggressive forms of pediatric epilepsy in normally developed children. The role of immune-mediated mechanisms or neuroinflammation has been attributed to selected pediatric epileptic syndromes, such as Rasmussen encephalitis (RE), a chronic progressive encephalopathy [4], and suspected in others in which the dominant feature is the presence of seizure disorders associated with either acute and rapidly progressive encephalopathies preceded by fever and/or infections, such as febrile infection-related epilepsy syndrome (FIRES) [5]. The purpose of this review is to describe the most important features and discuss the most up-to-date knowledge of pathogenic mechanisms involved in epileptogenesis in well-known pediatric epileptic syndromes, such as RE, IS, and FIRES.
Rasmussen Encephalitis
RE, also known as Rasmussen’s syndrome, is a rare, progressive, unihemispheric neurological disorder of previously normal children who develop intractable focal seizures, epilepsia partialis continua, and—as the disorder progresses—hemiparesis and progressive cognitive decline [4, 6, 7]. The rate of progression of intellectual and functional deterioration may range in duration from months to years. As the disease progresses, the outcome of children with RE is almost invariably a patient with a dense hemiparesis, substantial intellectual impairment, continued seizures, and a severely impaired quality of life [4, 8]. RE was originally described by Theodore Rasmussen in patients with chronic forms of epilepsy in which neuropathological studies showed cortical neuroinflammatory changes characterized by active encephalitis, perivascular cuffing, lymphocytes, and microglial nodules scattered throughout the gray and white matter, and marked neuronal loss [6]. Although considered a rare form of epilepsy, RE does not show a gender preference, and has been described in almost all regions of the world. Recent epidemiological studies from Germany and the UK estimate an annual incidence of 1.7–2.4 cases/107 people aged ≤ 16–18 years [9, 10].
The clinical profile of patients with RE is variable; however, characteristically, it is a chronic, progressive disorder that affects young children, but has also been described during adulthood [11]. The diagnosis of RE is clinical and based on unilateral seizures, motor involvement, neuroimaging, and electroencephalogram (EEG) studies, and may be supported histopathological changes in brain biopsy studies, although a “negative” biopsy may be misleading and will not exclude the possibility of RE [12]. A consensus for diagnostic criteria of RE was established by European groups in 2005 [13]—guidelines that have been validated and that are currently accepted for the evaluation and diagnosis of RE patients [14]. In most of the cases, RE may present with a “prodromal period” that may involve mild motor manifestations, such as hemiparesis, and seizures that evolve in an “acute stage” characterized by focal or multifocal seizures that remain mostly unilateral, and which may occasionally continue to evolve to epilepsia partialis continua or to bilateral convulsive seizures [4, 15]. Even with the spread of seizures throughout one side of the body, the seizures do not follow a Jacksonian pattern; unilateral partial seizures predominate, with only rare secondary generalization [7]. EEG abnormalities in RE are variable and reflect the stage of hemispheric disease, although interictal abnormalities may be seen over the nonaffected hemisphere. Such a pattern of contralateral activity does not indicate bilateral disease nor appear to reflect cognitive changes [16]. Patients in the late, or “residual”, stages of RE are frequently affected by severe cognitive deficits, hemiplegia, and in some language abnormalities, such as aphasias, if the dominant hemisphere is affected [4].
Neuroimaging studies in patients with RE demonstrate a characteristic unihemispheric involvement. Magnetic resonance imaging (MRI) in the early stages of the RE may show subtle T2 weighted (T2W) and fluid attenuated inversion recovery (FLAIR) signal intensity abnormalities in areas of the cerebral cortex or subcortical white matter. Those changes may progress to involve other areas of the cortex and white matter, widening of sulci, and enlargement of the ventricular system within the same hemisphere as an indicator of progression of cerebral atrophy. Frequently, involvement of basal ganglia structures is seen with fluid attenuated inversion recovery, or T2W signal abnormalities and/or atrophy (Fig. 1a) [17–19]. Although the rate of progression of atrophic changes is variable, volumetric studies indicate the hemispheric volume loss peaks in the first 8 months of disease [18, 20, 21]. Interestingly, topographic analysis of brain atrophy showed that the frontal lobe and the insula were preferentially involved by the atrophic process. The degree of hemispheric, parietal, and occipital atrophy was negatively correlated with the age at disease onset, indicating a more aggressive and outspread disease in young children than in adolescents [21]. Functional brain imaging with fluorodeoxyglucose-positron emission tomography scans frequently show hypometabolic activity within the affected hemisphere [22].
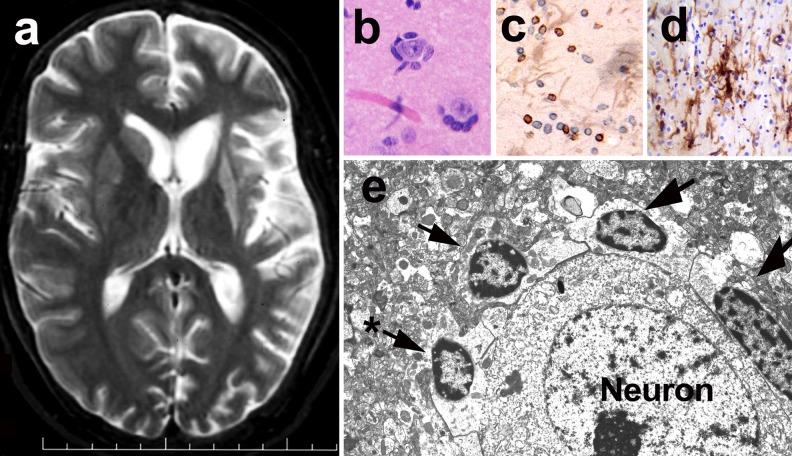
Fig. 1.
Magnetic resonance (MR) imaging and neuropathological abnormalities in Rasmussen encephalitis. (a) T2-weighted MR image shows cortical atrophy in the left hemisphere most prominent in the peri-insular region. Note the marked atrophy and increased signal intensity of head of caudate and putamen. (b) Perineuronal lymphocytic infiltration observed in the cerebral cortex as seen in a hematoxylin and eosin stain. (c) CD8+ (brown) and CD3+ (blue) T lymphocytes infiltrating the cerebral cortex. Immunostaining with specific antibodies for identification of T lymphocytes (CD3+) and CD8+ lymphocytes. Light brown cells represent reactive astrocytes. (d) Microglial activation (brown cells) and T lymphocyte infiltrates (blue cells) of the cerebral cortex. Immunostaining with anti-human leukocyte antigen-Dr (HLADr) antibodies for identification of microglial cells. (e) Electron microscope imaging of T lymphocytes (arrows) surrounding a neuron. Note the disruption of the neuronal membrane produced by one of the lymphocytes (*), which likely represents an early cytotoxic effect.
Immunopathogenesis and Mechanisms of Disease in RE
Neuropathological studies in RE disclose multifocal cortical inflammation confined to 1 hemisphere, a variable degree of neuronal loss, and gliosis. The most characteristic neuropathological hallmarks of RE are the presence of clusters of perineuronal lymphocytes (Fig. 1b, e) and microglial nodules (Fig. 1d), along with perivascular cuffing, neuronal cell loss, neuronophagia, and marked astroglial and microglial activation in the cerebral cortex [12, 23, 24]. T cell clusters and neuroglial infiltration may be seen also in white matter and deep gray nuclear structures, such as putamen and caudate (Fig. 1c). The patterns of cortical damage in RE include a “gyral pattern”, with involvement of large areas of the top or sulcal regions of a gyrus, and, less frequently, wedge-shaped or “punched out” lesions [12]. Focal neuroinflammatory changes may be surrounded by normal cerebral cortex, a finding that suggests sampling errors may occur frequently with brain biopsies obtained for diagnosis, leading to negative results that may be misleading in determining a precise diagnosis [12]. Four stages of neuropathological progression in the cerebral cortex have been described in RE, findings that demonstrate the progressive cortical damage mediated by T lymphocytes and neuroinflammation. Early stages of pathological changes disclose discrete focal T cell infiltrates and microglial activation that evolve to intermediate stages, and more extensive neuroinflammatory changes and neuronal loss. End-stage pathological changes include cortical cavitation, marked astroglioisis, and neuronal cell loss [12]. These neuropathological features are consistent with immune-mediated mechanisms that involve both adaptive immune reactions, with T lymphocyte responses, and innate immunity facilitated by both microglia and astroglia activation [12, 25].
The Role of Adaptive Immunity and T Cell-Mediated Immunopathogenesis in RE
Immunopathological studies have demonstrated that cytotoxic CD8+ T lymphocytes comprise the most frequent subset of T lymphocytes that infiltrates the cerebral cortex and white matter in RE (Fig. 1c). The cytotoxic mechanisms triggered by CD8+ T lymphocytes involve the release of granzyme B [25] and activation of inflammasome pathways, which includes the release of interleukin (IL)-1β and other inflammatory mediators [26] into the microenvironment of neurons and neuroglia—mechanisms that appear to play a critical role in the pathogenic neuroinflammatory process that drives the progression of RE. Interestingly, spectra-typing of the T lymphocytes found in brain tissues from RE patients demonstrated that these cells were expanded from discrete antigenic epitope-responding precursor T cells [27], a finding that supports the view that single, specific brain antigens drive such T cell responses. At present, the identity of such antigens remain unknown, but the fact that both neurons and astroglia appear to be the target of T lymphocyte injury suggests that either intrinsic autoantigens or foreign antigens from neuroinvasive pathogens (e.g., virus) may be present in these CNS cells and act as triggers of T cell cytotoxicity.
Recent studies of brain tissue obtained from RE have also demonstrated increased expression of messenger RNA of genes associated with chemokines associated with lymphocyte and monocyte trafficking, and cytokines involved with activation of helper/inducer and memory/effector T lymphocytes, such as CCL5, CXCL10, CCL22, CCL23, and CXCL9 and interferon-γ [28]. Similarly, the expression of several inflammasome-related genes, such as IL-1β, IL18, NLRP1, NLPR3, and CASP1 were significantly increased in brain RE tissues compared with mesial temporal sclerosis controls, and appeared to correlate with the magnitude of clinical involvement [26]. These findings support the view that adaptive immune responses are central to the disease process in RE. Unfortunately, therapeutic approaches that use immunomodulatory and immunosuppressive drugs have a limited effect on the final outcome of RE [29, 30], and many patients undergo hemispherectomy or surgical treatments as the only approach to control seizure activity, and the cognitive and neurological decline associated with disease progression [31–33]. The potential use of new immunomodulatory medications that modulate lymphocyte trafficking (e.g., Natalizumab) [34] into the CNS may be of benefit, but larger studies may be needed to determine the risks versus benefits for prolonged use in pediatric populations [15].
Autoantibodies in Pathogenesis of RE
The potential role of autoantibodies in the pathogenesis of epilepsy has been explored extensively in recent years [35]. Interestingly, the role of antineuronal membrane receptor antibodies was first noted in animal models and was proposed to be involved in pathogenesis of RE after demonstration of anti-GluR3 antibodies and transitory improvement of seizure frequency in a patient with RE treated with plasmapheresis [36]. This initial observation was not validated by subsequent studies, which showed that anti-GluR3 antibodies were not specific to RE and that only few RE patients possess such antibodies [37]. Similarly, other studies found antibodies against other neuronal antigens, such as the α-7 nicotinic acetylcholine receptor or Munc-18-1, in a few RE patients [38], but lack of replication of such observations decreased enthusiasm about their potential involvement. Interestingly, the recent explosion of interest around the discovery of anti-N-methyl-D-aspartate (NMDA) receptor antibody-mediated encephalitis [35] has rekindled interest in the search for autoantibodies in epilepsies, including RE. Antibodies to neuronal antigens, such as leucin-rich glioma inactivated 1 (LGI1), α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA), and gamma-aminobutyric acid (GABA) receptors, have been found in patients with limbic encephalitis, in which seizures may be a main component of the clinical profile [39]. The implications of these observations are particularly important for clinical diagnosis, as some of the neurological disorders mediated by these antibodies may mimic the initial stages of RE, as has been disclosed in a recent case of anti-NMDA encephalitis [40]. However, in the case of RE, the prevalence of anti-neuronal antibodies is low, and their presence likely represents an epiphenomenon secondary to the pathological process, rather than a causal factor. In addition, the lack of meaningful therapeutic response to antibody-depleting approaches such as plasmapheresis [29, 41] supports the view that antibody-mediated mechanisms in RE are not relevant to the pathogenic process. Empirical approaches with B lymphocyte-depleting therapies, such as rituximab, have been used in RE [42, 43], but, to date, no well-controlled clinical trial data that document efficacy are available.
Innate Immunity and Neuroglial Responses in RE
Although adaptive immune responses, such as those mediated by T lymphocytes, are an important effector of CNS injury, innate immune responses mediated by microglia and astroglia activation are also central to the pathogenic process in RE. Microglial activation is one of the main neuropathological features that have been observed in RE since its original description [6]. The magnitude of activation of microglia follows the pattern of progression of RE, parallels the magnitude of T lymphocyte infiltration, and it is observed in early stages of cortical involvement [12]. The role of microglia in early stages of pathogenesis remains uncertain, but likely represents the first line of innate immune response to the unknown factors that trigger neuronal dysfunction in RE. Microglia represent an important source of proinflammatory immune mediators and elements of the inflammasome, such as proinflammatory cytokines [44]. Microglia release IL-1β, a cytokine shown to be increased in regions of the cerebral cortex in RE brains [26], and which is critical for epileptogenic mechanisms [45, 46]. Interestingly, microglia are also critical participants in the mechanisms of synaptic plasticity and dendritic modeling, thus the assumption that microglia only have a unique proinflammatory role in RE may be incorrect, considering their role in synaptic pruning and plasticity [47, 48].
In addition to the microglial responses, astroglial activation is quite prominent in all stages of RE cortical involvement, and parallels both microglial activation and T lymphocyte infiltration [12]. Astrocytes have important roles in neuronal–neuroglial interactions during epileptogenesis [49], and are central to mechanisms of glutamate detoxification, as they participate in processes of glutamate processing and transport [50]. Immunopathological studies suggest that astrocytes as neurons may be the target of T cell-mediated cytotoxicity, and their numbers may be reduced during the disease process in RE [51]. Disruption of neuronal–neuroglial interactions, and particularly of the astrocyte function, may carry important consequences for excitotoxicity mechanisms and neuronal injury [52].
Infection and Viral Hypothesis in RE
Since the original description of RE, the presence of chronic encephalitis changes has prompted the search for pathogen-infected cells in areas of cortical damage of the brain. Because of the pathological features and chronicity of the neuroinflammatory processes, viruses, in particular, are the first suspects among pathogens that may cause RE. A localized and slowly progressive viral infection may explain the unihemispheric encephalitic changes in RE. However, extensive studies to investigate the presence of viruses associated with well-known encephalitis (e.g., herpes simplex virus, cytomegalovirus, Epstein–Barr virus, enterovirus) have failed to demonstrate causal involvement in RE [53–58]. It is very likely that new nucleic acid deep sequencing technologies, microbiome, and metagenomic studies of the brain will revisit issues related to viral infections in RE.
Genetics Factors and “Double Pathology” in RE
Studies about the role of genetic factors in RE are lacking, and there is no evidence of familial inheritance. One study discovered a mutation in the SCN1A gene in a patient with some clinical features resembling, but without pathological confirmation of, RE [59]. Interestingly, the association of gene mutations in STXBP1 (MUNC-18-1) with some epileptic syndromes and childhood encephalopathies, and the presence of autoantibodies against Munc-18 in a subset of patients with RE [38, 60–62] may raise the possibility that mutations in STXBP1 may be present in patients with RE. Although the rarity of RE makes it difficult to perform genetic studies, a potential approach should focus on the assessment of genetic susceptibility to immunological responses as a potential predisposing factor in patients with RE. This issue is particularly important as previous reports showed a subset of patients with RE with associated secondary pathologies, such as tuberous sclerosis (TSC) [63] and underlying focal cortical dysplasias [64, 65], findings that suggest that genetic developmental disorders or postmigratory cortical abnormalities may precede the development of neuroinflammation and/or trigger cascades of responses responsible for epileptogenesis. Larger clinical and neuropathological studies are needed to determine the validity of these potential associations.
The Issue of Unihemispheric Involvement and Future Directions
The enigma of unilateral hemispheric involvement in RE is central in understanding the pathogenesis of RE. The possibility that local factors within the affected hemisphere trigger specific immune responses is supported by evidence that once the “inciting” areas are removed after early hemispherectomy treatment, patients stop the process of deterioration and cognitive decline [31, 66]. Several hypotheses may emerge from these observations. First, that cerebral cortex abnormalities determined by somatic mutations leading to unilateral neurodevelopmental abnormalities or disorders of cortical lamination, dysgenesis, or dysplasia trigger seizures and subsequent immunological responses in people genetically predisposed to develop such an “aggressive” immune activation. Second, unihemispheric viral infections that trigger the development of chronic encephalitis changes that will eventually perpetuate the recurrence of seizure activity and further progression of degenerative changes within the affected hemisphere may be involved. Hypothesis of primary autoimmune responses against the CNS or systemic immunological disease are not supported by the characteristic unihemispheric involvement and the fact that patients do well after surgical or hemispherectomy treatments.
Infantile Spasms
IS are epileptic seizures that usually herald the presence of infantile epileptic encephalopathies with poor epilepsy and developmental outcomes [1, 67]. The triad of IS, neurodevelopmental delay, and a chaotic, multifocally epileptic, and disorganized interictal EEG (hypsarrhythmia) comprise the West syndrome [67, 68], named after William James West, the first person to report, in his own son, IS [69]. IS have a characteristic age specificity, usually appearing in the first year of life, although late-onset cases have also been reported. In most cases (60–90 %), a known underlying etiology is identified, whether genetic or other structural/metabolic; only in a minority of infants with IS does the etiology remain unknown [1].
Etiology and Pathogenic Mechanisms of IS
The causes and pathologies underlying IS are numerous. IS are distinct from other seizures in that the recommended initial therapies include adrenocorticotropic hormone (ACTH) and high-dose steroids, or the GABA aminotransferase inhibitor vigabatrin, whereas antiseizure drugs like phenytoin or carbamazepine show no effect [70–72]. Occasional reports of response to certain antiepileptic treatments (e.g., ketogenic diet, valproic acid, topiramate) exist, but these need further evaluation in prospective, controlled studies. Genetic, developmental, and immunological mechanisms have been postulated as potential pathogenic factors, and animal models are available for testing such mechanisms.
Genetic Etiologies Associated with IS
Numerous genes have been associated with early infantile epileptic encephalopathies with IS, although the overall frequency of occurrence of each of these individual genetic abnormalities in infants with IS is quite low [2, 3]. The true overall incidence of genetic defects in patients with IS is not well known, as genetic studies are done in a subset of infants without any identified etiology, depending on the availability of genetic tests. It has been reported that among infants with IS of unknown etiology approximately 7–8 % have copy number variant abnormalities [73, 74]. In addition, genetic mutations or defects appear de novo, or are transmitted in an autosomal recessive or dominant or X-linked pattern. It should be noted, however, that genetic defects can be identified in patients with metabolic or certain types of structural etiologies (e.g., brain malformations or tumors).
The affected genes are involved in neurotransmission, vesicular release or organelle trafficking, cytoskeletal or plasma membrane interactions, tumor suppression, intracellular metabolism, DNA repair, and brain development. Clearly, functional disruption of these genes may potentially increase brain excitability directly or indirectly owing to the ensuing brain malformations and/or tumors that may predispose to IS. Among the genes associated with IS, only the aristaless X-linked homeobox gene (ARX) genes have been studied, and are reported to manifest epileptic spasms in mouse models of IS. ARX is a transcription factor that is involved in ventral telencephalon morphogenesis, migration of GABAergic neuronal progenitors, and early commitment of cholinergic neurons [75]. Seven transgenic mouse models with ARX loss-of-function or knockin mutations have been produced [76–79]. However, only two of these mouse strains exhibit epileptic spasms, suggesting a heterogeneity in the phenotype, similar to humans, which could be due to either the severity of genetic dysfunction stemming for the gene defect or other modifiers. The conditional deletion of ARX in ganglionic eminence-derived neurons produces an epilepsy phenotype, with stage 5 seizures in the infantile period (postnatal day 14–17), and epileptic spasms only appearing in adulthood [79]. In contrast, the knockin ARX mouse model (ARX(GCG)10+7) with expansion of the first polyalanine repeat of ARX manifests spasms between postnatal days 7 and 20, later occurrence of other seizures, and neurodevelopmental deficits [78]. An important common element of the underlying pathology in ARX mice is the loss of GABAergic interneurons from the cortex and, in some cases, hippocampus and striatal regions, which prompted use of the term “interneuronopathy”. Interneuronopathy has been found in all ARX mice, with only one exception [76]. Loss of GABAergic interneurons for acquired causes has also been reported in the multiple-hit rat model of IS [80], and is currently one of the investigated “common pathways” for other models of IS. Interestingly, in heterozygous mice for STXBP1 (syntaxin binding protein 1; MUNC18-1), which has also been associated with IS in humans, high-frequency stimulation causes a preferential depression of synaptic transmission in GABAergic neurons, rather than glutamatergic neurons, which was attributed to reduction in the readily releasable pool of synaptic vesicles [81]. However, there has been no report of epilepsy in these STXBP1+/– mice.
IS have also been reported in up to a third of patients with Down syndrome, and can be responsive—initially, at least—to medical treatments [82–85]. Recently, an acute animal model of IS was developed in a transgenic mouse strain of Down syndrome through induction with gamma-butyrolactone, which eventually activates GABAB receptors [86]. This study focused on the potential involvement of GABAB receptors in the expression of IS in Down syndrome. However, there is no evidence that GABAB receptor activation in Down syndrome is sufficient to reproduce the chronic phenotype of the syndrome.
The Mammalian Target of Rapamycin Pathway in Genetic and Nongenetic Etiologies of IS
Among the genetic etiologies, TSC is probably one of the leading causes of IS. Among patients with TSC, 38 % manifest IS, whereas 5–10 % of infants with IS are diagnosed with TSC [73, 87–92]. Furthermore, a number of genetic disorders that lead to pathologic overactivation of the mammalian target of rapamycin (mTOR) pathway also manifest IS. Mutations in various genes along the phosphatidylinositol 3-kinase–AKT–mTOR pathway have been identified in hemimegalencephaly, patients with which often present with IS. These include mutations in the phosphatase and tensin homolog (PTEN) and AKT genes, or de novo somatic mutations of phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha, AKT3, or mTOR genes detected in the affected brain tissue, but not in peripheral blood [70, 93–96]. Polyhydramnios, microcephaly and symptomatic epilepsy syndrome can manifest with IS, and has been linked to mutations in STE20-related kinase adaptor alpha (STRAD-alpha) [97–99]. Loss of function of STRAD-alpha has been shown to lead to overactivation of the mTOR pathway, whereas rapamycin improved epilepsy in these patients [98].
Dysregulation of the mTOR pathway can also manifest in nongenetic causes of IS. In focal cortical dysplasias with balloon cells (type IIB; FCDIIB), loss of heterozygocity and polymorphisms of TSC1 were found [100]. Balloon cells from FCDIIB tissue show increased expression of downstream targets of the mTOR complex [phosphorylated S6 ribosomal protein (pS6) and eIF4G, but not phosphorylated 70S6 kinase], prompting the hypothesis that the overexpression of pS6 is due to other pathways than the classical mTORC1/phosphorylated 70S6 kinase [101, 102]. Among the acquired causes of mTOR overactivation, the potential role of viral-mediated activation of mTOR was recently proposed by Chen et al. [49]. They detected the E6 oncoprotein of the human papillomavirus 16 (HPV16) in balloon cells from FCDIIB that also overexpressed pS6. They also demonstrated that overexpression of E6 oncoprotein in the fetal brain produced cortical malformations [49]. These results, and prior evidence that E6 can activate the mTORC1 complex [103, 104], offer the possibility that FCDIIB could actually be caused by perinatal viral infections that could activate the mTOR pathway in selected vulnerable cells. The route of HPV invasion in the brain and the cellular factors that may render certain cells more vulnerable to HPV are yet unknown. However, further replication of these findings is warranted.
There has been no report that loss of function of TSC1 or TSC2 may lead to early life spasms in animal models of TSC. However, the presence of early-life epileptic spasms during the first 2 weeks has not been systematically looked at in these models. In the multiple-hit rat model of IS, which is induced in wild type rats by right intracerebral injection of lipopolysaccharide and doxorubicin on PN3, overexpression of the pS6 was only observed during the period of spasms [105]. Most interestingly, normalization of pS6 expression using high doses of rapamycin, an mTOR inhibitor, suppressed spasms in the multiple-hit model and also partially improved the learning performance later on, suggesting a potential disease-modifying effect. These observations suggest that mTOR signaling may be one of the pathways contributing to the expression of IS and subsequent cognitive decline, not only in genetic disorders of mTOR, but also in certain nongenetic causes of IS. Certainly, the ability of mTOR to respond to a variety of insults associated with IS (e.g., hypoxia, energy disorders, endocrine, growth factor and inflammatory signaling pathways, seizures) places it centrally, where these pathways could converge. However, not all infants with TSC develop IS, further attesting to the importance of individualized factors that could modify outcomes.
Neuroinflammatory Mechanisms
The beneficial response of IS to steroids and ACTH have supported hypotheses that link neuroinflammatory mechanisms with IS. Cytokines and other inflammatory markers have been investigated sporadically in patients with IS. Increased populations of CD3+ and CD4+ T lymphocytes, CD4+/8+ T cell ratio, and higher serum concentrations of IL-1β, IL-12, and macrophage inflammatory protein 1β were observed in the pre-ACTH treatment patient group compared with the post-ACTH treatment group [106]. Similarly, the IL receptor antagonist (IL-1ra) seems to be decreased during the active stage of IS [107] and increased after the resolution of symptoms of IS [108]. IL-2, interferon-α, and tumor necrosis factor-α were also reported to be increased in the serum of patients with IS [109]. These findings were equally relevant for both patients with IS of structural/metabolic or unknown etiology [108, 109]. However, cerebrospinal fluid (CSF) concentrations of the classical proinflammatory IL-6 were significantly lower in patients with IS than in patients with generalized tonic–clonic seizures due to infection or trauma [110]. Interestingly, among the animal models, inflammatory processes are one of the triggers of IS in the multiple-hit model [105, 111]. Moreover, recent studies have demonstrated that inflammatory insults alone may induce epileptic spasms in very young rats [112]. These clinical and preclinical findings indicate that inflammatory cascades may be a target for therapy discovery in IS. The anti-inflammatory effects of current (ACTH, steroids) and experimental (rapamycin) treatments certainly offer promise in this target pathway.
Viral or bacterial etiologies have been implicated in the induction of IS [113–115] and hypothesized to explain occasional cases with spontaneous remission [116]. However, the effects of inflammatory insults seem to be complex. Occasional beneficial effects of viral infections have been described [117], whereas re-activation of viral infections during the treatment of IS may also account for some seropositive cases, confounding attempts to provide causative links between viral illness and IS [118]. Autoimmune-mediated epilepsies with IS have also been reported [119, 120], including IS due to autoimmune cerebral folate deficiency [121].
Developmental Desynchronization and Hypsarrhythmia
Frost and Hrachovy [122] hypothesized that genetic or other insults could selectively disrupt the normal maturation of the specific targeted pathways or brain regions, and that this temporal desynchronization between the maturation of the affected and nonaffected developmental processes could cause the chaotic, epileptic background characteristic of hypsarrhythmia. To arrest focally the maturation of cortical and/or hippocampal regions, Lee et al. [123] developed the tetrodotoxin model, in which chronic infusion of tetrodotoxin (a sodium channel blocker that inhibits action potentials) in the cortical or hippocampal regions eventually triggers epileptic spasms, other seizures, and a hypsarrhythmia-like background. The behavioral features of the model were not age-specific, as they were observed in postpubertal and adult rats. Hypsarrhythmia has not been documented in animal models developed in younger rats, mainly owing to the technical difficulties in placing multiple electrodes in the very small and fragile skull of newborn or infantile rodents.
Glutamate NMDA Receptors and IS
An early report on behaviors associated with NMDA-induced seizures focused attention on “emprosthotonus”, or forward body tonic flexion [124]. NMDA spasms are responsive to both high-dose ACTH1–24 and vigabatrin [125–128]. Genetic associations have reported NMDA receptor 1 (GRIN1) de novo mutations in some patients with IS [3].
Stress and IS
The stress hypothesis of IS proposes that excessive activation of corticotropin-releasing hormone in response to early postnatal stressors could lead to IS [129]. When tested experimentally, intracerebroventricular corticotropin-releasing hormone was able to induce phenytoin-responsive seizures, but not spasms, in neonatal rats [130, 131]. More recently, perinatal manipulations aiming to activate stress pathways were used in the NMDA model to study their sensitivity to ACTH and susceptibility to NMDA-induced spasms. Prenatal betamethasone or restrain stress were shown to render NMDA spasms responsive to ACTH, when given at doses that had previously failed to control these seizures [128, 132]. Postnatal adrenalectomy, however, accelerated and exacerbated NMDA spasms, while, most interestingly, retaining their sensitivity to ACTH [127]. While these studies support the idea that stress may modify the phenotype and treatment response of IS, it is no yet known whether stress is sufficient to trigger spasms.
Structural Lesions in the Pathogenesis and Pharmacorefractoriness of IS
As stated earlier, structural/metabolic etiologies underlie the majority of cases of IS. These vary widely in their nature, location, or severity. However, their presence leads to poorer outcomes and increases the probability of treatment failure to the available drugs. Etiologies may include early life hypoxia–ischemia, malformations of brain development, structural lesions from TSC (e.g., tubers), CNS infections, perinatal strokes, metabolic disorders, or autoimmune conditions. IS are currently thought of as network disruption, involving cortical and subcortical structures [133]. Pathologic activation of the involved network at any trigger point could, theoretically, trigger spasms. So far, the only reported experimental animal model demonstrating structural lesions is the multiple-hit rat model [111]. Similar to the clinical syndrome, the induction of cortical and subcortical lesions by doxorubicin and lipopolysaccharide in the multiple-hit model generates a chronic phenotype, including an early period of cluster of spasms, subsequent emergence of other seizures, and neurodevelopmental and cognitive deficits. Furthermore, the multiple-hit is currently the only tested model that exhibits relative pharmacoresistance to the available treatments by not responding to ACTH and only transiently responding to vigabatrin or a vigabatrin analogue (CPP-115) [111, 134]. Ongoing studies are aimed at unraveling new therapy targets for treatment that could help control drug-resistant spasms and exhibit disease-modifying effects [105, 135, 136]. Among these, a promising target is the mTOR pathway.
FIRES
FIRES is characterized by refractory status epilepticus in previously healthy, school-aged children that presents itself with or just after a nonspecific febrile illness [137]. In order to differentiate this syndrome from viral encephalitis, it was previously described as devastating epilepsy in school age children [138] and later as febrile infection responsive epileptic encephalopathy of school age [137]. Most recently, the Commission of Classification and Terminology of the International League Against Epilepsy recognized the term FIRES at the last proposed revision [139]. Children with FIRES develop frequent focal seizures during the acute phase in the setting of, or immediately after, a nonspecific febrile illness. Seizures worsen and evolve rapidly to status epilepticus, which is highly pharmacoresistant and persists for several weeks or months [137, 138, 140, 141]. After the acute phase of status epilepticus, patients with FIRES enter a chronic stage of pharmacoresistant epilepsy and major cognitive decline that comes to the fore of the syndrome [137, 138, 140, 141].
In almost half of patients, fever disappears before the onset of the first seizures. The semiological features of the seizures suggest a focal onset. Autonomic features suggest a mesial temporal lobe involvement, and clonic movements of the mouth that extend to the limbs indicate spreading to opercular structures [138]. Seizures may rise from both hemispheres [137, 138]. EEG studies show focal discharges mainly in the temporal and frontal area, with diffuse slowing. Bilateral diffusion has been recorded frequently. Status epilepticus is highly pharmacoresistant during FIRES, and may persist despite conventional treatment with AEDs. The use of general anesthesia with pentobarbital appears to be inefficient, and in one report negative cognitive outcome correlated with the duration of therapy [142]. In addition, although seizures are controlled with suppression-burst coma approaches, they always reappear when barbiturates are weaned [142, 143]. A ketogenic diet appeared to stop status epilepticus in almost half of cases, even when administered late during the acute phase with status epilepticus [141, 143, 144]. In most refractory cases, death may occur after several weeks or months of ongoing seizures.
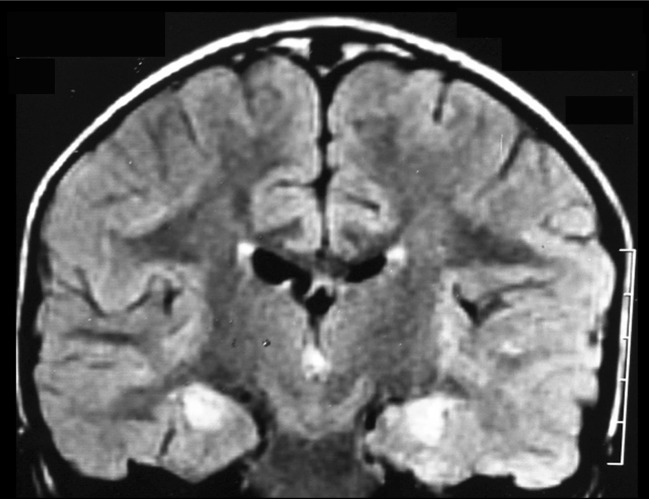
Early brain MRI obtained within the first weeks of FIRES reveals normal results in almost 55 % of patients [141]. In the other 45 %, a slight edema of the mesial temporal structures revealed by T2W signal hyperintensities has been reported [140, 141]. Brain MRI obtained at chronic stages, generally 6 months after the onset of FIRES, has revealed bilateral mesial temporal atrophy and T2W hyperintensities in both hippocampal structures in 50 % of patients (Fig. 2) [140, 141]. However, all patients with or without bilateral hippocampal lesions on MRI showed large areas of hypometabolism involving orbito-frontal and temporo-parietal regions bilaterally on fluorodeoxyglucose positron emission tomography scans performed in the chronic phase [145].
Fig. 2.
Magnetic resonance imaging abnormalities in febrile infection-related epilepsy syndrome (FIRES). Coronal FLAIR image shows bilateral hyperintensities in both hippocampal structures 3 weeks after onset of status epilepticus in a 3.5-year-old boy (image courtesy of Dr. M. Kara, Tripoli University Hospital, Tripoli, Lybia).
Hypothesized Pathogenic Mechanisms for FIRES
In the largest review of patients with FIRES (n = 77) [141], brain biopsies were performed in 13 patients; 7 biopsies revealed gliosis and 1 biopsy showed leptomeningeal inflammatory infiltrates, findings that may support the involvement of neuroinflammatory mechanisms. Although, to date, the etiology of FIRES has not been elucidated, several hypotheses that may focus the research on this devastating epileptic syndrome have been suggested.
Infectious Mechanisms and Encephalitis
Investigations to identify known infectious agents in CSF from patients with FIRES (bacterial, viral, and, in some instances, parasitic) through an extensive direct examination, polymerase chain reaction, and serological studies have been negative [141]. In patients with FIRES reported on in the first 2 series [137, 138], CSF did not show remarkable pleocytosis and, most importantly, extensive polymerase chain reaction studies for the identification of viruses were negative. In the largest review of patients with FIRES (n = 77), Kramer et al. [141] reported variable amounts of white cells in CSF (2–100 cells/mm3); however, in almost 75 % of the patients there was no evidence of pleocytosis in the initial CSF sample. These observations suggest that the potential presence of known viral encephalitis in FIRES is not supported by laboratory evidence, and the designation of this disorder as a form of viral encephalitis may be misleading. However, future prospective studies should take advantage of new deep nucleic acid sequencing, metagenomic, and microbiome techniques to determine, with more precision, the potential role of viral pathogens.
Metabolic Diseases and Mitochondrial Defects
The acute onset of status epilepticus in the setting of a febrile episode could also evoke diseases that involve mechanisms of energy defects, such as mitochondrial respiratory chain dysfunction, or in disorders arising from disruption of mitochondrial metabolic pathways, such as in fatty acid oxidation, or urea or citric acid cycle disorders [146]. Defects affecting the mitochondrial pathways may give rise to diseases that may be present at any age and involve tissues with high metabolic activity, including the CNS. As seizures are frequent in mitochondrial disorders that affect the CNS and lead to a significant risk of status epilepticus, some studies have focused on the potential role of mitochondrial disorders in FIRES through extensive metabolic assessment of sera and CSF. Evaluations have included studies of CSF lactic acid, biogenic amines, and amino acids, as well as blood assessment for ammonia, lactic acid, amino acids, carnitine and acyl-carnitine to investigate disorders of mitochondrial dysfunction. Many patients were also tested for very long chain fatty acids, vitamin B12, biotin, heavy metals, copper, ceruloplasmin, homocysteine, uric acid, transferrin, urine orotic acid, organic acids, and purine/pyrimidine ratio. This extensive metabolic work-up and imaging studies using brain MRI did not show evidence of mitochondrial respiratory chain defects [140, 141]. In addition, mitochondrial enzyme studies in muscles and fibroblasts performed in a few patients with FIRES did not show any abnormalities [138, 143]. Other studies that focused on POLG mutations also failed to find any causal mutation in a series of 15 patients [147]. All of these negative studies point out that the potential role of mitochondrial and/or metabolic disorders in FIRES remains uncertain.
Monogenic Epilepsy Genes and Rare Copy Number Variations in FIRES
De novo gene mutations are emerging as an important cause of childhood onset epileptic encephalopathies [148, 149]. In addition to single candidate genes, rare copy number variations (CNVs) have been established as risk factors for various epilepsies, including epileptic encephalopathies [150]. Patients with FIRES share common features with two emblematic monogenic early onset epilepsies: Dravet syndrome (DS) and patients with PCDH19 mutations. These two syndromes manifest with seizures and/or status epilepticus during a febrile illness in the absence of biomarkers for encephalitis or CNS infection in a previously healthy patient. Patients with these syndromes also show cognitive, slowing evolving to intellectual, disability, findings that may suggest a potential overlapping pathogenic mechanism with FIRES. However, clinically, these syndromes show several differences from FIRES, as outlined. DS occurs much earlier than FIRES, before 1 year of age. SCN1A mutations are reported in almost 80 % of patients. The long-lasting first seizure occurs during a febrile illness or after vaccine administration in DS. Although seizures might be focal, in many instances EEG is normal during the first 2 years, with generalized spikes and waves appearing on the recordings later. Intellectual disability usually appears 1–3 years after the onset and fever sensitivity persists for life [151]. Patients with PCDH19 mutation-related epilepsy phenotype are almost exclusively girls, which is in contrast to the male predominance in FIRES [141]. Patients present mainly with a cluster of seizures, rather than with status epilepticus during the febrile illness, as occurs in FIRES [148, 152]. Heterozygous PCDH19 mutations were initially identified in epilepsy and mental retardation limited to girls, a familial disorder with a singular mode of inheritance as only heterozygous girls are affected, whereas hemizygous boys are asymptomatic. Yet, mosaic boys can also be affected, supporting cellular interference as the pathogenic mechanism [137]. In some instances, patients with PCDH19 resemble patients with DS, and seizures occur earlier in the severe form of PCDH19 than in FIRES [153]. Although there appear to be some similarities between patients with FIRES and those with DS and PCDH19 gene mutations, molecular genetic studies in patients with FIRES showed no SCN1A or PCDH19 mutations [147, 154], a finding that suggests a lack of a molecular mechanism association of FIRES with those two syndromes.
In addition, other molecular genetic studies that included CNV screening performed in a series of 10 patients with FIRES showed no potentially pathogenic deletions in 9 of 10 patients. Only in 1 patient was a single novel duplication (chromosome 9: 6038553–6930898) overlapping 50 % with a known CNV (variation_0649) detected. However, this duplication was inherited from the unaffected mother and was not considered to be causal in the disease [147].
Autoimmunity and Autoantibodies
The presence of pre-existent immune systemic disease or the appearance of de novo autoimmune antibodies as a pathogenic mechanism for status epilepticus in patients with FIRES has been explored in previous studies [140, 141]. Patients underwent extensive immunological evaluations for biomarkers of systemic rheumatological and autoimmune disorders that included systemic lupus erythematosus, Wegener’s granulomatosis, vasculitis, Hashimoto’s thyroiditis and encephalitis, and celiac disease, among others [140, 141], with negative results.
Electrophoresis of CSF proteins yielded no significant findings in the vast majority of patients, and no evidence of CSF oligoclonal bands has been observed in FIRES [138, 155]. Furthermore, studies of autoantibodies against neuronal epitopes, such as glutamate receptors of type NMDA and type AMPA, GABAB-receptors, voltage-gated potassium channel-associated proteins (VGKC), LGI1 and contactin-associated protein like 2, and glutamic acid decarboxylase (GAD), associated with well-known immune-mediated neurological disorders and epilepsy, were also evaluated in patients with FIRES, yielding negative results [155]. Only a few patients in a large retrospective study disclosed positive anti- (VGKC) and anti- (GAD) and anti-GluR3 antibodies [141]. However, those observations were not replicated in a subsequent study [137] and their potential pathogenic role in FIRES is doubtful. In addition, patients with FIRES showed no beneficial response to steroids or immunotherapy treatments in the limited retrospective series [137, 140, 141], as is observed in patients with known autoimmune neurological disorders produced by antibodies against neuronal epitopes [39, 120]. These observations strongly suggest that not only are primary pathogenic mechanisms in FIRES not mediated by autoimmunity or by antibodies against neuronal epitopes, but that there is not a well supported rationale for the use of immunotherapy treatments.
In summary, the etiology of FIRES remains unknown and the underlying pathogenic mechanisms are not yet understood. Given the difficulties in treating patients with FIRES by conventional methods, insight into the underlying pathophysiology is clearly required in order to shape treatment strategies and improve outcome. Several approaches may be hypothesized and remain open for future research: an unknown viral agent not yet recognized; an unknown antineuronal or antiglial antibody that remains elusive to current techniques of antibody detection; a pattern of genetic susceptibility that may involve multiple genes with neuronal, immune, or even mitochondrial functions that could be identified by new exome sequencing technologies; or a neuroinflammatory disorder generated by neuronal–neuroglial disturbances during seizures. A major challenge for the future is to discover specific biomarkers of disease and pathogenesis detectable in CSF and/or serum. This might highlight novel targets for drug intervention aimed at interfering with the disease mechanisms, therefore providing putative disease-modifying treatments [156].
Conclusion and Future Directions
The mechanisms of epileptogenesis in pediatric epileptic syndromes such as RE, IS, and FIRES have been widely studied in the last few years (summarized in Table 1). Immune-mediated processes have been suspected to be involved in epileptogenesis of such syndromes. However, convincing evidence of such processes has only been demonstrated in RE, a disorder in which selective T cell responses against neurons and glia occurs, although the triggering factor that initiates such responses remains a mystery. Although primary immune factors and neuroinflammation have been postulated to be associated with IS and FIRES, current evidence is minimal and under investigation. In the case of IS, several etiopathogenic factors appear to be involved (including structural, metabolic, genetic, neuroimmune, or inflammatory factors). However, the response of IS to ACTH and high-dose steroids, as well as occasional clinical reports of cytokine abnormalities or of infectious and autoimmune etiologies, have raised the possibility that neuroinflammatory pathways may be involved in the pathogenesis of IS, at least in certain patients. Interestingly, the mTOR pathway appears to have a central role in the convergence of several signaling mechanisms, and may represent a promising avenue for therapeutic interventions in IS. Of the 3 syndromes discussed in this review, FIRES presents the major problem, as the underlying etiopathogenic factors remain unknown, although it is possible that a mixture of metabolic, genetic, and immune mechanisms converge to produce this complex and catastrophic epileptic syndrome. Further studies should focus on the identification of the triggering factor(s) associated with neuroinflammation in RE, and to determine whether an infection or autoimmune mechanism is present. Similarly, studies of FIRES should focus on the identification of genetic/metabolic abnormalities or potential infections that may be involved in its pathogenesis. New high-throughput deep-sequencing techniques, microbiome, and metagenomic studies would be useful in identifying host-related inflammatory and neuronal–neuroglial pathways associated with pathogenesis and potential pathogens that may be involved in pathogenesis of RE and FIRES. The challenge for future studies includes further clarification of etiopathogenic mechanisms and the introduction of potential therapeutic interventions to modify the course of these syndromes.
Table 1.
Mechanisms associated with epileptogenesis in pediatric epileptic syndromes
| Mechanisms/disorder | Adaptive immunity | Innate immunity | Genetic | Infection | Other/metabolic |
|---|---|---|---|---|---|
| Rasmussen encephalitis | CD8+ T cell cytotoxicity against neurons and glia Suspected autoantibodies |
Microglial and astroglial activation | Unknown | Suspected | Unknown |
| Infantile spasms | Disarrangements of T cell populations and increased cytokines in blood and cerebrospinal fluid Few cases with autoantibodies |
Suspected | Several known gene mutations | Few cases described | Structural, metabolic |
| Febrile infection-related epilepsy syndrome | Suspected, inconclusive evidence | Suspected | Suspected | Suspected | Metabolic (suspected, inconclusive evidence) |
Electronic supplementary material
(PDF 511 kb)
Acknowledgments
CAP was supported by The Bart McLean Fund for Neuroimmunology Research-Johns Hopkins Project Restore, and the RE Children’s Project. ASG was supported by a grant from the National Institute of Neurological Disorders and Stroke (NS078333), and by CURE, Autism Speaks, the Department of Defense, and the Heffer Family and Siegel Family Foundations. ASG has received royalties from Morgan & Claypool Publishers and John Libbey Eurotext Ltd, and a consultancy honorarium from Viropharma.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Pellock JM, Hrachovy R, Shinnar S, et al. Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51:2175–2189. doi: 10.1111/j.1528-1167.2010.02657.x. [DOI] [PubMed] [Google Scholar]
- 2.Paciorkowski AR, Thio LL, Dobyns WB. Genetic and biologic classification of infantile spasms. Pediatr Neurol. 2011;45:355–367. doi: 10.1016/j.pediatrneurol.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epi4K Consortium, Epilepsy Phenome/Genome P. Allen AS, et al. De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bien CG, Widman G, Urbach H, et al. The natural history of Rasmussen's encephalitis. Brain. 2002;125:1751–1759. doi: 10.1093/brain/awf176. [DOI] [PubMed] [Google Scholar]
- 5.Nabbout R. FIRES and IHHE: Delineation of the syndromes. Epilepsia. 2013;54(Suppl.) 6:54-56 [DOI] [PubMed]
- 6.Rasmussen T, Olszewski J, Lloydsmith D. Focal seizures due to chronic localized encephalitis. Neurology. 1958;8:435–445. doi: 10.1212/wnl.8.6.435. [DOI] [PubMed] [Google Scholar]
- 7.Freeman JM. Rasmussen's syndrome: progressive autoimmune multi-focal encephalopathy. Pediatr Neurol. 2005;32:295–299. doi: 10.1016/j.pediatrneurol.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Gordon N. Rasmussen's encephalitis. Develop Med Child Neurol. 1997;39:133–136. doi: 10.1111/j.1469-8749.1997.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 9.Bien CG, Tiemeier H, Sassen R, et al. Rasmussen encephalitis: incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia. 2013;54:543–550. doi: 10.1111/epi.12042. [DOI] [PubMed] [Google Scholar]
- 10.Lamb K, Scott W, Mensah A, et al. Incidence, prevalence and clinical outcome of Rasmussen encephalitis in children from the United Kingdom. Epilepsia 54(Suppl.3):4.
- 11.Granata T, Andermann F. Rasmussen encephalitis. Handbook Clin Neurol. 2012;111:511–519. doi: 10.1016/B978-0-444-52891-9.00054-3. [DOI] [PubMed] [Google Scholar]
- 12.Pardo CA, Vining EP, Guo L, Skolasky RL, Carson BS, Freeman JM. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia. 2004;45:516–526. doi: 10.1111/j.0013-9580.2004.33103.x. [DOI] [PubMed] [Google Scholar]
- 13.Bien CG, Granata T, Antozzi C, et al. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain. 2005;128:454–471. doi: 10.1093/brain/awh415. [DOI] [PubMed] [Google Scholar]
- 14.Olson HE, Lechpammer M, Prabhu SP, et al. Clinical application and evaluation of the Bien diagnostic criteria for Rasmussen encephalitis. Epilepsia. 2013;54:1753–1760. doi: 10.1111/epi.12334. [DOI] [PubMed] [Google Scholar]
- 15.Varadkar SM, Bien CG, Kruse CK, et al. Rasmussen encephalitis: current concepts and therapeutic advances. Lancet Neurol 2014 (in press). [DOI] [PMC free article] [PubMed]
- 16.Longaretti F, Dunkley C, Varadkar S, Vargha-Khadem F, Boyd SG, Cross JH. Evolution of the EEG in children with Rasmussen’s syndrome. Epilepsia. 2012;53:1539–1545. doi: 10.1111/j.1528-1167.2012.03565.x. [DOI] [PubMed] [Google Scholar]
- 17.Yacubian EM, Marie SK, Valerio RM, Jorge CL, Yamaga L, Buchpiguel CA. Neuroimaging findings in Rasmussen's syndrome. J Neuroimag. 1997;7:16–22. doi: 10.1111/jon19977116. [DOI] [PubMed] [Google Scholar]
- 18.Bien CG, Urbach H, Deckert M, et al. Diagnosis and staging of Rasmussen's encephalitis by serial MRI and histopathology. Neurology. 2002;58:250–257. doi: 10.1212/wnl.58.2.250. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Park YD, Pillai JJ, Lee MR, Smith JR. A longitudinal MRI study in children with Rasmussen syndrome. Pediatr Neurol. 2002;27:282–288. doi: 10.1016/s0887-8994(02)00437-x. [DOI] [PubMed] [Google Scholar]
- 20.Bien CG, Gleissner U, Sassen R, Widman G, Urbach H, Elger CE. An open study of tacrolimus therapy in Rasmussen encephalitis. Neurology. 2004;62:2106–2109. doi: 10.1212/01.wnl.0000128044.94294.87. [DOI] [PubMed] [Google Scholar]
- 21.Wagner J, Schoene-Bake J-C, Bien CG, Urbach H, Elger CE, Weber B. Automated 3D MRI volumetry reveals regional atrophy differences in Rasmussen encephalitis. Epilepsia. 2012;53:613–621. doi: 10.1111/j.1528-1167.2011.03396.x. [DOI] [PubMed] [Google Scholar]
- 22.Fiorella DJ, Provenzale JM, Coleman RE, Crain BJ, Al-Sugair A. 18F-fluorodeoxyglucose positron emission tomography and MR imaging findings in Rasmussen encephalitis. AJNR Am J Neuroradiol. 2001;22:1291–1299. [PMC free article] [PubMed] [Google Scholar]
- 23.Farrell MA, DeRosa MJ, Curran JG, et al. Neuropathologic findings in cortical resections (including hemispherectomies) performed for the treatment of intractable childhood epilepsy. Acta Neuropathol. 1992;83:246–259. doi: 10.1007/BF00296786. [DOI] [PubMed] [Google Scholar]
- 24.Farrell MA, Droogan O, Secor DL, Poukens V, Quinn B, Vinters HV. Chronic encephalitis associated with epilepsy: immunohistochemical and ultrastructural studies. Acta Neuropathol. 1995;89:313–321. doi: 10.1007/BF00309624. [DOI] [PubMed] [Google Scholar]
- 25.Bien CG, Bauer J, Deckwerth TL, Wiendl H, Deckert M, Wiestler OD, et al. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen's encephalitis. Ann Neurol. 2002;51:311–318. doi: 10.1002/ana.10100. [DOI] [PubMed] [Google Scholar]
- 26.Ramaswamy V, Walsh JG, Sinclair DB, et al. Inflammasome induction in Rasmussen's encephalitis: cortical and associated white matter pathogenesis. J Neuroinflammation. 2013;10:152. doi: 10.1186/1742-2094-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwab N, Bien CG, Waschbisch A, et al. CD8+ T-cell clones dominate brain infiltrates in Rasmussen encephalitis and persist in the periphery. Brain. 2009;132:1236–1246. doi: 10.1093/brain/awp003. [DOI] [PubMed] [Google Scholar]
- 28.Owens GC, Huynh MN, Chang JW, et al. Differential expression of interferon-gamma and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response. J Neuroinflammation. 2013;10:56. doi: 10.1186/1742-2094-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernasconi P, Antozzi C, Granata T, Spreafico R, Mantegazza R. Rasmussen's encephalitis: update on pathogenesis and treatment. Exp Rev Neurother. 2003;3:835–843. doi: 10.1586/14737175.3.6.835. [DOI] [PubMed] [Google Scholar]
- 30.Bien CG, Schramm J. Treatment of Rasmussen encephalitis half a century after its initial description: promising prospects and a dilemma. Epilepsy Res. 2009;86:101–112. doi: 10.1016/j.eplepsyres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Vining EP, Freeman JM, Brandt J, Carson BS, Uematsu S. Progressive unilateral encephalopathy of childhood (Rasmussen's syndrome): a reappraisal. Epilepsia. 1993;34:639–650. doi: 10.1111/j.1528-1157.1993.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 32.Pulsifer MB, Brandt J, Salorio CF, Vining EP, Carson BS, Freeman JM. The cognitive outcome of hemispherectomy in 71 children. Epilepsia. 2004;45:243–254. doi: 10.1111/j.0013-9580.2004.15303.x. [DOI] [PubMed] [Google Scholar]
- 33.Carson BS, Javedan SP, Freeman JM, et al. Hemispherectomy: a hemidecortication approach and review of 52 cases. J Neurosurg. 1996;84:903–911. doi: 10.3171/jns.1996.84.6.0903. [DOI] [PubMed] [Google Scholar]
- 34.Bittner S, Simon OJ, Göbel K, Bien CG, Meuth SG, Wiendl H. Rasmussen encephalitis treated with natalizumab. Neurology. 2013;81:395–397. doi: 10.1212/WNL.0b013e31829c5ceb. [DOI] [PubMed] [Google Scholar]
- 35.Armangue T, Petit-Pedrol M, Dalmau J. Autoimmune encephalitis in children. J Child Neurol. 2012;27:1460–1469. doi: 10.1177/0883073812448838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers SW, Andrews PI, Gahring LC, et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen's encephalitis. Science. 1994;265:648–651. doi: 10.1126/science.8036512. [DOI] [PubMed] [Google Scholar]
- 37.Wiendl H, Bien CG, Bernasconi P, et al. GluR3 antibodies: prevalence in focal epilepsy but no specificity for Rasmussen's encephalitis. Neurology. 2001;57:1511–1514. doi: 10.1212/wnl.57.8.1511. [DOI] [PubMed] [Google Scholar]
- 38.Yang R, Puranam RS, Butler LS, et al. Autoimmunity to munc-18 in Rasmussen's encephalitis. Neuron. 2000;28:375–383. doi: 10.1016/s0896-6273(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 39.Vincent A, Irani SR, Lang B. The growing recognition of immunotherapy-responsive seizure disorders with autoantibodies to specific neuronal proteins. Curr Opin Neurol. 2010;23:144–150. doi: 10.1097/WCO.0b013e32833735fe. [DOI] [PubMed] [Google Scholar]
- 40.Greiner H, Leach JL, Lee K-H, Krueger DA. Anti-NMDA receptor encephalitis presenting with imaging findings and clinical features mimicking Rasmussen syndrome. Seizure. 2011;20:266–270. doi: 10.1016/j.seizure.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Granata T, Fusco L, Gobbi G, et al. Experience with immunomodulatory treatments in Rasmussen’s encephalitis. Neurology. 2003;61:1807–1810. doi: 10.1212/01.wnl.0000099074.04539.e0. [DOI] [PubMed] [Google Scholar]
- 42.Laxer KD, Wilfong A, Morris III G, Andermann F. Pilot study of Rituximab to treat chronic focal encepahlitis. American Epilepsy Society Annual Conference 2008, Abst 1.277.
- 43.Thilo B, Stingele R, Knudsen K, et al. A case of Rasmussen encephalitis treated with rituximab. Nat Rev Neurol. 2009;5:458–462. doi: 10.1038/nrneurol.2009.98. [DOI] [PubMed] [Google Scholar]
- 44.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Ann Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 45.Vezzani A, Balosso S, Ravizza T. Inflammation and epilepsy. Handbook Clin Neurol. 2012;107:163–175. doi: 10.1016/B978-0-444-52898-8.00010-0. [DOI] [PubMed] [Google Scholar]
- 46.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ransohoff RM, Stevens B. Neuroscience. How many cell types does it take to wire a brain? Science. 2011;333:1391–1392. doi: 10.1126/science.1212112. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Tsai V, Parker WE, Aronica E, Baybis M, Crino PB. Detection of human papillomavirus in human focal cortical dysplasia type IIB. Ann Neurol. 2012;72:881–892. doi: 10.1002/ana.23795. [DOI] [PubMed] [Google Scholar]
- 50.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bauer J, Elger CE, Hans VH, et al. Astrocytes are a specific immunological target in Rasmussen's encephalitis. Ann Neurol. 2007;62:67–80. doi: 10.1002/ana.21148. [DOI] [PubMed] [Google Scholar]
- 52.Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen T. Further observations on the syndrome of chronic encephalitis and epilepsy. Appl Neurophysiol. 1978;41:1–12. doi: 10.1159/000102395. [DOI] [PubMed] [Google Scholar]
- 54.Walter GF, Renella RR. Epstein-Barr virus in brain and Rasmussen's encephalitis. Lancet. 1989;1:279–280. doi: 10.1016/s0140-6736(89)91292-0. [DOI] [PubMed] [Google Scholar]
- 55.Power C, Poland S, Blume W, Girvin J, Rice G. Cytomegalovirus and Rasmussen's encephalitis. Lancet. 1990;336:1282–1284. doi: 10.1016/0140-6736(90)92965-k. [DOI] [PubMed] [Google Scholar]
- 56.Vinters HV, Wang R, Wiley CA. Herpesviruses in chronic encephalitis associated with intractable childhood epilepsy. Human Pathol. 1993;24:871–879. doi: 10.1016/0046-8177(93)90137-6. [DOI] [PubMed] [Google Scholar]
- 57.Jay V, Becker LE, Otsubo H, et al. Chronic encephalitis and epilepsy (Rasmussen's encephalitis): detection of cytomegalovirus and herpes simplex virus 1 by the polymerase chain reaction and in situ hybridization. Neurology. 1995;45:108–117. doi: 10.1212/wnl.45.1.108. [DOI] [PubMed] [Google Scholar]
- 58.Atkins MR, Terrell W, Hulette CM. Rasmussen's syndrome: a study of potential viral etiology. Clin Neuropathol. 1995;14:7–12. [PubMed] [Google Scholar]
- 59.Ohmori I, Ouchida M, Kobayashi K, et al. Rasmussen encephalitis associated with SCN 1 A mutation. Epilepsia. 2008;49:521–526. doi: 10.1111/j.1528-1167.2007.01411.x. [DOI] [PubMed] [Google Scholar]
- 60.Alvarez-Baron E, Bien CG, Schramm J, Elger CE, Becker AJ, Schoch S. Autoantibodies to Munc18, cerebral plasma cells and B-lymphocytes in Rasmussen encephalitis. Epilepsy Res. 2008;80:93–97. doi: 10.1016/j.eplepsyres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Deprez L, Weckhuysen S, Holmgren P, et al. Clinical spectrum of early-onset epileptic encephalopathies associated with STXBP1 mutations. Neurology. 2010;75:1159–1165. doi: 10.1212/WNL.0b013e3181f4d7bf. [DOI] [PubMed] [Google Scholar]
- 62.Weckhuysen S, Holmgren P, Hendrickx R, et al. Reduction of seizure frequency after epilepsy surgery in a patient with STXBP1 encephalopathy and clinical description of six novel mutation carriers. Epilepsia. 2013;54:e74–e80. doi: 10.1111/epi.12124. [DOI] [PubMed] [Google Scholar]
- 63.Hart YM, Andermann F, Robitaille Y, Laxer KD, Rasmussen T, Davis R. Double pathology in Rasmussen's syndrome: a window on the etiology? Neurology. 1998;50:731–735. doi: 10.1212/wnl.50.3.731. [DOI] [PubMed] [Google Scholar]
- 64.Takei H, Wilfong A, Malphrus A, et al. Dual pathology in Rasmussen's encephalitis: a study of seven cases and review of the literature. Neuropathology. 2010;30:381–391. doi: 10.1111/j.1440-1789.2009.01079.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang D, Blumcke I, Gui Q, et al. Clinico-pathological investigations of Rasmussen encephalitis suggest multifocal disease progression and associated focal cortical dysplasia. Epileptic Disord. 2013;15:32–43. doi: 10.1684/epd.2013.0555. [DOI] [PubMed] [Google Scholar]
- 66.Vining EP, Freeman JM, Pillas DJ, et al. Why would you remove half a brain? The outcome of 58 children after hemispherectomy-the Johns Hopkins experience: 1968 to 1996. Pediatrics. 1997;100:163–171. doi: 10.1542/peds.100.2.163. [DOI] [PubMed] [Google Scholar]
- 67.Dulac O. What is West syndrome? Brain Dev. 2001;23:447–452. doi: 10.1016/s0387-7604(01)00268-6. [DOI] [PubMed] [Google Scholar]
- 68.Hrachovy RA. West's syndrome (infantile spasms). Clinical description and diagnosis. Adv Exp Med Biol. 2002;497:33–50. [PubMed] [Google Scholar]
- 69.West WJ. On a peculiar form of infantile convulsions. Lancet. 1841;35:724–725. [Google Scholar]
- 70.Mackay MT, Weiss SK, Adams-Webber T, et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology. 2004;62:1668–1681. doi: 10.1212/01.wnl.0000127773.72699.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Go CY, Mackay MT, Weiss SK, et al. Evidence-based guideline update: medical treatment of infantile spasms. Report of the Guideline Development Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2012;78:1974–1980. doi: 10.1212/WNL.0b013e318259e2cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mytinger JR, Joshi S. The current evaluation and treatment of infantile spasms among members of the Child Neurology Society. J Child Neurol. 2012;27:1289–1294. doi: 10.1177/0883073812455692. [DOI] [PubMed] [Google Scholar]
- 73.Osborne JP, Lux AL, Edwards SW, et al. The underlying etiology of infantile spasms (West syndrome): information from the United Kingdom Infantile Spasms Study (UKISS) on contemporary causes and their classification. Epilepsia. 2010;51:2168–2174. doi: 10.1111/j.1528-1167.2010.02695.x. [DOI] [PubMed] [Google Scholar]
- 74.Mefford HC, Yendle SC, Hsu C, et al. Rare copy number variants are an important cause of epileptic encephalopathies. Ann Neurol. 2011;70:974–985. doi: 10.1002/ana.22645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Friocourt G, Parnavelas JG. Mutations in ARX result in several defects involving GABAergic neurons. Front Cell Neurosci. 2010;4:4. doi: 10.3389/fncel.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beguin S, Crepel V, Aniksztejn L, et al. An epilepsy-related ARX polyalanine expansion modifies glutamatergic neurons excitability and morphology without affecting GABAergic neurons development. Cereb Cortex. 2013;23:1484–1494. doi: 10.1093/cercor/bhs138. [DOI] [PubMed] [Google Scholar]
- 77.Olivetti PR, Noebels JL. Interneuron, interrupted: molecular pathogenesis of ARX mutations and X-linked infantile spasms. Curr Opin Neurobiol. 2012;22:859–865. doi: 10.1016/j.conb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Price MG, Yoo JW, Burgess DL, et al. A triplet repeat expansion genetic mouse model of infantile spasms syndrome, Arx(GCG)10+7, with interneuronopathy, spasms in infancy, persistent seizures, and adult cognitive and behavioral impairment. J Neurosci. 2009;29:8752–8763. doi: 10.1523/JNEUROSCI.0915-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marsh E, Fulp C, Gomez E, et al. Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain. 2009;132:1563–1576. doi: 10.1093/brain/awp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galanopoulou AS. Basic mechanisms of catastrophic epilepsy—overview from animal models. Brain Dev. 2013;35:748–756. doi: 10.1016/j.braindev.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toonen RF, Wierda K, Sons MS, et al. Munc18-1 expression levels control synapse recovery by regulating readily releasable pool size. Proc Natl Acad Sci U S A. 2006;103:18332–18337. doi: 10.1073/pnas.0608507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nabbout R, Melki I, Gerbaka B, Dulac O, Akatcherian C. Infantile spasms in Down syndrome: good response to a short course of vigabatrin. Epilepsia. 2001;42:1580–1583. doi: 10.1046/j.1528-1157.2001.13501.x. [DOI] [PubMed] [Google Scholar]
- 83.Lujic L, Bosnjak VM, Delin S, Duranovic V, Krakar G. Infantile spasms in children with Down syndrome. Coll Antropol. 2011;35(Suppl. 1):213–218. [PubMed] [Google Scholar]
- 84.Sanmaneechai O, Sogawa Y, Silver W, Ballaban-Gil K, Moshe SL, Shinnar S. Treatment outcomes of West syndrome in infants with Down syndrome. Pediatr Neurol. 2013;48:42–47. doi: 10.1016/j.pediatrneurol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Goldberg-Stern H, Strawsburg RH, Patterson B, et al. Seizure frequency and characteristics in children with Down syndrome. Brain Dev. 2001;23:375–378. doi: 10.1016/s0387-7604(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 86.Cortez MA, Shen L, Wu Y, et al. Infantile spasms and Down syndrome: a new animal model. Pediatr Res. 2009;65:499–503. doi: 10.1203/PDR.0b013e31819d9076. [DOI] [PubMed] [Google Scholar]
- 87.Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sidenvall R, Eeg-Olofsson O. Epidemiology of infantile spasms in Sweden. Epilepsia. 1995;36:572–574. doi: 10.1111/j.1528-1157.1995.tb02569.x. [DOI] [PubMed] [Google Scholar]
- 89.Curatolo P, Seri S, Verdecchia M, Bombardieri R. Infantile spasms in tuberous sclerosis complex. Brain Dev. 2001;23:502–507. doi: 10.1016/s0387-7604(01)00300-x. [DOI] [PubMed] [Google Scholar]
- 90.Riikonen R. Epidemiological data of West syndrome in Finland. Brain Dev. 2001;23:539–541. doi: 10.1016/s0387-7604(01)00263-7. [DOI] [PubMed] [Google Scholar]
- 91.Karvelas G, Lortie A, Scantlebury MH, Duy PT, Cossette P, Carmant L. A retrospective study on aetiology based outcome of infantile spasms. Seizure. 2009;18:197–201. doi: 10.1016/j.seizure.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. 2010;14:146–149. doi: 10.1016/j.ejpn.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Cross JH. Neurocutaneous syndromes and epilepsy-issues in diagnosis and management. Epilepsia. 2005;46(Suppl. 10):17–23. doi: 10.1111/j.1528-1167.2005.00353.x. [DOI] [PubMed] [Google Scholar]
- 94.Poduri A, Evrony GD, Cai X, et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron. 2012;74:41–48. doi: 10.1016/j.neuron.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baek ST, Gibbs EM, Gleeson JG, Mathern GW. Hemimegalencephaly, a paradigm for somatic postzygotic neurodevelopmental disorders. Curr Opin Neurol. 2013;26:122–127. doi: 10.1097/WCO.0b013e32835ef373. [DOI] [PubMed] [Google Scholar]
- 96.Lee JH, Huynh M, Silhavy JL, et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Orlova KA, Parker WE, Heuer GG, et al. STRADalpha deficiency results in aberrant mTORC1 signaling during corticogenesis in humans and mice. J Clin Invest. 2010;120:1591–1602. doi: 10.1172/JCI41592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parker WE, Orlova KA, Parker WH, et al. Rapamycin prevents seizures after depletion of STRADA in a rare neurodevelopmental disorder. Sc Transl Med. 2013;5:182ra53. doi: 10.1126/scitranslmed.3005271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Puffenberger EG, Strauss KA, Ramsey KE, et al. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130:1929–1941. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- 100.Becker AJ, Urbach H, Scheffler B, et al. Focal cortical dysplasia of Taylor's balloon cell type: mutational analysis of the TSC1 gene indicates a pathogenic relationship to tuberous sclerosis. Ann Neurol. 2002;52:29–37. doi: 10.1002/ana.10251. [DOI] [PubMed] [Google Scholar]
- 101.Baybis M, Yu J, Lee A, et al. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol. 2004;56:478–487. doi: 10.1002/ana.20211. [DOI] [PubMed] [Google Scholar]
- 102.Miyata H, Chiang AC, Vinters HV. Insulin signaling pathways in cortical dysplasia and TSC-tubers: tissue microarray analysis. Ann Neurol. 2004;56:510–519. doi: 10.1002/ana.20234. [DOI] [PubMed] [Google Scholar]
- 103.Lu Z, Hu X, Li Y, et al. Human papillomavirus 16 E6 oncoprotein interferences with insulin signaling pathway by binding to tuberin. J Biol Chem. 2004;279:35664–35670. doi: 10.1074/jbc.M403385200. [DOI] [PubMed] [Google Scholar]
- 104.Spangle JM, Munger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol. 2010;84:9398–9407. doi: 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43:322–329. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shiihara T, Miyashita M, Yoshizumi M, Watanabe M, Yamada Y, Kato M. Peripheral lymphocyte subset and serum cytokine profiles of patients with West syndrome. Brain Dev. 2010;32:695–702. doi: 10.1016/j.braindev.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Haginoya K, Noguchi R, Zhao Y, et al. Reduced levels of interleukin-1 receptor antagonist in the cerebrospinal fluid in patients with West syndrome. Epilepsy Res. 2009;85:314–317. doi: 10.1016/j.eplepsyres.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 108.Yamanaka G, Kawashima H, Oana S, et al. Increased level of serum interleukin-1 receptor antagonist subsequent to resolution of clinical symptoms in patients with West syndrome. J Neurol Sci. 2010;298:106–109. doi: 10.1016/j.jns.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 109.Liu ZS, Wang QW, Wang FL, Yang LZ. Serum cytokine levels are altered in patients with West syndrome. Brain Dev. 2001;23:548–551. doi: 10.1016/s0387-7604(01)00313-8. [DOI] [PubMed] [Google Scholar]
- 110.Tekgul H, Polat M, Tosun A, Serdaroglu G, Kutukculer N, Gokben S. Cerebrospinal fluid interleukin-6 levels in patients with West syndrome. Brain Dev. 2006;28:19–23. doi: 10.1016/j.braindev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 111.Scantlebury MH, Galanopoulou AS, Chudomelova L, Raffo E, Betancourth D, Moshe SL. A model of symptomatic infantile spasms syndrome. Neurobiol Dis. 2010;37:604–612. doi: 10.1016/j.nbd.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ono T, Briggs SW, Chudomelova L, Raffo E, Moshé SL, Galanopoulou AS. Intracortical injection of lipopolysaccharide induces epileptic spasms in neonatal rats: another animal model of symptomatic infantile spasms. Epilepsia. 2012;12:300. [Google Scholar]
- 113.Riikonen R. Infantile spasms: infectious disorders. Neuropediatrics. 1993;24:274–280. doi: 10.1055/s-2008-1071556. [DOI] [PubMed] [Google Scholar]
- 114.Ohtaki E, Yamaguchi Y, Shiotsuki Y, et al. A case with infantile spasms due to herpes simplex type 1 virus encephalitis. No To Hattatsu. 1987;19:502–506. [PubMed] [Google Scholar]
- 115.Hackney JR, Harrison DK, Rozzelle C, Kankirawatana S, Kankirawatana P, Palmer CA. Chronic granulomatous herpes encephalitis in a child with clinically intractable epilepsy. Case Rep Pediatr. 2012;2012:849812. doi: 10.1155/2012/849812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hattori H. Spontaneous remission of spasms in West syndrome—implications of viral infection. Brain Dev. 2001;23:705–707. doi: 10.1016/s0387-7604(01)00278-9. [DOI] [PubMed] [Google Scholar]
- 117.Ono J, Imai K, Tanaka-Taya K, Kurahashi H, Okada S. Decreased frequency of seizures in infantile spasms associated with lissencephaly by human herpes virus 7 infection. Pediatr Int. 2002;44:168–170. doi: 10.1046/j.1328-8067.2001.01511.x. [DOI] [PubMed] [Google Scholar]
- 118.Bonkowsky JL, Filloux FM, Byington CL. Herpes simplex virus central nervous system relapse during treatment of infantile spasms with corticotropin. Pediatrics. 2006;117:e1045–8. doi: 10.1542/peds.2005-1867. [DOI] [PubMed] [Google Scholar]
- 119.Mota NG, Rezkallah-Iwasso MT, Peracoli MT, Montelli TC. Demonstration of antibody and cellular immune response to brain extract in West and Lennox-Gastaut syndromes. Arq Neuropsiquiatr. 1984;42:126–131. doi: 10.1590/s0004-282x1984000200004. [DOI] [PubMed] [Google Scholar]
- 120.Suleiman J, Brenner T, Gill D, Troedson C, Sinclair AJ, Brilot F, et al. Immune-mediated steroid-responsive epileptic spasms and epileptic encephalopathy associated with VGKC-complex antibodies. Dev Med Child Neurol. 2011;53:1058–1060. doi: 10.1111/j.1469-8749.2011.04096.x. [DOI] [PubMed] [Google Scholar]
- 121.Steele SU, Cheah SM, Veerapandiyan A, Gallentine W, Smith EC, Mikati MA. Electroencephalographic and seizure manifestations in two patients with folate receptor autoimmune antibody-mediated primary cerebral folate deficiency. Epilepsy Behav. 2012;24:507–512. doi: 10.1016/j.yebeh.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 122.Frost JD, Jr, Hrachovy RA. Pathogenesis of infantile spasms: a model based on developmental desynchronization. J Clin Neurophysiol. 2005;22:25–36. doi: 10.1097/01.wnp.0000149893.12678.44. [DOI] [PubMed] [Google Scholar]
- 123.Lee CL, Frost JD, Jr, Swann JW, Hrachovy RA. A new animal model of infantile spasms with unprovoked persistent seizures. Epilepsia. 2008;49:298–307. doi: 10.1111/j.1528-1167.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 124.Mares P, Velisek L. N-methyl-D-aspartate (NMDA)-induced seizures in developing rats. Brain Res Develop Brain Res. 1992;65:185–189. doi: 10.1016/0165-3806(92)90178-y. [DOI] [PubMed] [Google Scholar]
- 125.Kabova R, Liptakova S, Slamberova R, Pometlova M, Velisek L. Age-specific N-methyl-D-aspartate-induced seizures: perspectives for the West syndrome model. Epilepsia. 1999;40:1357–1369. doi: 10.1111/j.1528-1157.1999.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 126.Kabova R, Veresova S, Velisek L. West syndrome model: seek and you will find. Sb Lek. 1997;98:115–126. [PubMed] [Google Scholar]
- 127.Wang YJ, Zhang Y, Liang XH, Yang G, Zou LP. Effects of adrenal dysfunction and high-dose adrenocorticotropic hormone on NMDA-induced spasm seizures in young Wistar rats. Epilepsy Res. 2012;100:125–131. doi: 10.1016/j.eplepsyres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 128.Velisek L, Jehle K, Asche S, Veliskova J. Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann Neurol. 2007;61:109–119. doi: 10.1002/ana.21082. [DOI] [PubMed] [Google Scholar]
- 129.Baram TZ. Pathophysiology of massive infantile spasms: perspective on the putative role of the brain adrenal axis. Ann Neurol. 1993;33:231–236. doi: 10.1002/ana.410330302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baram TZ, Schultz L. Corticotropin-releasing hormone is a rapid and potent convulsant in the infant rat. Brain Res Develop Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baram TZ, Schultz L. ACTH does not control neonatal seizures induced by administration of exogenous corticotropin-releasing hormone. Epilepsia. 1995;36:174–178. doi: 10.1111/j.1528-1157.1995.tb00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yum MS, Chachua T, Veliskova J, Velisek L. Prenatal stress promotes development of spasms in infant rats. Epilepsia. 2012;53:e46–e49. doi: 10.1111/j.1528-1167.2011.03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lado FA, Moshe SL. Role of subcortical structures in the pathogenesis of infantile spasms: what are possible subcortical mediators? Int Rev Neurobiol. 2002;49:115–140. doi: 10.1016/s0074-7742(02)49010-1. [DOI] [PubMed] [Google Scholar]
- 134.Briggs SW, Mowrey W, Hall CB, Galanopoulou AS. CPP-115, a vigabatrin analogue, suppresses spasms in the multiple-hit rat model of infantile spasms. Epilepsia. 2014;55:94–102. doi: 10.1111/epi.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ono T, Moshe SL, Galanopoulou AS. Carisbamate acutely suppresses spasms in a rat model of symptomatic infantile spasms. Epilepsia. 2011;52:1678–1684. doi: 10.1111/j.1528-1167.2011.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jequier Gygax M, Klein BD, White HS, Kim M, Galanopoulou AS. Efficacy and tolerability of the galanin analog NAX 5055 in the multiple-hit rat model of symptomatic infantile spasms. Epilepsy Res. 2014;108:98–108. doi: 10.1016/j.eplepsyres.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Baalen A, Hausler M, Boor R, Rohr A, Sperner J, Kurlemann G, et al. Febrile infection-related epilepsy syndrome (FIRES): a nonencephalitic encephalopathy in childhood. Epilepsia. 2010;51:1323–1328. doi: 10.1111/j.1528-1167.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 138.Mikaeloff Y, Jambaque I, Hertz-Pannier L, et al. Devastating epileptic encephalopathy in school-aged children (DESC): a pseudo encephalitis. Epilepsy Res. 2006;69:67–79. doi: 10.1016/j.eplepsyres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 139.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 140.Nabbout R, Vezzani A, Dulac O, Chiron C. Acute encephalopathy with inflammation-mediated status epilepticus. Lancet Neurol. 2011;10:99–108. doi: 10.1016/S1474-4422(10)70214-3. [DOI] [PubMed] [Google Scholar]
- 141.Kramer U, Chi CS, Lin KL, et al. Febrile infection-related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: a multicenter study on 77 children. Epilepsia. 2011;52:1956–1965. doi: 10.1111/j.1528-1167.2011.03250.x. [DOI] [PubMed] [Google Scholar]
- 142.Kramer U, Chi CS, Lin KL, et al. Febrile infection-related epilepsy syndrome (FIRES): does duration of anesthesia affect outcome? Epilepsia. 2011;52(Suppl. 8):28–30. doi: 10.1111/j.1528-1167.2011.03230.x. [DOI] [PubMed] [Google Scholar]
- 143.Nabbout R, Mazzuca M, Hubert P, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51:2033–2037. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 144.Villeneuve N, Pinton F, Bahi-Buisson N, Dulac O, Chiron C, Nabbout R. The ketogenic diet improves recently worsened focal epilepsy. Dev Med Child Neurol. 2009;51:276–281. doi: 10.1111/j.1469-8749.2008.03216.x. [DOI] [PubMed] [Google Scholar]
- 145.Mazzuca M, Jambaque I, Hertz-Pannier L, et al. 18F-FDG PET reveals frontotemporal dysfunction in children with fever-induced refractory epileptic encephalopathy. J Nucl Med. 2011;52:40–47. doi: 10.2967/jnumed.110.077214. [DOI] [PubMed] [Google Scholar]
- 146.Bindoff LA, Engelsen BA. Mitochondrial diseases and epilepsy. Epilepsia. 2012;53(Suppl. 4):92–97. doi: 10.1111/j.1528-1167.2012.03618.x. [DOI] [PubMed] [Google Scholar]
- 147.Appenzeller S, Helbig I, Stephani U, et al. Febrile infection-related epilepsy syndrome (FIRES) is not caused by SCN1A, POLG, PCDH19 mutations or rare copy number variations. Dev Med Child Neurol. 2012;54:1144–1148. doi: 10.1111/j.1469-8749.2012.04435.x. [DOI] [PubMed] [Google Scholar]
- 148.Depienne C, Bouteiller D, Keren B, et al. Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females. PLoS Genet. 2009;5:e1000381. doi: 10.1371/journal.pgen.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barcia G, Fleming MR, Deligniere A, et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat Genet. 2012;44:1255–1259. doi: 10.1038/ng.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Helbig I, Mefford HC, Sharp AJ, et al. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet. 2009;41:160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol. 2005;95:71–102. [PubMed] [Google Scholar]
- 152.Specchio N, Fusco L, Claps D, Vigevano F. Epileptic encephalopathy in children possibly related to immune-mediated pathogenesis. Brain Develop. 2010;32:51–56. doi: 10.1016/j.braindev.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 153.Specchio N, Fusco L, Vigevano F. Acute-onset epilepsy triggered by fever mimicking FIRES (febrile infection-related epilepsy syndrome): the role of protocadherin 19 (PCDH19) gene mutation. Epilepsia. 2011;52:e172–5. doi: 10.1111/j.1528-1167.2011.03193.x. [DOI] [PubMed] [Google Scholar]
- 154.Carranza Rojo D, Hamiwka L, McMahon JM, et al. De novo SCN1A mutations in migrating partial seizures of infancy. Neurology. 2011;77:380–383. doi: 10.1212/WNL.0b013e318227046d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Baalen A, Hausler M, Plecko-Startinig B, et al. Febrile infection-related epilepsy syndrome without detectable autoantibodies and response to immunotherapy: a case series and discussion of epileptogenesis in FIRES. Neuropediatrics. 2012;43:209–216. doi: 10.1055/s-0032-1323848. [DOI] [PubMed] [Google Scholar]
- 156.Ravizza T, Kostoula C, Vezzani A. Immunity activation in brain cells in epilepsy: mechanistic insights and pathological consequences. Neuropediatrics. 2013;44:330–335. doi: 10.1055/s-0033-1358601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 511 kb)