Abstract
In patients being evaluated for epilepsy and in animal models of epilepsy, electrophysiological recordings are carried to capture seizures to determine the existence of epilepsy. Electroencephalography recordings from the scalp, or sometimes directly from the brain, are also used to locate brain areas where seizure begins, and in surgical treatment help plan the area for resection. As seizures are unpredictable and can occur infrequently, ictal recordings are not ideal in terms of time, cost, or risk when, for example, determining the efficacy of existing or new anti-seizure drugs, evaluating potential anti-epileptogenic interventions, or for prolonged intracerebral electrode studies. Thus, there is a need to identify and validate other electrophysiological biomarkers of epilepsy that could be used to diagnose, treat, cure, and prevent epilepsy. Electroencephalography recordings in the epileptic brain contain other interictal electrophysiological disturbances that can occur more frequently than seizures, such as interictal spikes (IIS) and sharp waves, and from invasive studies using wide bandwidth recording and small diameter electrodes, the discovery of pathological high-frequency oscillations (HFOs) and microseizures. Of IIS, HFOs, and microseizures, a significant amount of recent research has focused on HFOs in the pathophysiology of epilepsy. Results from studies in animals with epilepsy and presurgical patients have consistently found a strong association between HFOs and epileptogenic brain tissue that suggest HFOs could be a potential biomarker of epileptogenicity and epileptogenesis. Here, we discuss several aspects of HFOs, as well as IIS and microseizures, and the evidence that supports their role as biomarkers of epilepsy.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-014-0259-0) contains supplementary material, which is available to authorized users.
Keywords: High frequency oscillation, Interictal spike, Microseizure, Epileptogenicity, Epileptogenesis, Ictogenesis
Introduction
The spontaneous occurrence of an epileptic seizure is the defining feature of the epileptic brain. In patients being evaluated for epilepsy and animal models of epilepsy, electrophysiological recordings are regularly carried out to capture seizures to identify the existence of epilepsy, identify a specific type of epileptic seizure, or epilepsy syndrome. In most cases, recordings are made from the scalp, but can also be obtained directly from brain parenchyma as in some presurgical diagnostic studies when subdural or depth electrode recordings are needed to localize the brain area responsible for generating spontaneous seizures. Electrophysiological recordings like these are also being used to identify biomarkers of epilepsy that are needed to prevent the development of epilepsy (“epileptogenesis”), and reverse the progression and ultimately cure epilepsy after it is established [1, 2].
Epileptic seizures themselves are a specific biomarker of epileptogenesis and epileptogenicity (the presence and severity of an epileptic condition), although owing to their unpredictable nature and irregular rate of occurrence, ictal electroencephalography (EEG) recordings are not ideal in terms of time, cost, or risk for evaluating, for example, the efficacy of existing or new anti-seizure drugs, potential anti-epileptogenic interventions, or presurgical evaluation. However, EEG recordings in individuals with epilepsy contain other transient electrophysiological disturbances that occur between ictal episodes that can occur more frequently than seizures, such as interictal EEG spikes and sharp waves (IIS), pathological high-frequency oscillations (HFOs) between 80 and 600 Hz [3, 4], and, more recently, electrographic seizure-like events and focal periodic epileptiform discharges occurring on submillimeter spatial scales termed “microseizures” and “micro-periodic epileptiform discharges” respectively [5, 6]. HFOs and microseizures were discovered using wide bandwidth recordings from small diameter electrodes or microelectrodes. Intracerebral recording such as these have helped accelerate technical improvements in clinical systems—electrodes, amplifiers, data digitization—that are now being incorporated more routinely into diagnostic presurgical evaluation, and that have expanded the spatial and temporal scales for studying abnormal brain electrical activity [7, 8].
Interictal EEG spikes occur in the epileptic brain and can be useful diagnostically [9], although IIS can be detected in the EEG of a small percentage of healthy volunteers and relatively larger percentage of patients without a history of seizures [10]. In addition, patient studies found that IIS do not consistently correspond with disease activity (e.g., occurrence in relation to seizures or changes in anti-seizure medication levels) [11–15] . However, recent work in animals has identified unique aspects of IIS that correspond with experimental epileptogenesis [16–18]. Similarly detailed studies like these on the mechanisms of IIS are needed that might distinguish different types of IIS involved with ictogenesis and epileptogenesis [19–21].
For over a decade basic research of HFOs in animal models of epilepsy and presurgical patients has consistently found a strong association between pathological HFOs and epileptogenic brain tissue. Based on evidence from retrospective studies, pathological HFOs could be a biomarker of epileptogenicity that would not only assist in localization of epileptogenic tissue for resective surgery [22], but help distinguish between epileptic and non-epileptic seizures, and reduce the cost and time of clinical trials of anti-seizure drugs and therapeutic devices [23]. As a biomarker of epileptogenesis, pathological HFOs could help identify individuals at risk for developing epilepsy after an epileptogenic insult and could be useful in the design of animal screening models for discovery of new anti-seizure and anti-epileptogenic drugs [23]. However, significant work remains to be done in order for HFOs (and similarly for IIS and microseizures) to fulfill their potential as biomarkers, including identifying strategies to reliably distinguish pathological from normal HFOs in the human epileptic brain [23–25]; developing non-invasive methods to record HFOs, which are now best detected using intracerebral EEG recording; efficient and accurate detection methods and automated tools for unbiased quantification of HFOs in wide bandwidth recordings across patients [8]; and, ultimately, prospective studies that can incorporate individual patient HFO data in planning the area for resection and follow patients to determine postsurgical seizure freedom [26]. Here we discuss HFOs, microseizures, and IIS, although a significant portion of this review focuses on HFOs based on the recent number of studies supporting their potential role as an electrophysiological biomarker of epilepsy.
HFOs
Terminology
A formal definition of HFOs and electromagnetic oscillations described in terms of a central spectral frequency that can extend from < 1 Hz to over 1000 Hz does not exist. EEG nomenclature and frequency band limits are often subject to individual interpretation, but EEG oscillations are generally labeled as slow (< 1 Hz), delta (1–4 Hz), theta (> 4–8 Hz), alpha and mu (> 8–13 Hz), beta (> 13–30 Hz), gamma (> 30–80 Hz), ripple (> 80–200 Hz), fast ripple (> 200–600), and sigma (≥ 600 Hz). Consistent with recommendations to standardize the description of HFOs [27], much HFO research in the epileptic brain has focused on transient, sinusoid-like events that contain spectral power between 80 and 600 Hz (Fig. 1), and occur spontaneously in the hippocampal formation and neocortex during the onset of some seizures, but most frequently during interictal episodes, primarily during slow wave sleep or waking quiescence when rates of HFOs are highest [28–30]. Discussion of the studies that follow include descriptive details to continue the effort to distinguish the likely different types of HFOs, as well as their mechanisms and function, in the normal and epileptic mammalian brain [23].
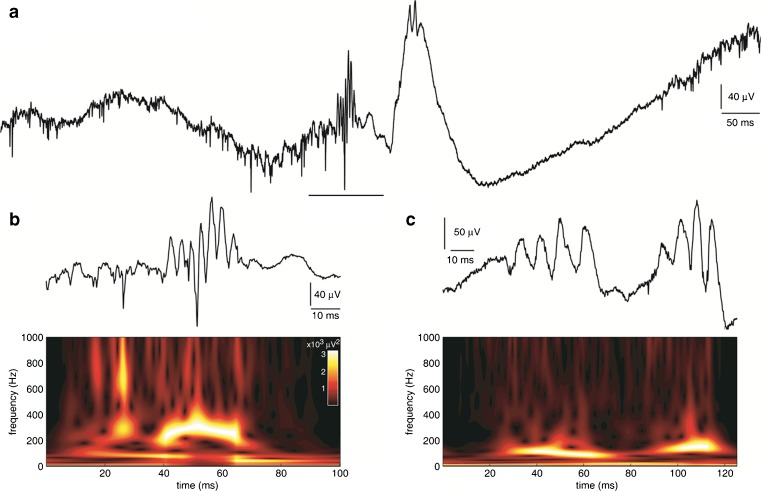
Fig. 1.
(A) Interictal wide bandwidth (1 Hz–3 kHz) depth electroencephalography (EEG) recorded from a microelectrode positioned in entorhinal cortex of a presurgical patient with suspected temporal lobe epilepsy. Neuronal spike firing and higher amplitude fast ripple-frequency high-frequency oscillation [HFO; underlined segment, shown in (B)] precedes interictal EEG spike that also contains HFO activity. (B) Example of fast ripple-frequency HFO (top) and corresponding time-frequency spectrogram (bottom). Note predominant spectral power between 200 and 400 Hz (color-coded white) during HFO and vertical streaks (yellow and red) extending towards higher frequencies that likely reflect power associated with neuronal spike firing. (C) Examples of ripple-frequency HFO (top trace) recorded from microelectrode positioned in anterior hippocampus of another patient. Spectral power largely between 80 and 200 Hz during HFOs (bottom spectrogram)
Neuronal Mechanisms of HFOs
Several reviews detail the experimental evidence for the single neuron and network basis generating HFOs [27, 31–33], and there could be important differences in the involvement of inhibitory processes between normal and pathological HFOs. In the normal mammalian brain, local inhibitory cells appear to play a prominent role in physiological HFOs. During CA1 hippocampal sharp wave-associated ripples [34], as well as neocortical ripple-frequency HFOs [28] and sensory-evoked fast ripple-frequency HFOs [35], spike firing from various types of interneurons (e.g., hippocampal basket cells and neocortical fast spiking cells) regularly occurs during the trough of the extracellularly-recorded HFO [28, 36–39]. It is believed that synchronous gamma-aminobutyric acid (GABA)-A receptor-mediated inhibitory postsynaptic potentials (IPSPs) imposed upon principal cells (i.e., in hippocampus, pyramidal cells; in neocortex, regular spiking, intrinsically bursting, and fast rhythmic bursting cells) provides precise temporal windows that regulate their firing and coordinate excitatory synaptic transmission onto postsynaptic targets. The tightly-orchestrated pattern of firing between interneurons and principal cells during normal HFOs has been implicated with encoding information [40], sensorimotor integration [41], and memory consolidation [42].
In contrast to HFOs in the normal mammalian brain, in the epileptic hippocampal formation, fast ripple-frequency HFOs reflect brief bursts of population spikes believed to be generated from a cluster of pathologically interconnected neurons (PIN cluster) [4, 43, 44]. Other studies observed bursts of populations spikes with ripple-frequency spectral power in the epileptic dentate gyrus, an area that does not normally generate such events in the naïve rat, which led to the term “pathological” HFOs to include bursts of population spikes within ripple- and fast ripple-frequency bands in the epileptic hippocampal formation [24, 44, 45]. In vitro results generally agree with in vivo data, which found that recurrent excitatory connections among CA3 pyramidal cells can generate synchronous firing and bursts of population spikes [46]. Recordings in dentate gyrus and hippocampus revealed that pathological HFOs persist after suppression of GABA-A receptor-mediated transmission [47, 48], while another study found that significant increases in principal cell spike firing and reduced interneuron firing coincide with spontaneous population spike in dentate gyrus of pilocarpine-treated epileptic mice [49]. In addition, neuronal recordings in neocortex of cats found that the precise spike firing from fast spiking cells in relation to the extracellular HFO waveform was lost during abnormal ripple-frequency HFOs compared with normal HFOs [28, 50], yet the spike firing from these cells, as well as pyramidal cells, significantly increased during abnormal compared with normal HFOs. Results from these studies suggest a reduced role of interneuron-mediated IPSPs in coordinating neuronal spike firing during some hippocampal and neocortical pathological HFOs, although it is not clear from these data whether inhibitory postsynaptic currents (IPSCs) are a significant source of the extracellular pathological HFO field potential. Results from one study recording from neocortical slices bathed in low Mg2+ medium provide evidence when IPSCs could significantly contribute to the pathological HFO [51]. In this work, under conditions when pyramidal cell firing was suppressed by strong inhibitory activity, as might occur during interictal or pre-ictal episodes, synchronous IPSCs onto principal cells were strongly correlated with the local HFO field potential. However, when inhibitory restraint weakened and principal cell spike firing increased (e.g., during onset and propagation of ictal discharges) the correlation between IPSCs and HFO field potential was significantly lower, suggesting there are periods when IPSCs could be a source of pathological HFOs compared with other times when excitatory postsynaptic currents predominate in the local HFO field potential.
Synchronous principal cell firing, and in some cases interneuron firing as noted in the preceding paragraph, could explain pathological HFOs with central frequencies up to 300 Hz [46, 48, 52], yet in patients there is little evidence that single neurons fire at frequencies greater than 300 Hz and thus it is more difficult for this neuronal mechanism to explain the occurrence of fast-ripple frequency HFOs up to 600 Hz [53–55]. Recent computational models provide evidence for the role of axonal gap junctions during fast ripple-frequency HFOs, as well as ripple-frequency and ripple-to-fast ripple transition [56]. Others have proposed that fast ripple-frequency HFOs emerge from the out-of-phase firing between small groups of neurons with individual neurons discharging at low frequencies and rarely firing during successive waves of the HFO [48, 52, 57]. Results from a recent modeling study of fast ripple-frequency HFOs in a hippocampal CA1 network are consistent with this proposal [58], which seemingly better explains the precision of spike firing, as well as the frequency spectra of pathological HFO [32, 46, 48, 52]. Interestingly, the study by Demont-Guignard et al. [58] also demonstrated that, when simulated, afferent input from CA3 onto the CA1 network became more synchronous when the number of synchronously firing CA1 pyramidal cells and interneurons increased producing a large amplitude IIS instead of fast ripple-frequency HFO. It is possible that the cellular and network parameters used to model the transition from pathological HFOs to IIS in this latter work could also explain in vivo recordings that contain IIS superimposed with HFOs and those without HFOs. How out-of-phase firing between groups of neurons (PIN clusters) arises is not known, but weak ephaptic field effects, neuron loss, circuit reorganization, or irregular spread of activity throughout neuronal networks could contribute to the differential spatial activation of local clusters of neurons [32, 59].
Recording, Detection, and Quantification of Spontaneous HFOs
Wider bandwidth and higher sampling rates now available on many clinical EEG recording systems have facilitated studies of HFOs. An important component in any electrophysiological recording setup is the electrode, and whether there exists an optimal electrode contact size and configuration to record HFOs is not yet known owing to variability in the amount of tissue supporting their generation. Some of the first studies of ripple- and fast ripple-frequency HFOs in the epileptic brain used small diameter microelectrodes (~40–60 μm; area, 0.0013–0.0029 mm2) that indicated ≤ 1 mm3 of tissue could generate pathological HFOs [3, 4, 60]. Interestingly, studies using simulated neuronal networks consisting of ≥ 1000 neurons, but also as few as 120, can generate HFOs that contain spectral frequencies, duration, and amplitude similar to HFOs recorded experimentally [52, 58]. This is considerably fewer neurons than what would be expected based on in vivo studies, but might reflect a theoretical minimum that could generate HFOs under ideal network parameters, connectivity, and recording conditions. Subsequent patient studies using larger diameter electrodes than microelectrodes (surface area up 7.2 mm2) confirmed and extended the spatiotemporal properties of HFOs, including results that suggested some pathological HFO-generating networks could cover several square centimeters [61–64]. One study found a significant reduction in the number of fast ripple-frequency HFOs recorded with standard clinical electrodes (surface area 9.4 mm2) compared with microelectrodes [65]. In contrast, other experiments compared hippocampal ripple- and fast ripple-frequency HFOs recorded with electrodes of different contact sizes in epileptic rats and found no difference in HFO duration or spectral frequency with respect to electrode diameter, although the mean amplitude of all HFOs was significantly lower compared with the amplitude of HFOs recorded with microelectrodes [4, 66]. In subsequent work involving patients from this same group, similar rates of HFOs were found among electrodes with surface areas between 0.2 and 5 mm2 [67], suggesting that smaller contact diameter would not necessarily improve the detection of HFOs. If this conclusion is correct, then differences between the aforementioned studies could be due to the position of the electrodes in relation to location and size of tissue generating HFOs or the spatial averaging of uncorrelated HFO activity between distant PIN clusters that attenuates or cancels activity occurring at higher frequencies [58]. In addition, recent work has found that choice of electrode (e.g. high impedance microelectrode) in combination with amplifier input impedance significantly affects signal-to-noise levels that could contribute to unreliable detection of HFOs [68].
Animal and patient studies of HFOs are complicated by the fact that HFOs are typically brief in duration (10–100 ms) and lower in amplitude (10–1000 μV) than background EEG and other epileptiform phenomena [4]. Manual detection of HFOs, which is the gold standard of EEG review and the method often used in studies of HFOs, is time-intensive and subjective. A manual strategy imposes practical limitations on the amount of clinical EEG that can be reasonably reviewed. There is some evidence that accurate detection of HFOs can be achieved in short-duration EEG recordings (e.g., 5 mins during slow wave sleep) [69], although the rate of HFO occurrence can be quite variable over time, particularly during peri-ictal periods and with changes in anti-seizure medication [70, 71]. Therefore, the EEG sample for review must be selected carefully. Furthermore, scalp and intracranial EEG recordings contain physiological and epileptiform sharp transients and often artifacts (electrode noise, eye-, and muscle-related activity) that contain high frequency power, and digital filtering of these events could be incorrectly interpreted as HFOs [72, 73]. Studies have implemented supervised and unsupervised computer-automated algorithms, some that incorporate aspects other than spectral power to improve specificity [63, 74–77] and one for specific types of HFOs [78]. While all detection strategies thus far have demonstrated strengths and weaknesses, a feasible and accurate approach will likely include a combination of computer-automated methods to process large data sets and manual inspection of a randomly selected sample of HFOs by experienced investigators.
One of the anticipated clinical uses of interictal pathological HFOs is to localize the brain area responsible for generating epileptic seizures, and is currently supported by evidence from retrospective studies described in the following sections. In these studies, the significant association between interictal fast ripple- and, in some cases, ripple-frequency HFOs and the seizure onset zone (SOZ) derive from group-averaged statistics. However, their use in presurgical evaluation will require quantification in an individual patient owing to variability in the properties of HFOs, and differences in spatial sampling and recording electrodes between patients. While this methodology still needs to be developed, it is likely that any patient-based quantitative approach will need to consider the type of HFO, and possibly anatomical location, and use a statistically-derived threshold to identify significant HFO-generating sites. One retrospective study involving surgical pediatric patients used an approach that calculated rates of ripple- and fast ripple-frequency HFOs in combination with histogram and bootstrapping analysis to define a threshold to identify high-rate HFO sites [64]. In addition to an important result that found more complete resection of high-rate fast ripple-frequency HFO sites was associated with better surgical outcome, the novel patient-oriented HFO quantification used in this study could be appropriate for prospective studies of HFOs in presurgical evaluation.
Microseizures, HFOs, and Ictogenesis
Microseizures
Microseizures are a newly described electrophysiological phenomenon in the study of epilepsy [5, 79]. They are defined as interictal seizure-like local field potential events whose spatial extent is restricted to approximately the width of a neocortical column (~500 μm) [80]. The restricted radius of these phenomena makes their detection only possible with intracranial microwire (diameter < 100 μm) recordings, being inaccessible to standard intracranial macroelectrodes (typical diameter ≥ 4 mm). Microseizures are interictal events by definition, a practical restriction necessitated by the fact that seizure-like discharges occurring on microwires during a seizure are an established and expected phenomenon. On rare occasions, patients may report auras in association with microseizure events, but, in general, they are purely subclinical events. Microseizures frequently evolve in frequency and occasionally in spatial extent. Their frequency content is similar to that for macroelectrode seizures recorded intracranially. A related phenomenon, microperiodic epileptiform discharges (μPEDs), have similar restrictions in spatial extent, but morphologically resemble the macroelectrode phenomenon periodic lateralized epileptiform discharges that are nonspecifically associated with neural tissue damage [81]. μPEDs tend to continue for much longer periods of time than microseizures, and tend not to evolve in frequency.
Microseizures and μPEDs both spatially localize to the SOZ, though at this time the statistics are more robust for microseizures [5]. Spatially, microseizure domains display a fractured arrangement such that their occurrence is much more frequent in the brain regions generating seizures, but they are not contiguous and are surrounded by microdomains generating only normal-appearing physiology (interictally). Microseizures or μPEDs are seen leading directly into clinical seizures as much as 20 % of the time. Whether the phenomenon is more ubiquitous and has been missed owing to relatively sparse spatial sampling is a matter of ongoing investigation. Current data do not support the utility of microseizures as a clinical marker of impending seizure (seizure predictors), as their occurrence prior to seizure, while statistically significantly, is below the level of clinical utility. It is conceivable this may change with higher spatial sampling in the future.
Microseizures have been observed in multiple species (human, rat, pig), in multiple pathologies (mesial temporal sclerosis, trauma, tumor, cortical dysplasia), and even rarely in non-epileptic human controls. One hypothesis is that microseizures represent a final common physiological pathway in ictogenesis [5]. If this is validated, the concept that clinical seizures emerge without a physiological precursor can finally be put to rest, and equating the question of the mechanism of ictogenesis to that of the mechanisms underlying microseizures. This would be a better position for the field, as tools exist to investigate this question, but the neuronal networks underlying microseizure generation are unknown. However, based on their restricted spatial extent and the fact that they are local field potentials, one hypothesis is that the microseizure network is one of intracolumnar feedback. A single cortical column is a network of 103–104 neurons that is known to support sustained independent oscillatory activity [82]. In archicortex, such as hippocampus, which lacks the columnar organization of neocortex, PIN clusters described by Bragin et al. [43], may support the same function. Epileptogenesis, the process by which normal brain regions become susceptible to generating unprovoked seizures, may have as its anatomical substrate the accumulation of PIN clusters or “sick columns” generating microseizures [5, 43].
Ictal HFOs
Studies of ictal HFOs can also address basic mechanisms of ictogenesis and have important clinical implications, particularly if the presence of HFOs at ictal onset reflects the proximity of electrode to tissue responsible for generating the seizure and, therefore, more accurate localization of the epileptogenic zone (see the section “HFOs and Surgical Outcome”). Results from animal studies suggest that different neuronal mechanisms are associated with HFOs that precede or occur during ictal onset. For example, depth electrodes positioned in the dentate gyrus of epileptic rats found during the ictal onset consisting of rhythmic, large amplitude EEG spikes, often referred to as a hypersynchronous ictal EEG onset pattern, there was an increase in ripple- and fast ripple-frequency HFO power and duration that presumably reflects the expansion of individual PIN clusters (i.e., predominately principal cell spike firing; see the section “Neuronal Mechanisms of HFOs”) and their progressive coalescence [83]. A subsequent study by another group using pilocarpine-treated epileptic rats observed, during hypersynchronous ictal onsets, significantly higher rates of fast ripple- than ripple frequency-HFO predominately in the CA3 SOZ, as well as adjacent areas of seizure spread [84]. By contrast, another type of ictal EEG onset pattern termed low voltage fast was associated with a significant increase in HFOs between beta- and ripple-frequencies, but not fast ripple-frequency HFO [83, 84]. Interestingly, recordings in an isolated whole brain preparation found that the beta-frequency HFO during seizure onset in entorhinal cortex was associated largely with interneuron spike firing, but IPSPs progressively declined, and principal cell spike firing increased during the evolution of the seizure [85]. In neocortex of normal cats during ketamine-induced neocortical polyspike and wave seizures, larger ictal ripple-frequency HFO amplitude correlated with neuron depolarization and increased spike firing [50]. Pyramidal cell-like firing was time-locked with the ictal ripple-frequency HFO, but not fast spiking cells (presumably local GABAergic interneurons), which are typically temporally coupled with the trough of normal ripple-frequency HFOs during slow wave sleep.
A number of patient studies have detected HFOs or found an increase in HFO power, generally between beta- and fast ripple-frequencies, during or preceding ictal onset by several minutes to seconds (Fig. 2) [62, 86–89]. Changes in HFO power were chiefly detected in the primary SOZ and rarely in sites of secondarily generalization of patients with focal epilepsy [90]. Similar changes in HFOs have been observed during epileptic spasms that, in some cases, occurred before the clinical onset, and were observed spreading across cortex and encompassing large cortical territories [91–95]. None of the aforementioned patient studies examined different types of HFO in relation to ictal EEG onset pattern, which is an area of research that could yield electrophysiological biomarkers [2]. Furthermore, one study did not find a consistent change (i.e., increase) in HFO power or rates during the transition to ictus [96], although total rates of ripple- and fast ripple-frequency pre-ictal HFOs (≤ 15 mins to ictal onset) were highest inside compared with outside the SOZ, similar to the results of interictal HFOs in relation to the SOZ discussed in the next section. This latter study raises uncertainty on the reliability of HFOs in predicting seizures, while overall results from animal and patients studies suggest that pre-ictal HFOs could reflect only one of the many different mechanisms of ictogenesis.
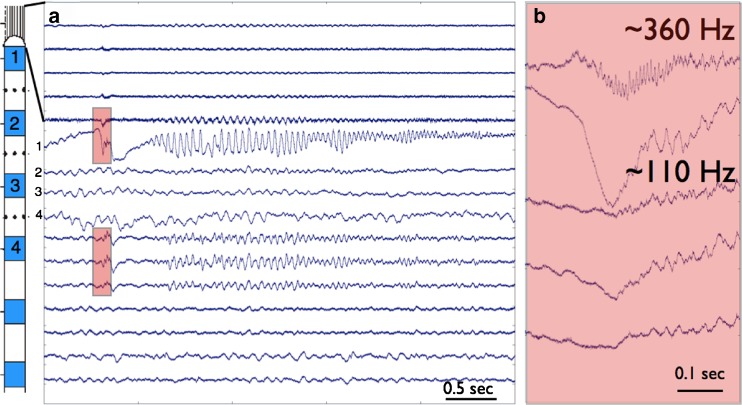
Fig. 2.
Wide bandwidth recording from hybrid depth implanted in human hippocampus. (A) The upper 5 tracings are from microwires protruding from the tip of the clinical depth electrode. The middle 4 tracings are from the numbered clinical macroelectrodes, and the 7 bottom tracings from microwires embedded in the shaft of the clinical depth between the clinical macroelectrodes. The seizure onset is associated with a paroxysmal sharp wave and associated ripple frequency (~110 Hz) discharge on the clinical macroelectrode and a fast ripple frequency (~360 Hz) discharge on the tip microwire (highlighted in red). (B) Expanded scale showing the highlighted channels in (A). The top trace is a microwire extending from the tip of the clinical depth, and at seizure onset shows a fast ripple HFO (~360 Hz) coinciding with the sharp wave seen on the clinical macroelectrode contact-1 in the second trace. Note the microwires are referenced to a local microwire and only show an attenuated version of the paroyxsmal slow wave seen at onset on the clinical macroelectrode that has a distant reference. The lower 3 traces are from the shaft microwires embedded between macroelectrodes 1 and 2 (shown as black dots on the schematic)
HFOs and Epileptogenicity
Interictal HFOs
A potential advantage of interictal HFOs as a biomarker of epileptogenicity for presurgical evaluation or assessing efficacy of therapeutic interventions is that such studies can be done without waiting for seizures to occur. However, to be useful as a biomarker, evidence is needed that interictal HFOs localize to epileptogenic brain tissue and their occurrence is associated with disease activity or severity of epilepsy. The initial studies of interictal HFOs in patients with mesial temporal lobe epilepsy (MTLE) found a strong association between microelectrode-recorded fast ripple-, but not ripple-, frequency HFOs and the SOZ [3, 4], results that were consistent with subsequent quantitative studies [29, 74]. In vitro and in vivo microelectrode recordings noted the limited volume of tissue (as small as 1 mm3) capable of supporting fast ripple-frequency HFOs [60, 97]. Studies relating interictal HFOs and pathology of MTLE found higher rates of fast ripple-frequency and lower rates of ripple-frequency HFOs correlated with hippocampal atrophy and reduced neuron densities [74, 98, 99]. These data suggest that morphological alterations associated with hippocampal sclerosis in MTLE could be an anatomical substrate for hippocampal fast ripple-frequency HFOs, but it also suggests that hippocampal damage could disrupt the generation of normal ripple-frequency HFOs.
A number of noteworthy results derive from studies using larger diameter electrodes and commercial clinical electrodes. For example, ripple- and fast ripple-frequency HFOs could be recorded using clinical macroelectrode, and the volume of tissue and/or spatial distribution associated with fast-ripple frequency HFOs was larger than predicted from microelectrode recordings [65, 66, 100]. The link between the rates of fast ripple-frequency HFOs and SOZ was also confirmed [65, 101, 102], and from these studies evidence for the association of ripple-frequency HFOs and the SOZ in MTLE and neocortical epilepsies was obtained, although fast ripple-frequency HFOs appear more specific to the SOZ, particularly in MTLE [63]. In addition, HFOs were observed in epileptogenic tissue extending beyond areas of pathology in other lesional epilepsies [102], and another study found HFOs in the SOZ of magnetic resonance imaging (MRI)-negative patients [103], although histological analysis of resected tissue indicated gliosis and neuron loss in many of these patients.
Multiple studies have reported that gamma-frequency HFOs [89] and ripple-frequency HFOs [14, 65, 101] are also increased in the SOZ. Differences in the association of ripple-frequency HFOs with the SOZ between micro- and macroelectrode studies reflect an outstanding issue of reliably distinguishing normal from pathological HFOs. It is likely that some of the ripple-frequency HFOs localizing with the SOZ are similar to pathological ripple-frequency HFOs found in the epileptic dentate gyrus [45]. Some have suggested a bias of larger-diameter electrodes to sample a larger volume of tissue and capture pathological (ripple-frequency) HFOs versus normal HFO [23, 63, 67]. Alternatively, recent work has described prolonged (> 500 ms in duration) episodes of high-frequency activity (80–250 Hz) in mesial temporal lobe and neocortex of presurgical patients [104, 105]. Continuous episodes of interictal high-frequency activity itself does not appear specific to the SOZ, but compared with irregular or sporadic episodes was associated with higher rates of IIS and bursts of HFOs, which could, in part, explain the higher rates of ripple-frequency HFO occurrence on larger electrodes. A recent study characterized physiological HFO by measuring frequency, duration, and power of HFO induced by a visual or motor task in single trial time frequency spectra [25]. These putative physiological HFOs were compared with putative pathological HFOs primarily associated with epileptiform sharp waves. Pathological HFOs were primarily characterized by higher mean power and longer duration than physiologically-induced HFOs. In individual patients, a support vector machine analysis correctly classified pathological HFOs with high sensitivities and specificities, with the exception of one patient with infrequent high power HFO arising in epileptic motor cortex just before movement onset [25].
Several of the studies mentioned in the previous paragraph also noted a strong spatial and temporal association between HFOs and IIS [61, 101]. A large percentage of IIS occur independently of HFOs and vice versa, although some IIS do contain HFOs that are not visible in wide bandwidth recordings unless the signal is filtered or the spectral power extracted using statistical time–frequency analysis [61, 106]. While prior work had found that IIS can extend beyond the SOZ depending on state of vigilance [107–109], recent evidence indicates that IIS containing HFOs, as well as HFOs alone, particularly ripple-frequency HFOs [110], more accurately localize to the SOZ than IIS alone [101, 111]. The mechanisms that give rise to IIS containing HFOs are not known, but studies have found important differences between IIS and HFOs associated with disease activity and cortical excitability that suggest the pathophysiological mechanisms are different. One study observed that after seizures rates of IIS increased, whereas rates of HFOs, as well as IIS containing HFOs, did not change [70]. In the same study, the rate and duration of HFOs increased after a reduction in medication, although there was no statistical change in rates of IIS alone or IIS containing HFOs. Another study by the same group found that the rate of HFOs increased from interictal to pre-ictal to ictal episodes, and electrodes from which HFOs were recorded remained consistent across these episodes [112]. In contrast, IIS increased from interictal (but not pre-ictal) to ictal episodes and were recorded on different electrodes during ictal compared to interictal episodes. Finally, high rates of HFOs correlated with lower threshold of electrical stimulation (ES)-evoked responses inside the SOZ, although in neocortex outside the SOZ high rates of fast ripple-frequency HFOs were also associated with lower thresholds of ES-evoked response [113]. The association between IIS, ES-evoked response, and SOZ was not evaluated in the latter study, but overall results suggest HFOs reflect levels of neuronal excitability inside the SOZ and possibly areas of epileptogenicity beyond where seizures begin.
Studies in epileptic rats found that a higher number of electrodes recording fast ripple-frequency HFOs correlated with seizure frequency [114], indicating that fast ripple-frequency HFOs could correspond with severity of epilepsy. One patient study did not find a correlation between seizure frequency and rates or proportion of electrodes with ripple- or fast ripple-frequency HFOs [112]. However, in the same study, higher seizure frequency correlated with a greater number of electrodes with high (> 20/min) rates of fast ripple-frequency HFOs in patients with temporal lobe epilepsy. Another study found that higher rates of fast ripple-, but not ripple-, frequency HFOs correlated with local areas of hippocampal atrophy in patients with MTLE [99], suggesting that higher rates and broader spatial distribution of fast ripple-frequency HFOs could reflect disease burden in some cases of temporal lobe epilepsy.
The preponderance of work on HFOs in epileptic animals and patients used direct brain recordings and, in spite of the advantages interictal HFO recordings might possess in terms of time, cost, and possibly accuracy for presurgical evaluation, it does not obviate the risk associated with invasive recording. Moreover, it is clear invasive recording is an obstacle for the use of HFOs as a biomarker of epileptogenesis. However, recent work has detected spontaneous ripple-frequency HFOs associated with spikes in scalp EEG from children with continuous spike and wave during slow wave sleep [115]. Two subsequent studies detected gamma- and ripple-frequency HFOs in the scalp EEG of adults with epilepsy [116, 117]. In these studies, HFOs were often associated with IIS, but some occurred independently of IIS, and rates of HFO and IIS were higher inside than outside the SOZ. Importantly, compared with IIS, the specificity and accuracy of HFO in localizing with the SOZ was higher, although sensitivity was lower. Results from these preliminary studies are exciting and raise the possibility that other non-invasive modalities, such as magnetoencephalography or functional MRI, either independently or in combination with EEG recordings, can capture HFO signals and advance studies of HFOs in patients at risk for developing epilepsy or those newly diagnosed with epilepsy.
HFOs and Surgical Outcome
Several retrospective studies that have followed up on patients after resective surgery found that removal of tissue generating interictal pathological HFOs correlated with a good outcome, that is, becoming seizure-free or having a significant reduction in disabling seizures (Table 1) [64, 111, 119–121]. One study using single pulse electrical stimulation found, in 2 patients, that complete or near complete removal of tissue that contained evoked fast ripple-frequency HFOs was associated with a seizure-free outcome, whereas in 7 patients who had removal of < 50 % of sites that contained evoked fast ripple-frequency HFOs, only 2 were seizure-free, while the remaining 5 patients continued to have seizures [122]. Additionally, studies of ictal or peri-ictal HFOs found, in most cases, a larger proportion of patients who had ictal HFOs had better postsurgical outcome than patients who did not have ictal HFOs [91, 95, 123, 124], but some did not find an association between fast ripple-frequency HFOs and outcome [125]. With respect to ictal HFO studies, if the area of seizure onset was completely removed in all patients, then the surgery outcome results suggest that in those patients who continued to have seizures, recording sites without ictal HFO could be distant from where seizures actually began.
Table 1.
Association between high-frequency oscillations (HFOs) and postsurgical outcome
| Study | Patients/n | Type of recording/seizures | Surgery | HFOs | Association w/ outcome | Outcomes, n (follow-up) |
|---|---|---|---|---|---|---|
| Ochi et al. [91] | Children/9 | Subdural/epileptic spasms | Focal, multi-lobe resection | Ictal R | R with seizure-free | IA, 4 vs IB-IVB, 5 (17) |
| RamachandranNair et al. [94]* | Children/5 | Subdural, depth/epileptic spasms | Focal resection | Ictal HFO (80–250 Hz) | Undetermined | I, 3 (22) III, 2 (15) |
| Nariai et al. [95] | Children/11 | Subdural/epileptic spasms | Focal resection, lobectomy | Ictal R, FR (210–300 Hz), β, γ | R with good outcome | I-IC, 6 (22) vs II, 5 (14) |
| Modur et al. [126]* | Adults/6 | Subdural, depth/focal, GTC | Focal, multi-lobe resection | Ictal HFO (70–300 Hz) | HFO with good outcome | I, 3 (22) II, 2 (27) III, 1 (38) |
| Fujiwara et al. [124] | Children/44 | Subdural | Focal, multi-lobe resection | Ictal HFOs (80–150, 150–300, 300–500 Hz) | HFO with seizure-free outcome | Full removal: I, 18 vs II-IV, 4 Partial removal: I, 4 vs II-IV, 15 |
| Park et al. [118] | Adults/23 | Subdural, depth/focal | Focal resection | Ictal high γ (60–99 Hz) | High γ with seizure-free | IA, 6 (39) vs IB-IV, 9 (28) |
| Usui et al. [125] | Adults/19 | Depth/focal | Focal resection | Ictal FR (200–300 Hz) | No correlation between FR and outcome | Sites with FR: I, 6 (31) vs II-IIIA, 3 (18) Sites without FR: I, 5 (37) vs II-IIIA, 3 (38) |
| Jacobs et al. [111] | Adults/20 | Depth | Focal resection | Interictal R, FR | R and FR with good outcome | I-II, 8 (20) vs III-IV, 12 (24) |
| Wu et al. [119] | Children/30 | ECoG intraoperative | Focal resection, hemispherectomy | Interictal FR | FR with seizure-free outcome | I, 19 vs II-IV, 5 |
| Akiyama et al. [64] | Children/28 | Subdural, depth/focal, GTC, epileptic spasms | Focal resection, lobectomy | Interictal R, FR | FR with seizure-free outcome | IA, 10 vs IB-II, 18 (> 24) |
| van’t Klooster et al. [122] | Children, Adults/13 | Subdural | Focal resection | SPES-evoked R, FR | Evoked FR with good outcome | > 94 % FR sites removed: I, 2 < 50 % FR sites removed: I, 2, and III-IV, 5 |
| Cho et al. [120] | Adults/7 | Subdural | Focal resection | Interictal R, FR | R and FR with good outcome | IA-B, 6 (7) vs IIIA, 1 (8) |
| Haegelen et al. [121] | Adults/37 | Subdural, depth | Focal resection | Interictal R, FR | R and FR with good outcome (TLE) | I-II, 9 (36) vs III-IV,12 (17) |
GTC = generalized tonic-clonic seizure; ECoG = electrocorticography; R = ripple-frequency HFO; FR = fast ripple-frequency HFO; SPES = single pulse electrical stimulation; TLE = temporal lobe epilepsy
*Study considered HFO information in the surgical resection plan. Outcomes data (based on Engel Outcome Classification; numbers denote patients in each outcome group and, in parentheses, mean follow-up in months) reflect basis for association between HFOs and postsurgical outcome
To date, there appears to be only two studies that included ictal HFO information in surgical planning (Table 1). In one study that included 5 patients with epileptic spasms, site(s) of seizure onset with ictal HFOs (80–250 Hz), as well as location of MRI lesion, magnetoencephalography spike source dipole, and eloquent cortex, was(were) used to determine cortical area for resection [94]. Of these 5 patients, 3 were seizure-free at follow up between 14 and 29 months, 1 had > 90 % reduction in seizures at 12 months, and the remaining patient had a 50–75 % reduction in seizures at 18 months. In the other study involving 6 patients with drug-resistant neocortical seizures, the SOZ was determined from the combination of all channels with seizures that contain HFOs at onset (≥ 70 Hz) and resection area defined as the contiguous region consisting of the SOZ, 1 cm of surrounding cortex, and sites of early spread within 2 s of onset [126]. Of these 6 patients, 3 had Engel class I outcome between 20 and 25 months, 2 had class II outcome at 24 and 30 months, and the remaining patient had class III outcome at 38 months. Owing to small sample sizes and absence of comparison surgical groups, no conclusion can be drawn on the efficacy of incorporating HFOs in resective surgery planning. Nonetheless, results from these two studies are consistent with retrospective data reviewed in the previous paragraph and indicate the need to carry out careful, rigorous prospective studies of HFOs in epilepsy surgery.
IIS, HFOs, and Epileptogenesis
Owing, in part, to the technical challenges and time required to carry out prolonged EEG recordings and data analysis in behaving animals, there have been far fewer studies of HFOs during epileptogenesis than on HFOs and epileptogenicity. Studies of epileptogenesis are frequently performed in chronic models of epilepsy that use either chemical or electrical stimulation to induce an episode of status epilepticus, which is an epileptogenic insult in many animals. After a variable delay of days to weeks spontaneous seizures can appear that often progress in number or severity, or both [127]. In one such model using unilateral intrahippocampal kainic acid injection that reproduces many features of human temporal lobe epilepsy, depth electrode recordings during epileptogenesis found shorter latencies to the first appearance of pathological HFOs correlated with shorter latencies to first spontaneous seizure [45]. After seizures appeared, brain areas generating pathological HFOs were typically stable over time (days to months), and a greater number of electrodes recording fast ripple-frequency HFOs correlated with a higher daily rate of seizure occurrence [114].
To date, no studies have replicated these results, although one study using a systemically injected pilocarpine model found that a higher rate of IIS containing fast ripple-, but not ripple-, frequency HFOs in CA3 correlated with a higher daily rate of seizure occurrence in epileptic rats [128]. Interestingly, other animal studies of epileptogenesis have found that the appearance of IIS and subsequent changes in the pattern of occurrence and shape features of IIS, for example slow wave component, were associated with development of spontaneous seizures [16–18]. Furthermore, in vitro work and computation modeling indicate that a reduction in GABA-mediated dendritic inhibition could explain changes in IIS waveform morphology [18], which might also contribute to the hyperexcitability within a PIN cluster supporting pathological HFOs [60]. Collectively these data suggest that the occurrence of pathological HFOs and IIS, either alone or in combination with each other, could predict the development of seizures and possibly severity of an epileptic condition in chronic animal models. Clearly, much more experimental work in animals remains to be done, and patient studies could contribute to this body of work if preliminary work on scalp EEG-recorded HFOs and epileptogenicity are substantiated.
Conclusions and Future Perspective
The experimental animal and patient studies described here provide compelling evidence that supports HFOs as a biomarker of epileptogenic brain tissue. Recent studies identifying differences in IIS waveform morphology and an association between some IIS, with or without HFOs, and development of seizures will likely stimulate future research on IIS and epileptogenesis. While microseizures, as well as HFOs and IIS, could be useful in studies of ictogenesis. Many of the conclusions drawn from interictal and ictal studies of HFOs in relation to epileptogenicity and postsurgical outcome derive from group-averaged data between patients and electrodes. It is anticipated that improvements in electrode design and configuration, new strategies to differentiate normal from pathological HFOs and quantify the extent of HFOs on an individual patient basis will help clarify questions relating HFOs to epileptogenicity and help design prospective trials to evaluate HFO in planning resective surgery. Continued research on HFO using scalp EEG combined with intracerebral EEG, magnetoencephalography, or functional MRI should provide new information on the mechanisms and spatial distribution of networks supporting HFOs. Furthermore, non-invasive recordings would make it feasible to study HFOs in relation to disease activity and evaluate HFOs as a biomarker of epileptogenesis in patients at risk for developing epilepsy, as well as those newly diagnosed with epilepsy.
Electronic supplementary material
(PDF 1.19 mb)
(PDF 1.19 mb)
Acknowledgments
RJS is funded by NINDS NS R01-071048, MS NINDS NS R01-78136, and GAW NINDS NS R01-63039.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Pitkanen A. Therapeutic approaches to epileptogenesis—hope on the horizon. Epilepsia. 2010;51(Suppl. 3):2–17. doi: 10.1111/j.1528-1167.2010.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engel J, Jr, Pitkanen A, Loeb JA, et al. Epilepsy biomarkers. Epilepsia. 2013;54(Suppl. 4):61–69. doi: 10.1111/epi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bragin A, Engel J, Jr, Wilson CL, et al. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Bragin A, Engel J, Jr, Wilson CL, et al. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 5.Stead M, Bower M, Brinkmann BH, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–2797. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schevon CA, Goodman RR, McKhann G, Jr, Emerson RG. Propagation of epileptiform activity on a submillimeter scale. J Clin Neurophysiol. 2010;27:406–411. doi: 10.1097/WNP.0b013e3181fdf8a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanhatalo S, Voipio J, Kaila K. Full-band EEG (FbEEG): an emerging standard in electroencephalography. Clin Neurophysiol. 2005;116:1–8. doi: 10.1016/j.clinph.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Worrell GA, Jerbi K, Kobayashi K, et al. Recording and analysis techniques for high-frequency oscillations. Prog Neurobiol. 2012;98:265–278. doi: 10.1016/j.pneurobio.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirrell EC. Prognostic significance of interictal epileptiform discharges in newly diagnosed seizure disorders. J Clin Neurophysiol. 2010;27:239–248. doi: 10.1097/WNP.0b013e3181ea4288. [DOI] [PubMed] [Google Scholar]
- 10.So EL. Interictal epileptiform discharges in persons without a history of seizures: what do they mean? J Clin Neurophysiol. 2010;27:229–238. doi: 10.1097/WNP.0b013e3181ea42a4. [DOI] [PubMed] [Google Scholar]
- 11.Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann Neurol. 1985;17:597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- 12.Gotman J, Koffler DJ. Interictal spiking increases after seizures but does not after decrease in medication. Electroencephalogr Clin Neurophysiol. 1989;72:7–15. doi: 10.1016/0013-4694(89)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Spencer SS, Goncharova II, Duckrow RB, et al. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia. 2008;49:1881–1892. doi: 10.1111/j.1528-1167.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- 14.Worrell G, Gotman J. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomark Med. 2011;5:557–566. doi: 10.2217/bmm.11.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinhoff BJ, Scholly J, Dentel C, Staack AM. Is routine electroencephalography (EEG) a useful biomarker for pharmacoresistant epilepsy? Epilepsia. 2013;54(Suppl. 2):63–66. doi: 10.1111/epi.12187. [DOI] [PubMed] [Google Scholar]
- 16.White A, Williams PA, Hellier JL, et al. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia. 2010;51:371–383. doi: 10.1111/j.1528-1167.2009.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chauviere L, Doublet T, Ghestem A, et al. Changes in interictal spike features precede the onset of temporal lobe epilepsy. Ann Neurol. 2012;71:805–814. doi: 10.1002/ana.23549. [DOI] [PubMed] [Google Scholar]
- 18.Huneau C, Benquet P, Dieuset G, et al. Shape features of epileptic spikes are a marker of epileptogenesis in mice. Epilepsia. 2013;54:2219–2227. doi: 10.1111/epi.12406. [DOI] [PubMed] [Google Scholar]
- 19.Staley KJ, White A, Dudek FE. Interictal spikes: Harbingers or causes of epilepsy? Neurosci Lett. 2011;497:247–250. doi: 10.1016/j.neulet.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avoli M, de Curtis M, Kohling R. Does interictal synchronization influence ictogenesis? Neuropharmacology. 2013;69:37–44. doi: 10.1016/j.neuropharm.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staba RJ, Bragin A. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: underlying mechanisms. Biomark Med. 2011;5:545–556. doi: 10.2217/bmm.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs J, Staba R, Asano E, et al. High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol. 2012;98:302–315. doi: 10.1016/j.pneurobio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engel J, Jr, da Silva FL. High-frequency oscillations - where we are and where we need to go. Prog Neurobiol. 2012;98:316–318. doi: 10.1016/j.pneurobio.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engel J, Jr, Bragin A, Staba RJ, Mody I. High-frequency oscillations: What is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto A, Brinkmann BH, Stead SM, et al. Pathological and physiological high frequency oscillations in focal human epilepsy. J Neurophysiol. 2013;110:1958–1964. doi: 10.1152/jn.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gloss D, Nolan SJ, Staba R. The role of high-frequency oscillations in epilepsy surgery planning . Cochrane Database Syst Rev 2014, Issue 1. Art. No.: CD010235. doi:10.1002/14651858.CD010235.pub2 [DOI] [PMC free article] [PubMed]
- 27.Jefferys JG, de la Prida LM, Wendling F, et al. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol. 2012;98:250–64. doi: 10.1016/j.pneurobio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80-200 Hz) in neocortex and their neuronal correlates. J Neurophysiol. 2001;86:1884–1898. doi: 10.1152/jn.2001.86.4.1884. [DOI] [PubMed] [Google Scholar]
- 29.Staba RJ, Wilson CL, Bragin A, et al. High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- 30.Bagshaw AP, Jacobs J, LeVan P, et al. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2008;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bragin A, Engel J, Jr, Staba RJ. High-frequency oscillations in epileptic brain. Curr Opin Neurol. 2010;23:151–156. doi: 10.1097/WCO.0b013e3283373ac8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menendez de la Prida L, Trevelyan AJ. Cellular mechanisms of high frequency oscillations in epilepsy: On the diverse sources of pathological activities. Epilepsy Res. 2011;97:308–317. doi: 10.1016/j.eplepsyres.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Staba RJ. Normal and pathologic high-frequency oscillations. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies. 4th ed. Bethesda, MD. Oxford University Press, Inc. 2012;202-212.
- 34.Buzsaki G, Horvath Z, Urioste R, et al. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 35.Jones MS, Barth DS. Spatiotemporal organization of fast (>200 Hz) electrical oscillations in rat Vibrissa/Barrel cortex. J Neurophysiol. 1999;82:1599–1609. doi: 10.1152/jn.1999.82.3.1599. [DOI] [PubMed] [Google Scholar]
- 36.Csicsvari J, Hirase H, Czurko A, et al. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ylinen A, Bragin A, Nadasdy Z, et al. Sharp wave-associated high-frequency oscillation (200Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klausberger T, Magill PJ, Marton LF, et al. Brain-state and cell-type specific firing of hippocampal interneurons in vivo. Nature. 2003;42:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 39.Jones MS, MacDonald KD, Choi B, et al. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2000;84:1505–1518. doi: 10.1152/jn.2000.84.3.1505. [DOI] [PubMed] [Google Scholar]
- 40.Singer W. Synchronization of Cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- 41.Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A. 1992;89:5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buzsaki G. The Hippocampo-neortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 43.Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: A hypothesis. Epilepsia. 2000;41(Suppl. 6):S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 44.Bragin A, Wilson CL, Engel J., Jr Voltage depth profiles of high-frequency oscillations after kainic acid-induced status epilepticus. Epilepsia. 2007;48:35–40. doi: 10.1111/j.1528-1167.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- 45.Bragin A, Wilson CL, Almajano J, et al. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 46.Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci. 2004;24:8896–8906. doi: 10.1523/JNEUROSCI.3112-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Antuono M, de Guzman P, Kano T, Avoli M. Ripple activity in the dentate gyrus of disinhibited hippocampus-entorhinal cortex slices. J Neurosci Res. 2005;80:92–103. doi: 10.1002/jnr.20440. [DOI] [PubMed] [Google Scholar]
- 48.Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:930–941. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 49.Bragin A, Benassi SK, Kheiri F, Engel J., Jr Further evidence that pathologic high-frequency oscillations are bursts of population spikes derived from recordings of identified cells in dentate gyrus. Epilepsia. 2011;52:45–52. doi: 10.1111/j.1528-1167.2010.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80–200 Hz) during seizures: intracellular correlates. J Neurophysiol. 2003;89:841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- 51.Trevelyan AJ. The direct relationship between inhibitory currents and local field potentials. J Neurosci. 2009;29:15299–15307. doi: 10.1523/JNEUROSCI.2019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ibarz JM, Foffani G, Cid E, et al. Emergent dynamics of fast ripples in the epileptic hippocampus. J Neurosci. 2010;30:16249–16261. doi: 10.1523/JNEUROSCI.3357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colder BW, Wilson CL, Frysinger RC, et al. Interspike intervals during interictal periods in human temporal lobe epilepsy. Brain Res. 1996;719:96–103. doi: 10.1016/0006-8993(96)00107-2. [DOI] [PubMed] [Google Scholar]
- 54.Staba RJ, Wilson CL, Fried I, Engel J., Jr Single neuron burst firing in the human hippocampus during sleep. Hippocampus. 2002;12:724–734. doi: 10.1002/hipo.10026. [DOI] [PubMed] [Google Scholar]
- 55.Staba RJ, Wilson CL, Bragin A, et al. Sleep states differentiate single neuron activity recorded from human epileptic hippocampus, entorhinal cortex, and subiculum. J Neurosci. 2002;22:5694–5704. doi: 10.1523/JNEUROSCI.22-13-05694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon A, Traub RD, Vladimirov N, et al. Gap junction networks can generate both ripple-like and fast ripple-like oscillations. Eur J Neurosci. 2014;39:46–60. doi: 10.1111/ejn.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiruska P, Csicsvari J, Powell AD, et al. High-Frequency network activity, global increase in neuronal activity, and synchrony expansion precede epileptic seizures in vitro. J Neurosci. 2010;30:5690–5701. doi: 10.1523/JNEUROSCI.0535-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demont-Guignard S, Benquet P, Gerber U, et al. Distinct hyperexcitability mechanisms underlie fast ripples and epileptic spikes. Ann Neurol. 2012;71:342–352. doi: 10.1002/ana.22610. [DOI] [PubMed] [Google Scholar]
- 59.Kohling R, Staley K. Network mechanisms for fast ripple activity in epileptic tissue. Epilepsy Res. 2011;97:318–323. doi: 10.1016/j.eplepsyres.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100-500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- 62.Khosravani H, Mehrotra N, Rigby M, et al. Spatial localization and time-dependant changes of electrographic high frequency oscillations in human temporal lobe epilepsy. Epilepsia. 2008;50:605–616. doi: 10.1111/j.1528-1167.2008.01761.x. [DOI] [PubMed] [Google Scholar]
- 63.Crepon B, Navarro V, Hasboun D, et al. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133:33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- 64.Akiyama T, McCoy B, Go CY, et al. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia. 2011;52:1802–11. doi: 10.1111/j.1528-1167.2011.03199.x. [DOI] [PubMed] [Google Scholar]
- 65.Worrell GA, Gardner AB, Stead SM, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chatillon CE, Zelmann R, Bortel A, et al. Contact size does not affect high frequency oscillation detection in intracerebral EEG recordings in a rat epilepsy model. Clin Neurophysiol. 2011;122:1701–1705. doi: 10.1016/j.clinph.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chatillon CE, Zelmann R, Hall JA, et al. Influence of contact size on the detection of HFOs in human intracerebral EEG recordings. Clin Neurophysiol. 2013;124:1541–1546. doi: 10.1016/j.clinph.2013.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stacey WC, Kellis S, Greger B, et al. Potential for unreliable interpretation of EEG recorded with microelectrodes. Epilepsia. 2013;54:1391–1401. doi: 10.1111/epi.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zelmann R, Zijlmans M, Jacobs J, et al. Improving the identification of high frequency oscillations. Clin Neurophysiol. 2009;120:1457–1464. doi: 10.1016/j.clinph.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zijlmans M, Jacobs J, Zelmann R, et al. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pearce A, Wulsin D, Blanco JA, et al. Temporal changes of neocortical high-frequency oscillations in epilepsy. J Neurophysiol. 2013;110:1167–1179. doi: 10.1152/jn.01009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benar CG, Chauviere L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol. 2010;121:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 73.Worrell G. High-frequency oscillations recorded on scalp EEG. Epilepsy Curr. 2012;12:57–58. doi: 10.5698/1535-7511-12.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Staba RJ, Wilson CL, Bragin A, et al. Quantitative analysis of high-frequency oscillations (80-500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 75.Gardner AB, Worrell GA, Marsh E, et al. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin Neurophysiol. 2007;118:1134–1143. doi: 10.1016/j.clinph.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blanco JA, Stead M, Krieger A, et al. Data mining neocortical high-frequency oscillations in epilepsy and controls. Brain. 2011;134:2948–2959. doi: 10.1093/brain/awr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zelmann R, Mari F, Jacobs J, et al. A comparison between detectors of high frequency oscillations. Clin Neurophysiol. 2012;123:106–116. doi: 10.1016/j.clinph.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Birot G, Kachenoura A, Albera L, et al. Automatic detection of fast ripples. J Neurosci Methods. 2013;213:236–249. doi: 10.1016/j.jneumeth.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 79.Schevon CA, Ng SK, Cappell J, et al. Microphysiology of epileptiform activity in human neocortex. J Clin Neurophysiol. 2008;25:321–330. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mountcastle VB. An organization principle for cerebral function: the unit module and the distributed system. In: Petsche H, Hughes JR, editors. The mindful brain. Cambridge, MA: MIT Press; 1978. [Google Scholar]
- 81.Ebersole JS, Pedley TA. Current practice of clinical electroencephalography. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 82.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bragin A, Azizyan A, Almajano J, et al. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46:1592–1598. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 84.Levesque M, Salami P, Gotman J, Avoli M. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci. 2012;32:13264–13272. doi: 10.1523/JNEUROSCI.5086-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gnatkovsky V, Librizzi L, Trombin F, de Curtis M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol. 2008;64:674–686. doi: 10.1002/ana.21519. [DOI] [PubMed] [Google Scholar]
- 86.Fisher RS, Webber WR, Lesser RP, et al. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- 87.Allen PJ, Fish DR, Smith SJ. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1992;82:155–159. doi: 10.1016/0013-4694(92)90160-j. [DOI] [PubMed] [Google Scholar]
- 88.Traub RD, Whittington MA, Buhl EH, et al. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia. 2001;42:153–170. doi: 10.1046/j.1528-1157.2001.26900.x. [DOI] [PubMed] [Google Scholar]
- 89.Worrell GA, Parish L, Cranstoun SD, et al. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 90.Jirsch JD, Urrestarazu E, LeVan P, et al. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- 91.Ochi A, Otsubo H, Donner EJ, et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 92.Akiyama T, Otsubo H, Ochi A, et al. Focal cortical high-frequency oscillations trigger epileptic spasms: Confirmation by digital video subdural EEG. Clin Neurophysiol. 2005;116:2819–2825. doi: 10.1016/j.clinph.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 93.Akiyama T, Otsubo H, Ochi A, et al. Topographic Movie of ictal high-frequency oscillations on the brain surface using subdural EEG in neocortical epilepsy. Epilepsia. 2006;47:1953–1957. doi: 10.1111/j.1528-1167.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 94.RamachandranNair R, Ochi A, Imai K, et al. Epileptic spasms in older pediatric patients: MEG and ictal high-frequency oscillations suggest focal-onset seizures in a subset of epileptic spasms. Epilepsy Res. 2008;78:216–224. doi: 10.1016/j.eplepsyres.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 95.Nariai H, Nagasawa T, Juhasz C, et al. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011;52:63–74. doi: 10.1111/j.1528-1167.2010.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacobs J, Zelmann R, Jirsch J, et al. High frequency oscillations (80–500 Hz) in the preictal period in patients with focal seizures. Epilepsia. 2009;50:1780–1792. doi: 10.1111/j.1528-1167.2009.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bragin A, Wilson CL, Staba RJ, et al. Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 98.Staba RJ, Frighetto L, Behnke EJ, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 99.Ogren JA, Wilson CL, Bragin A, et al. Three dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66:783–791. doi: 10.1002/ana.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Urrestarazu E, Jirsch JD, Le Van P, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 101.Jacobs J, LeVan P, Chander R, et al. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jacobs J, Levan P, Chatillon CE, et al. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Andrade-Valenca L, Mari F, Jacobs J, et al. Interictal high frequency oscillations (HFOs) in patients with focal epilepsy and normal MRI. Clin Neurophysiol. 2012;123:100–105. doi: 10.1016/j.clinph.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mari F, Zelmann R, Andrade-Valenca L, et al. Continuous high-frequency activity in mesial temporal lobe structures. Epilepsia. 2012;53:797–806. doi: 10.1111/j.1528-1167.2012.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Melani F, Zelmann R, Mari F, Gotman J. Continuous high frequency activity: a peculiar SEEG pattern related to specific brain regions. Clin Neurophysiol. 2013;124:1507–1516. doi: 10.1016/j.clinph.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobayashi K, Jacobs J, Gotman J. Detection of changes of high-frequency activity by statistical time-frequency analysis in epileptic spikes. Clin Neurophysiol. 2009;120:1070–1077. doi: 10.1016/j.clinph.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wieser HG, Bancaud J, Talairach J, et al. Comparative value of spontaneous and chemically and electrically induced seizures in establishing the lateralization of temporal lobe seizures. Epilepsia. 1979;20:47–59. doi: 10.1111/j.1528-1157.1979.tb04775.x. [DOI] [PubMed] [Google Scholar]
- 108.Lieb JP, Joseph JP, Engel J, Jr, et al. Sleep state and seizure foci related to depth spike activity in patients with temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1980;49:538–557. doi: 10.1016/0013-4694(80)90396-x. [DOI] [PubMed] [Google Scholar]
- 109.Sammaritano M, Gigli GL, Gotman J. Interictal spiking during wakefulness and sleep and the localization of foci in temporal lobe epilepsy. Neurology. 1991;41:290–297. doi: 10.1212/wnl.41.2_part_1.290. [DOI] [PubMed] [Google Scholar]
- 110.Wang S, Wang IZ, Bulacio JC, et al. Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia. 2013;54:370–376. doi: 10.1111/j.1528-1167.2012.03721.x. [DOI] [PubMed] [Google Scholar]
- 111.Jacobs J, Zijlmans M, Zelmann R, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–220. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zijlmans M, Jacobs J, Kahn YU, et al. Ictal and interictal high frequency oscillations in patients with focal epilepsy. Clin Neurophysiol. 2011;122:664–671. doi: 10.1016/j.clinph.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jacobs J, Zijlmans M, Zelmann R, et al. Value of electrical stimulation and high frequency oscillations (80–500 Hz) in identifying epileptogenic areas during intracranial EEG recordings. Epilepsia. 2010;51:573–582. doi: 10.1111/j.1528-1167.2009.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bragin A, Wilson CL, Engel JJ. Spatial stability over time of brain areas generating fast ripples in the epileptic rat. Epilepsia. 2003;44:1233–1237. doi: 10.1046/j.1528-1157.2003.18503.x. [DOI] [PubMed] [Google Scholar]
- 115.Kobayashi K, Watanabe Y, Inoue T, et al. Scalp-recorded high-frequency oscillations in childhood sleep-induced electrical status epilepticus. Epilepsia. 2010;51:2190–2194. doi: 10.1111/j.1528-1167.2010.02565.x. [DOI] [PubMed] [Google Scholar]
- 116.Andrade-Valenca LP, Dubeau F, Mari F, et al. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011;77:524–531. doi: 10.1212/WNL.0b013e318228bee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Melani F, Zelmann R, Dubeau F, Gotman J. Occurrence of scalp-fast oscillations among patients with different spiking rate and their role as epileptogenicity marker. Epilepsy Res. 2013;106:345–356. doi: 10.1016/j.eplepsyres.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park SC, Lee SK, Che H, et al. Ictal high-gamma oscillation (60-99 Hz) in intracranial electroencephalography and postoperative seizure outcome in neocortical epilepsy. Clin Neurophysiol. 2012;123:1100-1110. [DOI] [PubMed]
- 119.Wu JY, Sankar R, Lerner JT, et al. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010;75:1686–1694. doi: 10.1212/WNL.0b013e3181fc27d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cho JR, Joo EY, Koo DL, et al. Clinical utility of interictal high-frequency oscillations recorded with subdural macroelectrodes in partial epilepsy. J Clin Neurol. 2012;8:22–34. doi: 10.3988/jcn.2012.8.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haegelen C, Perucca P, Chatillon CE, et al. High-frequency oscillations, extent of surgical resection, and surgical outcome in drug-resistant focal epilepsy. Epilepsia. 2013;54:848–857. doi: 10.1111/epi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van ’t Klooster MA, Zijlmans M, Leijten FS, et al. Time-frequency analysis of single pulse electrical stimulation to assist delineation of epileptogenic cortex. Brain. 2011;134:2855–2866. doi: 10.1093/brain/awr211. [DOI] [PubMed] [Google Scholar]
- 123.Wetjen NM, Marsh WR, Meyer FB, et al. Intracranial electroencephalography seizure onset patterns and surgical outcomes in nonlesional extratemporal epilepsy. J Neurosurg. 2009;110:1147–1152. doi: 10.3171/2008.8.JNS17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fujiwara H, Greiner HM, Lee KH, et al. Resection of ictal high-frequency oscillations leads to favorable surgical outcome in pediatric epilepsy. Epilepsia. 2012;53:1607–1617. doi: 10.1111/j.1528-1167.2012.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Usui N, Terada K, Baba K, et al. Clinical significance of ictal high frequency oscillations in medial temporal lobe epilepsy. Clin Neurophysiol. 2011;122:1693–1700. doi: 10.1016/j.clinph.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 126.Modur PN, Zhang S, Vitaz TW. Ictal high-frequency oscillations in neocortical epilepsy: implications for seizure localization and surgical resection. Epilepsia. 2011;52:1792–1801. doi: 10.1111/j.1528-1167.2011.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Williams PA, White AM, Clark S, et al. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Levesque M, Bortel A, Gotman J, Avoli M. High-frequency (80–500 Hz) oscillations and epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2011;42:231–241. doi: 10.1016/j.nbd.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1.19 mb)
(PDF 1.19 mb)