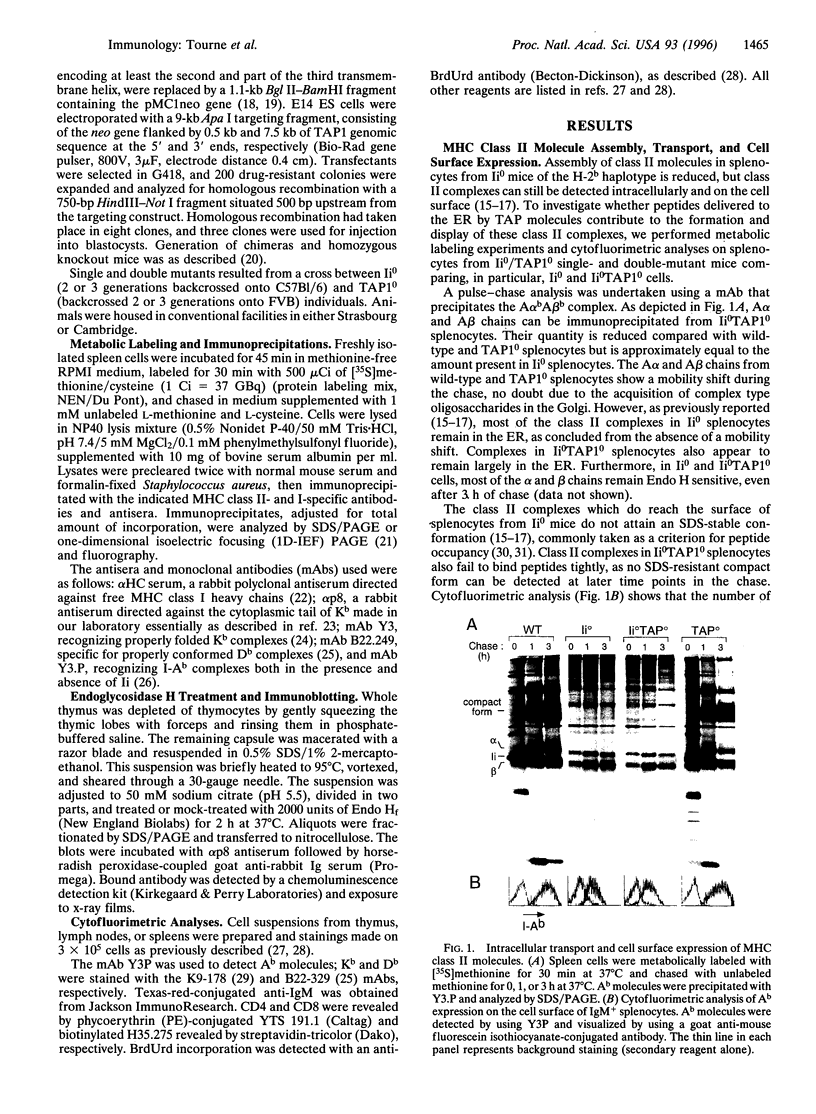
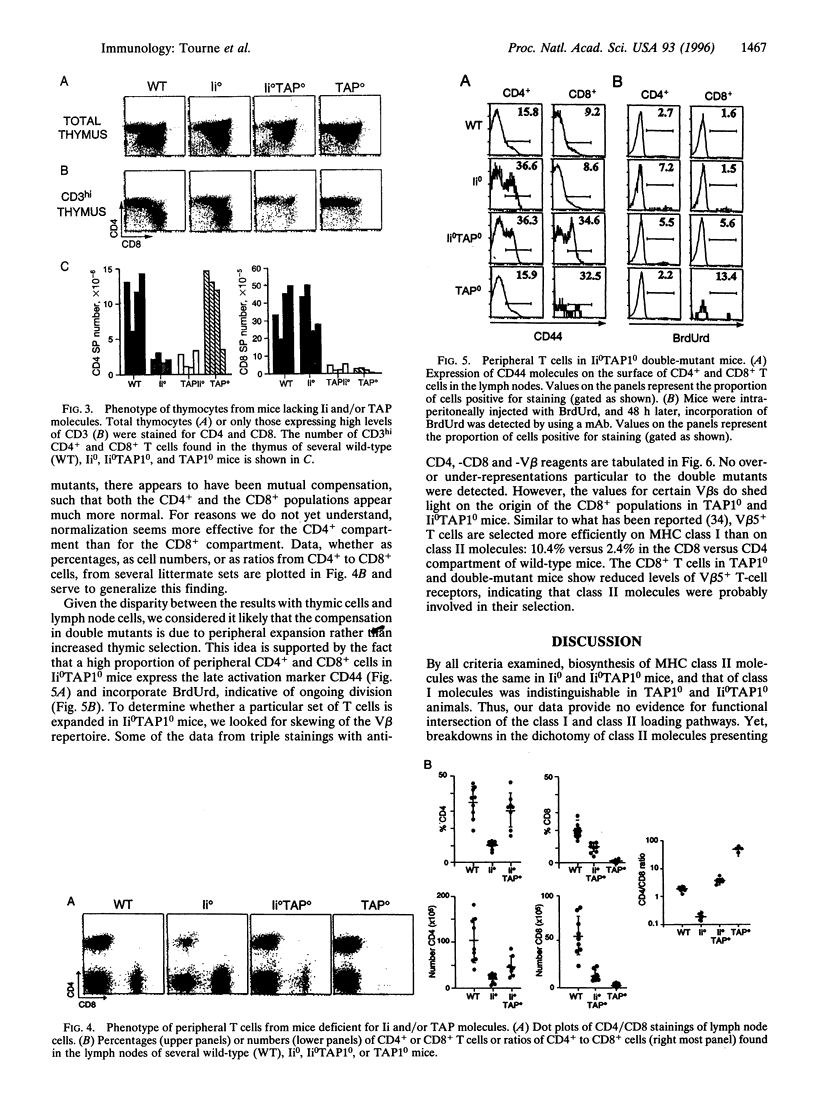
Abstract
Major histocompatibility complex (MHC) class I and II molecules are loaded with peptides in distinct subcellular compartments. The transporter associated with antigen processing (TAP) is responsible for delivering peptides derived from cytosolic proteins to the endoplasmic reticulum, where they bind to class I molecules, while the invariant chain (Ii) directs class II molecules to endosomal compartments, where they bind peptides originating mostly from exogenous sources. Mice carrying null mutations of the TAP1 or Ii genes (TAP10) or Ii0, respectively) have been useful tools for elucidating the two MHC/peptide loading pathways. To evaluate to what extent these pathways functionally intersect, we have studied the biosynthesis of MHC molecules and the generation of T cells in Ii0TAP10 double-mutant mice. We find that the assembly and expression of class II molecules in Ii0 and Ii0TAP10 animals are indistinguishable and that formation and display of class I molecules is the same in TAP10 and Ii0TAP10 animals. Thymic selection in the double mutants is as expected, with reduced numbers of both CD4+ CD8- and CD4- CD8+ thymocyte compartments. Surprisingly, lymph node T-cell populations look almost normal; we propose that population expansion of peripheral T cells normalizes the numbers of CD4+ and CD8+ cells in Ii0TAP10 mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., Fraser J., Flyer D., Calvin S., Flavell R. Beta 2-microglobulin is not required for cell surface expression of the murine class I histocompatibility antigen H-2Db or of a truncated H-2Db. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7447–7451. doi: 10.1073/pnas.83.19.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikoff E. K., Huang L. Y., Episkopou V., van Meerwijk J., Germain R. N., Robertson E. J. Defective major histocompatibility complex class II assembly, transport, peptide acquisition, and CD4+ T cell selection in mice lacking invariant chain expression. J Exp Med. 1993 Jun 1;177(6):1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix M., Raulet D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. J Exp Med. 1992 Sep 1;176(3):829–834. doi: 10.1084/jem.176.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer H., Viville S., Benoist C., Mathis D. Diversity of endogenous epitopes bound to MHC class II molecules limited by invariant chain. Science. 1994 Mar 4;263(5151):1284–1286. doi: 10.1126/science.7510069. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Carbone F. R., Bevan M. J. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990 Feb 1;171(2):377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardell S., Tangri S., Chan S., Kronenberg M., Benoist C., Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med. 1995 Oct 1;182(4):993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerundolo V., Elliott T., Elvin J., Bastin J., Townsend A. Association of the human invariant chain with H-2 Db class I molecules. Eur J Immunol. 1992 Sep;22(9):2243–2248. doi: 10.1002/eji.1830220910. [DOI] [PubMed] [Google Scholar]
- Chan S. H., Cosgrove D., Waltzinger C., Benoist C., Mathis D. Another view of the selective model of thymocyte selection. Cell. 1993 Apr 23;73(2):225–236. doi: 10.1016/0092-8674(93)90225-f. [DOI] [PubMed] [Google Scholar]
- Chen B. P., Madrigal A., Parham P. Cytotoxic T cell recognition of an endogenous class I HLA peptide presented by a class II HLA molecule. J Exp Med. 1990 Sep 1;172(3):779–788. doi: 10.1084/jem.172.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Crooks M. E., Littman D. R. Disruption of T lymphocyte positive and negative selection in mice lacking the CD8 beta chain. Immunity. 1994 Jul;1(4):277–285. doi: 10.1016/1074-7613(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Elliott E. A., Drake J. R., Amigorena S., Elsemore J., Webster P., Mellman I., Flavell R. A. The invariant chain is required for intracellular transport and function of major histocompatibility complex class II molecules. J Exp Med. 1994 Feb 1;179(2):681–694. doi: 10.1084/jem.179.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung W. P., Kündig T. M., Ngo K., Panakos J., De Sousa-Hitzler J., Wang E., Ohashi P. S., Mak T. W., Lau C. Y. Reduced thymic maturation but normal effector function of CD8+ T cells in CD8 beta gene-targeted mice. J Exp Med. 1994 Sep 1;180(3):959–967. doi: 10.1084/jem.180.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung W. P., Schilham M. W., Rahemtulla A., Kündig T. M., Vollenweider M., Potter J., van Ewijk W., Mak T. W. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991 May 3;65(3):443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Hendrix L. R. MHC class II structure, occupancy and surface expression determined by post-endoplasmic reticulum antigen binding. Nature. 1991 Sep 12;353(6340):134–139. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Rüsch E., Tada N., Kimura S., Hämmerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Conrad P. J., Lerner E. A., Babich J., Wettstein P., Murphy D. B. Monoclonal antibodies specific for Ia glycoproteins raised by immunization with activated T cells: possible role of T cellbound Ia antigens as targets of immunoregulatory T cells. J Immunol. 1984 Feb;132(2):662–667. [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Clark K., Benacerraf B., Rock K. L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao N. S., Maltzman J., Raulet D. H. Positive selection determines T cell receptor V beta 14 gene usage by CD8+ T cells. J Exp Med. 1989 Jul 1;170(1):135–143. doi: 10.1084/jem.170.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljunggren H. G., Oudshoorn-Snoek M., Masucci M. G., Ploegh H. L. High-resolution one-dimensional isoelectric focusing of mouse MHC class I antigens. Identification of natural and experimentally induced class I antigens. Immunogenetics. 1990;32(6):440–450. doi: 10.1007/BF00241639. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Van Kaer L., Ashton-Rickardt P. G., Tonegawa S., Ploegh H. L. Differential reactivity of residual CD8+ T lymphocytes in TAP1 and beta 2-microglobulin mutant mice. Eur J Immunol. 1995 Jan;25(1):174–178. doi: 10.1002/eji.1830250129. [DOI] [PubMed] [Google Scholar]
- Machold R. P., Andrée S., Van Kaer L., Ljunggren H. G., Ploegh H. L. Peptide influences the folding and intracellular transport of free major histocompatibility complex class I heavy chains. J Exp Med. 1995 Mar 1;181(3):1111–1122. doi: 10.1084/jem.181.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnati M. S., Marti M., LaVaute T., Jaraquemada D., Biddison W., DeMars R., Long E. O. Processing pathways for presentation of cytosolic antigen to MHC class II-restricted T cells. Nature. 1992 Jun 25;357(6380):702–704. doi: 10.1038/357702a0. [DOI] [PubMed] [Google Scholar]
- Martien van Santen H., Woolsey A., Rickardt P. G., Van Kaer L., Baas E. J., Berns A., Tonegawa S., Ploegh H. L. Increase in positive selection of CD8+ T cells in TAP1-mutant mice by human beta 2-microglobulin transgene. J Exp Med. 1995 Feb 1;181(2):787–792. doi: 10.1084/jem.181.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuchtern J. G., Biddison W. E., Klausner R. D. Class II MHC molecules can use the endogenous pathway of antigen presentation. Nature. 1990 Jan 4;343(6253):74–76. doi: 10.1038/343074a0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Rahemtulla A., Fung-Leung W. P., Schilham M. W., Kündig T. M., Sambhara S. R., Narendran A., Arabian A., Wakeham A., Paige C. J., Zinkernagel R. M. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991 Sep 12;353(6340):180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990 Aug 24;249(4971):918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., Germain R. N. A role for peptide in determining MHC class II structure. Nature. 1991 Sep 12;353(6340):167–170. doi: 10.1038/353167a0. [DOI] [PubMed] [Google Scholar]
- Schilham M. W., Fung-Leung W. P., Rahemtulla A., Kuendig T., Zhang L., Potter J., Miller R. G., Hengartner H., Mak T. W. Alloreactive cytotoxic T cells can develop and function in mice lacking both CD4 and CD8. Eur J Immunol. 1993 Jun;23(6):1299–1304. doi: 10.1002/eji.1830230617. [DOI] [PubMed] [Google Scholar]
- Smith M. H., Parker J. M., Hodges R. S., Barber B. H. The preparation and characterization of anti-peptide heteroantisera recognizing subregions of the intracytoplasmic domain of class I H-2 antigens. Mol Immunol. 1986 Oct;23(10):1077–1092. doi: 10.1016/0161-5890(86)90006-4. [DOI] [PubMed] [Google Scholar]
- Sugita M., Brenner M. B. Association of the invariant chain with major histocompatibility complex class I molecules directs trafficking to endocytic compartments. J Biol Chem. 1995 Jan 20;270(3):1443–1448. doi: 10.1074/jbc.270.3.1443. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Tourne S., Nakano N., Viville S., Benoist C., Mathis D. The influence of invariant chain on the positive selection of single T cell receptor specificities. Eur J Immunol. 1995 Jul;25(7):1851–1856. doi: 10.1002/eji.1830250709. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Cellular studies on antigen presentation by class II MHC molecules. Curr Opin Immunol. 1992 Feb;4(1):63–69. doi: 10.1016/0952-7915(92)90127-z. [DOI] [PubMed] [Google Scholar]
- Van Kaer L., Ashton-Rickardt P. G., Ploegh H. L., Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992 Dec 24;71(7):1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- Viville S., Neefjes J., Lotteau V., Dierich A., Lemeur M., Ploegh H., Benoist C., Mathis D. Mice lacking the MHC class II-associated invariant chain. Cell. 1993 Feb 26;72(4):635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. Cell biology of antigen processing and presentation to major histocompatibility complex class I molecule-restricted T lymphocytes. Adv Immunol. 1992;52:1–123. doi: 10.1016/s0065-2776(08)60875-5. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- te Riele H., Maandag E. R., Berns A. Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5128–5132. doi: 10.1073/pnas.89.11.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lugt N. M., Domen J., Linders K., van Roon M., Robanus-Maandag E., te Riele H., van der Valk M., Deschamps J., Sofroniew M., van Lohuizen M. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994 Apr 1;8(7):757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]