Decreased macrophage maturation following immunization, concomitant with increased IL-10 expression is associated with preferential activation of Th2 cells in male SJL mice.
Keywords: monocytes, cytokines, phagocytosis
Abstract
Injection of proteins and particulate antigens into the peritoneal cavity of male SJL mice preferentially activates T cells secreting Th2 cytokines. Identical immunizations of females activate T cells secreting Th1 cytokines. CD11b+F4/80hi LPM and CD11b+F4/80lo SPM populations were compared between naive males and females to define their role in supporting differential Th1 versus Th2 T cell activation. No sex-dependent differences in the expression of MHC class II, costimulatory molecules, and MR were detected. Immunization induced influx of CD11bloF4/80lo cells in both sexes. CD11bloF4/80lo cells consist predominantly of Ly6Chi monocytes, which mature into a Ly6C− SPM subset. Following immunization, equivalent frequencies of LPM had taken up antigen. However, the CD11bloF4/80lo population, which had taken up antigen, was decreased significantly in males compared with females. Similar to naïve macrophages, antigen-positive cells in immunized males and females exhibited no phenotypic differences. However, fewer Ly6C−F4/80+ cells were present in males compared with females, consistent with the reduced number of antigen-positive cells. Furthermore, CD11bloF4/80lo cells, which had taken up antigen in males, expressed increased IL-10 and limited IL-12 mRNA compared with the predominant IL-12 mRNA expression in female-derived, antigen-positive CD11bloF4/80lo cells. IL-10 blockade increased the frequency of Ly6C−F4/80+ cells in males to the frequency in females, suggesting that preferential activation of Th2 T cells in male SJL mice is associated with increased IL-10 expression and limited antigen presentation as a result of decreased macrophage maturation under the influence of IL-10.
INTRODUCTION
The peritoneal cavity is a classic site of immunization in mice as well as a source of macrophages, which are the predominant cell type within the peritoneal cavity, in addition to a substantial population of B cells, as well as minor populations of neutrophils, eosinophils, and mast cells [1]. Macrophages in various tissues are functionally and phenotypically heterogeneous [2–4], and the peritoneal cavity contains two distinct macrophage subsets [1]. CD11b+F4/80hi LPM efficiently take up antigen, express high levels of costimulatory molecules, but lack MHC class II expression, suggesting an inability to present antigen [1]. By contrast, CD11b+F4/80lo SPM take up antigen and express MHC class II, costimulatory molecules, and the CD62 ligand LN homing receptor [1, 5], suggesting an ability to take up and present antigen and to migrate to draining LNs. Inflammation or immunization induces an influx of Ly6ChiCD11bloF4/80lo inflammatory monocytes into the peritoneal cavity [1, 6], which differentiate into phagocytic Ly6C−MHC II+ SPM [1], supporting the concept that SPM are the primary phagocytic cells in the peritoneal cavity with the capacity to initiate immune responses.
Despite a variety of potential immune defects, SJL mice mount normal, protective immune responses to many viral and bacterial infections. Potential immune deficits include deficiencies in CD5+ B cells and NK cells, a limited TCR repertoire, reduced IgE responses, and a sex-dependent bias in the activation of Th1 versus Th2 cells [7]. Female SJL mice efficiently mount Th1-type responses following immunization via the i.p. and s.c. routes and are also susceptible to actively induced EAE [8–11]. By contrast, immunized male SJL mice (<8 weeks of age) preferentially mount Th2 responses, which manifest as a deficiency in Th1-mediated, DTH and resistance to EAE [8]. Preferential activation of Th2 cells by males following immunization with a variety of soluble and particulate antigen is reversed by adoptive transfer of CD11b+MHC II+ PEM derived from females prior to or concomitant with immunization [8, 12].
PEM, which mediate the novel, strain-dependent, differential Th1 versus Th2 activation between female and male SJL mice [8, 12, 13], are relatively deficient in stimulating T cells compared with splenic macrophages yet exhibit increased antigen uptake [4]. However, similar to the SPM population, PEM are derived from recruited Ly6Chi blood monocytes, suggesting that sex-dependent, differential immune responses and autoimmune disease susceptibility unique to SJL mice [12] may correlate with a differential function of APC within the peritoneal cavity. Therefore, this study was undertaken to define phenotypic and functional differences in peritoneal macrophages in male and female SJL mice. No difference in percentages or cell surface molecules was found comparing macrophage populations in naïve mice. However, the proportion of CD11bloF4/80lo cells, which take up antigen, was reduced by ∼50% in males. Analysis of the CD11bloF4/80lo cells following immunization demonstrated that maturation into Ly6C− macrophages is reduced in males compared with females.
Transfer of PEM from females, inhibition of IL-10 in males prior to immunization, as well as transfer of PEM from males following inhibition of IL-10 all resulted in Th1 activation [13]. Comparison of the SPM populations in naïve males and females demonstrated equivalent expression of IL-12 mRNA. IL-10 mRNA was near-detection limits in SPM derived from naïve females; however, SPM derived from naïve males expressed significantly higher levels of IL-10 mRNA. Increased IL-10 and limited IL-12 expression was sustained in the cells from males who had taken up antigen. IL-10 neutralization in males increased the frequency of mature Ly6C− macrophages, which had taken up antigen. These results suggest a correlation between the activation of T cells secreting a Th2 cytokine profile following i.p. antigen challenge in male SJL mice and IL-10 expression by the potential APCs, resulting in decreased macrophage maturation following antigen encounter.
MATERIALS AND METHODS
Mice
Male and female SJL mice were obtained at 5 weeks of age from The Jackson Laboratory (Bar Harbor, ME, USA) and the National Cancer Institute (Frederick, MD, USA), respectively, and used within 2 weeks of arrival. All procedures were performed in compliance with the protocols approved by the Institutional Animal Care and Use Committees of the Cleveland Clinic Foundation (Cleveland, OH, USA).
Analysis of peritoneal macrophages
Peritoneal cells were isolated by lavage with ice-cold RPMI 1640 containing 5 U/ml heparin (Sigma-Aldrich, St. Louis, MO, USA). For surface staining, cells were incubated for 15 min on ice with 1% mouse serum and 1% rat anti-mouse FcγIII/IIR in PBS containing 0.5% BSA (FACS buffer) to eliminate nonspecific binding. Cells were stained on ice for 30 min with anti-CD11b PerCP (M1/70), anti-F4/80 allophycocyanin or PE (Cl:A3-1), anti-CD80 PE (16-10A1), anti-CD86 PE (GL-1), anti-Ly6C PE (AL-21), anti-I-Aq Alexa Fluor 647 (KH 116), anti-CD19 PE Cy7 (ID3), and rat IgMκ PE, rat IgG2aκ PE, and hamster IgG2κ PE and mouse IgG2bκ Alexa Fluor 647 isotype controls. All mAb were obtained from BD Biosciences (San Diego, CA, USA), except anti-F4/80 and anti-I-Aq, which were from AbD Serotec (Raleigh, NC, USA) and BioLegend (San Diego, CA, USA), respectively. The cells were washed three times with FACS buffer prior to analysis. MR expression was examined by intracellular staining with anti-CD206 FITC (MR5D3) and rat IgG2a FITC isotype control antibody (AbD Serotec) using intracellular staining reagents and protocol from BD Biosciences. Briefly, after surface staining with anti-CD11b PerCP and anti-F4/80 allophycocyanin mAb, cells were fixed and permeabilized with cytofix/cytoperm for 20 min on ice. The cells were then washed twice with 1× perm/wash buffer and stained with anti-CD206 and isotype control antibodies in 1× perm/wash buffer for 30 min on ice. Cells were then washed three times with 1× perm/wash buffer and resuspended in FACS buffer. Samples were acquired on a FACSCalibur or FACSAria (Becton Dickinson, San Jose, CA, USA) and analyzed by FlowJo software (Tree Star, Ashland, OR, USA).
Antigen uptake and processing
To compare antigen uptake, mice were immunized i.p. with 100 μg FITC- conjugated OVA (Molecular Probes, Eugene, OR, USA) in 0.5 ml endotoxin-free Dulbecco's PBS (Irvine Scientific, Santa Ana, CA, USA). Mice were also immunized i.p. with 100 μg unlabeled OVA (Sigma-Aldrich) to establish gates for FITC-OVA+ cells. To compare in vivo antigen processing, mice were immunized i.p. with 100 μg cleavage-activated DQ-OVA (Molecular Probes) in 0.5 ml PBS. To examine the role of IL-10, male and female SJL mice were injected i.p. with anti-IL-10 (JES5-2A5; 0.5 mg/mouse) or an isotype control antibody specific for an unrelated antigen β-galactosidase (GL113; 0.5 mg/mouse) at the time of immunization.
RNA isolation and quantitative real-time PCR
Peritoneal cells obtained from naïve mice or mice immunized with FITC-OVA were stained with anti-CD11b PerCP, anti-F4/80 allophycocyanin, and anti-Ly6G PE (1A8) mAb as described above. Naïve SPM and FITC-OVA+ or FITC-OVA− CD11bloF4/80lo cells were purified using a BD FACSAria by excluding Ly6G+ cells and gating on desired SPM populations. RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and precipitated with isopropyl alcohol. After washing with 75% ethanol, RNA was resuspended in RNase-free water. cDNA was synthesized using 2 μg RNA, Moloney murine leukemia viurs RT (Invitrogen), and oligo-dT primers (Promega, Madison, WI, USA) after treatment with DNase (Ambion, Austin, TX, USA). Quantitative real-time PCR was performed using 4 μl cDNA and Syber Green Master Mix (Applied Biosystems, Foster City, CA, USA) in triplicate on a 7500 Fast Real-Time PCR System (Applied Biosystems) under the following conditions: 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, annealing at 60°C for 30 s, and an elongation at 72°C for 30 s. Real-time primer sequences are as follows: IL-10 sense, 5′-TTGAATTCCCTGGGTGAGAA-3′, and antisense, 5′-GCTCCACTGCCTTGCTCTTATT-3′; IL-12p40 sense, 5′-ACAGCACCAGCTTCTTCATCAG-3′, and antisense, 5′-TCTTCAAAGGCTTCATCTGCAA-3′; and GAPDH sense, 5′-CATGGCCTTCCGTGTTCCTA-3′, and antisense, 5′-ATGCCTGCTTCACCACCTTCT-3′. Transcripts were calculated relative to the housekeeping gene GAPDH using the following formula: 2[CT (GAPDH)−CT (target gene)] × 1000, in which CT is determined as the threshold cycle at which the fluorescent signal becomes significantly higher than background.
Statistical analysis
Statistical analysis was performed by Student's t test. Values of P ≤ 0.05 are considered significant. Results are expressed as mean ± sem.
RESULTS
Phenotype of peritoneal macrophages in naïve SJL mice
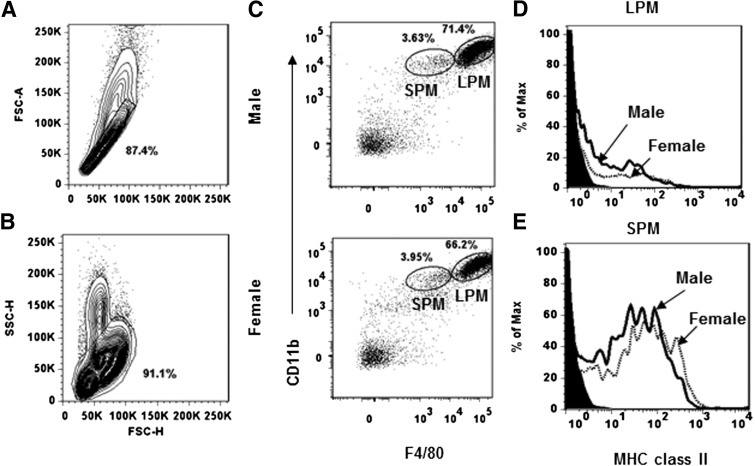
The peritoneal cavity of naïve females contained approximately twofold more cells compared with the peritoneal cavity of age-matched male SJL mice [4.9±0.4×106/female (n=9) vs. 2.8±0.3×106/male (n=9); P<0.05]. For phenotypic analysis of the peritoneal cells, single cells were gated based on forward scatter-height and forward scatter-area (Fig. 1A). Granular cells were excluded based on high side scatter (Fig. 1B), and CD19+ B cells were excluded as described previously [1]. Based on CD11b and F4/80 expression, two distinct macrophage subsets, CD11b+F4/80hi LPM and CD11b+F4/80lo SPM, were identified in mice of both genders with no significant differences in the proportions of the respective populations (Fig. 1C). MHC class II, CD80, and CD86 were compared on LPM and SPM from both sexes to confirm the differential expression defined recently using peritoneal cells derived from BALB/c mice [1] and to determine if expression were gender-dependent. CD80 and CD86 were expressed on 80–90% of the LPM derived from both sexes (data not shown), whereas the vast majority lacked MHC class II expression [male: 94±0.5% (n=7) vs. female: 95±0.8% (n=7; Fig. 1D)], supporting the concept that this population does not function as an APC [1]. By contrast, MHC class II was expressed by the majority of SPM from both sexes [male: 60.9±6% (n=5) vs. female: 66.3±5.8% (n=5; Fig. 1E)]. Although expression varied on individual cells, no differences were observed in the frequency of cells expressing MHC class II isolated from males and females. Similar to MHC class II, CD80 and CD86 expression on the SPM from both sexes was also heterogeneous and expressed by the majority of SPM (data not shown), supporting the notion that SPM have the capacity to activate antigen-specific T cells [1].
Figure 1. Phenotypic analysis of peritoneal macrophage populations in naïve male and female SJL mice.
Peritoneal cells from naïve male and female mice analyzed by flow cytometry. (A) Forward scatter-height (FSC-H) versus forward scatter-area (FSC-A) plot, demonstrating the gating used for analysis. (B) Forward scatter-height versus side scatter-height (SSC-H) plot demonstrating gating on nongranular cells. (C) Macrophage subsets were identified after gating out CD19+ cells as: CD11b+F4/80hi LPM and CD11b+F4/80lo SPM. (D and E) MHC class II expression on LPM and SPM from males and females. Filled histograms represent isotype controls, and solid lines and dotted lines represent data from males and females, respectively. Data are representative of greater than three experiments.
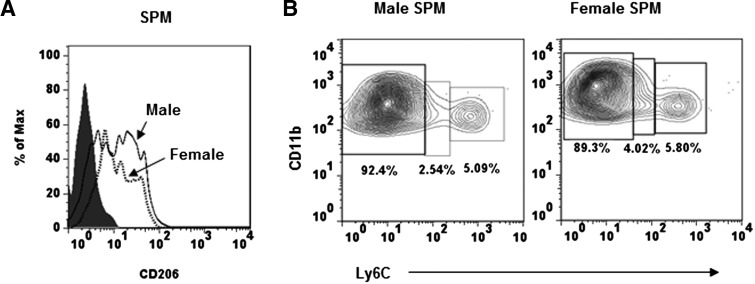
These data indicate that although there are approximately twofold more cells present in the peritoneal cavity of naïve female compared with male SJL mice, differences in the proportions of the macrophage populations appeared not to account for the preferential activation of Th2 cells in males. OVA immunization, which preferentially induces Th1 responses in female SJL and Th2 responses in males, was used to examine sex-dependent, differential antigen uptake and processing. Thus, first, we determined whether SPM from males and females differ in expression of MR, CD206, which is primarily used by macrophages to take up OVA [14]. CD206 expression by SPM, derived from males and females, was heterogeneous (Fig. 2A), similar to MHC class II expression (Fig. 1D). However, neither the frequency of CD206+ cells nor the average number of molecules expressed/cell (based on mean fluorescence intensity) differed between males and females (Fig. 2A).
Figure 2. MR expression and phenotype of SPM subset.
SPM peritoneal cells from male and female SJL mice were identified with anti-CD11b and anti-F4/80 mAb. (A) MR expression identified with anti-CD206. Filled histogram represents rat IgG2a isotype control mAb, solid lines represent CD206 expression by cells derived from males, and dotted lines represent CD206 expression by cells derived from females. (B) Three SPM subsets—Ly6Chi, Ly6Clo, and Ly6C−—in naïve mice. Data are representative of three experiments.
Ly6C expression distinguishes between mature macrophages and newly recruited monocytes, which are Ly6Chi, whereas mature macrophages are Ly6C− [1, 15]. Furthermore, Ly6C− macrophages have a higher antigen-uptaking capability compared with Ly6Chi monocytes [16]. Thus, we determined whether SPM from naïve males and females differ in Ly6C expression. The majority of SPM in the peritoneal cavity of both sexes was Ly6C− [males: 89.2±3.4% (n=3) vs. females: 88.3±2.3% (n=3; Fig. 2B)], consistent with a mature macrophage phenotype. In addition, small populations of Ly6Chi (∼8%) and ly6Clo (∼3%) are present in SPM from naïve mice of both sexes. Thus, although the environment prior to antigen challenge influences the activation of T cells secreting Th1- or Th2-associated cytokines in SJL mice [13], no phenotypic differences related to antigen acquisition and presentation were detected comparing the naive SPM populations from males and females.
Antigen-induced increase in CD11bloF4/80loLy6Chi cells in males
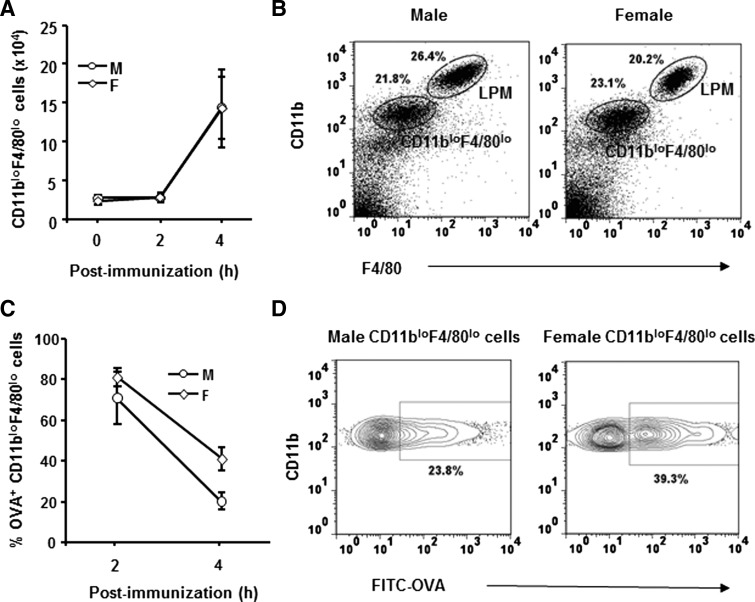
Males and females were immunized i.p. with FITC-OVA to determine the relative antigen-induced changes in the macrophage populations. The CD11bloF4/80lo population, comprised of cells present in the naïve peritoneal cavity, as well as newly recruited MHC class II−Ly6Chi monocytes [1, 6], increased following antigen challenge in males and females (Fig. 3A and B). By contrast, the LPM populations decreased (Fig. 3B, and data not shown), probably as a result of “macrophage disappearance reaction” [17]. The absence of an overall change in total peritoneal cells compared with naïve mice at 4 h p.i. (data not shown) was thus a result of an increase in CD11bloF4/80loLy6Chi cells (Fig. 3A) coupled with a decrease in the LPM population, consistent with a loss of LPM and recruitment of CD11bloF4/80loLy6Chi cells [1, 6].
Figure 3. Antigen uptake and antigen-induced alterations in peritoneal macrophages.
Male and female SJL mice were immunized i.p. with FITC-OVA. Peritoneal cells were isolated at indicated times p.i. (A) CD11bloF4/80lo cell numbers in males and females in naïve (0 h) and at 2 and 4 h p.i. Data represent three to seven individual mice analyzed/time-point. (B) CD11b and F4/80 expression identify LPM and CD11bloF4/80lo cells. Data represent at least three experiments with similar results. (C) Percentages of FITC-OVA+ CD11bloF4/80lo cells at indicated times (n=3). (D) Percentage of OVA+ cells at 4 h p.i. Data represent at least three experiments with similar results.
To compare rates of antigen uptake, male and female SJL mice were immunized i.p. with OVA and uptake compared at 2 and 4 h p.i. The majority of LPM from both sexes contained antigen at both time-points examined (data not shown), consistent with previous results [1]. However, LPM remained MHC class II−, even after 18 h p.i. (data not shown), supporting the notion that this population does not function as an APC. In the CD11bloF4/80lo population, antigen uptake was evident by 2 h p.i. with ∼75% of CD11bloF4/80lo cells from both sexes positive for antigen (Fig. 3C). At 4 h p.i., the frequency of antigen-positive CD11bloF4/80lo cells declined in both sexes (Fig. 3C) with ∼50% fewer CD11bloF4/80lo cells from males positive for antigen compared with females (Fig. 3C and D). These results suggested three possibilities: 1) increased egress of antigen-positive cells from the peritoneal cavity of males; 2) increased frequency of newly recruited Ly6Chi cells in males; or 3) increased antigen degradation by the SPM population in males.
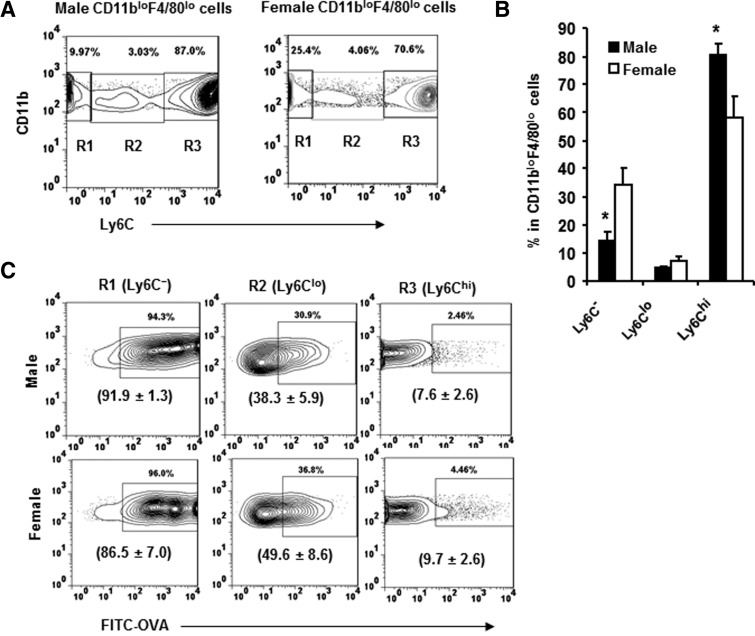
Few antigen-positive macrophages were detected in the spleen following immunization (data not shown). This suggests that the vast majority of antigen-positive cells resided within the peritoneal cavity at least at 4 h p.i., although we cannot completely rule out the possibility of recruitment to sites outside of the peritoneal cavity. To explore the possibility of an increased frequency of newly recruited Ly6Chi cells in males, the distribution of Ly6Chi and more differentiated Ly6C− cells within the CD11bloF4/80lo population was compared in males and females. The CD11bloF4/80lo populations in males and females contained an increased frequency of Ly6Chi cells (Fig. 4A and B) compared with naïve mice (Fig. 2B), indicating an active process of antigen-induced recruitment into the peritoneal cavity. However, the larger relative increase of Ly6Chi cells in males compared with females (Fig. 4A and B) indicated recent recruitment and/or decreased maturation. Antigen uptake within the Ly6C−, Ly6Clo, and Ly6Chi cells was examined to determine if maturation and/or recruitment differences contributed to the reduced frequency of antigen-positive cells in males. The majority of CD11bloF4/80lo cells within the peritoneal cavity of females, who had taken up antigen, was Ly6C− (Fig. 4C, left panel), with fewer cells expressing the Ly6Clo phenotype (Fig. 4C, middle panel), consistent with previous data examining the uptake of the particulate antigen Escherichia coli [1]. Few Ly6Chi cells with in CD11bloF4/80lo population had taken up antigen (Fig. 4C, right panel), consistent with recently recruited monocytes. Similar to females, the Ly6C− cells in males also represented the largest population, which had taken up antigen (Fig. 4C, left panel).
Figure 4. Antigen-induced alteration in CD11bloF4/80lo cell subsets.
Peritoneal cells were isolated from male and female SJL mice at 4 h p.i with FITC-OVA. The CD11bloF4/80lo cells analyzed for Ly6C expression identify three populations. (A) Ly6C− (R1), Ly6Clo (R2), and Ly6Chi (R3). Data represent at least three experiments with similar results. (B) Percentages of Ly6C−, Ly6Clo, and Ly6Chi SPM in males and females. *P ≤ 0.05 (n=4, male vs. female). (C) FITC-OVA+ cells within the Ly6C− (left panel), Ly6Clo (middle panel), and Ly6Chi (right panel) populations derived from male and female SJL mice. Average percentages ± sem of FITC-OVA+ cells in the Ly6C−, Ly6Clo, and Ly6Chi SPM in males and females (n=3) are presented in parentheses.
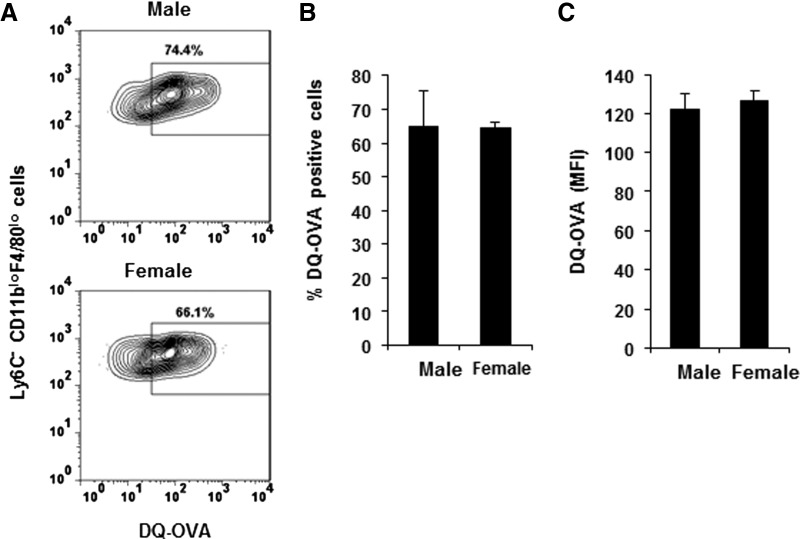
Male and female mice were injected with self-quenched DQ-OVA, which only fluoresces when degraded, reflecting a critical step in antigen processing [18] to address the possibility that differential antigen degradation contributes to the decrease in antigen-positive CD11bloF4/80lo cells in males compared with females (Fig. 3C and D). Similar to immunizations with FITC-OVA, immunization with DQ-OVA resulted in an increase in CD11bloF4/80lo cells in mice of both sexes (data not shown). However, at 4 h p.i., comparable percentages (Fig. 5) and absolute numbers [males: 3.3±1.9×103 (n=3) vs. females: 3.6±0.9×103 (n=3)] of Ly6C− CD11bloF4/80lo cells from males and females had degraded antigen (Fig. 5). In addition, similar mean fluorescence intensities within the Ly6C− populations (Fig. 5) suggest that although fewer Ly6C− CD11bloF4/80lo cells from males take up antigen, there is no sex-dependent difference in the efficiency of antigen degradation. Therefore, these data indicate that the overall reduced frequency of antigen-positive cells in males compared with females reflects the paucity of Ly6C− cells in the peritoneal cavity of males following immunization.
Figure 5. Antigen processing in male and female SJL mice.
Male and female SJL mice were immunized i.p. with DQ-OVA, and peritoneal cells were isolated at 4 h p.i. (A) Percentages of cells that processed DQ-OVA within the Ly6C− CD11bloF4/80lo population. Gates were established using CD11bloF4/80lo from mice injected with unlabeled OVA. Data represent two experiments using two to three mice analyzed individually. (B) Percentages of DQ-OVA+Ly6C− CD11bloF4/80lo cells (mean±sem; n=5). (D) Mean fluorescence intensity (MFI) of DQ-OVA+ Ly6C− CD11bloF4/80lo cells (mean±sem; n=5).
Preferential IL-10 expression in male SJL mice
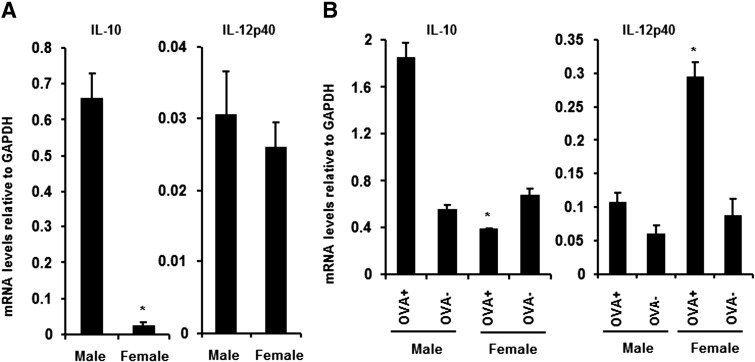
No sex-dependent difference in MHC class II expression was detected comparing SPM derived from naive mice (Fig. 1D and E) or on antigen-positive cells isolated following immunization (data not shown). Similarly, no IL-10-dependent decrease in MHC class II expression was apparent on the PEM comparing males and females [13, 19], although neutralization of IL-10 prior to immunization supported the induction of the Th1 cell in males [13, 19]. Therefore, SPM purified from naïve males and females were compared to determine whether they differed in IL-10 and IL-12 expression. SPM isolated from naïve males, which predominately consist of Ly6C− cells (Fig. 2B), express significantly increased IL-10 mRNA compared with the identical population isolated from females (Fig. 6A). By contrast, these two populations did not differ in IL-12 mRNA expression (Fig. 6A). To determine an association between preferential Th1/Th2 activation and potential macrophage functional activity, IL-10 and IL-12 mRNA expression were compared in CD11bloF4/80lo cells from males and females that had taken up antigen. CD11bloF4/80lo cells from males, which had taken up antigen, expressed significantly increased IL-10 mRNA compared with the same population derived from female mice (Fig. 6B). By contrast, antigen-negative cells derived from males did not differ in IL-10 expression from antigen-negative cells from females (Fig. 6B). In contrast to IL-10, IL-12 mRNA expression did not differ in antigen-positive and -negative SPM derived from males (Fig. 6B). However, the antigen-positive CD11bloF4/80lo cells derived from females showed a significant increase in IL-12 mRNA (Fig. 6B), consistent with the activation of Th1 cells. These results suggest that CD11bloF4/80lo cells within the peritoneal cavity, which take up protein antigen in males and females, differ in expression of the Th2-promoting cytokine IL-10 and Th1-inducing cytokine IL-12.
Figure 6. IL-10 and IL-12p40 mRNA expression by antigen-positive SPM.
(A) IL-10 and IL-12p40 mRNA expression in purified SPM from naïve males and females. (B) IL-10 and IL-12p40 mRNA expression in FITC-OVA+ and FITC-OVA− CD11bloF4/80lo cells obtained at 4 h p.i. from males and females (n=4). *P < 0.05 (comparison between naïve SPM from males and females and FITC-OVA+ CD11bloF4/80lo cells from males and females).
IL-10 prevents macrophage maturation in males
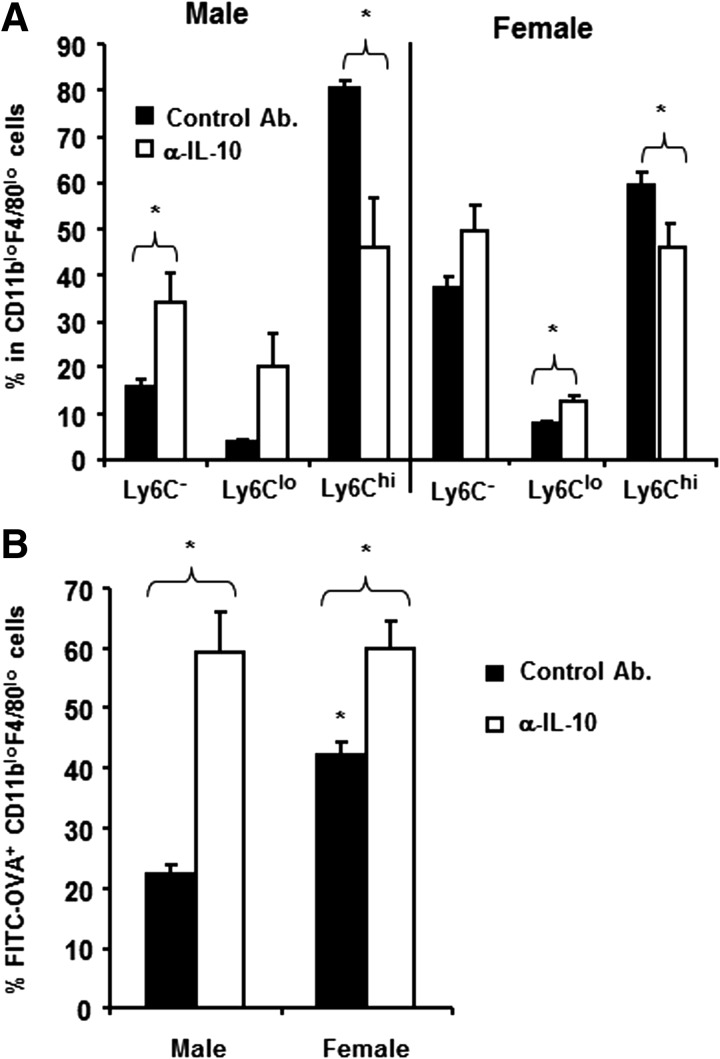
Increased IL-10 expression by antigen-positive CD11bloF4/80lo cells from males (Fig. 6B), coupled with the increased frequency of Ly6Chi cells in the CD11bloF4/80lo population, suggested an inverse correlation with CD11bloF4/80lo cell maturation into Ly6C− macrophages. To examine this possibility, male and female SJL mice were treated with anti-IL-10 at the time of immunization and CD11bloF4/80lo cell maturation into Ly6C− and Ly6Clo populations compared. Blockade of IL-10 significantly decreased (P<0.05) the proportion of Ly6Chi cells in males and females, associated with an increase in Ly6C− cells (Fig. 7A). However, the resulting populations in females were only slightly influenced by the blockade of IL-10, whereas the populations in males were converted to a frequency resembling females (Fig. 7A). Consistent with percentages, IL-10 blockade also significantly increased absolute numbers of Ly6C− (males: from 14.3±2.4×103–25.6±0.04×103, P<0.05; and females: 25.7±3.7×103–52.3±7.6×103, P<0.05), as well as Ly6Clo cells (males: 2.9±0.17×103–8.1±0.24×103, P<0.05; and females: 5.43±0.44×103–14.5±1.15×103, P<0.05) in peritoneal cavity of both sexes following immunization. Following IL-10 blockade, the Ly6Chi cells also increased (males: 65.8±13.2×103–176.1±16.5×103, P=0.18; and females: 43.3±5.4×103–62.8±12.8×103; P=0.20), which may be a result of ongoing recruitment of the Ly6Chi cell and/or the availability of limiting amounts of maturation factors. These data suggested that decreased uptake of antigen in males was influenced by endogenous IL-10 and was a result of the increase in antigen-negative Ly6Chi cells. To confirm this concept, the frequency of cells capable of taking up antigen was compared in control and anti-IL-10-treated males and females. Indeed, the percentage of CD11bloF4/80lo cells taking up antigen increased in the males and females. However, IL-10 blockade normalized the frequency of antigen-positive cells in the mice of both sexes (Fig. 7B), consistent with the concept that IL-10 prevented maturation of Ly6Chi cells to a more mature Ly6C− phenotype. Thus, antigen uptake enhances the IL-10 versus IL-12 bias in the peritoneal macrophages, already evident under steady-state conditions in male and female SJL mice.
Figure 7. IL-10 alters maturation and antigen uptake.
(A) Peritoneal cells were isolated from male and female SJL mice at 4 h p.i. with FITC-OVA plus anti (α)-IL-10 or control mAb treatment. The CD11bloF4/80lo cells analyzed for Ly6C expression identify three populations—Ly6C−, Ly6Clo, and Ly6Chi—as shown in Fig. 4A. (A) Percentages of Ly6C−, Ly6Clo, and Ly6Chi cells in control mAb and anti-IL-10-treated males and females. Data represent means ± sem of four mice in each group analyzed individually. (B) Percentages of FITC-OVA+ CD11bloF4/80lo cells in control mAb and anti-IL-10-treated mice. Data represent means ± sem of four mice in each group analyzed individually. *P < 0.05.
DISCUSSION
A variety of data suggests that the strain-dependent preferential activation of Th2 cells in male SJL mice [12] is related to APC function. For example, adoptive transfer of antigen-loaded CD11b+MHC II+ PEM from females, but not from males, induces Th1 responses in male recipients [20]. Furthermore, transfer of PEM derived from females to males prior to i.p. immunization results in the activation of Th1 cells in males [8, 12, 21]. Macrophages in the peritoneal cavity of naïve mice have recently been divided into two subsets, designated LPM and SPM [1]. These distinct populations are consistent with the concept that macrophages are heterogeneous, exhibit proinflammatory and anti-inflammatory activities, and not only vary with tissue site but also within a tissue [1–4]. Similar to SPM, PEM are derived from the recruited Ly6Chi blood monocytes [1] and are more efficient in antigen uptake yet express lower levels of MHC class II [19, 22] and thus, are less efficient in T cell activation compared with splenic macrophages [4]. Male and female SJL mice were compared to understand the interaction between antigen and peritoneal macrophages in regulating the preferential activation of Th1 versus Th2 cells at the initial site of antigen encounter. Similar to other strains of mice [1], macrophages comprise the major cell population found in the peritoneal cavity of male and female SJL mice. In addition, no sex-dependent differences in the phenotype of the LPM and SPM were detected. LPM populations from both SJL genders were also active in taking up and processing antigen and expressed costimulatory molecules but lacked MHC class II expression, even following antigen uptake, consistent with previous data analyzing BALB/c mice [1]. Thus, the absence of MHC class II expression supports the notion that the LPM subset is not involved directly in the development of CD4+ T cell-mediated immunity [1].
SPM derived from naïve mice of both sexes expressed equivalent costimulatory molecules as well as MHC class II, suggesting similar capacities to initiate and/or sustain antigen-driven T cell immune responses. The increase in the SPM population following immunization derives from recruited peripheral blood Ly6Chi monocytes [1, 6]. Following immunization, an equivalent increase in the SPM population in both sexes suggested that recruitment of Ly6Chi inflammatory monocytes into the peritoneal cavity is not impaired in male SJL mice. However, fewer cells in the peritoneal cavity of males exhibited the capacity to take up antigen (Fig. 3D). OVA, the model antigen, is a glycoprotein taken up primarily via the MR, CD206 [14]. Therefore, one possibility to explain reduced antigen uptake by SPM from males is a defect in CD206 expression. Although IL-4 and IL-10 up-regulate CD206 expression on PEM [23], suggesting increased expression on cells derived from males, equivalent CD206 expression was detected on SPM from both sexes. These data indicate that although Th2 cytokines present in males prior to antigen challenge influence the preferential activation of Th2 cells [13, 19], reduced antigen uptake by SPM from males was not a result of a cytokine-mediated alteration in MR expression. The SPM population in the peritoneal cavity of both sexes was composed of Ly6C−, Ly6Clo, and Ly6Chi cells following immunization. Ly6C down-regulation marks maturation into macrophages [1, 24], and the reduced number of cells that had taken up antigen in males reflected a decrease in the Ly6C− population. These data suggest that males have a delay in the transition of Ly6C+ cells into the Ly6C− population. Equivalent antigen uptake by SPM of both sexes at an early time-point (2 h) when there is low influx of inflammatory cells in the peritoneal cavity further suggests that reduced antigen uptake by male SPM is a result of decreased maturation into Ly6C− cells. Recently, similar kinetics of GR-1+ monocyte recruitment into peritoneal cavity of Leishmania major promastigote-infected mice has been reported [6]. In contrast to our results, increased L. major uptake was detected in GR-1+ monocytes. The reason for discrepancy between these two results is not known but may be a result of differential uptake of soluble OVA versus particulate L. major by cells of the monocyte lineage.
PEM derived from males and females are phenotypically identical; however, a higher frequency of macrophages from females produce IL-12 compared with those derived from males [19]. In addition, preferential Th2 activation in males is inhibited by adoptive transfer of PEM from males treated with anti-IL-10 prior to transfer [13]. Furthermore, rIL-12 preferentially activates Th1 cells in immunized males [25], suggesting that the relative expression of these proinflammatory and anti-inflammatory cytokines is critical in determining whether T cells secret Th1- or Th2-associated cytokines. Differentiation of SPM and PEM from recruited Ly6Chi blood monocytes [1] suggests that differential IL-10 and/or IL-12 expression may contribute to the preferential Th2 activation in males. The SPM populations derived from naïve male and female SJL mice expressed equivalent levels of IL-12p40 mRNA, suggesting the capability of supporting Th1 responses. However, IL-10 was differentially expressed with high levels in the SPM population derived from males, supporting the preferential activation of Th2 cells in males. To insure that the differential expression of IL-12 and IL-10 was maintained by the CD11bloF4/80lo cells taking up antigen, IL-12 and IL-10 expression was compared in cells that had taken up antigen or remained antigen-free. In cells from females that had taken up antigen, IL-12p40 mRNA increased dramatically. By contrast, cells derived from males that had taken up antigen expression of IL-10 mRNA increased dramatically. IL-10 is expressed by macrophages expressing the acquired deactivation phenotype [26]. However, comparison of SPM from naïve mice and antigen-positive CD11bloF4/80lo cells from immunized mice showed no differential expression of “found in the inflammatory zone”, Ym-1 (a mouse chitinase-like protein), or IL-4R antagonist mRNA (data not shown). These data suggest that the cells derived from males could not be categorized into the acquired deactivation or into an alternative activation phenotype and are consistent with a differential environment in male SJL mice, which results in the preferential activation of Th2 cells following immunization.
These results demonstrate that peritoneal macrophages in SJL mice of both sexes are equivalent in MHC class II expression, expression of costimulatory molecules, and their ability to take up and degrade protein antigen. Although the number of antigen-positive SPM was initially identical in males and females, the data demonstrate that a rapid change in the SPM composition resulted in a significant decrease in antigen-positive cells with time p.i. The present data cannot distinguish between increased recruitment of Ly6Chi cells into the peritoneal cavity of the male mice or a decreased maturation into Ly6C− cells. SPM in naïve males and females expressed equivalent levels of the Th1-inducing cytokine IL-12 mRNA; however, the SPM population in males also coexpressed high levels of the Th2-promoting cytokine, IL-10. Indeed, the subset of CD11bloF4/80lo cells, which had taken up antigen, exhibited high levels of IL-10 mRNA coupled with low levels of IL-12 mRNA expression, whereas the identical population from females showed exactly the opposite cytokine expression profile. A direct role of increased IL-10 secretion by the antigen-positive CD11bloF4/80lo cells in polarizing the T cell response is not clear; however, blocking IL-10 increased the Ly6C− subset of CD11bloF4/80lo cells, suggesting that endogenous IL-10 prevents macrophage maturation, possibly via alterations in GM-CSF. Macrophage maturation is dependent on GM-CSF, which is influenced adversely by IL-10 [27, 28]. In contrast to the inhibitory effect of IL-10 on macrophage maturation, a potentiating effect of IL-10 on macrophage maturation has been described [29, 30], including the increased differentiation of CD16-positive macrophages from human blood monocytes cultured in the presence of IL-10 [29]. Similarly, IL-10 enhanced macrophage maturation in blood monocyte cultures [30]. The reason for these differential effects of IL-10 on macrophage maturation is not clear but may reflect differences in culture conditions of the initial cell populations examined. Altogether, our data suggest that the reduced number of cells maturing into the Ly6C− phenotype in males under the influence of antigen, in addition to providing an initial source of IL-10 during T cell priming, may contribute to the preferential activation of Th2 cells by limiting the numbers of available antigen-loaded APCs [31].
ACKNOWLEDGMENTS
The authors thank Dr. Cornelia Bergmann for helpful discussions and critical reading of the manuscript; Jeffery Tarcy, Nermina Covic, and Kate Stenson for technical assistance; and Jennifer Powers for cell sorting. This work was supported by U.S. National Institutes of Health grant NS 53824.
Footnotes
- CT
- cycle threshold
- EAE
- experimental autoimmune encephalomyelitis
- LPM
- large peritoneal macrophage(s)
- MR
- mannose receptor
- PEM
- peritoneal exudate macrophage(s)
- p.i.
- postimmunization
- SPM
- small peritoneal macrophage(s)
AUTHORSHIP
S.H. designed research, performed experiments, and wrote the paper. S.A.S. designed research, wrote the paper, and secured funding.
REFERENCES
- 1. Ghosn E. E., Cassado A. A., Govoni G. R., Fukuhara T., Yang Y., Monack D. M., Bortoluci K. R., Almeida S. R., Herzenberg L. A. (2010) Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc. Natl. Acad. Sci. USA 107, 2568–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soehnlein O., Lindbom L. (2010) Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 10, 427–439 [DOI] [PubMed] [Google Scholar]
- 3. Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu G., Xia X. P., Gong S. L., Zhao Y. (2006) The macrophage heterogeneity: difference between mouse peritoneal exudate and splenic F4/80+ macrophages. J. Cell. Physiol. 209, 341–352 [DOI] [PubMed] [Google Scholar]
- 5. Xu H., Manivannan A., Crane I., Dawson R., Liversidge J. (2008) Critical but divergent roles for CD62L and CD44 in directing blood monocyte trafficking in vivo during inflammation. Blood 112, 1166–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goncalves R., Zhang X., Cohen H., Debrabant A., Mosser D. M. (2011) Platelet activation attracts a subpopulation of effector monocytes to sites of Leishmania major infection. J. Exp. Med. 208, 1253–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilcoxen S. C., Kirkman E., Dowdell K. C., Stohlman S. A. (2000) Gender-dependent IL-12 secretion by APC is regulated by IL-10. J. Immunol. 164, 6237–6243 [DOI] [PubMed] [Google Scholar]
- 8. Cua D. J., Hinton D. R., Kirkman L., Stohlman S. A. (1995) Macrophages regulate induction of delayed-type hypersensitivity and experimental allergic encephalomyelitis in SJL mice. Eur. J. Immunol. 25, 2318–2324 [DOI] [PubMed] [Google Scholar]
- 9. Cua D. J., Hinton D. R., Stohlman S. A. (1995) Self-antigen-induced Th2 responses in experimental allergic encephalomyelitis (EAE)-resistant mice. Th2-mediated suppression of autoimmune disease. J. Immunol. 155, 4052–4059 [PubMed] [Google Scholar]
- 10. Stohlman S. A., Pei L. D. J., Cua, Li Z., Hinton D. R. (1999) Activation of regulatory cells suppresses experimental allergic encephalomyelitis via secretion of IL-10. J. Immunol. 163, 6338–6344 [PubMed] [Google Scholar]
- 11. Kirwin S. J., Dowdell K. C., Hindinger C., Feng N., Bergmann C. C., Hinton D. R., Stohlman S. A. (2006) Altered neuroantigen-specific cytokine secretion in a Th2 environment reduces experimental autoimmune encephalomyelitis. J. Neuroimmunol. 178, 30–39 [DOI] [PubMed] [Google Scholar]
- 12. Stohlman S. A., Matsushima G. K., Casteel N., Frelinger J. A. (1985) The defect in delayed-type hypersensitivity of young adult SJL mice is due to a lack of functional antigen-presenting cells. Eur. J. Immunol. 15, 913–916 [DOI] [PubMed] [Google Scholar]
- 13. Cua D. J., Coffman R. L., Stohlman S. A. (1996) Exposure to T helper 2 cytokines in vivo before encounter with antigen selects for T helper subsets via alterations in antigen-presenting cell function. J. Immunol. 157, 2830–2836 [PubMed] [Google Scholar]
- 14. Burgdorf S., Lukacs-Kornek V., Kurts C. (2006) The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J. Immunol. 176, 6770–6776 [DOI] [PubMed] [Google Scholar]
- 15. Lenzo J. C., Turner A. L., Cook A. D., Vlahos R., Anderson G. P., Reynolds E. C., Hamilton J. A. (2011) Control of macrophage lineage populations by CSF-1 receptor and GM-CSF in homeostasis and inflammation. Immunol. Cell Biol., Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 16. Lee P. Y., Li Y., Kumagai Y., Xu Y., Weinstein J. S., Kellner E. S., Nacionales D. C., Butfiloski E. J., van Rooijen N., Akira S., Sobel E. S., Satoh M., Reeves W. H. (2009) Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am. J. Pathol. 175, 2023–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barth M. W., Hendrzak J. A., Melnicoff M. J., Morahan P. S. (1995) Review of the macrophage disappearance reaction. J. Leukoc. Biol. 57, 361–367 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez G. M., Diment S. (1995) Destructive proteolysis by cysteine proteases in antigen presentation of ovalbumin. Eur. J. Immunol. 25, 1823–1827 [DOI] [PubMed] [Google Scholar]
- 19. Cua D. J., Stohlman S. A. (1997) In vivo effects of T helper cell type 2 cytokines on macrophage antigen-presenting cell induction of T helper subsets. J. Immunol. 159, 5834–5840 [PubMed] [Google Scholar]
- 20. Matsushima G. K., Stohlman S. A. (1988) Maturation of the delayed-type hypersensitivity response in SJL mice: absence of effector cell induction. Eur. J. Immunol. 18, 1411–1416 [DOI] [PubMed] [Google Scholar]
- 21. Matsushima G. K., Stohlman S. A. (1991) Distinct subsets of accessory cells activate Thy-1+ triple negative (CD3−, CD4−, CD8−) cells and Th-1 delayed-type hypersensitivity effector T cells. J. Immunol. 146, 3322–3331 [PubMed] [Google Scholar]
- 22. Chan J., Leenen P. J., Bertoncello I., Nishikawa S. I., Hamilton J. A. (1998) Macrophage lineage cells in inflammation: characterization by colony-stimulating factor-1 (CSF-1) receptor (c-Fms), ER-MP58, and ER-MP20 (Ly-6C) expression. Blood 92, 1423–1431 [PubMed] [Google Scholar]
- 23. Martinez-Pomares L., Reid D. M., Brown G. D., Taylor P. R., Stillion R. J., Linehan S. A., Zamze S., Gordon S., Wong S. Y. (2003) Analysis of mannose receptor regulation by IL-4, IL-10, and proteolytic processing using novel monoclonal antibodies. J. Leukoc. Biol. 73, 604–613 [DOI] [PubMed] [Google Scholar]
- 24. Sunderkötter C., Nikolic T., Dillon M. J., Van Rooijen N., Stehling M., Drevets D. A., Leenen P. J. (2004) Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172, 4410–4417 [DOI] [PubMed] [Google Scholar]
- 25. Kim S., Voskuhl R. R. (1999) Decreased IL-12 production underlies the decreased ability of male lymph node cells to induce experimental autoimmune encephalomyelitis. J. Immunol. 162, 5561–5568 [PubMed] [Google Scholar]
- 26. Colton C. A. (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 4, 399–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. (1991) Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174, 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oehler L., Foedinger M., Koeller M., Kollars M., Reiter E., Bohle B., Skoupy S., Fritsch G., Lechner K., Geissler K. (1997) Interleukin-10 inhibits spontaneous colony-forming unit-granulocyte-macrophage growth from human peripheral blood mononuclear cells by suppression of endogenous granulocyte-macrophage colony-stimulating factor release. Blood 89, 1147–1153 [PubMed] [Google Scholar]
- 29. Calzada-Wack J. C., Frankenberger M., Ziegler-Heitbrock H. W. (1996) Interleukin-10 drives human monocytes to CD16 positive macrophages. J. Inflamm. 46, 78–85 [PubMed] [Google Scholar]
- 30. Allavena P., Piemonti L., Longoni D., Bernsconi S., Stoppacciaro A., Ruco L., Mantovani A. (1998) IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur. J. Immunol. 28, 359–371 [DOI] [PubMed] [Google Scholar]
- 31. Hosken N. A., Shibuya K., Heath A. W., Murphy K. M., O'Garra A. (1995) The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-α β-transgenic model. J. Exp. Med. 182, 1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]