Abstract
Brain-derived neurotrophic factor (BDNF) levels in dopaminergic (DA) cells within the ventral tegmental area (VTA)/nucleus accumbens (NAc) circuitry appear to be a candidate mechanism for the neuroadaptive changes that follow stress and reward responses in animal models. However, the role of the BDNF gene variants in responses to salient cues through DA neurotransmission in humans remains unexplored. Here, we studied the effect of the common functional BDNF Val66Met (rs6265) polymorphism on rewarding experiences in the striatum and DA-mediated responses to stress. Seventy-two healthy controls were genotyped for the BDNF Val66Met polymorphism and underwent the monetary incentive delay task during an functional magnetic resonance imaging (fMRI) session. Forty-nine of them also underwent a sustained pain challenge with and without placebo administration with potential analgesic properties during PET measures of DA D2/3-receptor-mediated neurotransmission. Neuroimaging results revealed a significant effect of BDNF (Met66 carriers > Val/Val) on brain responses during the anticipation of monetary losses, baseline D2/3 receptor availability, and pain-stress-induced DA release in the NAc. Conversely, BDNF Met66 carriers showed no activation in response to monetary gains and a blunted DA response to the analgesic placebo in the NAc. These results provide initial human evidence regarding the effect of the BDNF Val66Met polymorphism on DA-mediated responses to stress, its cognitive regulation by positive expectations, and the anticipatory responses to monetary gains and losses in the VTA-NAc pathway. Our results are of relevance to the neurobiology of stress and reward interactions and the pathophysiology of stress-related disorders.
Keywords: BDNF Val66Met, dopamine, nucleus accumbens, pain, reward, stress
Introduction
Brain-derived neurotrophic factor (BDNF) plays a key role in synaptic plasticity and the survival and function of dopaminergic (DA) neurons within the ventral tegmental area–nucleus accumbens (VTA-NAc) pathway. In animal models, striatal in vivo infusions of BDNF locally augment spontaneous electrical activity of midbrain DA neurons (Shen et al., 1994), enhance DA turnover (Altar et al., 1992), and elevate DA-activity-dependent release (Goggi et al., 2002). BDNF levels in DA cells in the VTA-NAc pathway also seem to be involved in the neuroadaptive changes occurring after intermittent brief episodes of social defeat stress, persistent chronic subordination (Berton et al., 2006; Krishnan et al., 2007; Miczek et al., 2011), and responses to rewarding stimuli (Horger et al., 1999; Pierce and Bari, 2001; Lu et al., 2004). BDNF injections into the VTA or the NAc enhance cocaine-induced locomotion (Pierce and Bari, 2001) and responses to cocaine cues for up to 30 d after the cessation of cocaine use (Lu et al., 2004). In addition, BDNF heterozygote knock-out mice are also less responsive to cocaine's rewarding effects (Horger et al., 1999; Hall et al., 2003).
In humans, mesolimbic activity and DA neurotransmission in the NAc are robustly engaged during the anticipation of a number of salient cues regardless of valence (Knutson et al., 2000; Scott et al., 2006; Scott et al., 2007b; McCabe et al., 2009; Spreckelmeyer et al., 2009). However, a potential role of BDNF on the processing of salient cues of different valences as it relates to DA neurotransmission has not been studied in humans. A common single-nucleotide polymorphism (SNP) in the BDNF human gene, Val66Met (rs6265), codes a substitution from valine (Val) to methionine (Met) at codon 66. It is postulated that Met substitution leads to inefficient trafficking of BDNF to secretory granules and reduced activity-dependent BDNF release (Egan et al., 2003; Chen et al., 2004; Chen et al., 2006). This functional polymorphism has been associated with interindividual variations in cognitive function (Egan et al., 2003), stress reactivity (Duman and Monteggia, 2006; Frielingsdorf et al., 2010; Colzato et al., 2011), and reward processing (Gasic et al., 2009).
Here, we studied the effect of the BDNF Val66Met functional polymorphism on anticipatory responses to monetary gains and losses during the fMRI monetary incentive delayed (MID) task and striatal DA neurotransmission during two challenges of opposite valence known to activate DA release, a stress-pain challenge (Scott et al., 2006), and placebo administration with expectations of analgesia (Scott et al., 2008) using PET and the DA D2/3-selective receptor radiotracer [11C]-raclopride. Based on the animal literature and assuming effects regardless of valence, we initially hypothesized that, compared with Val homozygotes, decreased BDNF-dependent activity in Met carriers would result in lower functional responses during the anticipation of monetary gains and losses, lower baseline DA tone with a compensatory upregulation of D2/3 receptors at baseline, and potentially lower DA release during both a painful stressor and placebo administration. The effects of BDNF Val66Met were expected within the VTA DA projections to the NAc, medial prefrontal cortex (mPFC), hippocampus (Hipp), and amygdala (AMY; Russo and Nestler, 2013) during the MID and in the NAc in the DA PET studies.
Materials and Methods
Subjects
Seventy-two subjects (34 females), age 20–40 years (mean ± SE, 26 ± 0.5) were genotyped for BDNF Val66Met SNP (rs6265) and completed the MID task. Pain reports were collected (see “Experimental Design”) in a subsample of 49 subjects (28 females, age 26.45 ± 0.7 years) that participated in two PET scanning sessions during a pain-stress challenge with and without placebo administration as described previously (Scott et al., 2008). Results on 20 of the 49 subjects in the present sample were part of a previous study examining the effects of placebo administration on μ-opioid and DA neurotransmission (Scott et al., 2008). Main effects of placebo in the present sample replicated those in the previous study (Scott et al., 2008) and therefore are not reported here. BDNF Val66Met gene effects were not studied in this sample (Scott et al., 2008) due to insufficient power. In addition to completing physical and neurological examinations, study participants underwent screening using the nonpatient version of the Structured Clinical Interview for DSM-IV. Participants had no history of or current medical, neurological, or psychiatric illnesses, including substance abuse or dependence and had an alcohol intake of <5 drinks per week. Participants also had no first-degree family history of psychiatric illness. Women had regular menstrual cycles of 26–32 d duration and had not used hormonal birth control for at least 1 year. We attempted to study women during the follicular phase using menstrual cycle diaries at screening and confirmatory progesterone levels at each PET scanning session (progesterone levels of ≤1 ng/ml were confirmed in 23/28 women). Protocols were approved by the University of Michigan Investigational Review Board and the Radioactive Drug Research Committee and written informed consent was obtained from all subjects.
Genotyping
BDNF Val66Met SNP (rs6265) was genotyped in all subjects using the Illumina Golden Gate Assay platform, the Addictions Array content of 130 genes (1350 SNPs), and 186 Ancestry Informative Markers (AIMs), which have been described previously (Hodgkinson et al., 2008). Genotyping accuracy was confirmed by replicate genotyping of 10% of the total sample with a completion rate of >93% (mean 99.4%, median 100%) and replicates showed no errors at this loci. Because the sample was predominantly Caucasian (75%) and most of the non-Caucasians were African Americans (18%), the European and African ethnic factors (European and African AIM scores) were included as a continuous covariate in statistical analyses to account for the variability in allele frequencies across ethnicities.
MID and fMRI data acquisition and analysis
MID.
Seventy-two participants completed a version of the MID (Knutson et al., 2000). Each subject completed 2 runs consisting of 72 trials lasting 6 s each. A trial consisted of a cue representing a monetary value (small: ±$0.20, medium: ±$1.00, and large: ±$5.00, null: $0), followed by an anticipation phase and a neutral target requiring button press with their right thumb. Subjects were then informed of their success on the preceding trial, in which they either gained or avoided losing the cued amount of money for monetary gain or loss trials, respectively. In the null trials, subjects experienced no monetary gain or loss but were still instructed to respond to the target. Subjects successfully hit the target an average of 45 ± 24% of the gain trials and 44 ± 24% of the loss response trials. Their average reaction time on gain trials was (165 ± 41 ms) and on loss trials was (165 ± 43 ms).
fMRI data acquisition.
The blood oxygenation level-dependent (BOLD) signal was measured using a Signa 3-tesla scanner (General Electric) with standard RF coil using a T2* weighted pulse sequence (single-shot combined spiral in/out, gradient echo; repetition time = 2 s; echo time = 30 ms; flip-angle = 90 deg; field-of-view = 20 cm; 64-by-64 image matrix; slice thickness = 4 mm; 29 oblique-axial slices; Glover and Law, 2001). This imaging protocol was selected to minimize signal loss due to magnetic susceptibility effects (Noll, 2002). Field map images were collected to correct for B0 inhomogeneities (Noll et al., 1991).
Data were reconstructed offline, slice-time corrected to the middle slice (Aguirre and D'Esposito, 1999), and realigned to the first volume of each run to correct for intrascan movement using Statistical Parametric Mapping (SPM)-based algorithms (Friston et al., 1995). Each session was visually inspected for artifacts and screened for excessive head movement (mean translational or rotational instantaneous head movement >0.55 mm). High resolution anatomical MRI studies were also acquired using an axial spoiled gradient recall T1-weighted sequence (echo time, 3.4 ms; repetition time, 10.5 ms; inversion time, 200 ms; flip angle, 25°; number of excitations, 1; using 124 contiguous images, 1.5 mm thickness).
To allow comparisons between individuals, a subject's MRI and functional images were coregistered and anatomically normalized by warping the anatomical T1-weighted image to a standard stereotactic space (Montreal Neurological Institute, MNI) using SPM8 (Wellcome Department of Cognitive Neurology, University College, London). Finally, functional images were smoothed with a Gaussian kernel (full width at half maximum 6 mm) to reduce residual interindividual variability. Smoothed functional images were band-pass filtered with a 128 s high-pass filter to eliminate low-frequency signals.
Pain and placebo experimental design
Forty-nine subjects underwent two PET scans with [11C]-raclopride. The experimental design, in the absence or presence of placebo, consisted of a painful condition starting at minute 45 and maintained for 20 min after radiotracer administration by a computer-controlled delivery system through the infusion of medication-grade hypertonic saline solution (5%) into the left masseter muscle. In this model of sustained deep somatic pain, the intensity of the painful stimulus is standardized across subjects (Zhang et al., 1993; Stohler and Kowalski, 1999). Briefly, volunteers are asked to rate their pain intensity every 15 s from 0 (no pain) to 100 (most intense pain imaginable) using an electronic 0–100 visual analog scale (VAS) placed in front of the scanner gantry during the pain condition. Initially, the subject-specific settings of the closed-loop system for maintaining muscle pain were established. This consisted of measuring each subject's response to a standard 0.15 ml bolus of 5% sodium chloride injected over a 15 s period as an impulsive input while recording the subject's pain intensity response every 15 s. A suitable infusion rate for the maintenance of pain over time was then estimated by comparing the subject's response to the mean response of 65 subjects of the same age range exposed to the same bolus. From that point on, the adaptive controller depended on feedback from subjects. The subject ratings of pain intensity every 15 s were fed back to the computer via an analog-digital board, which then changed the infusion rate to maintain pain at similar levels over time. The same individual infusion profiles generated during the pain challenges were used for the studies with placebo administration (Scott et al., 2008).
During the placebo condition, subjects were given the following instructions before administration of the placebo: “We are studying the effect of a pain relief medication. This medication is thought to have analgesic effects through the activation of natural brain systems that suppress pain.” The placebo condition consisted of the introduction of 1 ml of 0.9% isotonic saline into 1 of the intravenous ports every 4 min, with the volunteer being made aware that this was the case, starting 2 min before the pain challenges and lasting for 15 s each time. Subjects were aware that the study drug was to be administered because they were alerted by a computer-generated human voice recording, followed by a second-by-second count of the infusion timing (15 s). Based on our prior experience using a fully randomized design (Zubieta et al., 2005), the placebo administration followed the pain challenge without placebo; this provided the subjects with a frame of reference for the expectation of analgesic effects. Each subject underwent four pain challenges, two of them with placebo administration, as described previously (Scott et al., 2008), but only the results of two of the sets are reported here, those associated with [11C]-raclopride PET scanning. The order of each pair of pain and pain + placebo studies was randomized. Pain and pain + placebo studies were separated by 2 h (changes in nondisplaceable binding potential [BPND] do not persist in subsequent scans after the pain stressor; Scott et al., 2007a).
Immediately after the pain and the pain + placebo challenges, subjects completed the McGill Pain Questionnaire (MPQ; Melzack and Torgerson, 1971). This measure, together with a 0–100 VAS pain intensity rating acquired every 15 s in the absence and presence of placebo, was used as the primary end point for the assessment of placebo responses. Levels of expectancy and effectiveness of placebo were rated before and after the placebo administration, respectively, with the following questions: for expectancy, “From 0 to 100 how effective do you think the treatment will be?”; for effectiveness, “From 0 to 100 how effective was the treatment?”
PET data acquisition and preprocessing
The two 90 min PET studies per subject were acquired (HR+ scanner; Siemens) in 3D mode (reconstructed full-width/half-maximum resolution, ∼5.5 mm in plane and 5.0 mm axially), with the septa retracted and scatter correction. Participants were positioned in the PET scanner gantry and two intravenous (antecubital) lines were placed. A light forehead restraint was used to eliminate intrascan head movement. [11C]-raclopride was synthesized at high specific activity by the reaction of O-desmethyl raclopride with [11C]-methyl triflate. Then, 15.0 ± 2.2 mCi was administered in each of the imaging procedures, with a mass of raclopride of 0.20 ± 0.15 μg/kg per image. These levels ensured that the compounds were administered in tracer quantities; that is, subpharmacological doses occupying <1% of the available receptors. Fifty percent of the [11C]-raclopride dose was administered as a bolus, with the remainder delivered as a continuous infusion by a computer-controlled automated pump to more rapidly achieve steady-state tracer levels. For each study, 21 sets of dynamic scans were acquired with an increasing duration (4 30 s frames, 3 1 min frames, 2 2.5 min frames, 8 5 min frames, and 4 10 min frames). Images were reconstructed using iterative algorithms (brain mode; Fourier rebinning algorithm with ordered-subsets expectation maximization, four iterations, and 16 subsets; no smoothing) into a 128 × 128 pixel matrix in a 28.8-cm-diameter field of view. Attenuation correction was performed through a 6 min transmission scan (Ge68 source) obtained before the PET study and with iterative reconstruction of the blank/transmission data, followed by segmentation of the attenuation image. Small head motions during PET were corrected by an automated computer algorithm for each subject before analysis and the images were coregistered with the same software (Minoshima et al., 1993). Time points were then decay corrected during reconstruction of the PET data. Image data were transformed on a voxel-by-voxel basis into two sets of parametric maps, a tracer transport measure (K1 ratio) and a receptor-related measure (BPND), the latter using data obtained from 35–45 min (baseline) or 45–90 min (pain stress ± placebo) after tracer administration. This measure was obtained using the ratio of brain activity to activity in the cerebellum minus 1 (Carson et al., 1997; Watabe et al., 2000). Using the bolus-continuous infusion protocol described in the experimental design, the slope of the Logan plot becomes linear ∼5–7 min after tracer administration and is proportional to the receptor concentration divided by its affinity for the radiotracer as follows: [(f2Bmax/Kd) +1], where f2Bmax/Kd is the BPND (Innis et al., 2007) or receptor availability in vivo, Bmax is the receptor concentration, and Kd is the receptor-ligand dissociation constant. The term f2 refers to the concentration of free radiotracer in the extracellular fluid and is considered to represent a constant and very small value.
The K1 and BPND images for each experimental period and the MRIs acquired on the 3 tesla scanner described in fMRI data acquisition, above, were coregistered to each other and to the International Consortium for Brain Mapping stereotactic atlas orientation (Meyer et al., 1997).
Three receptor-related measures were calculated: (1) DA D2/3 BPND during the pain challenge (45–90 min., scan 1); (2) DA D2/3 BPND during the pain + placebo challenge (45–90 min., scan 2); and (3) baseline DA D2/3 BPND at equilibrium (35–45 min., scan 1). DA release during pain and placebo administration was assessed by calculating the difference between baseline (control) and pain conditions and between pain and pain + placebo conditions. Reductions in the in vivo availability of DA receptors after an acute challenge are thought to reflect DA release and competition between radiotracer and endogenous ligand for the receptor sites (Narendran and Martinez, 2008). A mask that included only regions with specific DA D2/3-receptor-binding potential (BPND > 0.2) was used.
Statistical analyses
For the fMRI data, statistical analysis proceeded in two stages. At the first level, a general linear model was used to generate individual subjects' activation maps. The anticipation phase for each of the different monetary values was modeled separately as regressors of interest and was convolved with the hemodynamic response function. Six regressors modeling residual effects of head motion were included as nuisance parameters. The contrasts of interest generated with the model were: (1) anticipation of large gain versus neutral and (2) anticipation of large loss versus neutral. At the second level, contrast images were place into MNI space using the transformation matrix derived from the linear and nonlinear warping transformation matrices and random-effects analysis was used to determine group effects, resulting in statistical parametric (t or F) maps. Statistical test were also applied to the two primary contrasts of interest, large gain minus neutral and large loss minus neutral conditions. A mask excluded the cerebellum and brainstem below the midbrain because these regions were not well represented. A voxel-by-voxel t test (fMRI) and mixed model of variance (PET) analysis were performed using SPM8 (Wellcome Department of Cognitive Neurology, University College, London) and MATLAB software (The MathWorks). To account for differences in sample sizes, we chose the unequal variance option in SPM for statistical analysis. Sex and European and African AIM factors were included as covariates. No global normalization was applied to the data.
The effects of BDNF Val66Met were hypothesized to take place within the VTA dopaminergic projections to the NAc, mPFC, Hipp, and AMY (Russo and Nestler, 2013) during the MID and within the NAc in the DA PET studies (because of the selective binding of [11C]-raclopride in the striatum). The summary statistical maps in these regions were thresholded at p < 0.001 uncorrected for multiple comparisons (Friston, 1997), with a voxel extent >10 voxels; for other regions, p < 0.05 false discovery rate (FDR)/FWE correlation was considered significant. These data were extracted for quantification of regional changes in BOLD activation and BPND, plotting, examination of potential outliers, and further statistical analyses using SPSS version 20.0 statistical software. ANCOVA models were performed on the psychophysical and extracted imaging data. Data are shown as the mean ± 1 SD. Genotype was included as the between-subject factors and sex and European and African AIMs were included as the covariates of no interest. Statistical significance was considered at p < 0.05.
We used the SPSS 20.0 macro Mediate.sbs (http://afhayes.com/spss-sas-and-mplus-macros-and-code.html) to estimate the path coefficients and the size of the indirect effect of the mediator model X (BDNF Val66Met) on Y (MPQ scores immediately after the pain challenge) through Z (BDNF Val66Met effect on NAc DA release during pain).
Results
Genotyping
Seventy-two healthy volunteers were genotyped for the BDNF Val66Met polymorphism (18 were Met66 carriers and 54 Val/Val homozygotes) and completed the MID fMRI task. Forty-nine of these volunteers were scanned with PET and completed pain reports; 11 subjects carried at least one Met allele and 38 were homozygotes for the Val allele. The BDNF Val66Met genotype distribution was in Hardy-Weinberg equilibrium in the two samples (n = 72, χ2 = 0.1, p = 0.7; n = 49: χ2 = 2; p = 0.16) and there were no significant differences between the two genotype groups with respect to sex, age, or European and African AIM scores.
Main effect of the MID and Val66Met (rs6265) on anticipation of gains and losses during the MID
Consistent with previous reports (Knutson et al., 2000), the anticipation of large monetary gains in the whole-brain analysis was associated with activation in the following regions (for all regions, p < 0.05, FDR corrected): the NAc bilaterally (with an area of activation that extended to the medial thalamus): MNI peak coordinates 8, −16, 10; cluster size 12.160 mm3; Z-score 5.1; the anterior cingulate cortex: MNI peak coordinates 0, −4, 50; cluster size 8.696 mm3; Z-score = 5; the occipital cortex: MNI peak coordinates 32, −94, 8; cluster size 23.008 mm3; Z-score = 5.8; and the primary motor area: MNI peak coordinates −48, −8, 50; cluster size 3.256 mm3; Z-score = 4.6. Anticipation of large monetary losses also induced regional activation in (for all regions, p < 0.05, FDR corrected): the NAc bilaterally (extending to the medial thalamus): MNI peak coordinates −10, 10, 0; cluster size 25.840 mm3; Z-score = 6.3; in the anterior cingulate cortex: MNI peak coordinates 0, 10, 44; cluster size 3.872 mm3; Z-score = 4.2; in the AMY: MNI peak coordinates 30, 0, −14; cluster size 440 mm3; Z-score = 4.4; in the occipital cortex: MNI peak coordinates −4, −86, −10; cluster size 17.632 mm3; Z-score = 5.5; and in the primary motor area: MNI peak coordinates −46, −2, 50; cluster size 3.208 mm3; Z-score = 4.1.
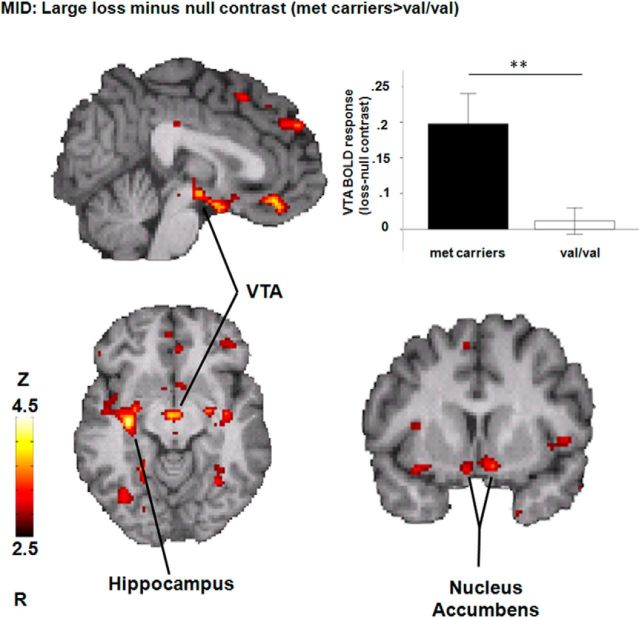
During the anticipation of monetary loss (large loss minus null condition), a whole-brain analysis showed a significant effect of BDNF Val66Met (Met66 varriers > Val/Val; for all regions, p < 0.001; Fig. 1, Table 1) in the mPFC, the VTA, the NAc and its extention to the hypothalamic region, the Hipp, and the parahippocampus. No significant effects were found for the opposite contrast (Val/Val > Met66) or during anticipation of reward (large gain minus null condition).
Figure 1.
BDNF Val66Met effects during the anticipation of large monetary loss minus null condition (for all regions, p < 0.001; n = 72). The bar graph shows regional BOLD activation in the VTA for the large monetary loss minus null condition for each genotype (**p < 0.005).
Table 1.
Effects of BDNF Val66Met during the anticipation of losses, stress, and placebo-induced DA release and D2/3 receptor availability
| Cluster sizea | Z-scoreb | Coordinatesc | |
|---|---|---|---|
| Anticipation of losses (Met66 > Val/Val) | |||
| mPFC | 464 | 3.55 | −2, 36, −14 |
| VTA | 216 | 3.46 | 2, −12, −10 |
| NAc/hypothalamic region | 104 | 3.42 | −8, 8, −14 |
| −2, 0, −16 | |||
| Hipp | 744 | 3.9 | 34, −16, −10 |
| Parahippocampus | 152 | 3.55 | 30, 4, −14 |
| Pain-induced DA release (Met66 > Val/Val) | |||
| NAc | 38 | 3.58 | −8, 6, −3 |
| 25 | 3.23 | 13, 11, −4 | |
| Placebo-induced DA release (Val/Val > Met66) | |||
| NAc | 237 | 4.19 | 14, 7, −1 |
| 56 | 3.75 | −12, 6, −5 | |
| D2/D3 receptor availability (Met66 > Val/Val) | |||
| NAc | 402 | 3.46 | −19, 8, −13 |
aCluster size in cubic millimeters.
bTwo-sided voxel-level Z-score at peak voxel (for all regions, p < 0.001, uncorrected).
cMNI coordinates of peak voxel.
Val66Met (rs6265) effects on D2/3 receptor availability at baseline and DA release during pain stress and placebo analgesia
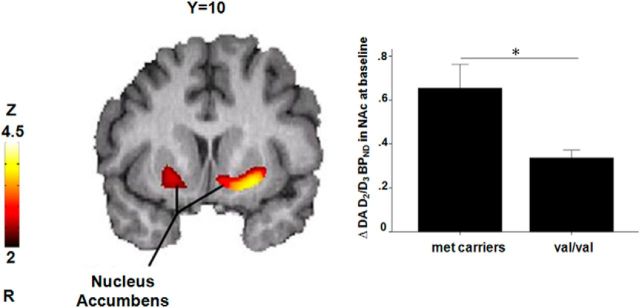
A voxel-by-voxel analysis within the striatum during the control condition revealed a significant effect of BDNF Val66Met (n = 49, Met66 carriers > Val/Val) on baseline D2/3 receptor BPND in the left ventral striatum (MNI peak coordinates −19, 8, −13; cluster size 402 mm3; Z-score = 3.46) and right ventral striatum, although at a lower threshold (Fig. 2, Table 1). Baseline D2/3 receptor BPND in the ventral striatum was not associated with pain ratings or changes in pain ratings during placebo administration with expectation of analgesia.
Figure 2.
Effects of BDNF Val66Met (Met66 carriers > Val/Val) on DA D2/3 receptor BPND in the bilateral ventral striatum (n = 49). Data are expressed as means ± SEM. Significant differences between genotype groups are marked with asterisks (*p < 0.05).
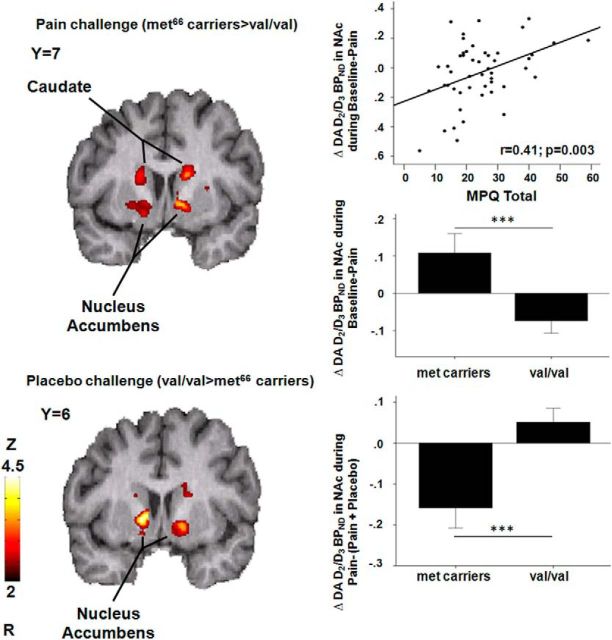
During the pain challenge, a voxel-by-voxel analysis within the striatum showed a significant effect of BDNF Val66Met (n = 49, Met66 carriers > Val/Val) on pain-induced D2/3 system activation maps in the NAc bilaterally [left NAc: MNI peak coordinates −8, 6, −3; cluster size 38 mm3; Z-score = 3.58; in the right NAc: MNI peak coordinates 13, 11, −4; cluster size 25 mm3; Z-score = 3.23 (Fig. 3, Table 1)]. Results remained significant after controlling for baseline DA BPND in the NAc.
Figure 3.
Effects of BDNF Val66Met on DA release. Top, Effects of BDNF Val66Met on stress-induced DA release in the caudate bilaterally and the NAc (n = 49). DA release in the left NAc was positively correlated with the MPQ total score. The bar graph shows BDNF Val66Met effects (Met66 carriers > Val/Val) on DA activation during the pain challenge (means ± SEM). Significant differences between genotype groups are marked with asterisks (***p < 0.001). Bottom, Effects of BDNF Val66Met on DA release during placebo administration during and experimental pain in the NAc bilaterally (n = 49). Reductions in the average momentary pain intensity ratings acquired every 15 s were negatively correlated with placebo-induced DA release in the right NAc in Met66 carriers (r = −0.65, p = 0.03), but not in Val homozygotes (r = −0.025, p = 0.9). The bar graph shows BDNF Val66Met effects (Met66 carriers in black < Val/Val in white) on DA release during placebo administration in the right NAc. Data are expressed as means ± SEM. Significant differences between genotype groups are marked with asterisks (***p < 0.001).
The magnitude of DA release in the NAc by the pain stressor was positively correlated with MPQ scores during the pain condition in the left NAc (MPQ total: r = 0.41; p = 0.003; MPQ sensory: r = 0.39; p = 0.006; MPQ pain effect: r = 0.30; p = 0.04; Fig. 3). We then conducted a mediation analysis to test whether DA release in the NAc mediates the effect of BDNF Val66Met on the subjective pain experience, measured by the MPQ. The mediation analysis confirmed the BDNF Val66Met effect on DA release in the NAc during the sustained pain challenge (coefficient = −0.18, t = −2.72, p = 0.009) and the relationship between stress-induced DA release in the NAc and MPQ ratings (coefficient = 23.8, t = 3.2, p = 0.002). The mean indirect effect (coefficient = 0.13) from the bootstrap analysis was significant, with a 95% confidence interval excluding zero (−1.13 to −8.12) and the direct effect (coefficient = 0.3.5) of BDNF Val66Met on MPQ scores was not significant (p = 0.0.33). This suggests an indirect-only mediation (Zhao et al., 2010), a form of mediation that is consistent with full mediation in Baron and Kenny's procedure (Baron and Kenny, 1986). These results therefore show that the effect of BDNF Val66Met on pain reports is mediated by DA release in the NAc during pain.
During placebo administration, the imaging data analysis showed a significant effect of BDNF Val66Met, in this case, Val/Val> Met66 carriers (n = 49), on placebo-induced D2/3 system activation maps in the NAc bilaterally, where Met66 carriers showed an overall deactivation of the DA system during placebo administration compared with Val carriers (n = 49, Fig. 3, Table 1). Results remained significant after controlling for baseline DA BPND in the NAc. Placebo-induced changes in DA neurotransmission in the NAc were not associated with changes in the subjective experience of pain during placebo administration.
Val66Met (rs6265) effect on pain ratings and placebo effectiveness
At a psychophysical level, we found no significant, direct effect of BDNF Val66Met on pain ratings during or immediately after the pain challenge (n = 49, average VAS scores acquired every 15 s during the challenge or MPQ scores). The volume of hypertonic saline to achieve average target pain ratings, a measure of pain sensitivity, was also not different between genotype or sex groups, nor was the ratio between VAS and volume infused. During the introduction of the placebo, we found no effect of BDNF Val66Met on psychophysical placebo responses.
Discussion
The present study examined the effects of BDNF Val66Met on basal ganglia DA receptor availability and responses to the anticipation of monetary gains and losses, as well as psychophysical and DA responses to a pain stressor, in the absence and presence of a placebo with analgesic properties. Compared with Val/Val homozygotes, BDNF Met66 carriers showed increased BOLD responses during anticipation of monetary losses (but not gains) in the VTA-NAc-mPFC circuit and greater DA release in the NAc during a pain challenge. Sustained pain-induced DA release in the NAc was positively correlated with subjective pain ratings. Moreover, a mediation analysis confirmed that BDNF Val66Met effects on the subjective pain experience were mediated by the magnitude of DA release in the NAc during the pain challenge. Conversely, compared with Val/Val homozygotes, an overall reduction in DA neurotransmission was observed in Met66 carriers during placebo-induced DA release in the NAc. Finally, BDNF Met66 carriers also showed increased D2/3 BPND at baseline compared with Val/Val homozygotes.
Evidence from animal (Berridge and Robinson, 1998; Ikemoto and Panksepp, 1999) and human in vivo imaging studies have shown that DA neurons in the mesolimbic system, especially in the ventral striatum, increase their firing and DA release in response to reward (Wise, 2004; Schultz, 2006; Peciña et al., 2012), but also to psychological (Pruessner et al., 2004; Montgomery et al., 2006; Soliman et al., 2008; Kobiella et al., 2010), physical (Scott et al., 2006; Scott et al., 2008; Peciña et al., 2012), or pharmacological stressors (Treadway et al., 2012). BDNF plays a key role in the survival and function of DA neurons, including neurons in the VTA that project to the NAc. Animal studies have demonstrated that the rodent striatum is rich in BDNF protein supplied from afferent midbrain DA and corticostriatal glutamate neurons (Altar et al., 1992; Conner et al., 1997; Kolbeck et al., 1999). Consistent with the neurotrophic role of BDNF in DA neurons in animal models, we observed a significant effect of BDNF Val66Met on DA receptor availability, in which BDNF Met66 carriers showed greater baseline D2/3 BPND than Val/Val homozygotes. This is likely a consequence of an upregulation of this receptor sites in the presence of chronic lower DA tone, as would be expected by the lower BDNF function in this group.
Exogenous BDNF has been shown to promote the survival and differentiation of cultured DA neurons (Hyman et al., 1991; Spina et al., 1992) and is associated with an overall increase in tyrosine hydroxylase activity, DA release, DA transporter uptake capacity, and DA tissue content (Hyman et al., 1991; Knüsel and Hefti, 1991; Beck et al., 1993; Zhou et al., 1994; Blöchl and Sirrenberg, 1996; Hoover et al., 2007). Consistent with this crucial role of BDNF in regulating DA release, we observed that the BDNF Val66Met polymorphism had effects on regional activation during the anticipation of monetary loss and pain- and placebo-induced DA release. However, BDNF Met66 carriers, who had lower levels of BDNF and potentially lower activity-dependent release of DA, had a selective increase in brain response to the anticipation of monetary losses (but not rewards) in the VTA-NAc-mPFC cortex and DA release in the NAc during the pain challenge (the latter correlating with subjective pain ratings), but an overall reduction in DA activity in the bilateral NAc during placebo administration compared with Val homozygotes. These results suggest a valence-selective effect of BDNF in regulating DA-mediated aversive stimuli. During the anticipation of monetary losses, greater BOLD responses were also observed in the Hipp in Met66 carriers, a region involved in the regulation of DA neural activity via a Hipp-NAc-ventral pallidum-VTA pathway (Floresco et al., 2001), and responses to aversive stimuli (Valenti et al., 2011). Consistent with the data presented here, BDNF Met66 has been robustly associated with impaired fear extinction learning and increased risk for anxiety disorders (Soliman et al., 2010).
This pattern of greater BOLD responses to the anticipation of monetary loss, but no effect during the anticipation of monetary gain, as well as increases in stress-induced DA release and a blunted DA response during positive expectations (i.e., of pain relief) seems to point to a vulnerability phenotype. This vulnerability phenotype is in agreement with associations between low levels of BDNF and the development of depressive-like anhedonic responses in animal stress models (Duman and Monteggia, 2006) and persistent suppression of cocaine and saccharine reward after intermittent social stress in mice (Miczek et al., 2011). These findings might also explain the neurobiology underlying the frequent comorbidity between mood and substance abuse disorders (Brady and Sinha, 2005). On the contrary, animal models of stress have suggested that preventing BDNF signaling to the NAc may be a key molecular mechanism of stress resiliency (Berton et al., 2006; Krishnan et al., 2007). Susceptibility to an avoidant phenotype was induced by upregulation of VTA neuronal activity, which resulted in increased BDNF signaling within the NAc (Berton et al., 2006). Moreover, although Val/Val and Met/Met mice showed comparable baseline responses in forced swim and sucrose preference tests (Chen et al., 2006), a differential phenotype became evident after chronic social defeat: whereas Val/Val mice demonstrated a significant reduction in social interaction after defeat, Met/Met mice displayed an unsusceptible phenotype (Krishnan et al., 2007). As acknowledged by the investigators (Russo and Nestler, 2013), an important caveat regarding that work is that most chronic stress paradigms in BDNF knock-out mice have relied solely on data acquired in males (Berton et al., 2006; Krishnan et al., 2007). Data in female mice suggests that they may have increased stress vulnerability after chronic unpredictable stress (CUS; Autry et al., 2009). In the present study, loss of BDNF in female mice was associated with increases in anxiety and depression-like behaviors after CUS compared with wild-type littermates, effects that were not observed in males. It has also been suggested that organizational differences in the development of reward-related neural circuits might predispose women to depression (Blehar, 2006). Further studies would have to carefully consider a potential gene × sex interaction modulating stress resiliency mechanisms in humans. The data presented here are not adequately powered to examine those interactions.
The present work provides initial evidence regarding the effect of BDNF Val66Met polymorphism modulating DA-mediated stress and reward responses in the human striatum. We identify a potential vulnerability phenotype in BDNF Met66 carriers defined by: (1) greater BPND in the NAc at baseline than Val homozygotes, suggesting chronic lower DA tone; (2) greater BOLD responses in the VTA-NAc-mPFC circuit during the anticipation of monetary losses, but not during gains; and (3) greater DA release in the NAc during a pain stressor, but a blunted DA response to the administration of a placebo with potential analgesic properties. Interindividual variability within the BDNF human gene therefore appears to be involved in the neuroplastic changes that follow responses to stress and might be involved in the vulnerability and recovery from stressful experiences, potentially affecting the pathophysiology and chronicity of stress related disorders.
Footnotes
This work was supported by The National Institutes of Health–National Institute of Drug Abuse (Grants R01 DA 022520 and R01 DA 027494 to J.K.Z.), the Phil F. Jenkins Foundation, the Spanish Ministry of Education (Grant AP2008-03742 to M.M.-J.), and the Spanish Ministry of Science and Innovation and European (Regional Development Fund Grant PSI2010-19372 to P.M.). We thank the technologists of the PET Center and the fMRI laboratory at the University of Michigan.
The authors declare no competing financial interests.
References
- Aguirre GK, D'Esposito M. Experimental design for brain fMRI. In: Moonen C, Bandettini PA, editors. Functional MRI. Berlin: Springer-Verlag; 1999. pp. 369–380. [Google Scholar]
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Cheng P, Monteggia LM. Gender-specific impact of brain-derived neurotrophic factor signaling on stress-induced depression-like behavior. Biol Psychiatry. 2009;66:84–90. doi: 10.1016/j.biopsych.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck KD, Knüsel B, Hefti F. The nature of the trophic action of brain-derived neurotrophic factor, des(1–3)-insulin-like growth factor-1, and basic fibroblast growth factor on mesencephalic dopaminergic neurons developing in culture. Neuroscience. 1993;52:855–866. doi: 10.1016/0306-4522(93)90534-M. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Blehar MC. Women's mental health research: the emergence of a biomedical field. Annu Rev Clin Psychol. 2006;2:135–160. doi: 10.1146/annurev.clinpsy.2.022305.095344. [DOI] [PubMed] [Google Scholar]
- Blöchl A, Sirrenberg C. Neurotrophins stimulate the release of dopamine from rat mesencephalic neurons via Trk and p75Lntr receptors. J Biol Chem. 1996;271:21100–21107. doi: 10.1074/jbc.271.35.21100. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, Der MG, Pickar D, Eckelman WC. Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab. 1997;17:437–447. doi: 10.1097/00004647-199704000-00009. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF; Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Van der Does AJ, Kouwenhoven C, Elzinga BM, Hommel B. BDNF Val66Met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology. 2011;36:1562–1569. doi: 10.1016/j.psyneuen.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann N Y Acad Sci. 2010;1208:150–157. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Imaging cognitive anatomy. Trends Cogn Sci. 1997;1:21–27. doi: 10.1016/S1364-6613(97)01001-2. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Hum Brain Mapp. 1995;2:165–189. [Google Scholar]
- Gasic GP, Smoller JW, Perlis RH, Sun M, Lee S, Kim BW, Lee MJ, Holt DJ, Blood AJ, Makris N, Kennedy DK, Hoge RD, Calhoun J, Fava M, Gusella JF, Breiter HC. BDNF, relative preference, and reward circuitry responses to emotional communication. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:762–781. doi: 10.1002/ajmg.b.30944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Goggi J, Pullar IA, Carney SL, Bradford HF. Modulation of neurotransmitter release induced by brain-derived neurotrophic factor in rat brain striatal slices in vitro. Brain Res. 2002;941:34–42. doi: 10.1016/S0006-8993(02)02505-2. [DOI] [PubMed] [Google Scholar]
- Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Everett CV, Sorkin A, Zahniser NR. Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J Neurochem. 2007;101:1258–1271. doi: 10.1111/j.1471-4159.2007.04522.x. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/S0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Knüsel B, Hefti F. Trophic actions of IGF-I, IGF-II and insulin on cholinergic and dopaminergic brain neurons. Adv Exp Med Biol. 1991;293:351–360. doi: 10.1007/978-1-4684-5949-4_31. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kobiella A, Vollstädt-Klein S, Bühler M, Graf C, Buchholz HG, Bernow N, Yakushev IY, Landvogt C, Schreckenberger M, Gründer G, Bartenstein P, Fehr C, Smolka MN. Human dopamine receptor D2/D3 availability predicts amygdala reactivity to unpleasant stimuli. Hum Brain Mapp. 2010;31:716–726. doi: 10.1002/hbm.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbeck R, Bartke I, Eberle W, Barde YA. Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J Neurochem. 1999;72:1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Torgerson WS. On the language of pain. Anesthesiology. 1971;34:50–59. doi: 10.1097/00000542-197101000-00017. [DOI] [PubMed] [Google Scholar]
- Meyer CR, Boes JL, Kim B, Bland PH, Zasadny KR, Kison PV, Koral K, Frey KA, Wahl RL. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med Image Anal. 1997;1:195–206. doi: 10.1016/S1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, Berger KL, Taylor SF, Frey KA, Kuhl DE. Automated detection of the intercommissural line for stereotactic localization of functional brain images. J Nucl Med. 1993;34:322–329. [PubMed] [Google Scholar]
- Montgomery AJ, Mehta MA, Grasby PM. Is psychological stress in man associated with increased striatal dopamine levels?: a [11C]raclopride PET study. Synapse. 2006;60:124–131. doi: 10.1002/syn.20282. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Noll DC. Rapid MR image acquisition in the presence of background gradients. IEEE International Symposium on Biomedical Imaging; July 7–10; Washington, DC. 2002. paper WA-SS-1.4. [Google Scholar]
- Noll DC, Meyer CH, Pauly JM, Nishimura DG, Macovski A. A homogeneity correction method for magnetic resonance imaging with time-varying gradients. IEEE Trans Med Imaging. 1991;10:629–637. doi: 10.1109/42.108599. [DOI] [PubMed] [Google Scholar]
- Peciña M, Mickey BJ, Love T, Wang H, Langenecker SA, Hodgkinson C, Shen PH, Villafuerte S, Hsu D, Weisenbach SL, Stohler CS, Goldman D, Zubieta JK. DRD2 polymorphisms modulate reward and emotion processing, dopamine neurotransmission and openness to experience. Cortex. 2012;49:877–890. doi: 10.1016/j.cortex.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Koeppe RA, Zubieta JK. Time-course of change in [11C]carfentanil and [11C]raclopride binding potential after a nonpharmacological challenge. Synapse. 2007a;61:707–714. doi: 10.1002/syn.20404. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007b;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Shen RY, Altar CA, Chiodo LA. Brain-derived neurotrophic factor increases the electrical activity of pars compacta dopamine neurons in vivo. Proc Natl Acad Sci U S A. 1994;91:8920–8924. doi: 10.1073/pnas.91.19.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman A, O'Driscoll GA, Pruessner J, Holahan AL, Boileau I, Gagnon D, Dagher A. Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology. 2008;33:2033–2041. doi: 10.1038/sj.npp.1301597. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina MB, Squinto SP, Miller J, Lindsay RM, Hyman C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. J Neurochem. 1992;59:99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Kircher T, Gründer G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc Cogn Affect Neurosci. 2009;4:158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohler CS, Kowalski CJ. Spatial and temporal summation of sensory and affective dimensions of deep somatic pain. Pain. 1999;79:165–173. doi: 10.1016/S0304-3959(98)00171-7. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE. Measurement of dopamine release with continuous infusion of [11C]raclopride: optimization and signal-to-noise considerations. J Nucl Med. 2000;41:522–530. [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ashton-Miller JA, Stohler CS. A closed-loop system for maintaining constant experimental muscle pain in man. IEEE Trans Biomed Eng. 1993;40:344–352. doi: 10.1109/10.222327. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, Qimei C. Reconsidering Baron and Kenny: myths and truths about mediation analysis. Journal of Consumer Research. 2010;37:197–206. doi: 10.1086/651257. [DOI] [Google Scholar]
- Zhou J, Bradford HF, Stern GM. The stimulatory effect of brain-derived neurotrophic factor on dopaminergic phenotype expression of embryonic rat cortical neurons in vitro. Brain Res Dev Brain Res. 1994;81:318–324. doi: 10.1016/0165-3806(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]