Abstract
Takotsubo cardiomyopathy (TC) is a disease that can be misinterpreted as a more serious acute coronary syndrome. Its clinical characteristics resemble those of a myocardial infarct, while its imaging characteristics are critical on correctly characterizing and diagnosing the disease. From angiography, where coronary anatomy is evaluated, to cardiac magnetic resonance (CMR), where morphology and tissue characterization is assessed, the array of imaging options is quite extent. In particular, CMR has achieved great improvements (stronger magnetic fields, better coils, etc.) in the last decade which in turn has made this imaging technology more attractive in the evaluation and diagnosis of TC. With its superior soft tissue resolution and dynamic imaging capabilities, CMR is currently, perhaps, the most useful imaging technique in TC as apical ballooning or medio-basal wall motion abnormalities (WMA), presence of wall edema and late gadolinium enhancement (LGE) characteristics are critical in the diagnosis and characterization of this pathology. In this review, CMRs role in TC will be evaluated in light of the current available evidence in medical literature, while also revising the clinical and physiopathologic characteristics of TC.
Keywords: Broken heart syndrome, chest pain, MRI, reversible cardiomyopathy, stress cardiomyopathy, takotsubo
Introduction
Takotsubo cardiomyopathy (TC), also known as transient apical ballooning, stress cardiomyopathy or broken heart syndrome, was first described in 1991 by Dote et al. (1) and received its name due to the similarity of the shape of the left ventricle (LV) with a traditional Japanese octopus trap. The importance of knowing and identifying this disease relies in its clinical similarity with other acute coronary syndromes (ACS) that require acute treatment and have a worse prognosis.
Many publications and case reports have been published analyzing the different aspects of this disease, but none has yet elucidated the pathophysiology of it (2-8). Despite increasing evidence that many pathologic conditions increase the risk for developing TC (e.g., chronic liver disease), in regard to the pathophysiology there are only theories with different degrees of evidentiary, but inconclusive, support. Among the many theories explaining the cause of the disease, four are the ones that have been studied the most: spontaneous thrombolysis of a coronary thrombus, multiple coronary vasospasm, microcirculatory dysfunction and catecholamine stunning.
TC has been reported in up to 2.2% of patients presenting with an ACS to the hospital (9), and as physicians become more aware of the disease, this percentage might increase with the detection of cases that were previously misdiagnosed. Among the different diagnostic tools available for the cardiologists and clinicians one that has been gaining an increasing role is cardiac MR (CMR). CMR allows not only the morphologic and dynamic assessment of the heart, as does echocardiography, but also myocardial inflammation can be visualized and when Gadolinium is used, scarring can be detected (10).
The objective of this review is to present the evidence available in the current medical literature of the different benefits of the use of CMR in the diagnosis of TC, as well as provide a short summary of the disease and its clinical aspects.
Pathophysiology
Some authors suggest the spontaneous thrombolysis of a coronary thrombus (11) as a cause of this disease. This would result in a transient episode of cardiac ischemia that isn’t harmful enough to cause cardiac scarring. In this regard, the extent of the wall motion abnormalities (WMA) associated with the syndrome, which extends beyond a single coronary area of perfusion is a major counterpoint for this theory.
Another theory is the presence of multiple coronary vasospasm which would generate a large area of ischemia (2,9). However, as pharmacologically induced vasospasm can only be performed successfully in some TC patients; and the existence of literature showing that some drugs that have a vasodilator effect, such as Dobutamine, are known to have triggered a TC syndrome (4,8), this theory is also regarded as flawed.
A third theory suggests microcirculatory dysfunction with transient ischemia (Figure 1). Furthermore some authors suggest a key role of an excess of catecholamines (4,12) in this phenomenon thus overlapping with a final theory of catecholamine stunning of the myocardium due to excess of this hormone (9,13). These two latter theories have been the most supported thus far and can explain the broad spectrum of diseases that TC has been associated to: chronic liver disease, drug abuse, anxiety disorders, mood disorders, malignancy, hyperthyroidism, cerebrovascular accidents and sepsis (12). This last condition, for example, has been hypothesized to be iatrogenic secondary to the treatment used for the associated hemodynamic instability (12).
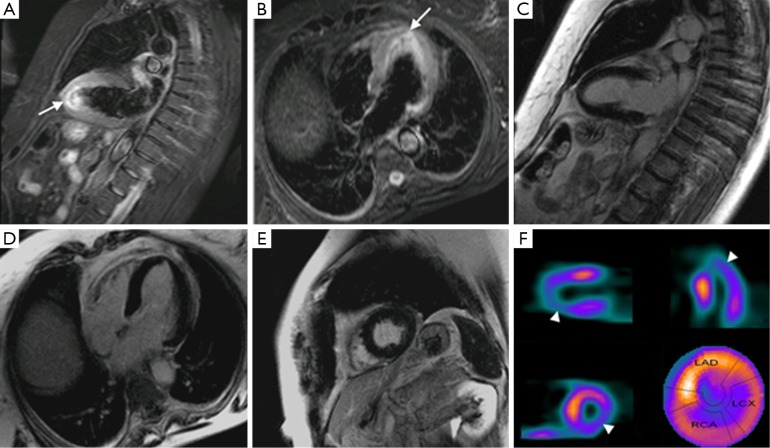
Figure 1.
Long axis, four chamber and short axis in STIR (A,B) and IR (C,D,E) sequences of a patient with TC where edema is visualized (arrows) and no LGE. However, a nuclear scan (F) shows hypoperfusion (arrowheads) of the affected area in MR. These findings support the presence of some sort of microcirculatory dysfunction leading to ischemia. Abbreviations: IR, inversion-recovery; TC, takotsubo cardiomyopathy; LGE, late gadolinium enhancement.
Clinical presentation
TC usually presents in menopausal women after an emotional or physical stress. Although the sex prevalence cannot be explained so far, there are theories relating it to the estrogen shortage (14). Meanwhile some authors have used this sex preference as a counterargument to the catecholamine theory (6) as they state that if the theory were to be true, no sex prevalence should be present. Also, stress might not always be present or recalled by the patient, so the absence of a stressful event cannot be used to discard TC.
The syndrome characterizes as chest pain, ECG alterations with ST-segment elevation and/or dynamic T-wave inversion predominantly affecting the precordial leads, slight raise in cardiac blood markers (CK-mb, troponin) and apical LV dyskinesia, all of which are reversible after a couple of days/months and are not consequence of coronary artery disease (CAD) (2,3,7,12). The Mayo criteria (Table 1) are used in many places as a diagnostic tool and all the criteria must be present in order to properly diagnose a TC. However some authors suggest also the use of imaging criteria, more specifically CMR at the symptomatic stage and follow up, to show the reversibility (9) (Figure 2) and the modification of some aspects of the established Mayo criteria, as they state that TC can coexist with CAD and the presence of CAD should not exclude TC (2). An example of the workflow we have established in our institution can be seen in Figure 3.
Table 1. Mayo Clinic criteria (11).
| 1 | Transient, reversible regional wall-motion abnormalities of the LV extending beyond a single epicardial vascular distribution |
| 2 | Absence of angiographic evidence of flow-limiting coronary disease or acute plaque rupture |
| 3 | New changes in electrocardiogram (ST-segment elevation or T-wave inversion) |
| 4 | No recent significant head trauma, intracranial bleeding, pheochromocytoma, obstructive epicardial CAD, myocarditis, and hypertrophic cardiomyopathy |
Abbreviation: LV, left centricle.
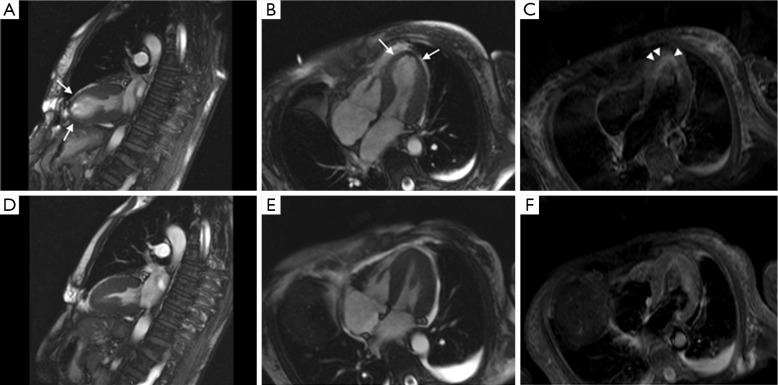
Figure 2.
Symptomatic (A,B,C) and follow-up (D,E,F) MR scan of a patient with TC. Notice the apical ballooning (arrows) as visualized during systole in the long and short axis, as well as the edema (arrowhead). Follow-up images show complete reversibility of the findings. Abbreviation: TC, takotsubo cardiomyopathy.
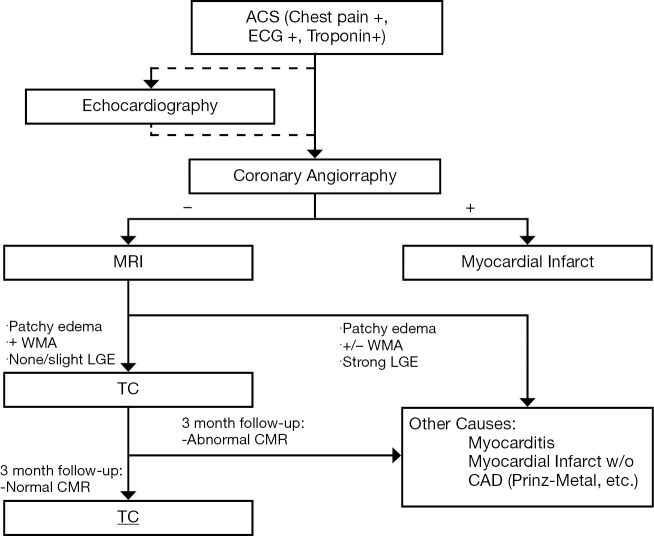
Figure 3.
Established workflow at our institution. When a patient presents with a confirmed ACS (Chest pain, ECG + and Troponin +) a coronary angiography is performed. Before the angiography and at the patient’s bedside an echocardiography is usually performed (although considered optional) in order to start risk stratifying the patient and coming up with possible differentials. If the angiography is concordant with the clinical and echocardiographic findings an MI is diagnosed and further studying of the patient ceased. If the angiography findings are discordant an MR is performed. In light of the MR findings the patient will be classified as a probable TC, to be confirmed through a three months follow-up MR, or will be classified into another set of diseases that can present as an ACS. Abbreviations: ACS, acute coronary syndromes; MI, myocardial infarction; TC, takotsubo cardiomyopathy.
As TC became increasingly studied and detected, many different reports started appearing on variant forms of the disease. So far, cases of mid-ventricular dyskinesia combined, or not, with basal dyskinesia and the presence of basal dyskinesia alone, which is referred to as inverted takotsubo, have been reported (5,15,16). Furthermore, some authors have found epidemiological differences between patients presenting with typical, versus those presenting with atypical TC. For example inverse TC has been seen to happen in younger patients (5,15) and to have higher cardiac blood markers (CK-mb, troponin, NT-proBNP) (5), although the latter might be explained by the amount of cardiac muscle involved in one another, as LVEF was not different amongst groups (5). It has also been seen that the stressful event is present in all patients with inverted TC, while it is not the rule in typical TC (5). Finally, it has been reported that mitral regurgitation and higher NT-proBNP are associated more with apical or mid-ventricular TC than inverted TC (5).
Different diseases have been associated with an increased risk of suffering TC (Table 2). The importance of differentiating TC from other ACS and inflammatory conditions of the heart, requires the physicians to be aware of these associations. The diseases that have an association with TC, either increase the sympathetic tone or the catecholamine concentration in blood. The first physiopathologic mechanism is believed to be responsible for the higher prevalence of malignancy in TC than in comparable population (5,12). All of these associations, although none clearly understood, support the general hypothesis that catecholamines play a major role in the pathophysiology of TC.
Table 2. Diseases with higher risk of suffering TC (5).
| Hyperthiroidism |
| Sepsis |
| Chronic liver disease |
| Drug abuse |
| Anxiety disorders |
| Mood disorders |
| Malignancies |
Abbreviation: TC, takotsubo cardiomyopathy.
In general TC is treated conservatively with symptomatic support and follow-up. Some reports even suggest that some complications such as ventricular thrombus can be left without aggressive treatment and they will resolve without further complications (9). It usually has an in-hospital mortality of 1.1-1.7% (2), excellent prognosis, with a 4-year survival study showing no difference between TC patients and an age and gender matched population (9), and a reported recurrence rate of 10% (2). Complete recovery in 96% of patients occurs within 7-37 days (2).
Possible complications in TC include: left heart failure with or without pulmonary edema, acute pericarditis, mitral valve regurgitation, ventricular arrhythmias, intramural thrombus, stroke, LV free wall rupture and death (2).
MR imaging protocol
MR cardiac imaging in TC and any other ACS needs not only to assess the presence or absence of structural abnormalities, but also needs to evaluate the presence of myocardial edema and scarring. MR scanners should be 1.5 T or higher with dedicated cardiac coils for proper imaging.
For structural abnormalities, T1 gated sequences in short and long axis and a four chamber plane, as well as free precession breath-hold cines in short and long axis and outflow tract are useful. For myocardial edema, T2 weighted images with triple inversion recovery (IR) in the short and long axis, as well as a four chamber acquisition is recommended. Finally, to assess for the presence/absence of scarring tissue, ten minutes after Gd injection, short and long axis acquisitions identical to the ones done in cine mode are performed using an IR gradient echo sequence. The description of the sequences we use clinically can be seen in Table 3.
Table 3. MR sequences.
| Protocol | Sequences | LV plane | Tips |
|---|---|---|---|
| Black-blood T2-weighted images | Black-blood triple inversion recovery | Short-axis plane (basal-medial-apical) Optional vertical long-axis plane, three and four-chamber view) |
Fat suppression enhances the edema hypersignal; note whether edema has a vascular location |
| Cine gradient-echo | Standard True-Fisp (fast imaging steady-state free precession) for functional images | Multiphase images in short-axis plane, (11 to 15 slices), 8 mm in thickness with a 2-mm interslice gap, achieving full ventricular coverage, and two three and four chamber views | It allows assessment of the contractility and ejection fraction of LV |
| Late enhancement | Inversion-recovery turbo field echo with an inversion pre-pulse and fat suppression |
15 slices can be obtained in a breath-hold short-axis plane, vertical long-axis plane, and three and four-chamber view are recommended | The signal of normal myocardium is suppressed |
Abbreviation: LV, left ventricle.
Images obtained are usually evaluated in dedicated workstations were LV and RV volumes and other measurements are performed and recorded for later reporting. In our institution, cardiac MRs are read in a radiology-cardiology collaborative effort.
Imaging findings
Edema
A direct result of an inflammatory process is edema, and as endomyocardial biopsies have already proven (9), inflammation is present in TC (Figure 4). The area of edema usually corresponds to the area of WMA and cannot be differentiated from edema secondary to other causes, such as myocarditis. To make this differentiation late gadolinium enhancement (LGE) is usually relied upon, although myocarditis without LGE can happen, thus precluding from a complete dismissal of this entity when LGE is not seen. In the latter case, laboratory findings, the age of the patient and other imaging findings may help in the differential. Another indicator of the role inflammation plays in TC, is the slight association between wall edema and pericardial effusion (6).
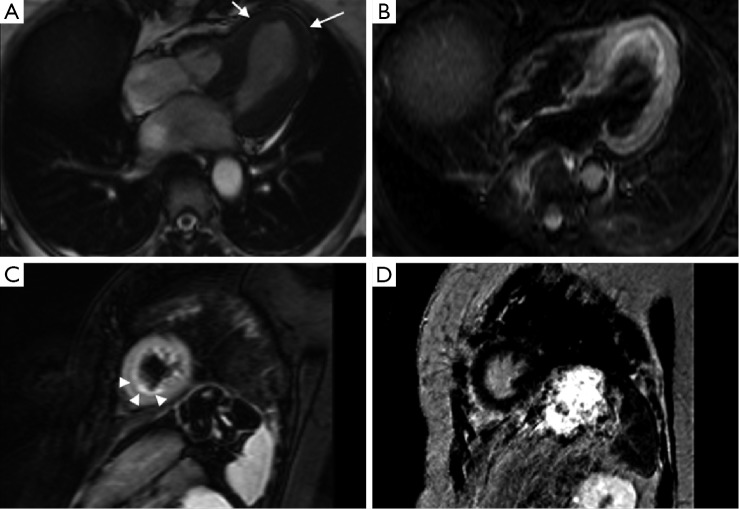
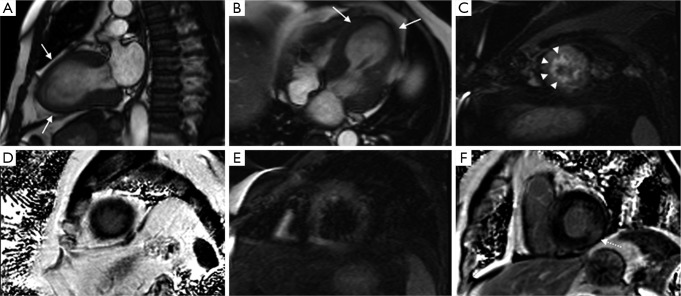
Figure 4.
Typical findings in TC. Cine sequence (A) shows apical ballooning during systole (arrow). STIR sequence in four chamber (B) and short axis (C) planes show wall edema on the same area (arrowheads). Finally, IR sequence in short axis (D) ten minutes after GD injection shows absence of LGE. Abbreviations: TC, takotsubo cardiomyopathy; IR, inversion-recovery; LGE, late gadolinium enhancement.
Even though T2 weighted triple IR is normally used in CMR to detect edema, some authors have suggested the use of T2-steady-state-free precession (SSFP) and a mapping technique to diagnose it. Those authors showed evidence that breathing motion can detrimentally affect the capability of the routinely used sequence to subjectively or objectively detect edema, while the sequence and mapping technique they propose seems to be more insensitive to motion (17).
Wall motion abnormality
WMA and the cine sequences in CMR that allow their visualization are a critical component in the diagnosis of TC and its different subtypes. It is critical in the diagnosis, because one of the most relevant criteria is the reversible regional wall-motion abnormalities of the LV that extend beyond a single epicardial vascular distribution. Regarding the identification of the different subtypes, it has been observed that typical and mid-ventricular TC have more severe heart failure symptoms (5). This latter finding is probably related to the higher frequency of reversible mitral valve regurgitation observed in these subgroups (5), a trait easily seen in cine sequences.
Another issue that has recently been seen with the use of CMR and its cine sequences is a significant percentage of patients (34-42%) with RV involvement, which in turn was associated with longer hospitalization, worse markers of heart failure (lower LVEF, etc.) and older age (7,9). Also, significant pleural effusion was seen to be associated with RV involvement, thus making it a reliable indicator for the presence of the latter (9).
LGE
This probably has been one of the most studied areas of CMR in TC. While initially it was believed that lack of LGE was a necessary condition to diagnose TC (9,16), and would thus help differentiate it from myocardial infarction (MI), where LGE is always present at some degree, and myocarditis, where 88% of patients show a patchy type of LGE (16). Eventually, some isolated reports (18) and, later on, more significant studies (7,13,19,20), showed that LGE can happen in the context of TC (Figure 5). Also, it should be kept in mind that TC and LGE due to a different pathology might co-exist (Figure 6). The critical difference with the LGE observed in MI and myocarditis is the cutoff value used to determine its presence. While MI and myocarditis tend to show signal intensities (SI) higher than five standard deviations of the SI observed in remote normal myocardium, different authors have suggested a cut-off value of 3-4 SD to detect LGE related to TC (7,19).
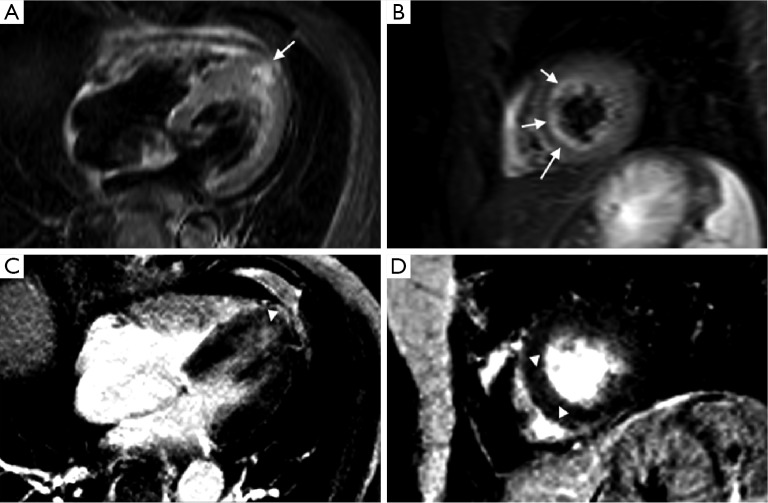
Figure 5.
MR scan of a patient with known cardiac sarcoidosis shows findings compatible with TC (A,B,C,D) and sarcoidosis (E,F). Cine sequences (A,B) show apical ballooning (arrows). STIR and IR sequences (C,D,E,F) show a patchy area of LGE (dotted arrow) with no associated edema (E,F) and an area of edema (arrowheads) with no associated LGE (C,D). Thus, coexistence of two overlapping heart diseases has to be taken into account and looked for when abnormal MR findings are observed. Abbreviations: TC, takotsubo cardiomyopathy; IR, inversion-recovery; LGE, late gadolinium enhancement.
Figure 6.
STIR (A,B) and IR (C,D) sequences in a patient with TC show minimal LGE in the apico-medial wall (arrowheads) in concordance to the area of edema (arrows). Abbreviations: TC, takotsubo cardiomyopathy; LGE, late gadolinium enhancement.
In concordance to these findings of LGE in TC patients, Rolf et al. performed multiple endomyocardial biopsies from areas of WMA with and without LGE showing a significantly higher extracellular matrix containing collagen-1 in areas with LGE in comparison to areas without LGE (21). Furthermore, repeat biopsies in the same areas after functional recovery showed normalization of the concentration of collagen-1. At the same time, necrosis and edema were not evidenced in any of the biopsies ruling out their role in LGE in TC patients (21). Thus, increased extracellular matrix, as a finding of transient fibrosis, might be accountable for the LGE observed in TC patients.
The importance of detecting LGE in TC patients relies on the observed association between the presence of LGE and poorer prognosis in both ischemic and non-ischemic cardiomyopathies (21). Furthermore, in a series of 20 patients with TC (20), it was observed that LGE was associated with an increased frequency of cardiogenic shock and longer day duration to ECG normalization. In that same series and in another study (19), it was also observed a longer period of time to recover from the WMA (19,20).
Complications
As it was stated before, the possible complications of TC are: left heart failure with or without pulmonary edema, acute pericarditis, mitral valve regurgitation, ventricular arrhythmias, intramural thrombus, stroke, LV free wall rupture and death (2). From that set of complications most of them can be diagnosed by MR.
Conclusions
TC is a complex and, probably, underdiagnosed disease. As it has a better prognosis and less aggressive treatment than other ACS, it is of high importance to properly identify the patients that are suffering from this condition, in order to avoid unnecessary interventions and expenditures. In that sense CMR, has shown great results in providing evidence to substantiate the diagnosis. Furthermore, CMR has shown the ability to properly classify the different subtypes of TC and identify certain imaging characteristics (RV involvement, LGE, etc.) that can also stratify the risk of developing complications or requiring closer follow up.
The usefulness of CMR has come to a point at which some authors suggest the inclusion of it, both at initial presentation and follow up, in an ideal workflow to diagnose TC (9). Although, there are still things to be clarified about certain aspects of CMR in this disease (e.g., how to best differentiate myocarditis from TC), evidence has shown that CMR has a critical role in diagnosing the disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Dote K, Sato H, Tateishi H, et al. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol 1991;21:203-14 [PubMed] [Google Scholar]
- 2.Pernicova I, Garg S, Bourantas CV, et al. Takotsubo cardiomyopathy: a review of the literature. Angiology 2010;61:166-73 [DOI] [PubMed] [Google Scholar]
- 3.Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004;141:858-65 [DOI] [PubMed] [Google Scholar]
- 4.Arias AM, Oberti PF, Pizarro R, et al. Dobutamine-precipitated Takotsubo cardiomyopathy mimicking acute myocardial infarction: a multimodality image approach. Circulation 2011;124:e312-5 [DOI] [PubMed] [Google Scholar]
- 5.Song BG, Chun WJ, Park YH, et al. The clinical characteristics, laboratory parameters, electrocardiographic, and echocardiographic findings of reverse or inverted takotsubo cardiomyopathy: comparison with mid or apical variant. Clin Cardiol 2011;34:693-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eitel I, Lücke C, Grothoff M, et al. Inflammation in takotsubo cardiomyopathy: insights from cardiovascular magnetic resonance imaging. Eur Radiol 2010;20:422-31 [DOI] [PubMed] [Google Scholar]
- 7.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011;306:277-86 [DOI] [PubMed] [Google Scholar]
- 8.Abdulla I, Ward MR. Tako-tsubo cardiomyopathy: how stress can mimic acute coronary occlusion. Med J Aust 2007;187:357-60 [DOI] [PubMed] [Google Scholar]
- 9.Eitel I, Behrendt F, Schindler K, et al. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J 2008;29:2651-9 [DOI] [PubMed] [Google Scholar]
- 10.Bohl S, Schulz-Menger J.Cardiovascular magnetic resonance imaging of non-ischaemic heart disease: established and emerging applications. Heart Lung Circ 2010;19:117-32 [DOI] [PubMed] [Google Scholar]
- 11.Schmalfuss C.Tako-tsubo cardiomyopathy and cardiac magnetic resonance imaging. Clin Cardiol 2011;34:145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Sayed AM, Brinjikji W, Salka S. Demographic and co-morbid predictors of stress (takotsubo) cardiomyopathy. Am J Cardiol 2012;110:1368-72 [DOI] [PubMed] [Google Scholar]
- 13.Avegliano G, Huguet M, Costabel JP, et al. Morphologic pattern of late gadolinium enhancement in Takotsubo cardiomyopathy detected by early cardiovascular magnetic resonance. Clin Cardiol 2011;34:178-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539-48 [DOI] [PubMed] [Google Scholar]
- 15.Ramaraj R, Movahed MR. Reverse or inverted takotsubo cardiomyopathy (reverse left ventricular apical ballooning syndrome) presents at a younger age compared with the mid or apical variant and is always associated with triggering stress. Congest Heart Fail 2010;16:284-6 [DOI] [PubMed] [Google Scholar]
- 16.Haghi D, Fluechter S, Suselbeck T, et al. Cardiovascular magnetic resonance findings in typical versus atypical forms of the acute apical ballooning syndrome (Takotsubo cardiomyopathy). Int J Cardiol 2007;120:205-11 [DOI] [PubMed] [Google Scholar]
- 17.Thavendiranathan P, Walls M, Giri S, et al. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging 2012;5:102-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruder O, Hunold P, Jochims M, et al. Reversible late gadolinium enhancement in a case of Takotsubo cardiomyopathy following high-dose dobutamine stress MRI. Int J Cardiol 2008;127:e22-4 [DOI] [PubMed] [Google Scholar]
- 19.Nakamori S, Matsuoka K, Onishi K, et al. Prevalence and signal characteristics of late gadolinium enhancement on contrast-enhanced magnetic resonance imaging in patients with takotsubo cardiomyopathy. Circ J 2012;76:914-21 [DOI] [PubMed] [Google Scholar]
- 20.Naruse Y, Sato A, Kasahara K, et al. The clinical impact of late gadolinium enhancement in Takotsubo cardiomyopathy: serial analysis of cardiovascular magnetic resonance images. J Cardiovasc Magn Reson 2011;13:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolf A, Nef HM, Möllmann H, et al. Immunohistological basis of the late gadolinium enhancement phenomenon in tako-tsubo cardiomyopathy. Eur Heart J 2009;30:1635-42 [DOI] [PubMed] [Google Scholar]