Abstract
We report an extremely rare case of primary squamous cell carcinoma of the stomach. A 69-year-old man was admitted to our hospital with a 2-month history of dysphagia and tarry stools. Endoscopic examination revealed a cauliflower-shaped protruding mass along the lesser curvature of the gastric cardia. Biopsy of the lesion revealed squamous cell carcinoma of the stomach. Computed tomography revealed a thickened stomach wall and a mass protruding into the gastric lumen. Total gastrectomy with splenectomy, distal pancreatectomy, and Roux-en-Y reconstruction was performed, together with a lower thoracic esophagectomy via a left thoracotomy. Histopathological examination of the specimen revealed well-differentiated squamous cell carcinoma of the stomach. Postoperative follow-up was uneventful for the first 18 months. However, multiple liver metastases and para-aortic lymph node metastasis developed subsequently. Despite systemic combination chemotherapy, the patient died because of progression of the recurrent tumors. Here, we review the characteristics of 56 cases of gastric squamous cell carcinoma reported in Japan.
Keywords: Gastric cancer, Squamous cell carcinoma
Introduction
Primary squamous cell carcinoma (SCC) of the stomach is extremely rare, with a worldwide incidence of 0.04% to 0.07% of all gastric cancers.1,2 The pathogenesis of this tumor remains unclear, and the optimal treatment strategy is controversial. Although the incidence of gastric cancer in Japan is much higher than that in Western countries, primary gastric SCC is still rare.3 To date, 56 cases, including the present one, have been reported in the Japanese literature. Here, we describe a resected case of SCC of the stomach and review previously reported Japanese cases.
Case Report
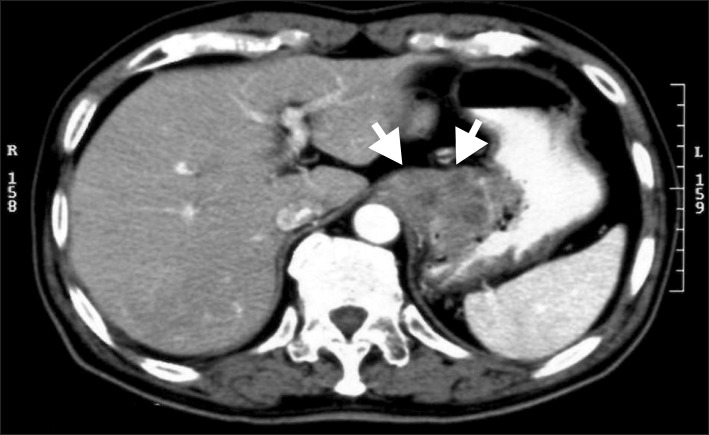
A 69-year-old Japanese man was admitted with a 2-month history of dysphagia and tarry stools. The results of physical examination were unremarkable. Routine laboratory tests revealed leukocytosis (11,900 cells/mm3). The tumor marker levels were as follows: SCC antigen, 2.5 ng/ml (normal range, 0~0.5 ng/ml) and CYFRA 21-1, 17 ng/ml (normal range, 0~1.5 ng/ml). Upper gastrointestinal endoscopy revealed erosive esophagitis and a cauliflower-shaped protruding mass located in the gastric cardia. The mass arose from the lesser curvature (Fig. 1A). Histological examination of biopsy specimens revealed a well-differentiated SCC. Several biopsy specimens were collected from the gastric mucosa surrounding the tumor. No Helicobacter pylori was detected, but the mucosa of the esophagogastric junction was mildly inflamed. An upper gastrointestinal barium series revealed an elevated mass arising from the lesser curvature of the gastric cardia (Fig. 1B). Computed tomography (CT) of the abdomen with intravenous administration of a contrast dye revealed a large heterogeneous mass on the dorsal wall of the stomach, with no evidence of metastatic disease outside the stomach (Fig. 2). Total gastrectomy with splenectomy, distal pancreatectomy, and Rouxen-Y reconstruction was performed, together with a lower thoracic esophagectomy via a left thoracotomy. There was no evidence of regional or distant metastasis. On opening the stomach, an ulcerated mass in the cardia, measuring 7.5×9.0 cm, was observed along the lesser curvature (Fig. 3A). Pathological examination of sections of the resected specimen stained with hematoxylin and eosin revealed a well-differentiated SCC with frequent typical keratin pearl formation, mosaic patterns of cell arrangement, and intracellular bridges (Fig. 3B). The esophageal and pyloric margins were free of tumor involvement, but tumor cells infiltrated the serosa of the dorsal margin. No nodal metastasis was apparent. The tumor had not invaded the mucosa of the esophagogastric junction. In addition, no squamous cells were detected in the normal portion of the stomach. The clinical stage was stage IIB (T4aN0M0), according to the TNM classification, version 7. Therefore, primary SCC of the stomach was diagnosed. The patient refused adjuvant chemotherapy. However, 18-months after the operation, a CT scan revealed a metastatic tumor that was 4.0 cm in diameter in segment 6 of the liver, accompanied by para-aortic lymph node metastasis. Although the patient immediately received systemic chemotherapy with 5-fluorouracil plus cisplatin and docetaxel plus cisplatin, the recurrent tumors progressed. Liver metastases developed subsequently, and the patient died 36 months after the first operation.
Fig. 1.
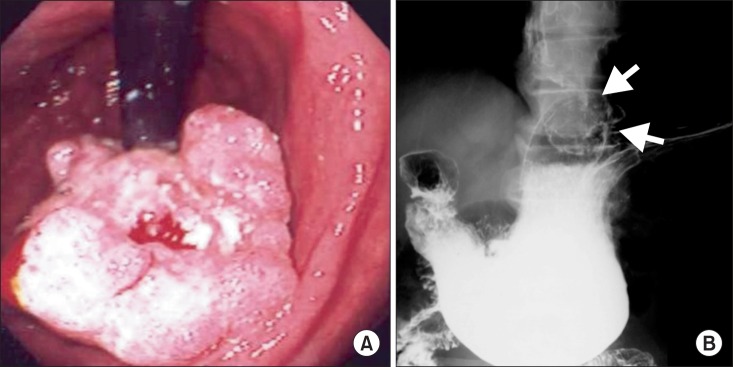
(A) Gastrointestinal endoscopic examination showing a cauliflower-shaped protruding mass involving the lesser curvature of the gastric cardia. (B) An upper gastrointestinal barium series showing an elevated mass arising in the lesser curvature of the gastric cardia (arrows).
Fig. 2.
A computed tomography showing a heterogeneous mass approximately 5.0 cm in diameter arising in the gastric cardia (arrows).
Fig. 3.
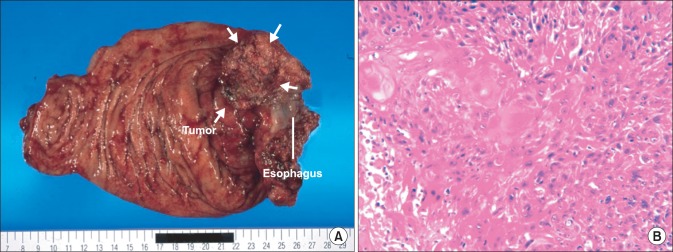
(A) Macroscopic examination of the resected specimens showing an ulcerated mass, measuring 7.5×9.0 cm, in the gastric cardia along the lesser curvature (arrows). (B) Histopathological examination showing a well-differentiated squamous cell carcinoma (H&E, ×200).
Discussion
Primary SCC of the stomach is defined according to the following diagnostic criteria proposed by the Japanese Classification of Gastric Carcinoma4: (1) all tumor cells are SCC cells, with no adenocarcinomatous components in any sections and (2) distinct evidence that SCC arises directly from the gastric mucosa. The pathogenesis of gastric SCC remains poorly understood because these tumors are very uncommon, and most published reports describe only single case. The pathogenesis of this tumor has been debated in many reports, and gastric SCC has been suggested to arise via several mechanisms: (1) squamous differentiation in a preexisting adenocarcinoma, (2) squamous metaplasia of the gastric mucosa before malignant transformation, (3) multipotent stem cells capable of giving rise to any cell type, and (4) nests of ectopic squamous epithelium in the gastric mucosa.2,5,6 In our patient, the tumor was mainly located along the lesser curvature of the cardia of the stomach. Histopathological analysis did not reveal any tumor cells in the mucosa of the esophagogastric junction. Furthermore, the tumor clearly originated in the stomach. All serial sections of resected specimens consisted of SCC cells without adenocarcinomatous components, and extensive evaluations, including thoracic CT and abdominal-pelvic CT, did not reveal any evidence of SCC in any other organ system. Therefore, a primary SCC of the stomach was diagnosed.
Primary SCC is a rare gastric tumor, with less than 100 cases reported in the English literature to date. A review of the Japanese literature, performed with the use of Ichushi-Web (http://login.jamas.or.jp/; NPO Japan Medical Abstracts Society), indicated that only 56 cases of primary SCC of the stomach, including the one we described here, have been reported in Japan (Table 1). The age at onset was 29 to 81 years (mean, 64.7±1.7 years), and the male-to-female ratio was 44 : 12, indicating that these tumors are more common in men. The most common tumor location was the upper third of the stomach (57.1%), followed by the lower third (21.4%) and the middle third (19.6%). Tumor diameter was 2.1 to 13 cm (mean, 6.6±0.3 cm). The most frequent macroscopic tumor type was type 2 (43%). The depth of invasion was T4a (n=8, 14%) or T4b (n=25, 45%) in more than 50% of all reported cases. Therefore, surgical curability tended to be poor (A: absent residual tumor [17%], B: microscopically present residual tumor [40%] and C: macroscopically present residual tumor [30%]). Previous reports indicate that radical surgical excision is the only potential cure for localized disease. For advanced-stage disease, surgical resection alone might be inadequate for the management of SCC of the stomach. Aggressive surgery plus adjuvant chemotherapy appears to result in a better outcome than surgery alone in terms of survival, although experience with this strategy is limited.1,6
Table 1.
Primary squamous cell carcinoma of the stomach in the Japanese cases
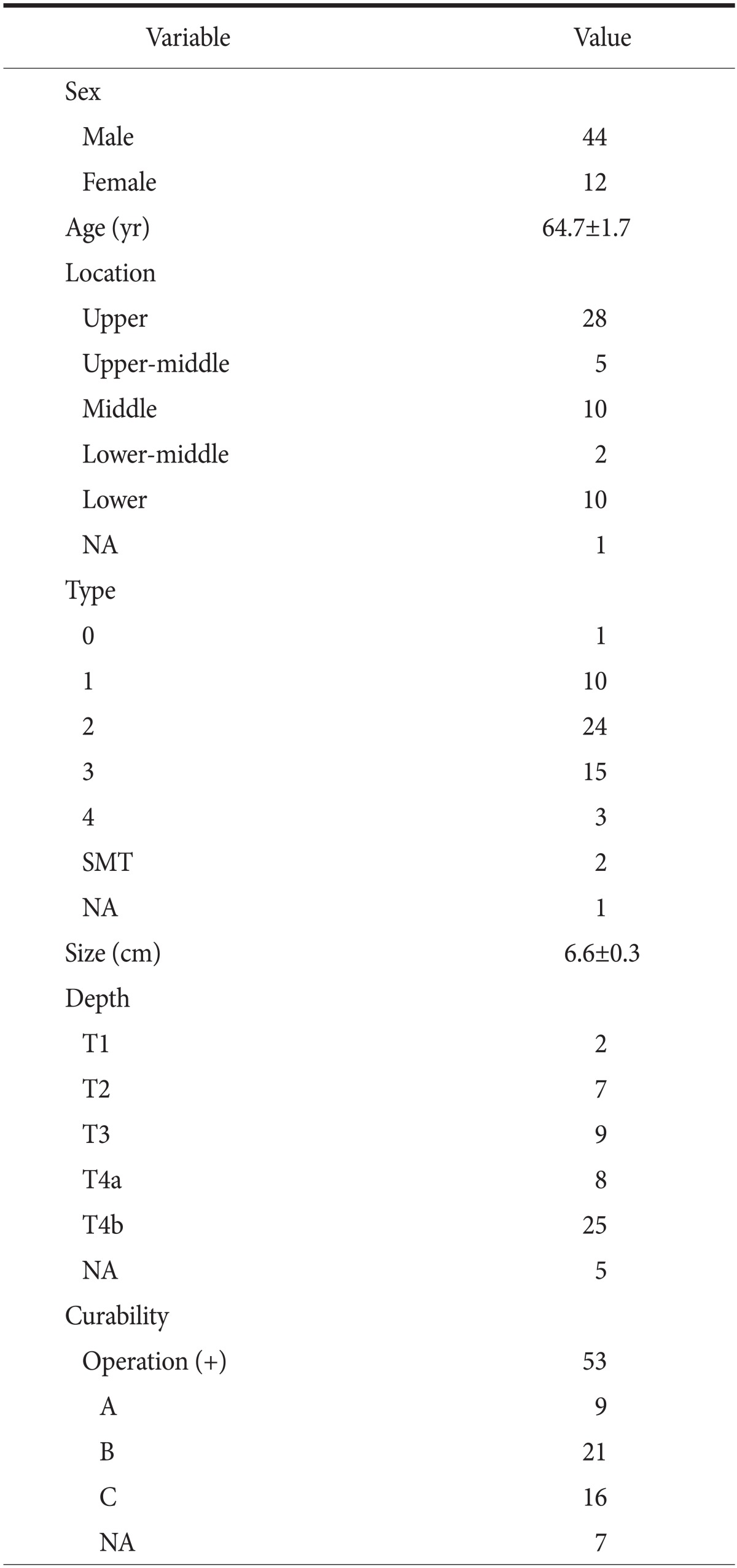
Values are presented as number, or mean±standard deviation. NA = not available. A = absent residual tumor; B = microscopically present residual tumor; C = macroscopically present residual tumor.
Some studies in the English literature1,2,7 have reported that primary gastric SCC has a better prognosis than gastric adenocarcinoma. However, gastric SCC is usually diagnosed at an advanced stage, and it aggressively metastasizes to the liver, lymph nodes, and other organs, generally leading to poor outcomes.3,8,9,10 To date, no standard chemotherapy or chemotherapeutic regimen has been defined for SCC of the stomach. Among the cases reported in Japan, only 1 patient previously received neoadjuvant chemotherapy, and only 19 patients received systemic chemotherapy postoperatively (Table 2). Therefore, whether chemotherapy is effective for gastric SCC remains to be determined. Our patient received definitive chemotherapy with 5-fluorouracil plus cisplatin and docetaxel plus cisplatin to manage the metastatic tumor in the liver and lymph node metastasis. However, he died of progressive disease 17 months after the initiation of chemotherapy. Therefore, it is necessary to develop new therapeutic strategies, including regimens for adjuvant and definitive chemotherapy or chemoradiotherapy, for advanced SCC of the stomach.
Table 2.
Adjuvant chemotherapy for primary squamous cell carcinoma of the stomach in the Japanese cases
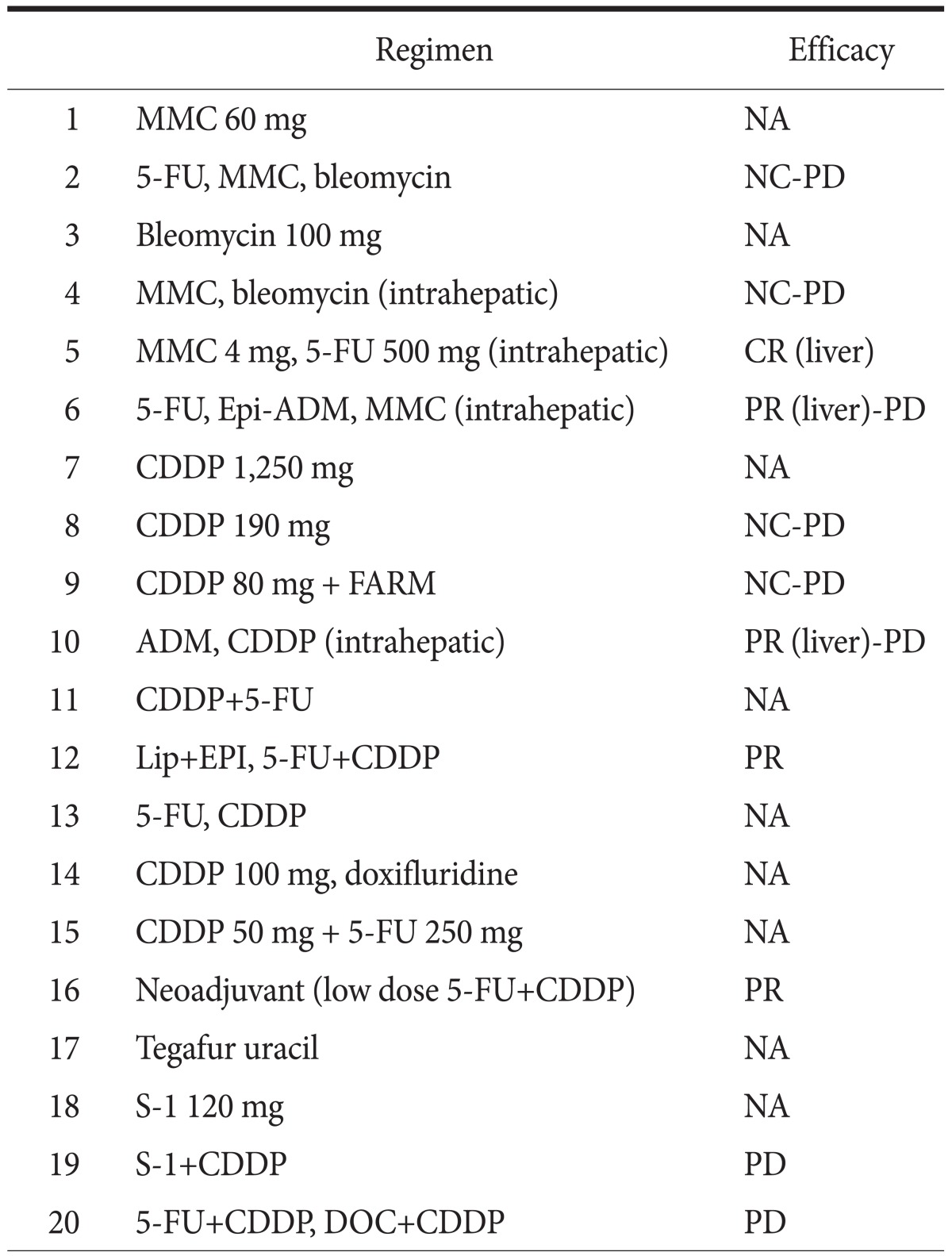
MMC = mitomycin; 5-FU = 5-fluorouracil; Epi-ADM = epirubicinadriamycin; CDDP = cisplatin; FARM = farmorubicin; Lip = lipiodol; EPI = farmorubicin; DOC = docetaxel; NA = not available; NC = no change; PD = progressive disease; CR = complete response; PR = partial response.
Acknowledgement
Department of Pathology, Nippon Medical School Tama-Nagayama Hospital performed the histological examination and played a crucial role in producing this paper.
References
- 1.Bonnheim DC, Sarac OK, Fett W. Primary squamous cell carcinoma of the stomach. Am J Gastroenterol. 1985;80:91–94. [PubMed] [Google Scholar]
- 2.Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985–995. doi: 10.1002/1097-0142(196911)24:5<985::aid-cncr2820240518>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Muto M, Hasebe T, Muro K, Boku N, Ohtsu A, Fujii T, et al. Primary squamous cell carcinoma of the stomach: a case report with a review of Japanese and Western literature. Hepatogastroenterology. 1999;46:3015–3018. [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 5.Amuluru K, Gupta H. Primary squamous cell carcinoma of the stomach: a case report. J Gastrointest Cancer. 2010;41:24–26. doi: 10.1007/s12029-009-9097-4. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt C, Schmid A, Lüttges JE, Kremer B, Henne-Bruns D. Primary squamous cell carcinoma of the stomach. Report of a case and review of literature. Hepatogastroenterology. 2001;48:1033–1036. [PubMed] [Google Scholar]
- 7.Altshuler JH, Shaka JA. Squamous cell carcinoma of the stomach. Review of the literature and report of a case. Cancer. 1966;19:831–838. doi: 10.1002/1097-0142(196606)19:6<831::aid-cncr2820190613>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Dursun M, Yaldiz M, Işikdoğan A, Yilmaz G, Canoruç F, Ormeci N, et al. Primary squamous cell carcinoma of the stomach: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2003;15:329–330. doi: 10.1097/01.meg.0000050011.68425.82. [DOI] [PubMed] [Google Scholar]
- 9.Mori M, Iwashita A, Enjoji M. Squamous cell carcinoma of the stomach: report of three cases. Am J Gastroenterol. 1986;81:339–342. [PubMed] [Google Scholar]
- 10.Volpe CM, Hameer HR, Masetti P, Pell M, Shaposhnikov YD, Doerr RJ. Squamous cell carcinoma of the stomach. Am Surg. 1995;61:1076–1078. [PubMed] [Google Scholar]