Abstract
Objective
To investigate the surgical and oncological outcomes of laparoscopic surgery compared with laparotomy for the treatment of early-stage ovarian cancer.
Methods
Data from patients who underwent surgical management for early-stage ovarian cancer between 2006 and 2012 were retrospectively reviewed. All patients presented with stage I or II disease, and underwent comprehensive staging surgery consisting of a total hysterectomy, bilateral salpingo-oophorectomy, pelvic and para-aortic lymphadenectomy, omentectomy, and peritoneal cytology.
Results
Seventy-seven patients who underwent laparoscopic surgery (24 patients) or laparotomy (53 patients) were identified. Surgery for none of the patients was converted from laparoscopy to laparotomy. The mean operation time was shorter and the estimated blood loss was lower in the laparoscopy group than in the laparotomy group, though the differences were not statistically significant (193 min vs. 224 min, p=0.127; 698 mL vs. 973 mL, p=0.127). There were no differences in the intraoperative or postoperative complications. During a mean follow-up period of 31 months, tumor recurrence occurred in 4 patients: 2 (8.3%) in the laparoscopy group and 2 (3.8%) in the laparotomy group. The mean disease-free survival was 59 months after laparoscopy and 66 months after laparotomy (p=0.367).
Conclusion
Laparoscopic surgery seems to be adequate and feasible for the treatment of early-stage ovarian cancer with comparable results to laparotomy in terms of the surgical outcomes and oncological safety.
Keywords: Early-stage ovarian cancer, Laparoscopy surgery, Laparotomy staging
INTRODUCTION
Ovarian cancer accounts for approximately only a quarter of all gynecological malignancies, but it is a leading cause of gynecological cancer death. Most cases of ovarian cancer are diagnosed incidentally during surgery for presumably benign adnexal masses observed with imaging techniques or after clinical examination. The standard treatment for early-stage ovarian cancer is primarily surgical management with or without chemotherapy. According to the International Federation of Gynecology and Obstetrics (FIGO) guidelines, the optimal staging procedures for ovarian cancer include total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, peritoneal biopsies, diaphragmatic scrapings, bilateral pelvic and para-aortic lymph node dissection, and maximal debulking effort with the intent of leaving "no visible and no palpable disease."
Laparoscopic surgery is considered the gold standard for the treatment of benign adnexal masses [1]. Although minimally invasive surgical techniques have dramatically improved over the last few years and are frequently applied in gynecologic surgery [2,3], many physicians continue to debate the use of laparoscopic surgery for ovarian cancer. A laparoscopic approach for the staging of ovarian cancer was first reported in 1994 [4]. Since then, many researchers have sought to demonstrate the advantages of laparoscopic surgery for early-stage ovarian cancer. In one of the largest series study, clinical evidence indicated that laparoscopic staging of ovarian cancer appeared to be feasible and comprehensive without compromising survival, supporting the use of laparoscopy in the management of early-stage ovarian cancer [5]. On the other hand, controversy remains concerning port-site metastasis, the spread of tumor cells owing to intra-abdominal CO2 pressure or tumor rupture, the inadequacy of staging, and insufficient nodal yield with laparoscopy. In particular, port-site metastasis has a widely varying reported incidence of 1%-16% [6,7]. Although the Cochrane database was published in 2008 and 2013 in order to evaluate the benefits and harms of laparoscopy for the surgical treatment of FIGO stage I ovarian cancer, it has not provided high-quality evidence, and the issue remains unclear [8,9]. In this study, we aimed to compare the surgical and oncological outcomes between laparoscopy and laparotomy staging for early-stage ovarian cancer.
MATERIALS AND METHODS
A retrospective analysis of all patients who underwent primary surgical management for ovarian cancer between October 2006 and December 2012 at the Cheil General Hospital and Women's Healthcare Center was performed after approval by the institutional review board. Patients eligible for inclusion in the study were those who underwent comprehensive laparoscopy or laparotomy staging surgery for early-stage ovarian cancer. As stated in the FIGO guidelines, comprehensive staging surgery included total hysterectomy, bilateral salpingooophorectomy, systemic pelvic and para-aortic lymph node dissection, omentectomy, peritoneal cytology, and multiple biopsies from the entire abdominal peritoneum, including the subdiaphragmatic area and the paracolic gutter. As a rule, appendectomy was performed in all cases of mucinous ovarian tumors, but it was only optionally performed in patients with other types of pathological tumors. Patients with borderline ovarian malignancy, advanced ovarian cancer of FIGO stage III or IV, or a concurrent malignancy of another organ were excluded. Patients referred from other hospitals after staging surgery or who had a history of fertility-sparing surgery were also excluded.
1. Patient selection and preoperative preparation
In our institution, although there is no clear indication for laparoscopic surgery in ovarian cancer, surgeons tend to prefer laparoscopy over laparotomy in cases of suspicious early-stage tumors according to the preoperative evaluation, including imaging studies, physical examination, and laboratory work-ups. However, the surgical modality is ultimately determined by the surgeon after considering the surgeons' skill and the patients' characteristics. As a preoperative preparation for both laparoscopy and laparotomy, the patients were placed on a liquid diet at least 2 days before the surgery, and their bowel was evacuated by using magnesium citrate so that the bowel was collapsed during the surgery. Graduated compression stockings were used in conjunction with an intermittent pneumatic venous compression device from immediately before the surgery to 24 hours after the surgery.
2. Surgical procedures
Laparotomy was performed in all cases via midline longitudinal incision by gynecologic specialists, according to our routine institutional practice. In laparoscopic surgery, a 10-mm 0° laparoscope was introduced at the umbilical site after pneumoperitoneum was established. Under direct vision, 3 ancillary trocars were positioned: one 12-mm suprapubic trocar for extraction of the retrieved lymph nodes and two 5-mm trocars at the lower abdomen lateral to the epigastric arteries. After employing this 4-trocar system, pelvic procedures including hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy were performed. Next, in order to perform para-aortic lymphadenectomy and omentectomy, the laparoscope was moved to and placed on the 12-mm suprapubic trocar, and an additional pair of 5-mm trocars was introduced 2 cm inferior to the costal margin and immediately medial to the left and right midclavicular line.
At the beginning of both laparoscopy and laparotomy staging, parietal and visceral peritoneal surfaces were carefully inspected, including the diaphragm, liver, gallbladder, small bowel and mesentery, rectosigmoid colon, pouch of Douglas, paracolic gutters, and abdominal wall. In the case of laparotomy, the peritoneum and organs in the abdomen and pelvis were palpated as well. In laparoscopic surgery, aside from the use of high-energy devices including either LigaSure (Covidien, Boulder, CO, USA) or PowerBlade (LiNA, Copenhagen, Denmark) that were used in particular for paraaortic lymphadenectomy, all procedures were performed with conventional laparoscopic instruments such as straight forceps, a suction and irrigation device, monopolar scissors, and a bipolar electrocoagulator. The retrieved lymph nodes were extracted from the intraperitoneal cavity by using an Endopouch. To reduce the risk of port site metastasis, incision sites were irrigated with large amounts of saline and povidone-iodine solution after removal of the trocars.
3. Postoperative management
In both laparoscopy and laparotomy, postoperative management was similar in terms of diet resumption and antibiotic use. Patients were allowed to drink water after they passed gas from the bowel, and thereafter, a liquid, soft, and normal regular diet was given on a daily basis until the patients had no complaints of gastrointestinal symptoms. Early ambulation was encouraged. In all patients, 3 kinds of antibiotics were used for at least 3 postoperative days; first- or second-generation cephalosporin was administered intravenously; aminoglycoside, intramuscularly; and metronidazole, intravenously.
4. Statistical analysis
Surgical outcomes included surgical findings, operative time, estimated blood loss, and perioperative complications; oncological outcomes included tumor recurrence and survival outcomes. According to our hospital policy, patients who received pelvic lymphadenectomy also received follow-up pelvic ultrasonography to rule out lymphocyst formation 2 weeks after surgery. The number of cases with lymphocysts >3 cm at the largest diameter was quantified. Postoperative febrile morbidity was defined as a temperature of ≥38℃.
Statistical analyses were performed by using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) statistical software package. Between the 2 groups, continuous variables were compared by using the Student t-test, and categorical variables were compared by using the 2-tailed chi-square test, as appropriate. Survival analyses were conducted by using the Kaplan-Meier method, and surviving patients were censored at the date of last follow-up. A p-value of <0.05 was considered statistically significant.
RESULTS
Seventy-seven patients who met the inclusion criteria were identified during the study period, and staging surgery was performed via laparoscopy in 24 patients and via laparotomy in 53 patients. Surgery for none of the patients was converted from laparoscopic surgery to laparotomy. Basic patient characteristics are shown in Table 1. Between the 2 groups, there was no significant difference in the mean age, parity, body mass index, preoperative CA-125 level, preoperative hemoglobin level, or previous surgical history. Referral for restaging surgery after initial surgical confirmation of ovarian cancer was more common in the laparoscopy group than in the laparotomy group (58.3% vs. 17.0%, p < 0.001). In these cases, the initial operation was most commonly cystectomy or unilateral salpingo-oophorectomy in both groups. All patients underwent comprehensive staging surgery according to the inclusion criteria, but appendectomy was performed more commonly with laparotomy than with laparoscopy (81.1% vs. 41.7%, p=0.001).
Table 1.
Patient characteristics and surgical procedures
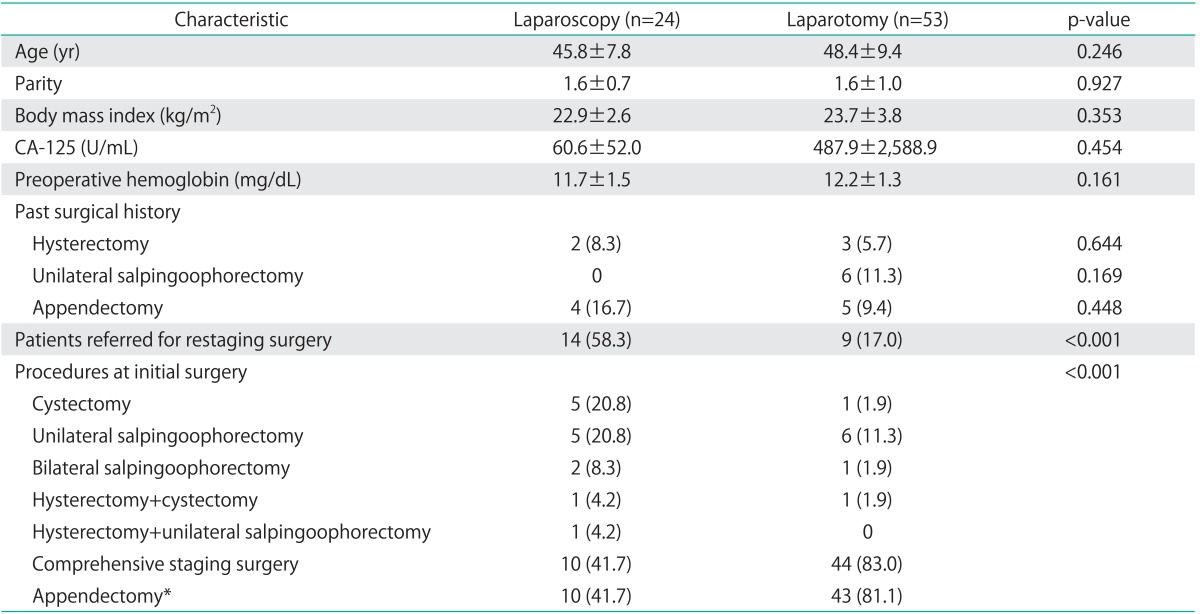
Values are presented as mean±SD or number (%).
*p=0.001.
The mean tumor size was 7.3 cm in the laparoscopy group and 11.2 cm in the laparotomy group (p=0.001) (Table 2). The differences in tumor stage, histological type, grade, and positive cytology between each surgical approach were not statistically significant. Tumor stage was distributed evenly in both the groups. The majority of the cases in both the groups were FIGO stage IA or IC (83.3% in the laparoscopy group and 81.2% in the laparotomy group), and stage II disease was diagnosed in 4 patients who underwent laparoscopy (16.7%) and 8 patients who underwent of laparotomy (18.9%). Although most of the cases were epithelial ovarian tumors, germ cell or sex-cord stromal tumors were found in 4 patients who underwent laparoscopy (16.7%) and 6 patients who underwent laparotomy (11.3%).
Table 2.
Surgical findings and pathology results
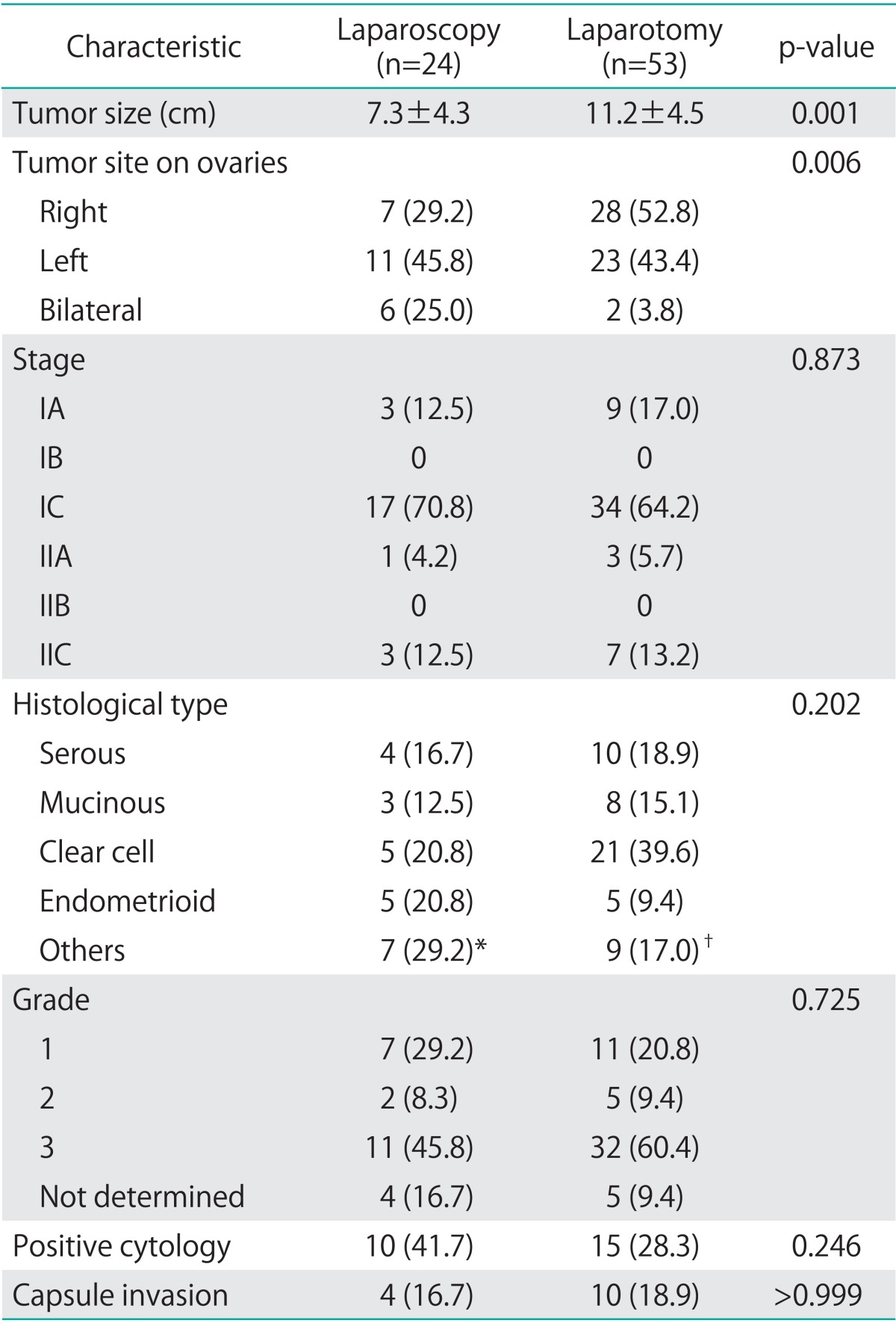
Values are presented as mean±SD or number (%).
*Consisted of mixed epithelial carcinoma (3), dysgerminoma (2), and malignant struma carcinoid tumor (2). †Consisted of granulosa cell tumor (2), transitional cell tumor (2), carcinosarcoma (2), immature teratoma (1), malignant struma carcinoid tumor (1), and mixed epithelial carcinoma (1).
Table 3 compares the main surgical outcomes of the 2 groups. Inadvertent rupture of the tumor during surgery occurred more often with laparoscopy than with laparotomy, but the difference was not statistically significant (54.2% vs. 39.6%, p=0.465). In addition, the number of pelvic and para-aortic lymph nodes retrieved was similar between the laparoscopy and laparotomy groups (26.8 vs. 27.8, respectively; 17.7 vs. 21.2, respectively). None of the cases in either group had visible residual disease after surgery.
Table 3.
Surgical outcomes of laparoscopy and laparotomy surgery in patients with early-stage ovarian cancer
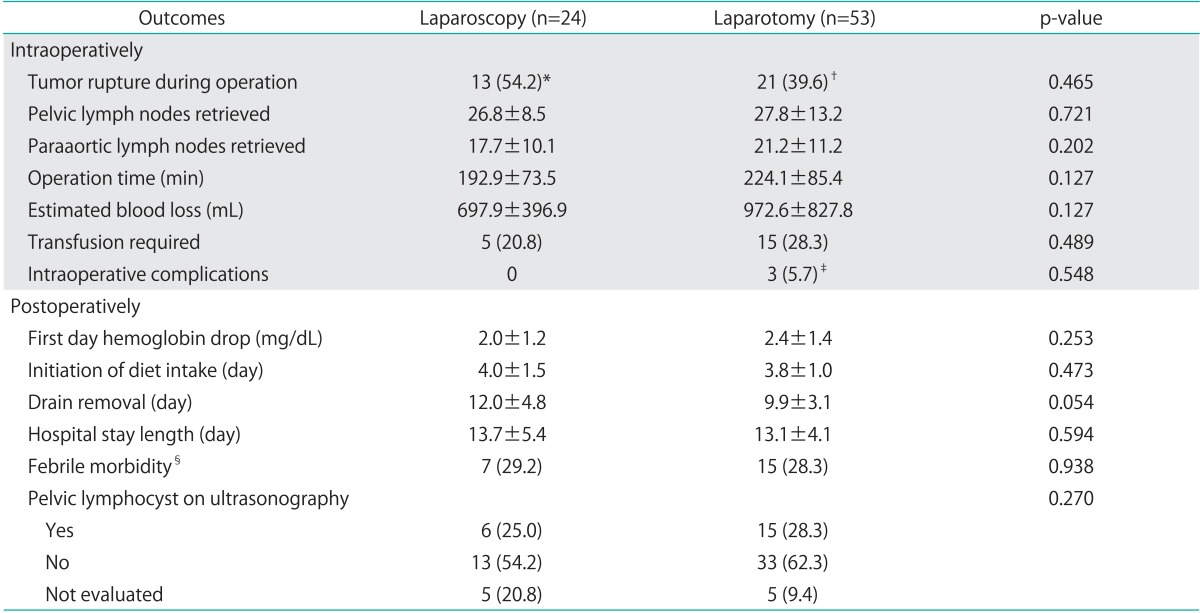
Values are presented as mean±SD or number (%).
In case of restaging, all initial surgery was performed *laparoscopically or †via laparotomy. ‡Consisted of ureter injury (1), perforation of sigmoid colon (1), and laceration of inferior vena cava (1). §Defined as >38.0℃.
The mean operative time was 192.9±73.5 min with laparoscopy and 224.1±85.4 min with laparotomy (p=0.127), and estimated blood loss was 697.9±396.9 mL in patients who underwent laparoscopy and 972.6±827.8 mL in patients who underwent laparotomy (p=0.127). An intraoperative or postoperative transfusion was performed in 20.8% of laparoscopy and 28.3% of laparotomy cases (p=0.489), and the mean drop in hemoglobin levels after surgery was 2.0 mg/dL in the laparoscopy group and 2.4 mg/dL in the laparotomy group (p=0.253). Between the 2 groups, the times to resumption of diet, drain removal, and hospital stay were nearly equal. Of interest, no patient in the laparoscopy group experienced intraoperative complications. However, 3 cases of intraoperative complications were identified in the laparotomy group, including ureteral injury, perforation of the sigmoid colon, and laceration of the inferior vena cava. The rate of postoperative complications such as febrile morbidity and pelvic lymphocyst was similar between the 2 groups (29.2% vs. 28.3%, p=0.938, respectively; 25% vs. 28.3%, p=0.270, respectively).
During the follow-up period, 2 patients in each group experienced tumor recurrence, but there were no cases of trocar-site metastasis (Table 4). In the recurrent cases, the disease-free survival was 13 months and 30 months for the 2 patients in the laparoscopy group, and 6 months and 27 months for those in the laparotomy group. A total of 4 patients showed recurrent cases of tumors in the pelvis, rectum, and sigmoid colon, which were confirmed pathologically. The types of adjuvant treatment and chemotherapy were not significantly different between the 2 groups. Most of the patients remained well without evidence of disease (91.6% in the laparoscopy group and 96.2% in the laparotomy group, p=0.231); however, one patient who underwent laparoscopic surgery died of recurrent ovarian cancer despite second-line chemotherapy and a second debulking surgery. The mean disease-free survival was 59.3 months after laparoscopy and 66.3 months after laparotomy (p=0.368) (Fig. 1).
Table 4.
Treatment method performed and survival outcomes
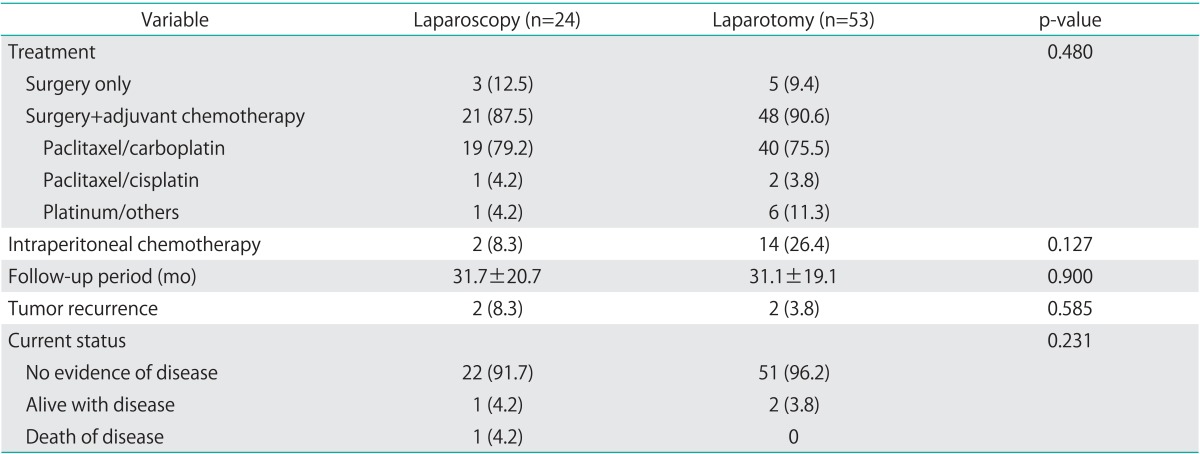
Values are presented as number (%) or mean±SD.
Fig. 1.
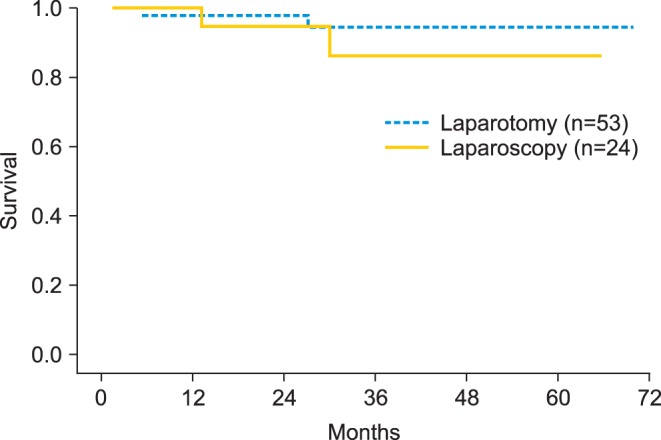
Kaplan-Meier analysis of the mean disease-free survival was 59.3 months (95% confidence interval [CI], 51.8 to 66.7) in the laparoscopy group and 66.3 months (95% CI, 62.8 to 69.9) in the laparotomy group with no statistically significant difference (p=0.367). The 3-year survival rate was 86.1% in the laparoscopy group and 94.7% in the laparotomy group.
DISCUSSION
Our results have provided evidence that laparoscopic surgery might be adequate and feasible for the treatment of early-stage (FIGO I or II) ovarian cancer with comparable surgical outcomes and oncological safety to laparotomy, while achieving the same comprehensive staging.
Recent advances in surgical techniques have led to the increasing utility of minimally invasive surgery, even in oncology. However, it is challenging for surgeons to use a laparoscopic approach for ovarian cancer, as the tumor may have metastasized throughout the peritoneal cavity at presentation. Any peritoneal surface suspected of harboring metastasis should be excised or biopsied, which is difficult with laparoscopy because of loss of the tactile sense and the need for proficient laparoscopic skill. Nevertheless, several studies indicating the advantages of laparoscopic surgery over laparotomy in early-stage ovarian cancer have been published recently [10,11,12]. One of the largest retrospective, comparative studies showed that complete surgical staging via laparoscopy was achieved in all 26 cases with reduced blood loss, earlier diet resumption, shorter hospital stay, and lower postoperative pain scores compared with staging via laparotomy among 113 patients with early-stage ovarian cancer [10].
On the other hand, several issues have been raised regarding laparoscopic staging of ovarian cancer. The main concerns are the increased risks of intraoperative tumor rupture, disease recurrence, and trocar-site metastasis, as well as reduced surgical adequacy and accuracy. The rate of tumor rupture or spillage during surgery may be affected by the surgical technique. In 2006, a study suggested that laparoscopic surgery increased the risk of tumor rupture (34.6% in patients who underwent laparoscopy vs. 6.6% in patients who underwent laparotomy) in a review of 113 cases of borderline ovarian tumors [13]; however, since the laparoscopy group contained a greater portion of cystectomies than adnexectomies, the risk of rupture was not properly evaluated. In our study, although half (54.2%) of the laparoscopy group experienced tumor rupture during surgery, the difference from that observed with laparotomy (39.6%) was not significant. We believe that tumor rupture can be avoided if proficient surgical skill is achieved, and initial adnexectomy is implemented for suspicious malignant tumors.
Port-site metastasis is another major risk of laparoscopic surgery. The incidence ranges widely, 1%-16% [6,7]. In a retrospective study, the authors reported that 31 patients (47%) showed abdominal wall metastasis among 66 patients who underwent histological examination of the port site [14]; most patients had an advanced cancer stage (FIGO IIIB-IV), and the authors concluded that abdominal wall metastasis did not have a dramatic impact on long-term survival. In several studies of early-stage ovarian cancer, no cases of port-site metastasis or recurrence in laparoscopy groups were reported [10,11,15]. Similarly, there were no cases of port-site metastasis in the present study, and the survival outcome was excellent in both groups, with high rates of patients with no evidence of disease and low rates of tumor recurrence (8.3% vs. 3.8%).
A study found that the mean number of pelvic and para-aortic nodes was 14 and 12, respectively, with low rates of complication in a series of 36 patients with early ovarian and fallopian tube cancers, indicating that laparoscopic staging is feasible and comprehensive [5]. However, their study included only a laparoscopic approach and did not compare the results with laparotomy. Moreover, the patient population was quite heterogeneous in terms of tumor histology. Borderline tumors were included along with invasive cancers, which may confound survival outcomes. In 2004, a multicenter comparative study of 105 cases of ovarian cancer reported that 14 and 78 patients were exclusively operated on via laparoscopy and laparotomy, respectively. Of these, surgery for 13 patients (12.4%) was converted from laparoscopy to laparotomy [13]. This study was limited because not all subjects underwent a complete staging surgery that included omentectomy. Specifically, the laparoscopy group underwent neither pelvic nor para-aortic lymphadenectomy, which hampered an accurate comparison of the surgical outcomes between laparoscopy and laparotomy. More recently, when comprehensive staging was performed in both groups, a study showed a similarly acceptable number of nodes obtained via laparoscopy (33.8 in laparoscopy vs. 42.7 in laparotomy, p=0.114) despite a shorter operative time [12]. This is consistent with the findings of our study.
The strength of our study lies in the consistency of our surgical procedures and the homogenous patient population achieved by excluding those with a history of fertility-sparing surgery or the presence of histologically borderline tumors. However, our study has several limitations. Patients were not randomly assigned to either laparoscopy or laparotomy staging; therefore, the initial tumor size was significantly larger in the laparotomy group. In addition, we failed to identify the reason for appendectomy more often in the laparotomy group, even though there was no difference in the previous history of appendectomy or the pathology type of ovarian cancer between the 2 groups. We assume that surgeons may feel more comfortable performing an appendectomy during laparotomy. Finally, after reviewing the detailed surgical findings in each case, we found that stage IIC tumors in the laparoscopy group were limited to solitary lesions on the uterine surface or uterosacral ligament, which are easily resectable. However, in the laparotomy group, the tumor often spread on the cul-de-sac or rectal wall as multiple nodules, which requires a more precise surgical technique, and leads to an increased risk of complications and worse survival outcomes.
Although laparoscopic surgery can provide better visualization and magnification of small lesions, it still has limitations in its access to critical areas such as the hepatophrenic ligament, lesser sac, porta hepatis, splenophrenic ligament, and hidden space in the folded intestine. Therefore, there is a possibility that the early stage of ovarian cancer in our laparoscopy group might be upstaged in patients who underwent laparotomy. In addition, the follow-up period of 31 months might not be long enough to determine the recurrence rate in terms of the oncological outcomes. Consequently, the current literature lacks the necessary power to conclude that laparoscopy and laparotomy surgery are equal in terms of surgical feasibility owing to the limitation of the retrospective study design and small sample size. Even so, our findings suggest that in a selective patient population, laparoscopic staging surgery performed by a skilled surgeon has at least equivalent surgical and oncological outcomes for the treatment of early-stage ovarian cancer, similar to laparotomy. Prospective randomized studies are required to confirm the safety of laparoscopic surgery and to determine the proper indication for laparoscopy as a treatment for ovarian cancer.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109–114. doi: 10.1016/s0002-9378(97)70447-2. [DOI] [PubMed] [Google Scholar]
- 2.Gowri V, Koliyadan SV, Al Hamdani A, Al Kindy N. Successful term pregnancies after laparoscopic excision of poorly differentiated Sertoli-Leydig cell tumor of the ovary. J Gynecol Oncol. 2012;23:201–204. doi: 10.3802/jgo.2012.23.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon A, Kim TJ, Lee WS, Kim BG, Bae DS. Single-port access laparoscopic staging operation for a borderline ovarian tumor. J Gynecol Oncol. 2011;22:127–130. doi: 10.3802/jgo.2011.22.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Querleu D, LeBlanc E. Laparoscopic infrarenal paraaortic lymph node dissection for restaging of carcinoma of the ovary or fallopian tube. Cancer. 1994;73:1467–1471. doi: 10.1002/1097-0142(19940301)73:5<1467::aid-cncr2820730524>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Nezhat FR, Ezzati M, Chuang L, Shamshirsaz AA, Rahaman J, Gretz H. Laparoscopic management of early ovarian and fallopian tube cancers: surgical and survival outcome. Am J Obstet Gynecol. 2009;200:83.e1–83.e6. doi: 10.1016/j.ajog.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Maneo A, Vignali M, Chiari S, Colombo A, Mangioni C, Landoni F. Are borderline tumors of the ovary safely treated by laparoscopy? Gynecol Oncol. 2004;94:387–392. doi: 10.1016/j.ygyno.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Childers JM, Aqua KA, Surwit EA, Hallum AV, Hatch KD. Abdominal-wall tumor implantation after laparoscopy for malignant conditions. Obstet Gynecol. 1994;84:765–769. [PubMed] [Google Scholar]
- 8.Medeiros LR, Rosa DD, Bozzetti MC, Rosa MI, Edelweiss MI, Stein AT, et al. Laparoscopy versus laparotomy for FIGO Stage I ovarian cancer. Cochrane Database Syst Rev. 2008;(4):CD005344. doi: 10.1002/14651858.CD005344.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Lawrie TA, Medeiros LR, Rosa DD, da Rosa MI, Edelweiss MI, Stein AT, et al. Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst Rev. 2013;2:CD005344. doi: 10.1002/14651858.CD005344.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Kim SW, Paek J, Lee SH, Yim GW, Kim JH, et al. Comparisons of surgical outcomes, complications, and costs between laparotomy and laparoscopy in early-stage ovarian cancer. Int J Gynecol Cancer. 2011;21:251–256. doi: 10.1097/IGC.0b013e318208c71c. [DOI] [PubMed] [Google Scholar]
- 11.Ghezzi F, Cromi A, Uccella S, Bergamini V, Tomera S, Franchi M, et al. Laparoscopy versus laparotomy for the surgical management of apparent early stage ovarian cancer. Gynecol Oncol. 2007;105:409–413. doi: 10.1016/j.ygyno.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, et al. Comparison of laparoscopy and laparotomy in surgical staging of early-stage ovarian and fallopian tubal cancer. Ann Surg Oncol. 2008;15:2012–2019. doi: 10.1245/s10434-008-9893-2. [DOI] [PubMed] [Google Scholar]
- 13.Romagnolo C, Gadducci A, Sartori E, Zola P, Maggino T. Management of borderline ovarian tumors: results of an Italian multicenter study. Gynecol Oncol. 2006;101:255–260. doi: 10.1016/j.ygyno.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Heitz F, Ognjenovic D, Harter P, Kommoss S, Ewald-Riegler N, Haberstroh M, et al. Abdominal wall metastases in patients with ovarian cancer after laparoscopic surgery: incidence, risk factors, and complications. Int J Gynecol Cancer. 2010;20:41–46. doi: 10.1111/IGC.0b013e3181c443ba. [DOI] [PubMed] [Google Scholar]
- 15.Park JY, Bae J, Lim MC, Lim SY, Seo SS, Kang S, et al. Laparoscopic and laparotomic staging in stage I epithelial ovarian cancer: a comparison of feasibility and safety. Int J Gynecol Cancer. 2008;18:1202–1209. doi: 10.1111/j.1525-1438.2008.01190.x. [DOI] [PubMed] [Google Scholar]


