Abstract
There has been significant progress in the field of heart transplantation over the last 45 years. The 1-yr survival rates following heart transplantation have improved from 30% in the 1970s to almost 90% in the 2000s. However, there has been little change in long-term outcomes. This is mainly due to chronic rejection, malignancy, and the detrimental side effects of chronic immunosuppression. In addition, over the last decade, new challenges have arisen such as increasingly complicated recipients and antibody-mediated rejection. Most, if not all, of these obstacles to long-term survival could be prevented or ameliorated by the induction of transplant tolerance wherein the recipient’s immune system is persuaded not to mount a damaging immune response against donor antigens, thus eliminating the need for chronic immunosuppression. However, the heart, as opposed to other allografts like kidneys, appears to be a tolerance-resistant organ. Understanding why organs like kidneys and livers are prone to tolerance induction, whereas others like hearts and lungs are tolerance-resistant, could aid in our attempts to achieve long-term, immunosuppression-free survival in human heart transplant recipients. It could also advance the field of pig-to-human xenotransplantation, which, if successful, would eliminate the organ shortage problem. Of course, there are alternative futures to the field of heart transplantation that may include the application of total mechanical support, stem cells, or bioengineered whole organs. Which modality will be the first to reach the ultimate goal of achieving unlimited, long-term, circulatory support with minimal risk to longevity or lifestyle is unknown, but significant progress in being made in each of these areas.
Long-term survival rates after heart transplantation could be improved by inducing transplant tolerance in the recipient. However, the heart, in contrast to other organs (e.g., kidneys), appears to be tolerance-resistant.
Although the first human-to-human heart transplant was performed in 1967, heart transplantation did not become the treatment of choice for patients with end-stage heart failure until the 1980s, when the use of cyclosporine (CyA) was extended to heart transplant recipients, resulting in a dramatic improvement in patient survival. One-year survival following heart transplantation in the era 1967–1973 was 30%, in the era 1974–1980, it was 60%, and in the current era, it approaches a remarkable 90% (Stehlik et al. 2012; Colvin-Adams et al. 2013). Although progress has clearly been made over the last 45 years, there are still serious challenges facing the field, which limit the application and the success of heart transplantation. Some barriers are well known, such as (1) the shortage of donor organs, which greatly limits the number of patients able to receive a heart transplant; (2) cardiac allograft vasculopathy (CAV) and malignancy, which compromise the long-term survival of heart transplant recipients; and (3) drug-induced complications from chronic immunosuppression including diabetes mellitus, kidney disease, hypertension, and obesity, which contribute to patient morbidity and mortality. Other challenges, such as increasingly complicated recipients and antibody-mediated rejection (AMR), have only become evident over the last decade as the recipient demographics have changed and the use of mechanical circulatory support (MSC) devices has increased (Hunt and Haddad 2008; Kobashigawa 2012). Together, these obstacles account for the fact that there has been no increase in the number of adult heart transplants performed over the last decade (∼4000 documented worldwide transplants/year) despite almost a 20% increase in the number of new adults on the waiting list (Colvin-Adams et al. 2013) and the fact that the 5-yr survival of patients lucky enough to receive a heart is still only ∼70%, with a disappointing median survival of 11 yr and an annual attrition rate of 3%–4%, which has not changed significantly in the last three decades (Stehlik et al. 2012; Colvin-Adams et al. 2013).
Strategies that have been and are being developed to overcome these challenges have focused on either controlling the human immune system more effectively and specifically with newer immunosuppressive agents such as rapamycin and rituximab or, alternatively, attempting to harness the immune system to achieve a state of transplant tolerance in which the recipient is induced not to mount a damaging immune response against the donor heart and remains free of chronic immunosuppression. In this article, we review how the field has changed over the last decade, focusing on the new and old barriers facing heart transplant recipients. We then discuss a particular avenue of research that exemplifies the potential for immune tolerance in overcoming these barriers and achieving long-term, immunosuppression-free heart allograft survival.
CHANGES AND CHALLENGES IN THE FIELD
Recipient Demographics
Over the last decade, the demographics of heart recipients have shifted in ways that have brought new challenges to transplant clinicians. A greater proportion of patients in their sixties and seventies along with their age-related comorbidities are being transplanted. These patients tend to have higher risks of infection and CAV (Kobashigawa 2012). At the other end of the spectrum, advances in congenital heart surgery have led to a greater proportion of younger patients with congenital heart disease (CHD) surviving past childhood and developing heart failure later in life. These patients can have complex cardiopulmonary anatomy and usually have undergone multiple previous median sternotomies, which increases the risk of postoperative bleeding and mortality. Indeed, CHD is one of the strongest risk factors for 1-yr mortality after heart transplantation in adults (Stehlik et al. 2012).
Immunosuppression
The past decade has seen changes in what is considered to be standard, triple-drug, maintenance immunosuppression for the conventional heart transplant recipient. Corticosteroids (usually prednisone) remain the backbone of most immunosuppressive regimens. However, mycophenolate mofetil (MMF) has replaced azathioprine as the most commonly used antiproliferative agent, and tacrolimus (TAC) has replaced CyA as the most commonly used calcineurin inhibitor (CNI). The MMF/TAC combination seems to possess the optimum risk–benefit ratio in preventing acute rejection (AR) and perhaps CAV even though it does not appear to improve long-term survival (Kobashigawa et al. 2006; Guethoff et al. 2013).
There are several important unanswered questions concerning immunosuppression for heart transplant recipients that require further study. For example, which recipients should receive induction therapy and using what agent? Although a survival benefit has not been clearly documented (Hershberger et al. 2005), half of all transplant programs currently use induction therapy, most commonly a short course of antithymocyte globulin (ATG) or anti-CD25 monoclonal antibody (basiliximab) (Stehlik et al. 2012). The general consensus is that the selective use of an induction agent is appropriate in highly sensitized patients or in patients with perioperative renal failure where delaying CNI therapy is beneficial. However, clear supporting data are lacking (Aliabadi et al. 2013).
The role for some of the newer immunosuppressive agents in heart transplantation is also being investigated. Several clinical trials have shown that inhibitors of the mammalian target of rapamycin (mTOR), such as sirolimus and everolimus, have been effective in preventing acute rejection (AR) (Eisen et al. 2003), mitigating CAV (Mancini et al. 2003), and improving outcomes in recipients with malignancies (Valantine 2007). They may allow for CNI minimization or elimination, which could avoid the progressive nephropathy associated with chronic CNI use (Zuckermann et al. 2012). Rituximab, a chimeric anti-CD20 (anti-B-cell) monoclonal antibody, has recently been shown to attenuate CAV in CNI-treated nonhuman primates (Kelishadi et al. 2010). An NIAID-sponsored trial (U01AI063623) is currently under way to determine whether preemptive rituximab will ameliorate CAV in human recipients. Bortezomib, a proteasome inhibitor that depletes plasma cells, has shown efficacy in the treatment of AMR and desensitization in kidney recipients (Walsh et al. 2012). In a recent pilot study, bortezomib and plasmapheresis appeared to decrease circulating antibodies in sensitized patients awaiting heart transplantation (Patel et al. 2011).
Antibody-Mediated Rejection
Antibody-mediated rejection (AMR) is a particularly challenging form of rejection in heart transplant recipients. The absence of practice guidelines for surveillance and diagnosis has resulted in it only recently being recognized as an important clinical entity. AMR results from alloantibody targeting donor antigens on capillary endothelium. It is increasingly recognized as a major cause of allograft failure and is associated with a greater risk of CAV and death (Nair et al. 2011). Prevention of AMR is dependent on identifying the sensitized patient before transplantation. This process has been assisted in recent years by the use of solid phase assays, which more accurately detects anti-HLA antibodies. In turn, this information permits virtual cross-matching, which identifies and rules out those prospective donors with HLA types that correspond to the specificities of the recipient’s high-level anti-HLA antibodies without the need for complement-dependent cytotoxicity assays (Stehlik et al. 2009). Advances in assessing anti-HLA antibodies in the recipient and the use of virtual cross-matching have allowed for better choices of suitable organ donors.
At present, the guidelines for the diagnosis of AMR rely solely on the presence of antibody-mediated injury on endomyocardial biopsy and not on the presence of circulating alloantibody, which may be bound to the donor tissue (Berry et al. 2011). The treatment of AMR depends on the patient’s presentation, the degree of cardiac dysfunction, and the detection of alloantibody (Kittleson and Kobashigawa 2012). Protocols differ by center because there is still a lack of randomized trials for AMR therapy (Kobashigawa et al. 2011). However, in most centers, patients with AMR and a significantly reduced ejection fraction are treated with intravenous corticosteroids and ATG. Patients presenting in cardiogenic shock can require plasmapheresis, intravenous immune globulin (IVIg), heparin, and mechanical support (Kittleson and Kobashigawa 2012). The long-term management of AMR is also complicated because patients can be left with a low ejection fraction, restrictive physiology, and accelerated CAV. Some institutions are treating these patients with rituximab, bortezomib, and photopheresis, and if necessary, redo transplantation (Kobashigawa et al. 2011).
Surgical Technique and Organ Preservation
The most significant technical advance in the heart transplantation surgery over the last decade has been related to the method of reestablishing systemic venous return. The original orthotopic heart transplant operation introduced by Lower and Shumway (1960) incorporated a biatrial technique in which cuffs of the left and right atria were preserved in the recipient and anastomosed to the corresponding atria of the donor heart. However, over the last decade, a bicaval method of systemic venous return has gained favor. The recipient’s right atrium is completely resected, and the remaining superior and inferior vena cavae are anastomosed directly to the corresponding donor structures. The reason for the switch is that the conventional biatrial technique puts the sinoatrial node at risk of injury, in addition to adversely impacting atrial hemodynamics and contributing to an increased risk of atrial arrhythmias in the postoperative period (Freimark et al. 1995; Leyh et al. 1995; Brandt et al. 1997). The bicaval technique eliminates the right atrial suture line, preserves right atrial morphology, and maintains the sinoatrial node and tricuspid valve function (Traversi et al. 1998; Aziz et al. 1999). A meta-analysis of 41 papers comparing bicaval to biatrial anastomoses found significant benefits for the bicaval technique in terms of early atrial pressure, tricuspid valve regurgitation, return to sinus rhythm, frequency of permanent pacemaker implantation, and even perioperative mortality. However, long-term outcomes were less disparate between the groups (Jacob and Sellke 2009).
In the area of donor heart preservation, a promising new technology is currently being evaluated in which normothermic perfusion provides continuous warm blood flow to the beating donor heart during transportation (Ghodsizad et al. 2012). This switch from conventional cold, static storage may not only decrease reperfusion injury and primary graft dysfunction but may also allow greater utilization of available organs.
TRANSPLANT TOLERANCE
As described above, despite improvements in early posttransplant survival over the last three decades, a relentless annual attrition rate continues to plague recipients of previously successful heart allografts, resulting in a median survival of only 11 yr (Stehlik et al. 2012). Although infection accounts for most recipient deaths 1-yr posttransplant, CAV and malignancy account for most cardiac recipient deaths after 5 yr (Stehlik et al. 2012). These sobering statistics emphasize the limitations of chronically administered immunosuppression and make clear the need for strategies that achieve long-term graft survival without the use of chronic immunosuppression. Inducing a state of tolerance has the potential to prevent or ameliorate the three greatest contributors to heart transplant recipient mortality, namely, infection, CAV, and cancer, while at the same time eliminating drug-specific morbidities.
Tolerance of kidney allografts has been achieved in nonhuman primates (NHPs) (Kawai et al. 1995, 1999, 2004) and in humans (Kawai et al. 2008) by using a combination of nonmyeloablative conditioning and donor bone marrow transplantation that results in transient mixed chimerism. However, mixed chimerism protocols that achieve long-term tolerance of kidney allografts in NHPs fail to induce tolerance in recipients of heart allografts (Kawai et al. 2002). The reasons for this organ-specific difference are not clear. However, it is clear that all transplanted organs are not created equal. Not only does the strength of the immune response to a particular organ vary with the organ transplanted, but also the nature of the response itself, rejection versus tolerance, varies from organ to organ. In most experimental models of transplantation, heart and lung allografts evoke a stronger rejection response than kidney and liver allografts. Moreover, under the right circumstances, kidney and liver allografts can promote a state of unresponsiveness instead of inciting an aggressive alloresponse and thus can be considered “tolerance-prone” organs. The same cannot be said for heart and lung allografts, which are, for the most part, “tolerance resistant.” Not only do tolerance-prone kidney and liver allografts appear to contribute to the actual process of tolerance induction, but also they possess the unique ability to confer unresponsiveness upon cotransplanted, tolerance-resistant organs like hearts. The mechanisms underlying this phenomenon are unclear, but understanding them could aid into our attempts to bring tolerance to the clinic. Below, we review organ-specific differences in allograft rejection and tolerance, focusing on ways we might harness the tolerogenicity of kidney allografts to achieve long-term, immunosuppression-free survival of more stringent heart allografts.
ORGAN-SPECIFIC DIFFERENCES IN REJECTION
The most extreme examples of organ-specific differences in transplantation are experimental models in which kidney and liver allografts are accepted spontaneously (without the use of immunosuppression), whereas other allografts such as heart, intestine, and skin transplanted across the same MHC barrier are rejected acutely (Russell et al. 1978; Dahmen et al. 1994; Qian et al. 1994; Zhang et al. 1996; Bickerstaff et al. 2001; Cook et al. 2008; Li et al. 2008; Miyajima et al. 2011; Wang et al. 2011). Zhang et al. (1996) compared liver, kidney, and heart transplantation in three different MHC disparate mouse strain combinations without treatment. The differences in the patterns of rejection between organs were remarkably consistent (Table 1). The majority of liver allografts in each strain combination were spontaneously accepted long term, whereas heart grafts transplanted across identical histocompatibility barriers were all rejected in <10 d. The pattern of kidney allograft rejection was mixed, with 20%–50% of organs surviving long term (Table 1) (Zhang et al. 1996). Our results (Madsen et al. 1997; Miyajima et al. 2011) and others (Bickerstaff et al. 2001; Cook et al. 2008; Wang et al. 2011) in mice support the fact that kidney allografts have a significantly prolonged survival compared with heart allografts transplanted across the same MHC barrier. Organ-specific differences in rejection responses extend to human transplantation. For example, the graft half-life for heart allografts is 11 yr (Stehlik et al. 2012), whereas the graft half-life for lung allografts is only 5 yr (Christie et al. 2012). Thus, the organ-specific differences in transplantation have clinical significance and deserve further study.
Table 1.
Proportion of liver, kidney, and heart allografts surviving >100 d in fully MHC disparate murine recipients
| Strain combination | Liver | Kidney | Heart |
|---|---|---|---|
| C57BL/6 into BALB/c (H-2b) (H-2d) | 72% | 20% | 0% |
| BALB/c into CBA (H-2d) (H-2k) | 57% | 33% | 0% |
| C57BL/6 into C3H/HeN (H-2b) (H-2k) | 73% | 50% | 0% |
Recipients received no treatment; n ≥ 6 recipients/group (from Zhang et al. 1996).
ORGAN-SPECIFIC DIFFERENCES IN TOLERANCE INDUCTION
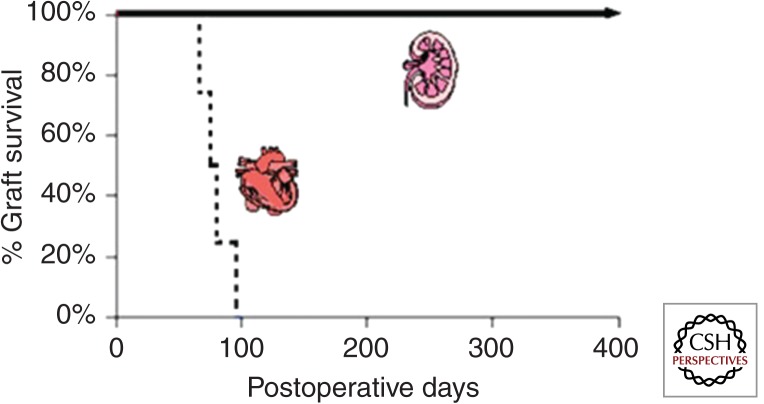
Our laboratory has compared the immunobiology of heart, kidney, and lung transplantation in MHC inbred miniature swine (Madsen 1998). These large animals provide the only preclinical model in which organ transplants can be performed across the same histocompatibility barrier reproducibly (Sachs 1992). In brief, when porcine recipients were transplanted with MHC class I disparate hearts and treated with 12 d of CyA, they all rejected within 55 d and showed the florid intimal proliferation of CAV on necropsy (Madsen et al. 1996). In contrast, when swine were transplanted with class I disparate kidney allografts and treated with the same course of CyA, they all became tolerant to donor antigen and maintained excellent renal function long term, in some instances for >2 yr (Fig. 1) (Rosengard et al. 1992). The survival of lungs transplanted across the same class I barrier with 12 d of CyA were in between that of hearts and kidneys, with graft survival ranging from 67 to >605 d and two-thirds developing obliterative bronchiolitis (Allan et al. 2002). A similar hierarchy was observed when organs were transplanted across a more rigorous two-haplotype full MHC barrier and FK506 was substituted for CyA. Long-term tolerance was induced in renal allografts (Utsugi et al. 2001), whereas lung survival ranged 100–500 d and hearts survived 55–134 d. Thus, there are also organ-specific differences that affect the process of tolerance induction. In swine, kidneys are easier to tolerize than lungs, which are easier to tolerize than hearts. This pattern seems to be true in most experimental transplant models. These observations emphasize that protocols designed to induce tolerance may not be directly transferable from one organ system to another and that the preclinical testing of tolerance protocols for human transplantation must proceed in an organ-specific manner (Massicot-Fisher et al. 2001).
Figure 1.
Heart versus kidney transplantation in MHC class I disparate swine treated with a 12-d course of CyA.
MECHANISMS OF KIDNEY- AND LIVER-SPECIFIC TOLERANCE INDUCTION
Murine models of spontaneous kidney and liver allograft acceptance have been well studied and are arguably our best source of information regarding potential mechanisms of organ-specific tolerance induction.
Kidney Allografts
Spontaneous renal allograft survival was first reported 35 years ago by Russell et al. (1978). Since then, several studies have shown that MHC-disparate kidney allografts transplanted across certain strain combinations are accepted spontaneously (Russell et al. 1978; Bickerstaff et al. 2001; Cook et al. 2008; Miyajima et al. 2011; Wang et al. 2011). We have recently shown that spontaneously accepted kidney allografts showed prominent periarterial lymphoid sheaths containing nodules of CD3+Foxp3+ T cells, CD4+ T cells, DCs, B cells, and indoleamine-pyrrole 2,3-dioxygenase (IDO)+ cells (Miyajima et al. 2011). The majority of the cells were CD3+, and ∼20%–30% of them were Foxp3+. These regulatory T-cell-rich organized lymphoid structures, which we term “TOLS,” are distinct from tertiary lymphoid structures (TLOs) found in chronic inflammation in that they lack high endothelial venules (MECA79−). Similar structures have been identified in tolerant pig and nonhuman primate kidney allografts (E Farkash, A Alessandrini, and RB Colvin, unpubl.). We have recently shown that Foxp3+ regulatory T cells (Tregs) are necessary to maintain unresponsiveness in spontaneously accepted MHC mismatched mouse kidney allografts (Miyajima et al. 2011). Administering diphtheria toxin (DT) to kidney recipients expressing the human diphtheria toxin receptor (DTR) under the control of the foxp3 gene (B6.Foxp3DTR) allowed us to transiently deplete Foxp3+ Tregs without morbidity (Kim et al. 2007). Treg depletion in long-term surviving kidney allograft recipients triggered acute cellular rejection, manifested by a sudden increase in BUN. The previously identified TOLS disintegrated after Treg depletion and was accompanied by widespread CD8+ interstitial mononuclear inflammation, tubulitis, and endarteritis, indicating acute cellular rejection (Miyajima et al. 2011). Although there is convincing evidence that Tregs play a role in mediating the spontaneous acceptance of renal allografts (Bickerstaff et al. 2001; Cook et al. 2008; Miyajima et al. 2011; Wang et al. 2011), the mechanisms by which cells or cell products intrinsic to kidney but not heart allografts promote or expand Tregs is unclear.
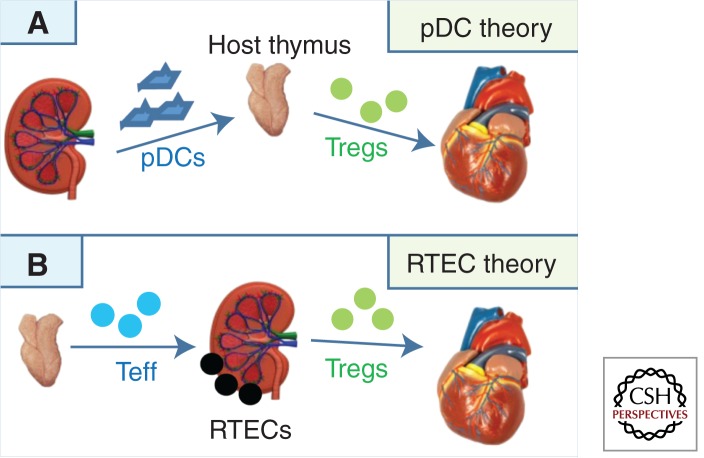
In addressing this question, we know that there are two cell populations present in kidneys with the capacity to down-regulate alloimmune responses: (1) plasmacytoid dendritic cells (pDCs), which have been shown to be capable of promoting the generation of Tregs and inducing tolerance to heart allografts in mice (Abe et al. 2005; Ochando et al. 2006; Gehrie et al. 2011); and (2) renal tubular epithelial cells (RTECs), which have been shown to be capable of inducing T-cell unresponsiveness to self- and alloantigens in mice and humans (Hagerty and Allen 1992; Kirby et al. 1993; Singer et al. 1993; Hadley et al. 1996; Deckers et al. 1997; Frasca et al. 1998). These cell types form the basis for two nonmutually exclusive hypotheses to explain spontaneous kidney allograft acceptance.
The pDC Hypothesis
The “pDC hypothesis” predicts that donor pDCs transferred within the kidney allograft traffic to the host thymus and lymph nodes, where they facilitate the activation/expansion of donor-specific Tregs (Fig. 2A). It is known that abundant CD11c+ DCs are present in normal mouse kidneys (Soos et al. 2006). We have recently shown that CD11c+ cells isolated from naïve, untransplanted kidneys (from DBA/2 and BALB/c mice) contain a subpopulation of PDCA-1+B220+ pDCs not found in hearts from the same animals (A Alessandrini and RB Colvin, unpubl.). The presence of PDCA-1+B220+ pDCs in DBA/2 kidneys but not hearts may explain why DBA/2 kidneys are spontaneously accepted when transplanted into B6 wild-type recipients, whereas DBA/2 hearts are uniformly rejected (Bickerstaff et al. 2001; Cook et al. 2008).
Figure 2.
Hypothetical models explaining kidney-induced cardiac allograft tolerance.
The RTEC Hypothesis
The “RTEC hypothesis” predicts that RTECs, intrinsic to the donor kidney, down-regulate or inactivate effector T cells emigrating from the host thymus or convert them to Tregs, thus shifting the balance of the immune response away from rejection and toward tolerance (Fig. 2B). There is precedent for this theory in that it has been previously shown that Foxp3+ cells are enriched in the tubules of mouse (Brown et al. 2007) and human (Veronese et al. 2007) renal allografts. Frasca and colleagues (1998) have shown that IFN-γ-treated human RTECs induce allospecific tolerance via a class II pathway. IFN-γ induces the T-cell inhibitor molecules PD-L1 and IDO on RTECs (Schoop et al. 2004; Mohib et al. 2007), and PD-L1 can induce the generation of allogeneic Foxp3+ Tregs (Krupnick et al. 2005), in part by down-regulating PTEN (Francisco et al. 2009). Importantly, Amarnath et al. (2011) have recently shown that PD1 signaling results in the conversion of human TH1 cells into Treg cells. Finally, RTECs are known to produce and activate TGF-β (Robertson et al. 2001), which is a major inducer of Foxp3+ Treg (Zheng 2008) and tolerogenic pDC (Pallotta et al. 2011) generation. High levels of TGF-β have been documented in accepted DBA/2 kidneys (Bickerstaff et al. 2001; Cook et al. 2008). Together, these findings support the possibility that RTECs contribute to spontaneous tolerance of kidney allografts.
Liver Allografts
Like kidney allografts, liver allografts are spontaneously accepted across certain mouse and rat strain combinations (Dahmen et al. 1994; Qian et al. 1994; Sriwatanawongsa et al. 1995; Zhang et al. 1996; Li et al. 2008). Like kidney allografts, depletion of recipient Tregs using anti-CD25 antibody induces acute liver allograft rejection (Li et al. 2008). And, like kidneys, the mechanism(s) by which Treg-mediated tolerance is induced by liver allografts is not known. However, the inherent tolerogenic properties of murine livers would suggest that, as in kidneys, cells or cell products intrinsic to the liver play a role. Three cell populations present in liver have been shown capable of suppressing an alloimmune response. They are (1) pDCs, located within the periportal areas and around the central veins (Sumpter et al. 2007); (2) liver sinusoidal endothelial cells (LSECs), which generate anti-inflammatory cytokines, such as IL-10 (Knolle et al. 1995), and diminish the survival CD8+ T cells (Limmer et al. 2000; Bowen et al. 2004; von Oppen et al. 2009); and (3) hepatic stellate cells (HSCs), which can promote the induction of Tregs (Fig. 3) (Yang et al. 2009).
Figure 3.
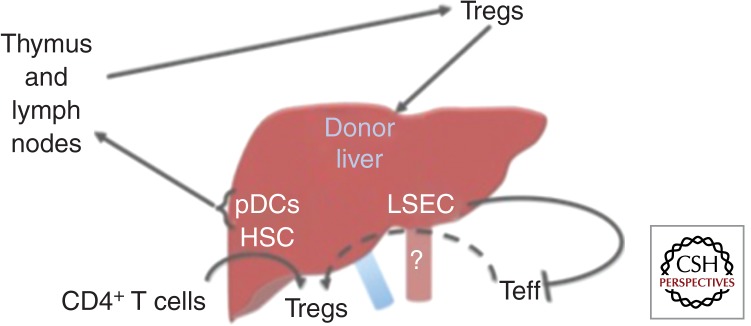
Hypothetical models explaining the spontaneous acceptance of liver allografts.
The pDC Hypothesis
The “pDC hypothesis” predicts that, like renal pDCs, liver pDCs migrate to the host thymus and lymph nodes, resulting in the activation/expansion of donor-specific Tregs. Support for this hypothesis comes from the observation that hepatic dendritic cells are less immunogenic than splenic dendritic cells and that only 5% of splenic DCs are made up of pDCs, whereas 19% of the liver DC population is made up of pDCs (Pillarisetty et al. 2004). Support also comes from that fact that circulating pDCs were increased relative to myeloid DCs (mDCs) in operationally tolerant pediatric liver allograft recipients as compared with patients maintained on chronic immunosuppression (Mazariegos et al. 2005).
The LSEC Hypothesis
The “LSEC hypothesis” predicts that these cells regulate the immune response by secreting IL-10 (Knolle et al. 1995). LSECs also constitutively express MHC class I molecules and, via cross-presentation of antigen to CD8+ T cells, are able to induce CD8+ T-cell tolerance rather than immunity (Limmer et al. 2000; von Oppen et al. 2009). LSEC-primed, naïve CD8+ T cells are initially induced to proliferate, to release cytokines, such as IL-2 and IFN-γ, and to express CD69 and CD25, but eventually begin to secrete low levels of the cytokines and show low cytotoxicity activity (Limmer et al. 2000). The induction of CD8+ T-cell tolerance has correlated with and has been shown to be dependent on the induction of the negative costimulatory molecule PD-L1 by LSECs (Diehl et al. 2008). Whether LSECs are able to induce Tregs or convert Teff to Tregs is not yet known, but their contribution to the down-regulation of an immune response begs further study in this area.
The HSC Hypothesis
Finally, the “HSC hypothesis” predicts that hepatic stellate cells (HSCs) are able to convert naïve or effector T cells to Tregs. HSCs store vitamin A and can produce TGF-β in response to inflammation and injury (Diehl et al. 2008; Tiegs and Lohse 2010). Vitamin A–derived retinoic acid and TGF-β have been shown to participate in the conversion of CD4+ T cells to Tregs (Xiao et al. 2008; Liu et al. 2011). It has been further shown that activated HSCs express PD-L1 (Yu et al. 2004). There is precedent for the HSC theory because HSCs are able to confer unresponsiveness and long-term survival to islet allografts by inducing Tregs (Yang et al. 2009) and myeloid-derived suppressor cells (Chou et al. 2011a,b).
TOLERANCE-PRONE ORGANS CAN CONFER UNRESPONSIVENESS UPON TOLERANCE-RESISTANT ORGANS
Although, as mentioned above, some organs are known to be tolerance prone (liver and kidney), whereas others are tolerance resistant (heart and lung), less is known about why tolerance-prone organs are able to confer a survival advantage upon another organ allograft procured from the same donor and cotransplanted into the same recipient. This phenomenon was first described in pigs and termed the “liver effect” by Calne et al. (1969). It is now clear that a similar effect occurs in human recipients of liver transplants (Calne et al. 1969; Hart et al. 1971; Calne and Davies 1994; Rasmussen et al. 1995; Praseedom et al. 2001) and to a lesser extent in kidney allograft recipients (see below).
Kidney-Induced Cardiac Allograft Tolerance (KICAT)
We have studied the phenomenon of kidney-induced cardiac allograft tolerance (KICAT) in MHC inbred miniature swine by taking advantage of the ability of kidney allografts to induce long-term stable tolerance of cotransplanted heart allografts, which, if transplanted alone, would reject acutely (Table 2, No. 1). Recipients cotransplanted with the heart and kidney from the same class I disparate donor all developed rapid and stable tolerance to the donor, which led to long-term survival of both kidney and heart allografts with no evidence of rejection on serial biopsies. In addition, there was no alloantibody formation, loss of antidonor responsiveness in cell-mediated lympholysis (CML) assays, and prevention of CAV (Table 2, No. 2). Neither heart nor kidney allograft survival was affected by the placement of donor-specific and third-party skin grafts despite the return of antidonor CML reactivity, which suggested the ongoing effects of regulatory T cells (Tregs) (Table 2, No. 3).
Table 2.
Results in class I disparate swine treated with 12 d of CyA
| No. | Experiment | Heart survivala | Heart rejection biopsiesb | CAVc | CML/Ab responsed | References |
|---|---|---|---|---|---|---|
| 1 | Heart alone | ++ | ++++ | ++++ | +/+ | Madsen et al. 1996 |
| 2 | Heart and kidney | ++++ | − | − | −/− | Madsen et al. 1998 |
| 3 | Heart and kidney and MHC-disparate skin | ++++ | − | − | +/− | Madsen et al. 1998 |
| 4 | Double hearts | ++++ | +++ | ++ | +/+ | Yamada et al. 2000 |
| 5 | Heart and kidney nephrectomized early | +++ | +++ | +++ | +/− | Mezrich et al. 2005 |
| 6 | Heart and kidney nephrectomized late | ++++ | − | ++ | −/− | Mezrich et al. 2005 |
| 7 | Irradiated heart and kidney | ++++ | +++ | +++ | +/− | Mezrich et al. 2003b |
| 8 | Irradiated heart and shielded kidney | ++++ | − | − | −/− | Mezrich et al. 2003b |
| 9 | Heart and kidney thymectomized early | +++ | +++ | +++ | +/− | Yamada et al. 1999 |
| 10 | Heart and kidney thymectomized late | ++++ | − | ++ | −/−− | Mezrich et al. 2005 |
aGraded on a relative scale from + (≤10 d) to ++++ (>100).
bGraded on a relative scale from + (ISHLT grade 2) to ++++ (ISHLT grade 4) on serial biopsies.
cGraded on a relative scale from + (rare, minimal luminal obstruction) to ++++ (frequent, significant luminal obstruction).
dGraded as + (response present) or – (response absent).
Kidney-Specific Elements Responsible for KICAT
Donor Antigen Load
To test whether KICAT was simply due to the additional donor antigen presented by the cotransplanted kidney, we compared heart/kidney recipients to recipients grafted with two class I disparate hearts. Although the allografts in recipients of two hearts survived longer than the allograft in recipients of one heart, (1) the double-heart allografts showed high-grade rejection with early and severe CAV on serial biopsies; (2) antidonor responsiveness in CML assays was maintained in the double-heart recipients; and (3) alloantibody was generated in the double-heart recipients (Table 2, No. 4). Thus, although augmentation of donor-antigen load could delay rejection, it could not induce tolerance.
Donor Nephrectomy
Donor kidney graftectomy on postoperative day (POD) 8 led to early cardiac allograft rejection. One recipient rejected its heart allograft on POD 29 with severe CAV. The other two showed prolonged heart allograft survival but showed high-grade interstitial rejection with severe CAV as early as POD 32 and never lost antidonor CML responsiveness (Table 2, No. 5). Furthermore, 2 wk after donor and third-party skin grafts were placed on the extended survivors, the heart allografts were acutely rejected. Thus, the donor kidney must remain in the recipient for >8 d to achieve KICAT. When donor nephrectomy was performed late (>100 d) rejection was not seen, although eventually mild CAV developed (Table 2, No. 6). However, within 2 wk of donor and third-party skin grafting, the heart allografts were rejected. These results suggest that the kidney allograft participates in the process of both tolerance induction and maintenance. More specifically, the skin grafting data suggest that elements associated with the donor kidney were able to actively suppress the antidonor response of circulating class I cytotoxic T lymphocyte precursors (CTLps), which were generated by the skin grafts (documented by CML) and which had the capacity to mediate acute rejection after the kidney allograft was removed (Madsen 1998; Christie et al. 2012).
Donor Kidney Irradiation
Cells of hematopoietic origin are extremely sensitive to radiation, with an LD50 estimated at 300–500 rads, but renal parenchymal cells are able to tolerate doses of 1000–2000 rads of unfractionated irradiation (Russell et al. 1978; Massicot-Fisher et al. 2001). Recipients of heart and kidney allografts from donors irradiated with 1000 rads before organ procurement failed to develop tolerance, maintaining strong antidonor CML responses despite having functioning, life-sustaining kidney allografts (Table 2, No. 7). Irradiating the donor heart while shielding the donor kidney before combined transplantation led to KICAT, indicating that it was unlikely that nonspecific irradiation-associated inflammation within the cardiac allograft was the reason for failure of KICAT. Instead, a radiosensitive, lymphohematopoietic cell population intrinsic to the donor kidney but not heart appeared necessary for the development of KICAT.
INITIAL ATTEMPTS TO DETERMINE THE ROLE FOR TREGS IN MEDIATING KICAT IN MINIATURE SWINE
Host Thymectomy
Regulatory T cells are primarily generated in the thymus (Wood and Sakaguchi 2003). To determine whether KICAT is dependent on an intact host thymus, total thymectomies were performed in recipients 21 d before heart and kidney transplants. Two of three thymectomized heart/kidney recipients rejected their heart grafts within 100 d, and the third animal had severe rejection when euthanized for seizures. Unlike the euthymic heart/kidney recipients, thymectomized heart/kidney recipients showed CAV, as well as persistent antidonor CML activity (Table 2, No. 9). The kidney grafts in these animals continued to function with stable creatinine values, but there was evidence of tubulitis and vasculopathy at necropsy. When the thymus was removed late (>100 d) no rejection was observed and CML assays remained unresponsive, although CAV eventually developed (Table 2, No. 10). These data suggest that, like the donor kidney, the host thymus plays a critical role during the induction phase of KICAT but has less influence late after transplantation.
Coculture Suppression Assays
We have found that primed naïve peripheral blood leukocytes (PBLs) cocultured with naïve cells augmented lysis of class I mismatched target cells. In contrast, primed PBLs from tolerant heart/kidney animals completely suppressed lysis of the same targets by naïve cells. Suppression was lost following removal of CD25+ T cells from the tolerant heart/kidney PBL population but was reestablished by incubation of naïve cells with CD25+ T cells from tolerant heart/kidney animals. In summary, these preliminary data suggest that CD25+ T cells in PBLs from tolerant swine contain regulatory T cells but that they require priming to fully suppress the response of naïve-matched T cells in coculture CML (Mezrich et al. 2003a).
Putative Intragraft Tregs
We have examined the phenotypes of graft infiltrating lymphocytes (GILs) retrieved from isolated kidneys transplanted across a class I barrier that accepted versus rejected (Giangrande et al. 1997). The number of cells expressing the phenotypic markers of Tregs (which in swine are CD4+CD8+CD25high) were substantially higher in acceptor than in rejector transplants at all time points.
KICAT in Fully MHC-Mismatched Swine
We have recently repeated these studies using donor/recipient pairs fully mismatched at the MHC and have again shown a dramatic difference in outcomes between recipients of isolated class I and II mismatched hearts, which reject their grafts by POD 35, and recipients of cotransplanted class I and II mismatched heart and kidneys, whose heart allografts continue to contract strongly, show no rejection on serial biopsies, and no evidence of alloantibody for >350 d (Madariaga et al. 2013).
KICAT in Nonhuman Primates
We have also extended these studies into cynomolgus monkeys using a mixed chimerism tolerance induction strategy and found that the majority of isolated heart recipients developed antidonor cellular and humoral immunity and lost their grafts to severe rejection with CAV by d 175. In contrast, recipients of heart and kidney allografts survived for >380–745 d with strongly contracting cardiac allografts (Tonsho et al. 2013). Impressively, every heart/kidney recipient that successfully completed its mixed chimerism conditioning regimen became tolerant. To our knowledge, these heart/kidney recipients represent the first nonhuman primates to become tolerant of cardiac allografts.
Summary
Together, these results underscore the robustness, consistency, and the clinical potential of kidney-induced cardiac allograft tolerance. Based on these findings and others from studies in isolated class I mismatched kidney transplant models (Ierino et al. 1999; Wu et al. 2003; Griesemer et al. 2008; Okumi et al. 2013), we hypothesize that cells or cell products intrinsic to kidney, but not heart allografts, promote the activation/expansion of host Tregs, which mediate tolerance of the heart grafts (for review, see Mezrich et al. 2004). The intrinsic mechanisms that allow a kidney but not a heart allograft to amplify regulatory mechanisms in the host following transplantation are unknown. Based on the findings in murine models described above, we are currently investigating whether pDCs or RTECs are the cells responsible for KICAT in large animals.
COMBINED ORGAN TRANSPLANTATION IN HUMAN RECIPIENTS
Review of Combined Heart and Kidney Transplantation in Patients
Given the remarkable effects a cotransplanted kidney allograft has on the survival of heart allografts in large animals undergoing tolerance-induction regimens, it is logical to expect to see a survival advantage in human recipients transplanted with combined heart and kidney allografts. However, the data are conflicting.
We have reviewed and summarized the world experience in combined heart and kidney transplantation in Table 3. The first clinical use of combined heart and kidney transplantation was reported in 1978 by Norman et al. (1978). Although combined heart and kidney transplantations account for only 1.5% of heart transplants performed annually, the last decade has seen a significant rise in the number of combined heart and kidney transplants conducted in humans, with excellent short-term successes (Cecka and Terasaki 2003). Indeed, since 1994, the number of patients in the United States requiring combined heart and kidney transplants has more than tripled to 78 recipients in 2012 (Stehlik et al. 2012).
Table 3.
Outcomes of heart versus heart/kidney transplantation in humans
| References | Year | Patients | Follow-up | Freedom from acute heart rejection | Overall survival |
|---|---|---|---|---|---|
| Czer et al. 2011 | 2011 | 30 | 5 yr | Heart/kidney: 96% at 1 mo 96% at 1 yr 91% at 5 yr |
Heart/kidney: 93% at 1 mo 87% at 1 yr 68% at 5 yr 51% at 10 yr |
| Heart: 99% at 1 mo 95% at 1 yr 88% at 5 yr 81% at 10 yr |
Heart: 98% at 1 mo 93% at 1 yr 76% at 5 yr 53% at 10 yr |
||||
| Conclusion: No significant difference |
Conclusion: No significant difference |
||||
| Gill et al. 2009 | 2009 | 263 | 4 yr | Heart/kidney: 85% at 1 yr |
Heart/kidney: 84% at 1 yr 77% at 4 yr |
| Heart: 69% at 1 yr |
Heart: 87% at 1 yr 77% at 4 yr |
||||
| Conclusion: Significantly less rejection in the combined group |
Conclusion: Significantly lower risk for combined group on multivariate analysis |
||||
| Bruschi et al. 2007 | 2007 | 9 | 10 yr | Heart/kidney: 56% at 3 mo |
Heart/kidney: 89% at 1 yr 78% at 5 yr 65% at 10 yr |
| Heart: 87% at 1 yr 78% at 5 yr 63% at 10 yr |
|||||
| Conclusion: No significant difference |
|||||
| Vermes et al. 2009 | 2009 | 67 | 10 yr | Heart/kidney: 73% at 1 yr 73% at 3 yr 73% at 5 yr 73% at 10 yr |
Heart/kidney: 62% at 1 yr 60% at 3 yr 53% at 5 yr 47% at 10 yr |
| Heart: 71% at 1 yr 65% at 3 yr 60% at 5 yr 47% at 10 yr |
|||||
| Conclusion: No significant difference |
|||||
| Hermsen et al. 2007 | 2007 | 19 | 5 yr | Heart/kidney: 57% at 1 yr 43% at 5 yr |
Heart/kidney: 90% at 1 yr 82% at 5 yr |
| Heart: 28% at 1 yr 20% at 5 yr |
Heart: 88% at 1 yr 75% at 5 yr |
||||
| Conclusion: Significantly less rejection in the combined group |
Conclusion: No significant difference |
||||
| Wang et al. 2006 | 2006 | 16 | 10 yr | Heart/kidney: 100% at 10 yr |
Heart/kidney: 83% at 1 yr 83% at 5 yr 55% at 10 yr |
| Heart: 63% at 5 yr 46% at 10 yr |
|||||
| Groetzner et al. 2005 | 2005 | 13 | 5 yr | Incidence of acute heart rejection episode Heart/kidney: 0.02/100 patient-days |
Heart/kidney: 92% at 1 yr 92% at 5 yr |
| Heart: 0.04/100 patient-days |
Heart: 88% at 1 yr 84% at 5 yr |
||||
| Conclusion: No significant difference |
Conclusion: No significant difference |
||||
| Trachiotis et al. 2003 | 2003 | 8 | 10 yr | Heart/kidney: 50% at 3 yr |
Heart/kidney: 88% at 1 yr 88% at 5 yr 88% at 10 yr |
| Heart: 50% at 4 yr |
Heart: 91% at 1 yr 74% at 5 yr 52% at 10 yr |
||||
| Conclusion: No significant difference |
|||||
| Luckraz et al. 2003 | 2003 | 13 | 10 yr | Incidence of acute heart rejection episodes per 100 patient-days was significantly lower in the combined group than that in the isolated heart transplant group | Heart/kidney: 77% at 1 yr 67% at 10 yr |
| Heart: 82% at 1 yr 58% at 10 yr |
|||||
| Conclusion: No significant difference |
|||||
| Leeser et al. 2001 | 2001 | 13 | 5 yr | Heart/kidney: 85% |
Heart/kidney: 92% at 1 mo 77% at 1 yr 60% at 5 yr |
| Heart: 66% |
Heart: 91% at 1 mo 80% at 1 yr 67% at 5 yr |
||||
| Conclusion: Significantly less rejection in the combined group |
Conclusion: No significant difference |
||||
| Vermes et al. 2001 | 2001 | 12 | 12 yr | Heart/kidney: 90% at 6 mo 70% at 1 yr |
Heart/kidney: 66% at 1 yr 55% at 5 yr 28% at 12 yr |
| Heart: 65% at 6 mo 49% at 1 yr |
Heart: 66% at 1 yr 44% at 5 yr 32% at 12 yr |
||||
| Conclusion: Significantly less rejection in the combined group |
Conclusion: No significant difference |
||||
| Blanche et al. 2001 | 2001 | 10 | 5 yr | Heart/kidney: 90% at 30 d 80% at 1 yr 80% at 2 yr |
Heart/kidney: 100% at 1 yr 88% at 2 yr 55% at 5 yr |
| Heart: 92% at 1 yr 84% at 2 yr 71% at 5 yr |
|||||
| Conclusion: Suggestive of lower rates of acute rejection than in hearts alone |
Conclusion: No significant difference |
||||
| Col et al. 1998 | 1998 | 6 | 2 yr | Heart/kidney: 66% at 2 yr |
Heart/kidney: 67% at 2 yr |
| Kocher et al. 1998 | 1998 | 9 | 4 yr | 67% at 4 yr | Heart/kidney: 89% at 4 yr |
| Conclusion: No significant difference |
Conclusion: No significant difference |
||||
| Narula et al. 1997 | 1997 | 82 | 2 yr | Heart/kidney: 88% at 1 mo 71% at 6 mo 66% at 12 mo |
Heart/kidney: 92% at 1 mo 76% at 12 mo 67% at 24 mo |
| Heart: 64% at 1 mo 44% at 6 mo 39% at 12 mo |
Heart: 92% at 1 mo 86% at 6 mo 83% at 12 mo 79% at 24 mo |
||||
| Conclusion: Significantly less rejection in the combined group |
Conclusion: No significant difference |
||||
| Colucci et al. 1997 | 1997 | 6 | 3.5 yr | Heart/kidney: 100% at 3.5 yr |
Heart/kidney: 100% at 3.5 yr |
| Gonwa et al. 1992 | 1992 | 3 | 6 mo | Heart/kidney: 100% at 3 yr |
Heart/kidney: 100% at 6 mo |
Data regarding long-term outcomes in recipients of these transplants are now becoming available. The recent publication by Czer et al. (2011) is the latest in a series of papers examining the results of heart and kidney cotransplantation. The investigators compared the outcomes of 30 patients receiving heart and kidney transplants with those of 440 patients receiving isolated heart allografts and found that the heart/kidney recipients had the same excellent long-term survival rates and low incidence of cellular rejection (ISHLT grade >2) as recipients of isolated hearts. Although there were no statistical differences between the two groups in terms of overall outcomes, cellular and antibody-mediated rejection of the cardiac allograft was not observed more than 5 yr following heart/kidney transplantation, whereas it was observed 5 yr after heart-alone transplantation (Table 3) (Czer et al. 2011). Thus, combined heart/kidney transplantation may confer a late immunological advantage.
In contrast, the most recent cohort analysis using the OPTN/UNOS database showed that the risk of death was 44% lower in heart/kidney recipients compared with heart-alone recipients after adjusting for potential confounders on multivariate analysis. Furthermore, acute rejection rates at 1 yr posttransplant were lower in heart/kidney recipients (Table 3) (Gill et al. 2009).
Taken together, the world’s literature suggests that recipients of combined heart and kidney allografts have less acute and possibly less chronic posttransplant rejection than recipients of isolated hearts (Rasmussen et al. 1995; Narula et al. 1997; Opelz et al. 2002; Goldfarb 2003; Luckraz et al. 2003; Hermsen et al. 2007). However, most studies do not show an increase in long-term survival following heart and kidney cotransplantation. The immunological advantage of heart and kidney recipients may not translate into a long-term survival benefit in human recipients because any tolerogenic effect engendered by the kidney allograft could be abrogated by the presence of continuous immunosuppression.
Review of Combined Heart and Liver Transplantation in Patients
Although many less patients have undergone heart and liver cotransplantation, the data that are available suggest that there is also an immunological benefit conferred upon the heart by the cotransplanted liver allograft. In a single-center study, Raichlin et al. (2009) reported that although 1-, 5-, and 10-yr survival rates in combined heart/liver recipients were similar to those in isolated heart recipients, the heart/kidney recipients showed significantly less acute cardiac allograft rejection. Moreover, heart/liver recipients showed no evidence of angiographic CAV, which was diagnosed in 38% of comparable isolated heart recipients (Raichlin et al. 2009). In a recent follow-up study using intravascular ultrasound (IVUS) to interrogate the coronary arteries, the same group showed that patients with heart/liver transplants had slower progression of CAV, which translated to better coronary-related clinical outcomes in unadjusted and adjusted analysis (Topilsky et al. 2013). These data suggest that the cotransplanted liver can modify the recipient’s immunological risk factors and improve the natural history of CAV. Finally, a protective “liver effect” can be documented by the fact that heart–liver recipients require less immunosuppression than recipients of isolated heart and still maintain low rates of acute rejection (Te et al. 2008).
Summary
Kidney-induced cardiac allograft tolerance has proven effective across two large animal models and across two different tolerance-induction protocols. Understanding how tolerance-prone organs confer unresponsiveness upon tolerance-resistant organs could provide important mechanistic information that is relevant to our attempts at inducing tolerance in human transplant recipients. Data from studies in mice, swine, and nonhuman primates suggest that cells or cell products resident within kidneys and livers but not hearts actively participate in the induction of tolerance by amplifying the contributions of Tregs. Isolating and harnessing these cells or cell products could provide an avenue toward the induction of tolerance in humans. Of course, cotransplanting a kidney with a heart allograft to induce tolerance in a recipient that does not have irreversible end-stage renal failure is an untenable strategy. Instead, one might imagine achieving tolerance of isolated heart allografts without sacrificing a donor kidney, possibly by obtaining the to-be-identified, KICAT-mediating cells via a simple kidney biopsy at the time of organ procurement and expanding them in vitro for subsequent use in a delayed tolerance protocol that our group described previously (Yamada et al. 2012). Indeed, this strategy could be applicable to all deceased-donor transplantation and to all tolerance-resistant organs/tissues including islets and vascularized composite allografts.
CONCLUSIONS
The future of heart transplantation must focus on the disappointing late outcomes that have not changed in decades and the donor organ shortage. Achieving tolerance could address both challenges by eliminating the need for chronic immunosuppression (Kawai et al. 2008) and by providing a potential avenue to pig-to-human xenotransplantation (Kuwaki et al. 2005). However, there are other exciting possibilities on the horizon that could achieve the same end including stem cell therapy (Shiba et al. 2012), advanced mechanical assistance (Abraham and Smith 2013), and whole-organ bioengineering (Ott et al. 2008). Which modality will be the first to reach the ultimate goal of achieving unlimited, long-term, circulatory support with minimal risk to longevity or life- style is unknown. However, significant advances are being made and will continue to be made in each of these areas.
ACKNOWLEDGMENTS
This work is supported in part by grants from the National Heart, Lung, and Blood Institute (P01HL18646) and the National Institute of Allergy and Infectious Disease (U01AI94374, R01AI081734-01A1) of the National Institutes of Health. We acknowledge C06RR020135-01 for construction of the facility used for production and maintenance of miniature swine and are indebted to J. Scott Arn for herd management and quality-control typing.
Footnotes
Editors: Laurence A. Turka and Kathryn J. Wood
Additional Perspectives on Transplantation available at www.perspectivesinmedicine.org
REFERENCES
- Abe M, Wang Z, de CA, Thomson AW 2005. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant 5: 1808–1819 [DOI] [PubMed] [Google Scholar]
- Abraham WT, Smith SA 2013. Devices in the management of advanced, chronic heart failure. Nat Rev Cardiol 10: 98–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliabadi A, Grommer M, Cochrane A, Salameh O, Zuckermann A 2013. Induction therapy in heart transplantation: Where are we now? Transpl Int 26: 684–695 [DOI] [PubMed] [Google Scholar]
- Allan JS, Wain JC, Schwarze ML, Houser SL, Benjamin LC, Madsen JC, Sachs DH 2002. Modeling chronic lung allograft rejection in miniature swine. Transplantation 73: 447–453 [DOI] [PubMed] [Google Scholar]
- Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, et al. 2011. The PDL1–PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med 3: 111ra120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz T, Burgess M, Khafagy R, Wynn Hann A, Campbell C, Rahman A, Deiraniya A, Yonan N 1999. Bicaval and standard techniques in orthotopic heart transplantation: Medium-term experience in cardiac performance and survival. J Thorac Cardiovasc Surg 118: 115–122 [DOI] [PubMed] [Google Scholar]
- Berry GJ, Angelini A, Burke MM, Bruneval P, Fishbein MC, Hammond E, Miller D, Neil D, Revelo MP, Rodriguez ER, et al. 2011. The ISHLT working formulation for pathologic diagnosis of antibody-mediated rejection in heart transplantation: Evolution and current status (2005–2011). J Heart Lung Transplant 30: 601–611 [DOI] [PubMed] [Google Scholar]
- Bickerstaff AA, Wang JJ, Pelletier RP, Orosz CG 2001. Murine renal allografts: Spontaneous acceptance is associated with regulated T cell-mediated immunity. J Immunol 167: 4821–4827 [DOI] [PubMed] [Google Scholar]
- Blanche C, Kamlot A, Blanche DA, Kearney B, Wong AV, Czer LS, Trento A 2001. Combined heart–kidney transplantation with single-donor allografts. J Thorac Cardiovasc Surg 122: 495–500 [DOI] [PubMed] [Google Scholar]
- Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P 2004. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest 114: 701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M, Harringer W, Hirt SW, Walluscheck KP, Cremer J, Sievers HH, Haverich A 1997. Influence of bicaval anastomoses on late occurrence of atrial arrhythmia after heart transplantation. Ann Thorac Surg 64: 70–72 [DOI] [PubMed] [Google Scholar]
- Brown K, Moxham V, Karegli J, Phillips R, Sacks SH, Wong W 2007. Ultra-localization of Foxp3+ T cells within renal allografts shows infiltration of tubules mimicking rejection. Am J Pathol 171: 1915–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruschi G, Busnach G, Colombo T, Radaelli L, Pedrazzini G, Garatti A, Sansalone CV, Frigerio M, Vitali E 2007. Long-term follow-up of simultaneous heart and kidney transplantation with single donor allografts: Report of nine cases. Ann Thorac Surg 84: 522–527 [DOI] [PubMed] [Google Scholar]
- Calne R, Davies H 1994. Organ graft tolerance: The liver effect. Lancet 343: 67–68 [DOI] [PubMed] [Google Scholar]
- Calne RY, Sells RA, Pena JR 1969. Induction of immunological tolerance by porcine liver allografts. Nature 223: 472–476 [DOI] [PubMed] [Google Scholar]
- Cecka JM, Terasaki PI 2003. Clinical transplants 2002. University of California, Los Angeles [Google Scholar]
- Chou HS, Hsieh CC, Yang HR, Wang L, Arakawa Y, Brown K, Wu Q, Lin F, Peters M, Fung JJ, et al. 2011a. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology 53: 1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HS, Hsieh CC, Charles R, Wang L, Wagner T, Fung JJ, Qian S, Lu LL 2011b. Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells. Transplantation 93: 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI, et al. 2012. The registry of the international society for heart and lung transplantation: 29th adult lung and heart–lung transplant report—2012. J Heart Lung Transplant 31: 1073–1086 [DOI] [PubMed] [Google Scholar]
- Col VJ, Jacquet L, Squifflet JP, Goenen M, Noirhomme P, Goffin E, Pirson Y 1998. Combined heart–kidney transplantation: Report on six cases. Nephrol Dial Transplant 13: 723–727 [DOI] [PubMed] [Google Scholar]
- Colucci V, Quaini E, Magnani P, Colombo T, De Carlis L, Grassi M, Merli M, Pellegrini A 1997. Combined heart and kidney transplantation: An effective therapeutic option—Report of six cases. Eur J Cardiothorac Surg 12: 654–658 [DOI] [PubMed] [Google Scholar]
- Colvin-Adams M, Smith JM, Heubner BM, Skeans MA, Edwards LB, Waller C, Snyder JJ, Israni AK, Kasiske BL 2013. OPTN/SRTR 2011 Annual Data Report: Heart. Am J Transplant 13: 119–148 [DOI] [PubMed] [Google Scholar]
- Cook CH, Bickerstaff AA, Wang JJ, Nadasdy T, Della Pelle P, Colvin RB, Orosz CG 2008. Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO. J Immunol 180: 3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czer LS, Ruzza A, Vespignani R, Jordan S, De Robertis MA, Mirocha J, Gallagher SP, Patel K, Schwarz ER, Kass RM, et al. 2011. Survival and allograft rejection rates after combined heart and kidney transplantation in comparison with heart transplantation alone. Transplant Proc 43: 3869–3876 [DOI] [PubMed] [Google Scholar]
- Dahmen U, Qian S, Rao AS, Demetris AJ, Fu F, Sun H, Gao L, Fung JJ, Starzl TE 1994. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation 58: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers JGM, Boonstr JG, Van der Kooij SW, Daha MR, van der Woude FJ 1997. Tissue-specific characteristics of cytotoxic graft-infiltrating T cells during renal allograft rejection. Transplantation 64: 178–181 [DOI] [PubMed] [Google Scholar]
- Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA 2008. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 47: 296–305 [DOI] [PubMed] [Google Scholar]
- Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sørensen K, Hummel M, Lind JM, et al. 2003. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med 349: 847–858 [DOI] [PubMed] [Google Scholar]
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH 2009. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 206: 3015–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca L, Marelli-Berg F, Imami N, Potolicchio I, Carmichael P, Lombardi G, Lechler R 1998. Interferon-γ-treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int 53: 679–689 [DOI] [PubMed] [Google Scholar]
- Freimark D, Silverman JM, Aleksic I, Crues JV, Blanche C, Trento A, Admon D, Queral CA, Harasty DA, Czer LS 1995. Atrial emptying with orthotopic heart transplantation using bicaval and pulmonary venous anastomoses: A magnetic resonance imaging study. J Am Coll Cardiol 25: 932–936 [DOI] [PubMed] [Google Scholar]
- Gehrie E, Van der Touw W, Bromberg JS, Ochando JC 2011. Plasmacytoid dendritic cells in tolerance. Methods Mol Biol 677: 127–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodsizad A, Bordel V, Ungerer M, Karck M, Bekeredjian R, Ruhparwar A 2012. Ex vivo coronary angiography of a donor heart in the organ care system. Heart Surg Forum 15: E161–E163 [DOI] [PubMed] [Google Scholar]
- Giangrande I, Yamada K, Arn S, Lorf T, Sachs DH, LeGuern C 1997. Selective increase in CD4-positive graft-infiltrating mononuclear cells among the infiltrates in class I disparate kidney grafts undergoing rejection. Transplantation 63: 722–728 [DOI] [PubMed] [Google Scholar]
- Gill J, Shah T, Hristea I, Chavalitdhamrong D, Anastasi B, Takemoto SK, Bunnapradist S 2009. Outcomes of simultaneous heart–kidney transplant in the US: A retrospective analysis using OPTN/UNOS data. Am J Transplant 9: 844–852 [DOI] [PubMed] [Google Scholar]
- Goldfarb DA 2003. Prolongation of long-term kidney graft survival by a simultaneous liver transplant: The liver does it, and the heart does it too. J Urol 169: 2435–2436 [PubMed] [Google Scholar]
- Gonwa TA, Husberg BS, Klintmalm GB, Mai ML, Goldstein RM, Capehart JE, Miller AH, Johnston SB, Alivizatos PA 1992. Simultaneous heart and kidney transplantation: A report of three cases and review of the literature. J Heart Lung Transplant 11: 152–155 [PubMed] [Google Scholar]
- Griesemer AD, LaMattina JC, Okumi M, Etter JD, Shimizu A, Sachs DH, Yamada K 2008. Linked suppression across an MHC-mismatched barrier in a miniature swine kidney transplantation model. J Immunol 181: 4027–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groetzner J, Kaczmarek I, Mueller M, Huber S, Deutsch A, Daebritz S, Arbogast H, Meiser B, Reichart B 2005. Freedom from graft vessel disease in heart and combined heart- and kidney-transplanted patients treated with tacrolimus-based immunosuppression. J Heart Lung Transplant 24: 1787–1792 [DOI] [PubMed] [Google Scholar]
- Guethoff S, Meiser BM, Groetzner J, Eifert S, Grinninger C, Ueberfuhr P, Reichart B, Hagl C, Kaczmarek I 2013. Ten-year results of a randomized trial comparing tacrolimus versus cyclosporine a in combination with mycophenolate mofetil after heart transplantation. Transplantation 95: 629–634 [DOI] [PubMed] [Google Scholar]
- Hadley GA, Rostapshova EA, Bartlett ST 1996. Dominance of tissue-restricted cytotoxic T lymphocytes in the response to allogeneic renal epithelial cell lines. Transplantation 62: 75–83 [DOI] [PubMed] [Google Scholar]
- Hagerty DT, Allen PM 1992. Processing and presentation of self and foreign antigens by the renal proximal tubule. J Immunol 148: 2324–2330 [PubMed] [Google Scholar]
- Hart AJ, Smellie WA, Calne RY 1971. Fate of kidney allografts from cadavers whose livers were also transplanted. Lancet 1: 103–105 [DOI] [PubMed] [Google Scholar]
- Hermsen JL, Nath DS, del Rio AM, Eickstaedt JB, Wigfield C, Lindsey JD, Edwards NM 2007. Combined heart–kidney transplantation: The University of Wisconsin experience. J Heart Lung Transplant 26: 1119–1126 [DOI] [PubMed] [Google Scholar]
- Hershberger RE, Starling RC, Eisen HJ, Bergh CH, Kormos RL, Love RB, Van Bakel A, Gordon RD, Popat R, Cockey L, et al. 2005. Daclizumab to prevent rejection after cardiac transplantation. N Engl J Med 352: 2705–2713 [DOI] [PubMed] [Google Scholar]
- Hunt SA, Haddad F 2008. The changing face of heart transplantation. J Am Coll Cardiol 52: 587–598 [DOI] [PubMed] [Google Scholar]
- Ierino FL, Yamada K, Hatch T, Rembert J, Sachs DH 1999. Peripheral tolerance to class I mismatched renal allografts in miniature swine: Donor antigen-activated peripheral blood lymphocytes from tolerant swine inhibit antidonor CTL reactivity. J Immunol 162: 550–559 [PubMed] [Google Scholar]
- Jacob S, Sellke F 2009. Is bicaval orthotopic heart transplantation superior to the biatrial technique? Interact Cardiovasc Thorac Surg 9: 333–342 [DOI] [PubMed] [Google Scholar]
- Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH 1995. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 59: 256–262 [PubMed] [Google Scholar]
- Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, Ko DS, Bartholomew A, Kimikawa M, Hong HZ, et al. 1999. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation 68: 1767–1775 [DOI] [PubMed] [Google Scholar]
- Kawai T, Cosimi AB, Wee SL, Houser S, Andrews D, Sogawa H, Phelan J, Boskovic S, Nadazdin O, Abrahamian G, et al. 2002. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation 73: 1757–1764 [DOI] [PubMed] [Google Scholar]
- Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, Andrews D, Nadazdin O, Koyama I, Sykes M, et al. 2004. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant 4: 1391–1398 [DOI] [PubMed] [Google Scholar]
- Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, et al. 2008. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 358: 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelishadi SS, Azimzadeh AM, Zhang T, Stoddard T, Welty E, Avon C, Higuchi M, Laaris A, Cheng XF, McMahon C, et al. 2010. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest 120: 1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol 8: 191–197 [DOI] [PubMed] [Google Scholar]
- Kirby JA, Rajasekar MR, Lin Y, Proud G, Taylor RM 1993. Interaction between T lymphocytes and kidney epithelial cells during renal allograft rejection. Kidney Int Suppl 39: S124–S128 [PubMed] [Google Scholar]
- Kittleson MM, Kobashigawa JA 2012. Antibody-mediated rejection. Curr Opin Organ Transplant 17: 551–557 [DOI] [PubMed] [Google Scholar]
- Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G 1995. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol 22: 226–229 [DOI] [PubMed] [Google Scholar]
- Kobashigawa JA 2012. The future of heart transplantation. Am J Transplant 12: 2875–2891 [DOI] [PubMed] [Google Scholar]
- Kobashigawa JA, Miller LW, Russell SD, Ewald GA, Zucker MJ, Goldberg LR, Eisen HJ, Salm K, Tolzman D, Gao J, et al. 2006. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-Year report. Am J Transplant 6: 1377–1386 [DOI] [PubMed] [Google Scholar]
- Kobashigawa J, Crespo-Leiro MG, Ensminger SM, Reichenspurner H, Angelini A, Berry G, Burke M, Czer L, Hiemann N, Kfoury AG, et al. 2011. Report from a consensus conference on antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 30: 252–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher AA, Schlechta B, Kopp CW, Ehrlich M, Ankersmit J, Ofner P, Langer F, Berlakovich GA, Grimm M, Wolner E, et al. 1998. Combined heart and kidney transplantation using a single donor: A single center’s experience with nine cases. Transplantation 66: 1760–1763 [DOI] [PubMed] [Google Scholar]
- Krupnick AS, Gelman AE, Barchet W, Richardson S, Kreisel FH, Turka LA, Colonna M, Patterson GA, Kreisel D 2005. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol 175: 6265–6270 [DOI] [PubMed] [Google Scholar]
- Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, et al. 2005. Heart transplantation in baboons using α-1,3-galactosyltransferase gene-knockout pigs as donors: Initial experience. Nat Med 11: 29–31 [DOI] [PubMed] [Google Scholar]
- Leeser DB, Jeevanandam V, Furukawa S, Eisen H, Mather P, Silva P, Guy S, Forster CE III 2001. Simultaneous heart and kidney transplantation in patients with end-stage heart and renal failure. Am J Transplant 1: 89–92 [DOI] [PubMed] [Google Scholar]
- Leyh RG, Jahnke AW, Kraatz EG, Sievers HH 1995. Cardiovascular dynamics and dimensions after bicaval and standard cardiac transplantation. Ann Thorac Surg 59: 1495–1500 [DOI] [PubMed] [Google Scholar]
- Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, Perkins JD 2008. New insights into mechanisms of spontaneous liver transplant tolerance: The role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant 8: 1639–1651 [DOI] [PubMed] [Google Scholar]
- Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA 2000. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med 6: 1348–1354 [DOI] [PubMed] [Google Scholar]
- Liu SM, Lee DH, Sullivan JM, Chung D, Jäger A, Shum BO, Sarvetnick NE, Anderson AC, Kuchroo VK 2011. Differential IL-21 signaling in APCs leads to disparate Th17 differentiation in diabetes-susceptible NOD and diabetes-resistant NOD.Idd3 mice. J Clin Invest 121: 4303–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lower RR, Shumway NE 1960. Studies on orthotopic homotransplantation of the canine heart. Surg Forum 11: 18–19 [PubMed] [Google Scholar]
- Luckraz H, Parameshwar J, Charman SC, Firth J, Wallwork J, Large S 2003. Short- and long-term outcomes of combined cardiac and renal transplantation with allografts from a single donor. J Heart Lung Transplant 22: 1318–1322 [DOI] [PubMed] [Google Scholar]
- Madariaga ML, Michel SG, Tasaki M, Villani V, La Muraglia GM II, Sihag S, Gottschall J, Farkash EA, Shimizu A, Allan JS, et al. 2013. Induction of cardiac allograft tolerance across a full MHC barrier in miniature swine by donor kidney cotransplantation. Am J Transplant 10.1111/ajt.12423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen JC 1998. Cardiac allograft vasculopathy in miniature swine: Utility of a large animal model. Graft 1: 41–44 [Google Scholar]
- Madsen JC, Sachs DH, Fallon JT, Weissman NJ 1996. Cardiac allograft vasculopathy in partially inbred miniature swine. I. Time course, pathology, and dependence on immune mechanisms. J Thorac Cardiovasc Surg 111: 1230–1239 [DOI] [PubMed] [Google Scholar]
- Madsen JC, Morris PJ, Wood KJ 1997. Immunogenetics of heart transplantation in rodents. Transplant Rev 11: 141–150 [Google Scholar]
- Madsen JC, Yamada K, Allan JS, Choo JK, Erhorn AE, Pins MR, Vesga L, Slisz JK, Sachs DH 1998. Transplantation tolerance prevents cardiac allograft vasculopathy in major histocompatibility complex class I—Disparate miniature swine. Transplantation 65: 304–313 [DOI] [PubMed] [Google Scholar]
- Mancini D, Pinney S, Burkhoff D, LaManca J, Itescu S, Burke E, Edwards N, Oz M, Marks AR 2003. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation 108: 48–53 [DOI] [PubMed] [Google Scholar]
- Massicot-Fisher J, Noel P, Madsen JC 2001. Recommendations of the NHLBI heart and lung tolerance working group. Transplantation 72: 1467–1470 [DOI] [PubMed] [Google Scholar]
- Mazariegos GV, Zahorchak AF, Reyes J, Chapman H, Zeevi A, Thomson AW 2005. Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. Am J Transplant 5: 314–322 [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Kesselheim JA, Johnston DR, Yamada K, Sachs DH, Madsen JC 2003a. The role of regulatory cells in miniature swine rendered tolerant to cardiac allografts by donor kidney cotransplantation. Am J Transplant 3: 1107–1115 [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Yamada K, Lee RS, Mawulawde K, Benjamin LC, Schwarze ML, Maloney ME, Amoah HC, Houser SL, Sachs DH, et al. 2003b. Induction of tolerance to heart transplants by simultaneous cotransplantation of donor kidneys may depend on a radiation-sensitive renal-cell population. Transplantation 76: 625–631 [DOI] [PubMed] [Google Scholar]
- Mezrich J, Yamada K, Sachs DH, Madsen JC 2004. Regulatory T cells generated by the kidney may mediate the beneficial immune effects of combining kidney with heart transplantation. Surgery 135: 473–478 [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Benjamin LC, Sachs JA, Houser SL, Vagefi PA, Sachs DH, Madsen JC, Yamada K 2005. Role of the thymus and kidney graft in the maintenance of tolerance to heart grafts in miniature swine. Transplantation 79: 1663–1673 [DOI] [PubMed] [Google Scholar]
- Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della Pelle P, Benichou G, Graham JA, Madsen JC, Russell PS, Colvin RB 2011. Early acceptance of renal allografts in mice is dependent on Foxp3+ cells. Am J Pathol 178: 1635–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohib K, Guan Q, Diao H, Du C, Jevnikar AM 2007. Proapoptotic activity of indoleamine 2,3-dioxygenase expressed in renal tubular epithelial cells. Am J Physiol Renal Physiol 293: F801–F812 [DOI] [PubMed] [Google Scholar]
- Nair N, Ball T, Uber PA, Mehra MR 2011. Current and future challenges in therapy for antibody-mediated rejection. J Heart Lung Transplant 30: 612–617 [DOI] [PubMed] [Google Scholar]
- Narula J, Bennett LE, DiSalvo TG, Hosenpud JD, Semigran MJ, Dec GW 1997. Outcomes in recipients of combined heart–kidney transplantation. Multi-organ, same-donor transplant study of the ISHLT/UNOS scientific registry. Transplantation 63: 861–867 [DOI] [PubMed] [Google Scholar]
- Norman JC, Brook MI, Cooley DA, Klima T, Kahan BD, Frazier OH, Keats AS, Hacker J, Massin EK, Duncan JM, et al. 1978. Total support of the circulation of a patient with post-cardiotomy stone-heart syndrome by a partial artificial heart (ALVAD) for 5 days followed by heart and kidney transplantation. Lancet 1: 1125–1127 [DOI] [PubMed] [Google Scholar]
- Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, et al. 2006. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 7: 652–662 [DOI] [PubMed] [Google Scholar]
- Okumi M, Scalea JR, Gillon BC, Tasaki M, Villani V, Cormack T, Hirakata A, Shimizu A, Sachs DH, Yamada K 2013. The induction of tolerance of renal allografts by adoptive transfer in miniature swine. Am J Transplant 13: 1193–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opelz G, Margreiter R, Dohler B 2002. Prolongation of long-term kidney graft survival by a simultaneous liver transplant: The liver does it, and the heart does it too. Transplantation 74: 1390–1394 [DOI] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA 2008. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat Med 14: 213–221 [DOI] [PubMed] [Google Scholar]
- Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, et al. 2011. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 12: 870–878 [DOI] [PubMed] [Google Scholar]
- Patel J, Everly M, Chang D, Kittleson M, Reed E, Kobashigawa J 2011. Reduction of alloantibodies via proteasome inhibition in cardiac transplantation. J Heart Lung Transplant 30: 1320–1326 [DOI] [PubMed] [Google Scholar]
- Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP 2004. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol 172: 1009–1017 [DOI] [PubMed] [Google Scholar]
- Praseedom RK, McNeil KD, Watson CJ, Alexander GJ, Calne RY, Wallwork J, Friend PF 2001. Combined transplantation of the heart, lung, and liver. Lancet 358: 812–813 [DOI] [PubMed] [Google Scholar]
- Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE 1994. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology 19: 916–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlin E, Daly RC, Rosen CB, McGregor CG, Charlton MR, Frantz RP, Clavell AL, Rodeheffer RJ, Pereira NL, Kremers WK, et al. 2009. Combined heart and liver transplantation: A single-center experience. Transplantation 88: 219–225 [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY 1995. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation 59: 919–921 [PubMed] [Google Scholar]
- Robertson H, Wong WK, Burt AD, Mohamed MA, Talbot D, Kirby JA 2001. Relationship between TGF-β1, intratubular CD103 positive T cells and acute renal allograft rejection. Transplant Proc 33: 1159. [DOI] [PubMed] [Google Scholar]
- Rosengard BR, Ojikutu CA, Guzzetta PC, Smith CV, Sundt TM III, Nakajima K, Boorstein SM, Hill GS, Fishbein JM, Sachs DH 1992. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation 54: 490–497 [DOI] [PubMed] [Google Scholar]
- Russell PS, Chase CM, Colvin RB, Plate JM 1978. Kidney transplants in mice. An analysis of the immune status of mice bearing long-term, H-2 incompatible transplants. J Exp Med 147: 1449–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs DH 1992. MHC homozygous miniature swine. In Swine as models in biomedical research (ed. Swindle MM, et al. ), pp. 3–15 Iowa State University Press, Ames, IA [Google Scholar]
- Schoop R, Wahl P, Le Hir M, Heemann U, Wang M, Wuthrich RP 2004. Suppressed T-cell activation by IFN-γ-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant 19: 2713–2720 [DOI] [PubMed] [Google Scholar]
- Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, et al. 2012. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489: 322–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer GG, Yokoyama H, Bloom RD, Jevnikar AM, Nabavi N, Kelley VR 1993. Stimulated renal tubular epithelial cells induce anergy in CD4+ T cells. Kidney Int 44: 1030–1035 [DOI] [PubMed] [Google Scholar]
- Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ 2006. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596 [DOI] [PubMed] [Google Scholar]
- Sriwatanawongsa V, Davies HS, Calne RY 1995. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nat Med 1: 428–432 [DOI] [PubMed] [Google Scholar]
- Stehlik J, Islam N, Hurst D, Kfoury AG, Movsesian MA, Fuller A, Delgado JC, Hammond ME, Gilbert EM, Renlund DG, et al. 2009. Utility of virtual crossmatch in sensitized patients awaiting heart transplantation. J Heart Lung Transplant 28: 1129–1134 [DOI] [PubMed] [Google Scholar]
- Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Hertz MI, et al. 2012. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant 31: 1052–1064 [DOI] [PubMed] [Google Scholar]
- Sumpter TL, Abe M, Tokita D, Thomson AW 2007. Dendritic cells, the liver, and transplantation. Hepatology 46: 2021–2031 [DOI] [PubMed] [Google Scholar]
- Te HS, Anderson AS, Millis JM, Jeevanandam V, Jensen DM 2008. Current state of combined heart–liver transplantation in the United States. J Heart Lung Transplant 27: 753–759 [DOI] [PubMed] [Google Scholar]
- Tiegs G, Lohse AW 2010. Immune tolerance: What is unique about the liver. J Autoimmun 34: 1–6 [DOI] [PubMed] [Google Scholar]
- Tonsho M, Benichou G, Boskovic S, Nadazdin O, Smith N, Colvin R, Sachs D, Cosimi A, Kawai T, Madsen J 2013. Successful tolerance induction of cardiac allografts in nonhuman primates through donor kidney co-transplantation. 2013 American Transplant Congress, Seattle, WA, May 18–22 [Google Scholar]
- Topilsky Y, Raichlin E, Hasin T, Boilson BA, Schirger JA, Pereira NL, Edwards BS, Clavell AL, Rodeheffer RJ, Frantz RP, et al. 2013. Combined heart and liver transplant attenuates cardiac allograft vasculopathy compared with isolated heart transplantation. Transplantation 95: 859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachiotis GD, Vega JD, Johnston TS, Berg A, Whelchel J, Smith AL, Lutz J, Kanter KR 2003. Ten-year follow-up in patients with combined heart and kidney transplantation. J Thorac Cardiovasc Surg 126: 2065–2071 [DOI] [PubMed] [Google Scholar]
- Traversi E, Pozzoli M, Grande A, Forni G, Assandri J, Viganò M, Tavazzi L 1998. The bicaval anastomosis technique for orthotopic heart transplantation yields better atrial function than the standard technique: An echocardiographic automatic boundary detection study. J Heart Lung Transplant 17: 1065–1074 [PubMed] [Google Scholar]
- Utsugi R, Barth RN, Lee RS, Kitamura H, LaMattina JC, Ambroz J, Sachs DH, Yamada K 2001. Induction of transplantation tolerance with a short course of tacrolimus (FK506). I: Rapid and stable tolerance to two-haplotype fully MHC-mismatched kidney allografts in miniature swine. Transplantation 71: 1368–1379 [DOI] [PubMed] [Google Scholar]
- Valantine H 2007. Is there a role for proliferation signal/mTOR inhibitors in the prevention and treatment of de novo malignancies after heart transplantation? Lessons learned from renal transplantation and oncology. J Heart Lung Transplant 26: 557–564 [DOI] [PubMed] [Google Scholar]
- Vermes E, Kirsch M, Houël R, Legouvelo S, Benvenuti C, Aptecar E, Le Besnerais P, Lang P, Abbou C, Loisance D 2001. Immunologic events and long-term survival after combined heart and kidney transplantation: A 12-year single-center experience. J Heart Lung Transplant 20: 1084–1091 [DOI] [PubMed] [Google Scholar]
- Vermes E, Grimbert P, Sebbag L, Barrou B, Pouteil-Noble C, Pavie A, Obadia JF, Loisance D, Lang P, Kirsch M 2009. Long-term results of combined heart and kidney transplantation: A French multicenter study. J Heart Lung Transplant 28: 440–445 [DOI] [PubMed] [Google Scholar]
- Veronese F, Rotman S, Smith RN, Pelle TD, Farrell ML, Kawai T, Benedict Cosimi A, Colvin RB 2007. Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant 7: 914–922 [DOI] [PubMed] [Google Scholar]
- von Oppen N, Schurich A, Hegenbarth S, Stabenow D, Tolba R, Weiskirchen R, Geerts A, Kolanus W, Knolle P, Diehl L 2009. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology 49: 1664–1672 [DOI] [PubMed] [Google Scholar]
- Walsh RC, Alloway RR, Girnita AL, Woodle ES 2012. Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int 81: 1067–1074 [DOI] [PubMed] [Google Scholar]
- Wang SS, Chou NK, Chi NH, Hsu RB, Huang SC, Chen YS, Yu HY, Ko WJ, Chu SH, Tsai MK, et al. 2006. Simultaneous heart and kidney transplantation for combined cardiac and renal failure. Transplant Proc 38: 2135–2137 [DOI] [PubMed] [Google Scholar]
- Wang C, Cordoba S, Hu M, Bertolino P, Bowen DG, Sharland AF, Allen RD, Alexander SI, McCaughan GW, Bishop GA 2011. Spontaneous acceptance of mouse kidney allografts is associated with increased Foxp3 expression and differences in the B and T cell compartments. Transpl Immunol 24: 149–156 [DOI] [PubMed] [Google Scholar]
- Wood KJ, Sakaguchi S 2003. Regulatory T cells in transplantation tolerance. Nat Rev 3: 199–210 [DOI] [PubMed] [Google Scholar]
- Wu A, Yamada K, Ierino FL, Vagefi PA, Sachs DH 2003. Regulatory mechanism of peripheral tolerance: In vitro evidence for dominant suppression of host responses during the maintenance phase of tolerance to renal allografts in miniature swine. Transpl Immunol 11: 367–374 [DOI] [PubMed] [Google Scholar]
- Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK 2008. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol 181: 2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Choo JK, Allan JS, Erhorn AE, Menard MT, Mawulawde K, Slisz JK, Aretz HT, Shimizu A, Sachs DH, et al. 1999. The effect of thymectomy on tolerance induction and cardiac allograft vasculopathy in a miniature swine heart/kidney transplantation model. Transplantation 68: 485–491 [DOI] [PubMed] [Google Scholar]
- Yamada K, Mawulawde K, Menard MT, Shimizu A, Aretz HT, Choo JK, Allison KS, Slisz JK, Sachs DH, Madsen JC 2000. Mechanisms of tolerance induction and prevention of cardiac allograft vasculopathy in miniature swine: The effect of augmentation of donor antigen load. J Thorac Cardiovasc Surg 119: 709–719 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, Ochiai T, Nadazdin O, Koyama I, Boenisch O, et al. 2012. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant 12: 330–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HR, Chou HS, Gu X, Wang L, Brown KE, Fung JJ, Lu L, Qian S 2009. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: A critical role of interferon-γ signaling. Hepatology 50: 1981–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S 2004. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 40: 1312–1321 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhu L, Quan D, Garcia B, Ozcay N, Duff J, Stiller C, Lazarovits A, Grant D, Zhong R 1996. Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation 62: 1267–1272 [DOI] [PubMed] [Google Scholar]
- Zheng SG 2008. The critical role of TGF-β1 in the development of induced Foxp3+ regulatory T cells. Int J Clin Exp Med 1: 192–202 [PMC free article] [PubMed] [Google Scholar]
- Zuckermann A, Keogh A, Crespo-Leiro MG, Mancini D, Vilchez FG, Almenar L, Brozena S, Eisen H, Tai SS, Kushwaha S 2012. Randomized controlled trial of sirolimus conversion in cardiac transplant recipients with renal insufficiency. Am J Transplant 12: 2487–2497 [DOI] [PubMed] [Google Scholar]