Abstract
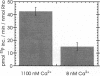
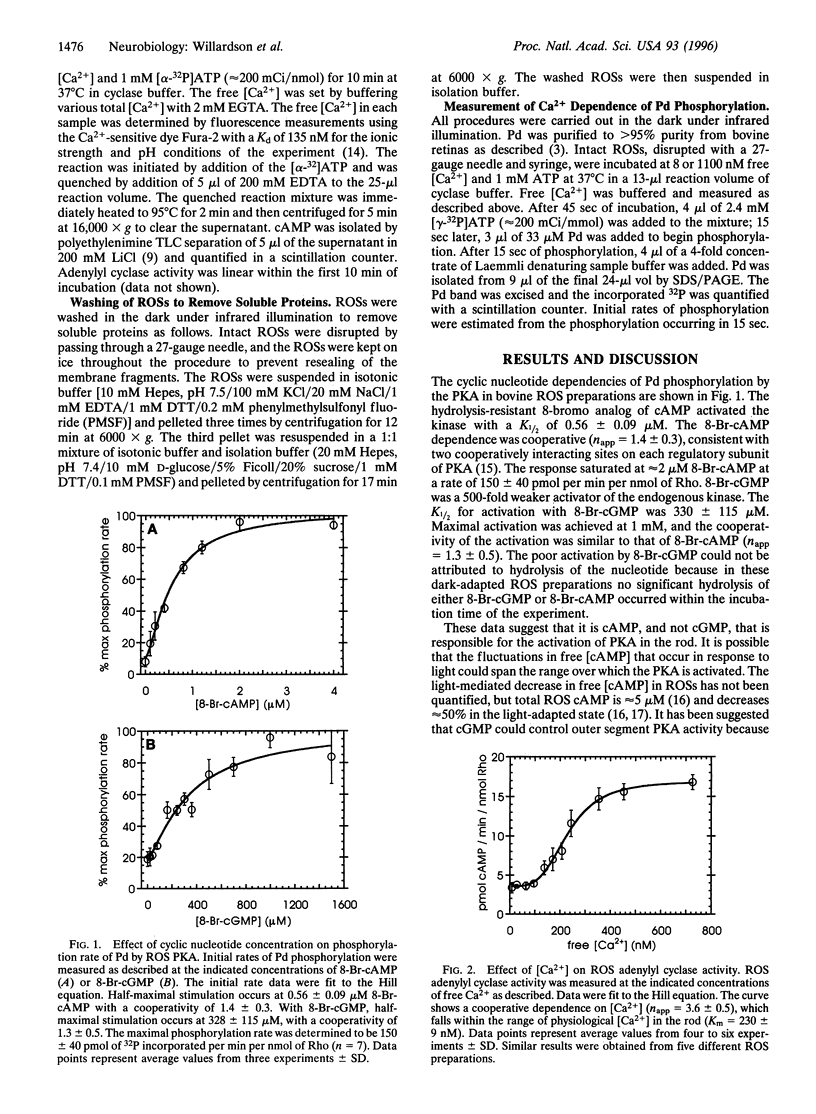
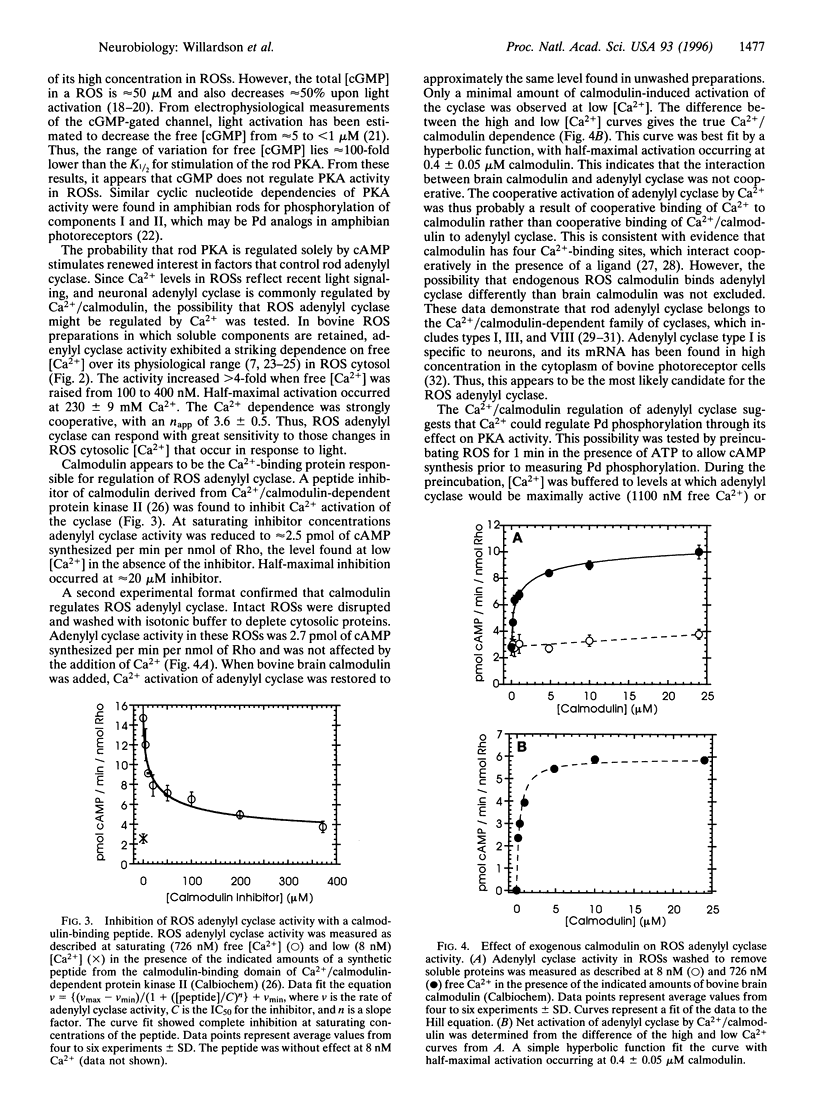
The phosphoprotein phosducin (Pd) regulates many guanine nucleotide binding protein (G protein)-linked signaling pathways. In visual signal transduction, unphosphorylated Pd blocks the interaction of light-activated rhodopsin with its G protein (Gt) by binding to the beta gamma subunits of Gt and preventing their association with the Gt alpha subunit. When Pd is phosphorylated by cAMP-dependent protein kinase, it no longer inhibits Gt subunit interactions. Thus, factors that determine the phosphorylation state of Pd in rod outer segments are important in controlling the number of Gts available for activation by rhodopsin. The cyclic nucleotide dependencies of the rate of Pd phosphorylation by endogenous cAMP-dependent protein kinase suggest that cAMP, and not cGMP, controls Pd phosphorylation. The synthesis of cAMP by adenylyl cyclase in rod outer segment preparations was found to be dependent on Ca2+ and calmodulin. The Ca2+ dependence was within the physiological range of Ca2+ concentrations in rods (K1/2 = 230 +/- 9 nM) and was highly cooperative (n app = 3.6 +/- 0.5). Through its effect on adenylyl cyclase and cAMP-dependent protein kinase, physiologically high Ca2+ (1100 nM) was found to increase the rate of Pd phosphorylation 3-fold compared to the rate of phosphorylation at physiologically low Ca2+ (8 nM). No evidence for Pd phosphorylation by other (Ca2+)-dependent kinases was found. These results suggest that Ca2+ can regulate the light response at the level of Gt activation through its effect on the phosphorylation state of Pd.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anholt R. R., Rivers A. M. Olfactory transduction: cross-talk between second-messenger systems. Biochemistry. 1990 May 1;29(17):4049–4054. doi: 10.1021/bi00469a004. [DOI] [PubMed] [Google Scholar]
- Bauer P. H., Müller S., Puzicha M., Pippig S., Obermaier B., Helmreich E. J., Lohse M. J. Phosducin is a protein kinase A-regulated G-protein regulator. Nature. 1992 Jul 2;358(6381):73–76. doi: 10.1038/358073a0. [DOI] [PubMed] [Google Scholar]
- Blazynski C., Cohen A. I. Cyclic nucleotide distribution in identified layers of suprafused rabbit retinas. Exp Eye Res. 1984 Mar;38(3):279–290. doi: 10.1016/0014-4835(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Blazynski C., Cohen A. I. Rapid declines in cyclic GMP of rod outer segments of intact frog photoreceptors after illumination. J Biol Chem. 1986 Oct 25;261(30):14142–14147. [PubMed] [Google Scholar]
- Cali J. J., Zwaagstra J. C., Mons N., Cooper D. M., Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem. 1994 Apr 22;269(16):12190–12195. [PubMed] [Google Scholar]
- Cote R. H., Biernbaum M. S., Nicol G. D., Bownds M. D. Light-induced decreases in cGMP concentration precede changes in membrane permeability in frog rod photoreceptors. J Biol Chem. 1984 Aug 10;259(15):9635–9641. [PubMed] [Google Scholar]
- DeVries G. W., Cohen A. I., Hall I. A., Ferrendelli J. A. Cyclic nucleotide levels in normal and biologically fractionated mouse retina: effects of light and dark adaptation. J Neurochem. 1978 Dec;31(6):1345–1351. doi: 10.1111/j.1471-4159.1978.tb06559.x. [DOI] [PubMed] [Google Scholar]
- Dizhoor A. M., Ray S., Kumar S., Niemi G., Spencer M., Brolley D., Walsh K. A., Philipov P. P., Hurley J. B., Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991 Feb 22;251(4996):915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Matthews H. R. Calcium and the mechanism of light adaptation in vertebrate photoreceptors. Trends Neurosci. 1990 Sep;13(9):378–384. doi: 10.1016/0166-2236(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Gorczyca W. A., Gray-Keller M. P., Detwiler P. B., Palczewski K. Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):4014–4018. doi: 10.1073/pnas.91.9.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Keller M. P., Detwiler P. B. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994 Oct;13(4):849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamm H. Regulation by light of cyclic nucleotide-dependent protein kinases and their substrates in frog rod outer segments. J Gen Physiol. 1990 Mar;95(3):545–567. doi: 10.1085/jgp.95.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes B. E., Touhara K., Kurose H., Lefkowitz R. J., Inglese J. Determination of the G beta gamma-binding domain of phosducin. A regulatable modulator of G beta gamma signaling. J Biol Chem. 1994 Nov 25;269(47):29825–29830. [PubMed] [Google Scholar]
- Hekman M., Bauer P. H., Söhlemann P., Lohse M. J. Phosducin inhibits receptor phosphorylation by the beta-adrenergic receptor kinase in a PKA-regulated manner. FEBS Lett. 1994 Apr 25;343(2):120–124. doi: 10.1016/0014-5793(94)80302-1. [DOI] [PubMed] [Google Scholar]
- Ikura M., Hasegawa N., Aimoto S., Yazawa M., Yagi K., Hikichi K. 113Cd-NMR evidence for cooperative interaction between amino- and carboxyl-terminal domains of calmodulin. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1233–1238. doi: 10.1016/0006-291x(89)91374-0. [DOI] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993 Apr 29;362(6423):855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Lee R. H., Brown B. M., Lolley R. N. Light-induced dephosphorylation of a 33K protein in rod outer segments of rat retina. Biochemistry. 1984 Apr 24;23(9):1972–1977. doi: 10.1021/bi00304a014. [DOI] [PubMed] [Google Scholar]
- Lee R. H., Brown B. M., Lolley R. N. Protein kinase A phosphorylates retinal phosducin on serine 73 in situ. J Biol Chem. 1990 Sep 15;265(26):15860–15866. [PubMed] [Google Scholar]
- Lee R. H., Lieberman B. S., Lolley R. N. A novel complex from bovine visual cells of a 33,000-dalton phosphoprotein with beta- and gamma-transducin: purification and subunit structure. Biochemistry. 1987 Jun 30;26(13):3983–3990. doi: 10.1021/bi00387a036. [DOI] [PubMed] [Google Scholar]
- Lee R. H., Ting T. D., Lieberman B. S., Tobias D. E., Lolley R. N., Ho Y. K. Regulation of retinal cGMP cascade by phosducin in bovine rod photoreceptor cells. Interaction of phosducin and transducin. J Biol Chem. 1992 Dec 15;267(35):25104–25112. [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne M. E., Fong Y. L., Ono T., Colbran R. J., Kemp B. E., Soderling T. R., Means A. R. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J Biol Chem. 1988 May 25;263(15):7190–7195. [PubMed] [Google Scholar]
- Ratto G. M., Payne R., Owen W. G., Tsien R. Y. The concentration of cytosolic free calcium in vertebrate rod outer segments measured with fura-2. J Neurosci. 1988 Sep;8(9):3240–3246. doi: 10.1523/JNEUROSCI.08-09-03240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp P. P., Klompmakers A. A., Daemen F. J. The isolation of stable cattle rod outer segments with an intact plasma membrane. Biochim Biophys Acta. 1979 Apr 19;552(3):379–389. doi: 10.1016/0005-2736(79)90182-2. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem. 1991 Jun 15;266(17):10711–10714. [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Adenylyl cyclases. Cell. 1992 Sep 18;70(6):869–872. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Krupinski J., Gilman A. G. Expression and characterization of calmodulin-activated (type I) adenylylcyclase. J Biol Chem. 1991 May 5;266(13):8595–8603. [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Woodruff M. L., Bownds M. D. Amplitude, kinetics, and reversibility of a light-induced decrease in guanosine 3',5'-cyclic monophosphate in frog photoreceptor membranes. J Gen Physiol. 1979 May;73(5):629–653. doi: 10.1085/jgp.73.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Choi E. J., Wang F., Blazynski C., Storm D. R. Type I calmodulin-sensitive adenylyl cyclase is neural specific. J Neurochem. 1993 Jan;60(1):305–311. doi: 10.1111/j.1471-4159.1993.tb05852.x. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature. 1985 Feb 14;313(6003):579–582. doi: 10.1038/313579a0. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Ikura M., Hikichi K., Ying L., Yagi K. Communication between two globular domains of calmodulin in the presence of mastoparan or caldesmon fragment. Ca2+ binding and 1H NMR. J Biol Chem. 1987 Aug 15;262(23):10951–10954. [PubMed] [Google Scholar]
- Yoshida T., Willardson B. M., Wilkins J. F., Jensen G. J., Thornton B. D., Bitensky M. W. The phosphorylation state of phosducin determines its ability to block transducin subunit interactions and inhibit transducin binding to activated rhodopsin. J Biol Chem. 1994 Sep 30;269(39):24050–24057. [PubMed] [Google Scholar]