Abstract
[Purpose] The aim of this study was to clarify the relationship between the muscle fiber conduction velocity (MFCV) obtained during muscle twitches from evoked potentials and the dynamic characteristics of muscular tension (muscle dynamic characteristics) by manipulating deep temperature. [Subjects] Subjects were 10 healthy adult men. Their mean age was 23.0 ± 3.9 years. [Methods] Measurement items were MFCV of the right tibialis anterior muscle and the force-time curve of right ankle dorsiflexion (muscle twitch). Measurements were made under conditions of ordinary (room) temperature, hot and cold. The rate of change in maximum torque was calculated from the force-time curve. [Results] In all subjects, MFCV increased significantly with heating and decreased significantly with cooling. A strong correlation was seen between MFCV and deep temperature. A strong correlation was also seen between MFCV and the rate of change in maximum torque. Stronger correlations were seen in the present results than in previous studies that conducted investigations using voluntary contractions. [Conclusion] The present results were not affected by psychological or other such factors, and are valuable as data with high physiological reliability. In conclusion, this study was able to clarify the relationship between MFCV from evoked potentials and muscle dynamic characteristics.
Key words: Muscle fiber conduction velocity, Dynamic characteristics of muscular tension, Muscle twitches
INTRODUCTION
Motor control in humans is accompanied by various peripheral uncertainties, one of which is muscle fiber conduction velocity (MFCV). MFCV is the velocity at which muscle fibers transmit action potentials prior to muscle contraction. Primarily, action potentials generated in end-plates are propagated toward the muscle fiber terminal1). MFCV is susceptible to influences from the type of muscle fiber, environment, and measurement method, and although a standard value cannot be clearly specified, in healthy adults, it is thought to be approximately 3–5 m·s−1 at ordinary temperature (room temperature)2). Various factors that alter MFCV have been suggested, including fundamental changes in muscle fibers due to muscular disease3,4,5), muscle fiber characteristics (muscle fiber diameter6,7,8,9,10,11,12), muscle fiber type13,14,15,16,17)), temperature (skin temperature, muscle temperature)5, 18), muscle atrophy7, 19) and muscle fatigue5, 20, 21), and these factors have been studied using various methods. In addition, motor control and contraction characteristics of muscle fibers suggest that changes in MFCV affect reaction time and the rate of change in maximum torque of the force-time curve, which indicates muscle dynamic characteristics. Thus, a decrease in MFCV slows the propagation of excitation, delaying muscle contraction time. Moreover, a delay in muscle contraction time would prolong the reaction time and force-peak time in the force-time curve decreasing the rate of change in maximum torque. This is thought to be followed by a decline in accuracy and speed of movement. Conversely, muscle dynamic characteristics are likely to be elevated when MFCV is increased, which is thought to lead to improved motor performance. Clarification of the relationship between MFCV and muscle dynamic characteristics is therefore important.
The relationship between MFCV and muscle dynamic characteristics has been reported for voluntary contractions of the tibialis anterior muscle22). When MFCV increases, muscle dynamic characteristics are improved; conversely, when MFCV decreases, muscle dynamic characteristics deteriorate. A previous study found a strong positive correlation between MFCV and muscle dynamic characteristics (standardized rate of change in maximum torque). However, there are numerous uncertainties, such as psychological factors, in voluntary contractions; thus, there is some doubt as to whether these parameters sufficiently reflect the true physiological characteristics of the muscle fibers. Resolving such doubts requires an investigation into the relationship between MFCV and muscle dynamic characteristics during muscle twitches from evoked potentials, which reflect the physiological characteristics of muscle fibers.
In this study, we induced changes in MFCV by deep temperature manipulation, similar to the method of Murakami et al22, 23). With decreased muscle temperature, the potassium ion concentration in the extracellular fluid rises, together with an increase in the resting membrane potential. When the extracellular potassium ion concentration rises, the repolarization time, which is related to the time constant of the membrane, becomes longer due to changes in the electrical gradient, and this is presumed to decrease MFCV1). When muscle temperature rises, this process is thought to reverse. Therefore, the purpose of the present study was to clarify the relationship between MFCV and muscle dynamic characteristics during muscle twitches due to evoked potentials are observed.
SUBJECTS AND METHODS
The subjects were 10 healthy adult men. Their mean age was 23.0 ± 3.9 years, their mean height was 172.0 ± 6.4 cm, and their mean body weight was 62.4 ± 8.5 kg. All measurements were performed within approximately one month. Written, informed consent was obtained from each subject prior to their participation in the study, and this study was conducted with the approval of the Research Ethics Committee of Tohoku Bunka University (11-10).
The measurement items were the force-time curve of right ankle dorsiflexion movement from muscle twitches induced by surface electrical stimulation, and MFCV of the right tibialis anterior muscle. In accordance with the method of Kondo5), measurements were performed under three conditions: ordinary temperature, hot and cold. The hot and cold conditions were not used on the same day to avoid confounding effects from temperature change. When measurements were performed under two temperature conditions on the same day, the measurement at the ordinary temperature was performed first.
In preparation for measurements, skin at the measurement site was treated by shaving above the right tibialis anterior muscle and removing dead skin by applying skin preparation gel for biological signal monitoring (Skinpure; Nihon Kohden Corp., Tokyo, Japan). Oil was also wiped from the area around the measurement site with alcohol cotton swabs. Subjects rested on a mat in a supine position and the right knee joint was fixed at 0°, and the ankle at 0°, with an ankle dorsiflexion dynamometer with an attached load cell. The ordinary temperature during the measurements was 23–26°C, and the deep temperature of the right tibialis anterior muscle was measured. The deep temperature was measured using a deep temperature monitor (Coretemp CM-210; Terumo Corp., Tokyo, Japan) and a deep temperature probe (PD-1; Terumo Corp., Tokyo, Japan). The deep temperature probe used the zero-heat flow method and a thermistor system, with a measurement range of 0–50°C and a measurement depth of 10 mm. The accuracy of the deep temperature probe is: ±0.1°C at 30–40°C, ±0.2°C at 0–30°C, and ±0.2 °C at 40–50°C. To confirm the location of the muscle belly of the tibialis anterior muscle at a depth of 10 mm at the measurement site, the distance from the skin surface to the tibialis anterior muscle (thickness of subcutaneous tissue) was measured using an ultrasound diagnostic system (LOGIQ α100; WIPRO GE, Bangalore, India). In deep temperature measurements at ordinary temperature, subjects rested in a supine position for 20 min after the probe was attached, and the value when the temperature stabilized was used. After deep temperature was measured at ordinary temperature, it was also measured under the hot or cold condition with the probe still attached while the subject was heated or cooled. At this time, the deep temperature probe was protected using shaped polystyrene foam to prevent it being affected by the hot or cold water. It was also waterproofed using MA film (A423880H; Hakujuji Co., Ltd., Tokyo, Japan) and Tegaderm Roll (NDC-8333-1600-60; 3 M Health Care Ltd., Tokyo, Japan). Deep temperature manipulations under the hot and cold conditions were performed using warm water or ice water in a whirlpool bath. At these times, the warm water was at 43°C, and the ice water to remain at 0°C. Subjects’ legs were immersed up to the head of the fibula, and the right lower leg and ankle were in the hot or cold water for 30 min. However, if a subject complained of distress from the hot or cold, the experiment was stopped, even if 30 min had not elapsed. After the completion of heating or cooling, MFCV and the force-time curve were measured.
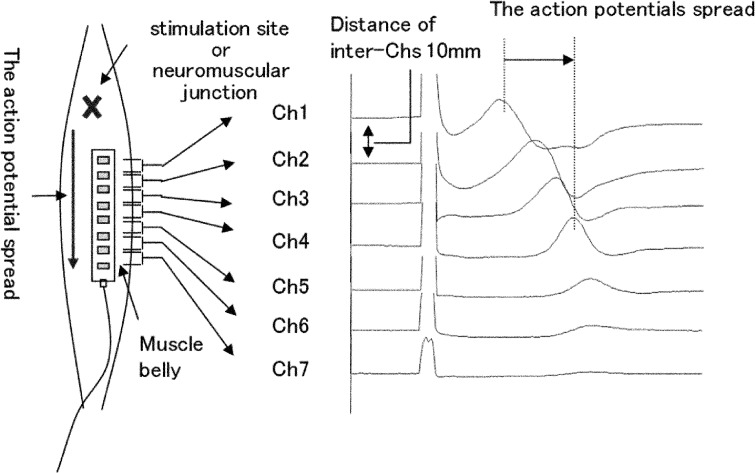
In order to measure the MFCV, subjects were instructed to lie in a resting supine position such that psychological factors affecting voluntary contraction were eliminated. In addition, we checked by palpation that there was no voluntary contraction. MFCV was measured non-invasively by surface electrical stimulation and surface derivation. The site of surface electrical stimulation was the most distant site from where the muscle twitched when induced by the electrical stimulation. Stimulation was performed using block pulses of 0.2 ms duration. The stimulation frequency was 1 Hz, and the stimulation intensity was 10–50 mA, at which waveforms with a positive peak showing a phase gap at fixed latent time intervals on channels 2–4 were obtained. We used array electrodes (TOG206-152; Unique Medical Co., Ltd., Tokyo, Japan), with eight silver chloride electrodes 1 mm in diameter and 10 mm in length arranged in parallel on a transparent silicon sheet. The distance between neighboring silver chloride electrodes was 10 mm. The array electrodes were arranged along the right tibialis anterior muscle at a site 2 cm proximal from the stimulation site. Surface electrical stimulation was performed, and using an evoked potential/electromyogram measuring system (Neuropack Σ; Nihon Kohden Corp., Tokyo, Japan) from the arranged array electrodes, seven channels were recorded simultaneously with bipolar leads from neighboring electrodes (Fig. 1). Signals were filtered by a 10 Hz-5 kHz band-pass filter and ten measurements, were summed and averaged. Subsequently, a channel with an obvious positive peak in the obtained waveform was selected, and MFCV was calculated by dividing the interchannel distance by the peak difference. A total of two trials were conducted for each subject.
Fig. 1.
The site of surface electrical stimulation and schematic diagram of electrode connection to the amplifiers
In order to measure the force-time curve, subjects were instructed to lie in a resting supine position such that muscle twitches were readily induced. Next, an ankle dorsiflexion movement was generated by muscle twitches induced after surface electrical stimulation to the right tibialis anterior muscle. Ankle dorsiflexion was induced twice. A load cell (LUR-A-SA1; Kyowa Electronic Instruments Co., Ltd., Tokyo, Japan) was attached to the ankle dorsiflexion dynamometer, and the rapid force generation process was amplified with a dynamic strain amplifier (DPM-711; Kyowa Electronic Instruments Co., Ltd., Tokyo, Japan). A/D conversion was then performed at 1 kHz with a 16-bit AD converter (Powerlab; ADInstruments Pty Ltd., Castle Hill, NSW2154, Australia) and the results were recorded on a personal computer. The force-time curve data were passed through a 6-Hz high-pass filter, and the rate of change of maximum torque was calculated.
For statistical analysis, two-way analysis of variance and Spearman’s rank correlation coefficient were used. Two-way analysis of variance was used to test for significant differences in each measurement item (deep temperature, rate of change in maximum torque and maximum torque) between the temperature conditions (ordinary temperature, hott and cold). For two-way analysis of variance, a test of normal distribution was performed in advance with the Shapiro-Wilk test. Subsequently, using a significance test for deep temperature and MFCV under the three temperature conditions (ordinary temperature, hot and cold), Bonferroni’s test (multiple comparison method) was used as a post-hoc test. Spearman’s rank correlation coefficient was used as a significance test for MFCV and each deep temperature measurement item (deep temperature, MFCV, rate of change in maximum torque and maximum torque).
Statistical significance was accepted for values of p < 5%. SPSS 13.0 J statistical analysis software was used for all statistical analyses.
RESULTS
No subjects prematurely terminated the experiment during the application of heat or cold. The thickness of subcutaneous tissue was 2.7 ± 0.67 mm (range, 2.0–4.0 mm). The values for MFCV and the rate of change of maximum torque of two trials were averaged for each subject and taken as representative values.
The mean deep temperature was 34.26 ± 0.95°C under ordinary temperature, 37.77 ± 0.34°C under the hot condition 30.06 ± 1.92°C under the cold condition. Significant differences were seen between each temperature condition (p < 0.01) (Table 1 (A)). The average of ordinary temperature MFCV was 3.94 ± 0.77 m·s−1, that of MFCV under the hot condition was 6.14 ± 0.44 m·s−1, and that of MFCV under the cold condition was 1.59 ± 0.37 m·s−1 (Table 1 (B)). In all subjects, MFCV increased together with rises in deep temperature of the tibialis anterior muscle, and decreased with falls in deep temperature. Significant differences were seen between each temperature condition (p < 0.01).
Table 1. Deep temperature (A) and muscle fiber conduction velocity (B) in each temperature condition (ordinary temperature, heat and cold).
| (A) Deep temperature | (B) Muscle fiber conduction velocity | |||||||
|---|---|---|---|---|---|---|---|---|
| ID | cold | ordinary | hot | ID | cold | ordinary | hot | |
| 1 | 32.5 | 36.5 | 37.8 | 1 | 1.28 | 3.43 | 6.01 | |
| 2 | 30.8 | 34.0 | 38.0 | 2 | 1.43 | 4.65 | 5.65 | |
| 3 | 31.0 | 33.5 | 37.7 | 3 | 1.41 | 3.12 | 5.96 | |
| 4 | 30.8 | 33.6 | 37.9 | 4 | 1.20 | 3.52 | 6.51 | |
| 5 | 26.8 | 33.6 | 37.2 | 5 | 1.32 | 3.70 | 5.95 | |
| 6 | 27.3 | 34.6 | 37.3 | 6 | 2.05 | 3.97 | 5.44 | |
| 7 | 31.3 | 34.3 | 37.7 | 7 | 2.01 | 5.70 | 6.86 | |
| 8 | 31.6 | 33.9 | 38.3 | 8 | 1.25 | 3.34 | 6.06 | |
| 9 | 28.3 | 33.5 | 37.7 | 9 | 2.14 | 3.66 | 6.33 | |
| 10 | 30.2 | 35.1 | 38.1 | 10 | 1.81 | 4.28 | 6.63 | |
| AVE | 30.06* | 34.26* | 37.77* | AVE | 1.59* | 3.94* | 6.14* | |
| SD | 1.92 | 0.95 | 0.34 | SD | 0.37 | 0.77 | 0.44 | |
| (°C) | (m·s−1) | |||||||
*Significant differences were seen between each temperature condition (p < 0.01)
MFCV and deep temperature thus showed a strong correlation (r = 0.89, p < 0.01); MFCV increased with a rise in deep temperature and decreased with a decrease in deep temperature. The regression equation was MFCV (m·s−1) = 0.51 × Temperature (°C) −13.50, and there was a change of 0.51 m·s−1 in MFCV per 1 °C of deep temperature.
A strong correlation was also observed between MFCV and the rate of change in maximum torque (r = 0.78), with the rate of change in maximum torque rising with increases in MFCV and decreasing with decreases in MFCV.
DISCUSSION
The results of this study, similar to a previous study using voluntary contraction, showed a strong correlation between MFCV and the rate of change in maximum torque. This suggests a relationship between MFCV and muscle dynamic characteristics. The correlation between muscle twitches and the rate of change in maximum torque was found to be stronger (r = 0.78) than that reported by Murakami et al. for MFCV and the rate of change in maximum torque from voluntary contraction (r = 0.44), and the rate of change in standardized maximum torque (r = 0.74). Thus, muscle twitches from evoked potentials directly reflect the physiological characteristics of muscle fibers, and uncertainties such as psychological factors with voluntary contractions were eliminated in this study. In addition, the strong correlation seen between MFCV and rate of change in maximum torque is likely related to the characteristics of action potential propagation between sarcomeres. With the method used in this study, the speed at which excitation is propagated increases with higher muscle temperature, and at the same time, a greater number of sarcomeres are thought to simultaneously contract. Thus, it is possible to produce contractile force in a shorter time and to produce greater tension by simultaneous contraction of a larger number of sarcomeres. Therefore, MFCV is directly related to the physiological characteristics (contractile characteristics) of muscle.
MFCV has been recognized as a general indicator for evaluating the disuse status of a muscle, or muscle fatigue5). From the present results, there appears to be a wide range of possibilities for the application to the evaluation of biological functions. For example, it may be possible to evaluate MFCV when performance is high in order to evaluate warming up before exercise. In physical therapy, it may be that muscle performance can be improved by performing heat therapy before kinesitherapy for patients, thereby allowing more efficient or more versatile movement. It is also conceivable that the postural control mechanism may work better when the muscle dynamic characteristics of the tibialis anterior muscle have been improved, thereby reducing the risk of falls.
In conclusion, in manipulation of deep temperature, which indicates muscle temperature, the rate of change of maximum torque increased when MFCV was augmented by heating, and the rate of change of maximum torque decreased when MFCV was decreased by cooling. Thus, a correlation between MFCV and muscle dynamic characteristics was demonstrated and a relationship between muscle dynamic characteristics from muscle twitches and MFCV was confirmed.
Issues for future study are investigation of muscle dynamic characteristics arising from differences in muscle fiber type, and the effects on postural control and related factors. By more clearly demonstrating the relationship between MFCV and muscle dynamic characteristics, we would like to determine whether MFCV is a factor in motor control.
REFERENCES
- 1.Mashima H: Physiology. Tokyo: Bunkoudo 1985, pp 49–75. [Google Scholar]
- 2.Murakami K: Fundamental of muscle fiber conduction velocity. Rigaku Ryoho no Ayumi, 2008, 20: 21–26.
- 3.Buchthal F, Rosenfakck P: Rate of impulse conduction velocity in denervated human muscle. Electroencephalograhy and clinical. Neurophysiology, 1958, 10: 521–526 [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Martínez A, Arpa J: Muscle fiber conduction velocity in situ (MFCV) in denervation, reinnervation and disuse atrophy. Acta Neurol Scand, 1999. 100: 337–340 [DOI] [PubMed] [Google Scholar]
- 5.Kondo K: Muscle fiber conduction velocity study as an indicator of muscle atrophy in the hemiplegia. Jpn J Rehabili Med, 1996, 36: 477–484 [Google Scholar]
- 6.Hakansoon CH: Conduction velocity and amplitude of the action potential as related to circumference in the isolated fiber of frog muscle. Acta Physiol Scand, 1956, 39: 291–312 [DOI] [PubMed] [Google Scholar]
- 7.Stalberg E: Propagation velocity in human muscle fibers in situ. Acta Physiol Scand Suppl, 1966, 287: 1–112 [PubMed] [Google Scholar]
- 8.Sadoyama T, Masuda T, Miyano H: Optimal conditions for the measurement of muscle fibre conduction velocity using surface electrode arrays. Med Biol Eng Comput, 1985, 23: 339–342 [DOI] [PubMed] [Google Scholar]
- 9.Troni W, Cantello R, Rainero I: Conduction velocity along human muscle fibers in situ. Neurology, 1983, 33: 1453–1459 [DOI] [PubMed] [Google Scholar]
- 10.Cruz Martínez A: Conduction velocity in human muscle fibers in situ. Study in upper and lower limbs in healthy adults. Electromyogr Clin Neurophysiol, 1989, 29: 363–368 [PubMed] [Google Scholar]
- 11.Rau G, Disselhorst-Klug C, Silny J: Noninvasive approach to motor unit characterization: muscle structure, membrane dynamics and neuronal control. J Biomech, 1997, 30: 441–446 [DOI] [PubMed] [Google Scholar]
- 12.Matsunaga S, Sadoyama T, Miyata H: The effects of strength training on muscle fiber conduction velocity of surface action potential. The Japanese society of physical fitness and sport medicine, 1990, 39: 99–105.
- 13.Miyata H, Sadoyama H, Katsuta S: Muscle fiber conduction velocity in human vastus lateralis during isometric contractions: relation to muscle fiber composition. The Japanese society of physical fitness and sport medicine, 1985, 34: 231–238.
- 14.Sadoyama T, Masuda T, Miyata H, et al. : Fibre conduction velocity and fibre composition in human vastus lateralis. Eur J Appl Physiol Occup Physiol, 1988, 57: 767–771 [DOI] [PubMed] [Google Scholar]
- 15.Kupa EJ, Roy SH, Kandarian SC, et al. : Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J Appl Physiol 1985, 1995, 79: 23–32 [DOI] [PubMed] [Google Scholar]
- 16.Grimby G, Danneskiold-Samsøe B, Hvid K, et al. : Morphology and enzymatic capacity in arm and leg muscles in 78-81 year old men and women. Acta Physiol Scand, 1982, 115: 125–134 [DOI] [PubMed] [Google Scholar]
- 17.Larsson L: Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand, 1983, 117: 469–471 [DOI] [PubMed] [Google Scholar]
- 18.Morimoto S, Umazume Y, Masuda M: Properties of spike potentials detected by a surface electrode in intact human muscle. Jpn J Physiol, 1980, 30: 71–80 [DOI] [PubMed] [Google Scholar]
- 19.Cruz-Martínez A, Ramírez A, Arpa J: Quadriceps atrophy after knee traumatisms and immobilization: electrophysiological assessment. Eur Neurol, 2000, 43: 110–114 [DOI] [PubMed] [Google Scholar]
- 20.Arendt-Nielsen L, Mills KR, Forster A: Changes in muscle fiber conduction velocity, mean power frequency, and mean EMG voltage during prolonged submaximal contractions. Muscle Nerve, 1989, 12: 493–497 [DOI] [PubMed] [Google Scholar]
- 21.Merletti R, Lo Conte LR, Cisari C: Age related change in surface myoelectric signals. Scand J Rehabil, 1992, 1992: 25–36 [PubMed] [Google Scholar]
- 22.Murakami K, Onobe J, Sato Y: Effect of deep temperature change on muscle fiber conduction velocity. Annu Rep The Tohoku Sect Jpn Phys Ther Assoc, 2009, 21: 63–68 [Google Scholar]
- 23.Murakami K, Sato Y, Onobe J: A study of the relationship between time-force characteristics of muscle force production and muscle fiber conduction velocity. Annu Rep The Tohoku Sect Jpn Phys Ther Assoc, 2010, 22: 20–25 [Google Scholar]