Abstract
Circadian rhythms generated by the suprachiasmatic nucleus (SCN) are entrained to the environmental light/dark cycle via intrinsically photosensitive retinal ganglion cells (ipRGCs) expressing the photopigment melanopsin and the neuropeptide PACAP. The ipRGCs regulate other non-image-forming visual functions such as the pupillary light reflex, masking behaviour and light induced melatonin suppression. To evaluate whether PACAP immunoreactive retinal projections are useful as a marker for central projection of ipRGCs in the monkey brain, we characterized the occurrence of PACAP in melanopsin expressing ipRGCs and in the retinal target areas in the brain visualized by the anterograde tracer Cholera Toxin subunit B (CtB) in combination with PACAP staining. In the retina, PACAP and melanopsin were found to be co-stored in 99% of melanopsin expressing cells characterized as inner and outer stratifying melanopsin RGCs. Two macaque monkeys were anesthetized and received a unilateral intravitreal injection of CtB. Bilateral retinal projections containing co-localized CtB and PACAP immunostaining were identified in the SCN, the lateral geniculate complex (LGN) including the pregeniculate nucleus (PrGC), the pretectal olivary nucleus (PON), the nucleus of the optic tract (NOT), the brachium of the superior colliculus (BSC), and the superior colliculus (SC). In conclusion, PACAP immunoreactive projections with co-localized CtB represent retinal projections of ipRGCs in the macaque monkey, and support previous retrograde tracer studies demonstrating that melanopsin containing retinal projections reach areas in the primate brain involved in both image and non-image-forming visual processing.
Keywords: Neurotransmitter, Circadian rhythms, RHT, Melanopsin, PACAP, VIP, Suprachiasmatic nucleus, LGN, PON
Introduction
The mammalian eyes are equipped with two functionally and anatomically different light detection systems. In addition to the classical image-forming system involving rods and cones, the eyes contain another system identified as an irradiance detection system, which relays non-image-forming visual information to the brain (Berson, 2003;Do and Yau, 2010). Non-image-forming photic information synchronizes the circadian clocks to the light/dark (LD) cycle and regulates pineal melatonin secretion, sleep/wake and pupillary constriction (Fu et al., 2005;Do and Yau, 2010;Gamlin et al., 2007). Non-image-forming visual information reaches areas in the brain including the circadian clock located in the hypothalamic suprachiasmatic nucleus (SCN) via a monosynaptic neuronal pathway designated as the retinohypothalamic tract (RHT) (Moore and Lenn, 1972;Moore et al., 1995). The RHT originates from a subset of retinal ganglion cells (RGCs) which are intrinsically photosensitive (ipRGCs) due to melanopsin, and which in rodent and humans co-store two neurotransmitters, glutamate and pituitary adenylate cyclase activating polypeptide (PACAP)(Hannibal et al., 2000;Hannibal et al., 2002;Hannibal et al., 2004). Central projections of ipRGCs have been demonstrated in mice using two different lines of melanopsin reporter mice; one line targeting the melanopsin gene by tau-lacZ under the control of the melanopsin promoter and one a Cre-based line (Hattar et al., 2006;Ecker et al., 2010). In rat, hamster and mouse, we have previously demonstrated melanopsin projections in the brain by staining for one of the RHT neurotransmitters, PACAP, in combination with the anterograde tracer, cholera toxin B (CtB) injected into the eye (Hannibal and Fahrenkrug, 2004;Bergström et al., 2003;Hannibal and Fahrenkrug, 2006). Knowledge of the circadian organization of behaviour in humans is essential to understanding human adaptation to the environment both in health and in diseases which may disrupt or disturb the circadian system. Since humans cannot be examined by the anatomical techniques necessary for a detailed mapping of neuronal connectivity, studies in other primates are essential for such purposes. However, at the moment it is not possible to use genetic based reporter systems in monkeys, and therefore classical retrograde tracing from the lateral geniculate complex and the pretectum has been used and identified these areas as a targets for the ipRGCs (Dacey et al., 2005). A more detailed characterization of central melanopsin projections in monkeys has, however, not been performed. To investigate whether immunohistochemical staining of PACAP in combination with staining for the anterograde tracer CtB, delivered by an intraocular injection, can be used to identify central ipRGC projections in the macaque brain we used this approach in two monkeys, one Macaca fascicularis (MF), and one Macaca mulatta (MM). This was performed after initially demonstrating that PACAP was found to be co-stored with melanopsin in ~99% of melanopsin expressing cells in the monkey eye.
Material and Methods
Animals
Two male macaque monkeys, one Macaca fascicularis (MF), aged 20, and one Macaca mulatta (MM1), aged 5, were used for the tracer study. Two male Macaca mulatta, one aged 8 (MM2) and one aged 2.5 years (MM3) were used for the detailed retina analyses. The animals were kept in the primate animal facility of the University of Alabama at Birmingham in 12:12 light:dark conditions. For the tracing study, MM1 and MF received a unilateral injection of 100 μl of 1% cholera toxin B (CtB) (List Biological) in sterile water into the vitreous body using a 1cc tuberculin syringe under isoflurane anaesthesia. Following a survival time of 7 days, the animals were deeply anesthetized with barbiturate and perfused through the aorta with 800 ml of warm (~37°C) 1% Sodium nitrite/0.9% Sodium chloride followed immediately by 4 L of cold (~4°C) 4% paraformaldehyde (PFA in PBS, pH 7.2). Eyes and brain were removed and the eyes were postfixed for 2 hours in PFA with anterior chamber removed during the fixation. The retinas were removed and placed in PBS followed by cryoprotectant (see below). The brains were placed in a solution of 30% sucrose in 0.1M phosphate buffer (PBS) for 48-96 hours. The brains were subsequently frozen, coronally sectioned at 40 μm using an AO 860 sliding microtome, and placed in PBS which was later replaced by cryoprotection solution (30 % sucrose, 1% Polyvinyl-Pyrrolidone (PVP-40), 30 % Ethylene glycol, 0.05 M Sodium phosphate buffer, pH 7.2) for better conservation and thereafter stored at −20 °C until immunohistochemically processed (see below). The anaesthesia used for MM2 and MM3 was initiated with Ketamine followed by Pentobarbital (200 mg/kg BW), and the animals were then perfused through the aorta with 2 L 1% Sodium nitrite/0.9% Sodium chloride and fixed with 3 L Stefani fixative for two 2h. Eyes were removed, the anterior chambers were removed, and they were post fixed overnight in PFA/Stefanini fixative. Subsequently, the retinas were dissected and placed in PBS followed by cryoprotectant.
Experimental procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC) and complied with the USPHS Policy on Humane Care and Use of Laboratory Animals.
Immunohistochemistry
Brain sections were treated by antigen retrieval solution for 16 h at 40 °C(DAKO ChemMate, code No. S 203120 in distilled water, pH 6) before processing for immunohistochemistry. Flat-mount retinas were treated with antigen retrieval solution for 1.5-2 h at 80 °C (DAKO ChemMate, code No. S 203120 in distilled water, pH 6) followed by immunostaining for PACAP (see below). Subsequently, the retinas received another treatment in antigen retrieval solution of 1.5 h at 80 °C when using the C-terminal anti-melanopsin antibody (see below) or no antigen retrieval when using the N-terminal anti-melanopsin antibody (see below)(DAKO ChemMate, code No. S2367 in distilled water, pH 9).
Antibody characterization (see also table 1)
Table 1.
Table of primary antibodies used
| Antigen | Immunogen | Manufacture | Dilution used |
|---|---|---|---|
| PACAP Code MabJHH1 |
PACAP1-38 | In-house | 1:5 |
| Melanopsin C- terminal Code no: 5J68 |
C-terminal part of human melanopsin (amino acid sequence of the fusion protein ; see methods) |
In-house | 1:10000 |
| Melanopsin N- terminal Code no: hNA |
peptide consisting of 19 amino acid residues, MNPPSGPRVPPSPTQEPSC |
In house by Dr. King-Wai Yau, John Hopkins University |
1:500 |
| anti-CtB antibody (code no. 703, Lot #7032E |
aB subunit pentamer of cholera toxin (choleragenoid) |
List Biologicals, Champbell, CA, USA, |
1:1000 |
The PACAP antibody used in the present study was a mouse monoclonal anti-PACAP antibody described in detail previously (Hannibal et al., 1995). The antibody (Code MabJHH1, diluted 1:2) was produced in mice immunized with PACAP38 and the antibody recognized an epitope located at PACAP6-16(Hannibal et al., 1995). Preabsorption with PACAP38, PACAP27 and PACAP6-27 but not PACAP16-38 eliminated all staining on brain tissue (Hannibal et al., 1995). No specific staining using the anti-PACAP antibody is seen in mice lacking the PACAP gene (our unpublished observation).
A commercially available anti-CtB antibody (code no. 703, Lot#7032E, List Biologicals, Champbell, CA, USA, diluted 1:1000) raised in goat was used for tracer visualization. According to the manufacturer the antigen was a B subunit pentamer of cholera toxin (choleragenoid). Omission of the primary antibody abolished all staining (not shown). The C-terminal anti-human melanopsin antibody was raised in-house in rabbit against a fusion protein representing the C-terminal part of human melanopsin (amino acid sequence of the fusion protein wasMRGSHHHHHHGMASMTGGQQMGRDLYDDDDKDHPFTHPKYRVAIAQHLPCLGVLLGVS RRHSRPYPSYRSTHRSTLISHTSNLSWISIRRRQESLGSESEVGWTHMEAAAVWGAAQQANGRSLY QGLEDLEAKAPPRPQGHEAETPGKTKGLIPSQDPRM) as described in detail previously (Hannibal et al., 2004;Jusuf et al., 2007). The C-terminal anti-melanopsin antibody (from rabbit no. 5J68, diluted 10,000) was characterized as described previously (Hannibal et al., 2004;Jusuf et al., 2007) by preabsorption of the antibody with the immunization material dialyzed against PBS with 0.1% Tween which abolished all staining. The rabbit anti-human melanopsin antiserum recognized human and monkey but not rodent melanopsin. An N-terminal anti-melanopsin antibody (a kind gift from Dr. King-Wai Yau, John Hopkins University) (code hNA diluted 1:500) which was raised in rabbit against a peptide consisting of 19 amino acid residues, MNPPSGPRVPPSPTQEPSC, from the N-terminus of the conceptually translated human melanopsin protein (NCBI accession number AAF24978) (Dacey et al., 2005) was used together with either PACAP or the C-terminal anti-melanopsin antibody. The C- and the N-terminal antibodies were used to clarify whether the C-terminal antibody showed different staining of melanopsin ipRGCs (see Results). The antibody against vasoactive intestinal polypeptide (VIP) was an in-house raised rabbit anti-VIP antibody (code no: 291E, diluted 1:1000) raised against human VIP1-28 described previously (Fahrenkrug et al., 1995). Preabsorption with VIP, but not PACAP, eliminated all staining.
Immunohistochemistry
Immunohistochemical detection of PACAP/CtB/VIP and PACAP/melanopsin was performed as described previously (Hannibal et al., 2001;Hannibal et al., 2004;Juhl et al., 2007) except that brain sections were incubated with primary antibodies for 72-96 h at 4 °C. For immunohistochemistry on flat-mounts a 72-84 h incubation period was used. For double and triple labelling a mixture of biotinylated donkey anti-mouse antiserum (code no. 715-065-151, Jackson Immunoresearch Laboratories, Inc., West Grove, Pennsylvania, USA, diluted 1:800), Texas Red®-conjugated donkey anti-goat antiserum (code no.705-075-147, Jackson Immunoresearch Laboratories, diluted 1:200) and CY5-conjugated donkey anti rabbit antiserum (code no.706-175-134, Jackson Immunoresearch Laboratories, diluted 1:100) or CY2/Alexa488 conjugated donkey anti rabbit antiserum (code no.711-226-152, Jackson Immunoresearch Laboratories, diluted 1:100/code no:A21206, Molecular Probes) were used in combination with streptavidin biotin-horseradish peroxidase complex (Vector, Burlingame, USA), biotinylated tyramide (Tyramide System amplification, DuPont NEN®, Boston, MA, USA) and streptavidin-CY2 (Amersham, Birkerød, Denmark). When using two primary antibodies raised in the same species we used the tyramide amplification system (Hundahl et al., 2012) originally described by Berghorn (Berghorn et al., 1994). Briefly, C-terminal melanopsin (diluted 1:10,000, five days at 4 °C) was visualized using Envision (code K4002, dilutes 1:2, DAKO) and tyramide conjugated Alexa594 (Molecular Probes) followed by blocking in 1% H2O2 to quench horseradish peroxidase, followed by incubation in N-terminal anti-melanopsin antibody (diluted 1:500, five days at 4 °C) and visualized using an Alexa488 conjugated donkey anti-rabbit antibody (code A21206, Molecular Probes). Images were obtained using an iMIC confocal microscope (Till Photonics, Germany) equipped with appropriate filter settings for detecting DAPI, CY2/Alexa488, Texas Red/Alexa561/594 and CY5. Co-localization was determined using the co-localization plug-in module in ImageJ/Fiji software (vers. 1.47q, NIH, USA) on digital images representing the retinal target areas in the brain. This plug-in highlights the co-localized points of two 8-bit images and co-localized points appear white (we used default value = 255). Pixels are considered co-localized if their intensities are higher than the threshold of their channels (we use threshold set at 50-100 depending on the background noise) and if the ratio of their intensity is higher than the ratio setting value (we use the default set at 50%). Melanopsin/PACAP cell counts were performed from pieces of retina photographed by the iMIC confocal microscope (Till Photonics, Germany) using the wide field camera and X10 objective. Each piece of retina was photographed and images covering the entire piece were stitched together using the LA Stitch plug-in in Fiji software (vers. 1.47q, NIH, USA). Each of these images were then analysed using the cell counter plug-in also in Fiji to mark and count which cells contain PACAP and melanopsin, only melanopsin or only PACAP. Cell counts were performed in areas in which cells were well stained with both antigens, PACAP and melanopsin. Areas in which either one or both immunoreactions were insufficient were excluded. Furthermore, the evaluation of the cell density was performed in central and more peripheral parts of the retina in which both the inner and outer melanopsin processes could be identified. In some areas the inner stratification plexus was not stained or only weekly stained and these areas were not used in the evaluation. For a more detailed visualization of the melanopsin immunoreactive network of outer and inner stratifying processes Z-stacks with a focal dept of 30-40 micron were generated and the Z-stacks of images were stitched as described above. For 3D analysis at the higher magnification, images were obtained by the spinning disk confocal part of the microscope. Z-stacks of typically 60-80 images separated in the Z-level by 0.5 μm were deconvolved in AutoQuant X (Version 3.02, Media Cybernetics, Inc, Rockville, USA) and the localization of dendritic processes and cell bodies were further analysed using IMARIS (version 7.6.4, Bitplane Scientific Software). Finally, images were corrected for brightness and contrast in Adobe Photoshop CS5 Extended and mounted into plates using Adobe Illustrator CS5.
Results
Melanopsin immunoreactivity demonstrated using N- and C-terminal directed anti-melanopsin antibodies
In mouse, melanopsin has been found in two isoforms, OPNL (long) and OPNS (short), differentiated by the length of the C-terminal of the protein (Pires et al., 2009). In humans and in monkeys such isoforms have so far not been identified. To ensure that our in-house C-terminal directed anti-melanopsin antibody detects all forms of melanopsin, double staining on the same piece of retina was performed using both N- and C-terminal directed antibodies. Both antibodies labelled the same population of cells, although the N-terminal antibody label appeared more robust (Fig. 1). The following results were obtained using either the C- or the N-terminal antibody.
Figure 1.
Melanopsin and PACAP immunoreactivity is found in inner and outer stratifying RGC’s. A-D. Outer (cell 1) and inner (cell 2) stratifying RGC’s expressing PACAP in magenta (A) and melanopsin identified using both a C-terminal antibody (B, green) and an N-terminal antibody (C, blue) and with the two antibodies shown merged (D). E. Extended view representing the same Z-stack of a total of 55 images of the cells shown in A-D presented in an XY (E), XZ (E1) and ZY (E2) plane to illustrate dendritic stratification. F. The same cells shown in A-E analysed for the localization of the dendritic processes using the Imaris filament tracer module (see Material and Methods). The majority of dendritic processes of cell 2 stratify in the most inner part of the inner plexiform layer (IPL)(magenta,F1-2F2). The dendrites of cell 1 stratify exclusively at the border of the INL/IPL (yellow, F1-2 F2) and its cell body is displaced to the INL (F1-2). Scale bars; 50 μm.
Melanopsin containing RGCs co-store PACAP immunoreactivity
Melanopsin immunoreactive RGCs were found throughout the entire macaque retina as described in detail previously (Jusuf et al., 2007;Dacey et al., 2005). Melanopsin immunoreactivity is located in the soma, axon and dendritic membrane (Figs. 1-3). Melanopsin immunoreactive cells form two distinct dendritic mosaics – one with dendrites stratified in the inner plexiform layer (IPL) close to the border of the ganglion cell layer (GCL) (Figs. 1-3) and one with dendrites stratified in the outer IPL close to the border of the inner nuclear layer (INL) (Figs.1-3). While inner stratifying cells have cell bodies exclusively located in the GCL, outer stratifying cell bodies were often displaced to the INL as previously described (Jusuf et al., 2007;Dacey et al., 2005) (Figs. 1-3). In both types of melanopsin cells PACAP was found to be co-stored (Figs. 1-3). In the melanopsin expressing RGCs the amount of melanopsin immunoreactivity varies in intensity (Figs. 1-3), and melanopsin cells with weak and strong intensity staining could be found among both inner and outer stratifying cells, although outer cells generally stain more strongly than inner stratifying cells (Figs. 2-3). Similarly, PACAP immunostaining in the two cell types varied in intensity but did not follow the intensity of the melanopsin staining (Figs. 2-3). In several cases melanopsin containing inner stratifying cells were often identified using PACAP immunostaining due to the weak staining of melanopsin (Figs. 2-3). Total cell counts were performed in pieces of retina in which immunostaining from both antigens was well preserved. We counted a total of 464 cells which contained both PACAP and melanopsin immunoreactivity. Three cells contained only melanopsin and 26 cells located in the GCL contained only PACAP immunoreactivity. Thus, 99% of the melanopsinlabeled cells co-stored PACAP whereas 95% of the PACAP labeled cells co-stored melanopsin. PACAP/melanopsin cell density varied with the lowest density of PACAP/melanopsin cells in the peripheral retina (3-5 cells/mm2)and the highest density in the more central retina (13-23 cells/mm2) as reported previously (Dacey et al., 2005).
Figure 3.
Melanopsin and PACAP immunoreactivity is found in inner and outer stratifying RGC’s. A. Three RGCs numbered 1-3 expressing both melanopsin (visualized using the N-terminal directed anti-melanopsin antibody) and PACAP, and one RGC stained only for PACAP (arrows), shown in an extended view representing a Z-stack of a total of 50 images presented in an XY (A), XZ (A1) and ZY (A2) plane. Co-localization of melanopsin and PACAP appears yellow. Note the weak expression of both melanopsin and PACAP in cell no. 2. Inner and outer stratifying dendritic processes are shown in the XZ (A1) and YZ (A2) planes. B. Focus is in the inner IPL near the GCL border, showing PACAP (green) and melanopsin (magenta) labelling of cells1 and.2 (same cells as shown in A). Arrow indicates the same PACAP labelled cell indicated by the arrow in A. Nuclei from other ganglion cells are stained with DAPI (blue). C. Focus is in the outer IPL near the INL border showing PACAP (green) and melanopsin (magenta) labelling of cell3 (same cell as shown in A). The cell body is displaced to the inner nuclear layer (INL). Co-localization of melanopsin and PACAP appears yellow. Nuclei from other cells in the INL stained with DAPI (blue). D-E. Extended view of the Z-stack of 50 images from A showing the melanopsin cells before (D) and after (E) being analysed for the localization of dendritic processes using the Imaris filament tracer module (see Materials and Methods). F. Focus as in B after Imaris filament tracer analysis showing cell 1 (yellow) and cell2 (green). The majority of dendrites of cell 1 are located in the most inner part of the IPL. Cell2 expressed low levels of melanopsin and the weakly stained proximal dendrites are also in the inner IPL. The axon of cell3 (magenta) can be seen descending through the IPL towards the GCL. G. Focus as in C after Imaris filament tracer analysis showing the cell body and outer stratifying dendrites of cell3 (magenta). Several dendrites from cell 1 (yellow) can be seen ascending to the outer IPL. H. Summary of the traced cells shown in E-G. Panel H1shows an extended view in the XZ plane and panel H2 shows an extended view in the YZ plane. The dendrites of cell3 are located exclusively in the outer IPL. GCL; ganglion cell layer, INL; inner nuclear layer. Scale bars; 50 μm.
Figure 2.
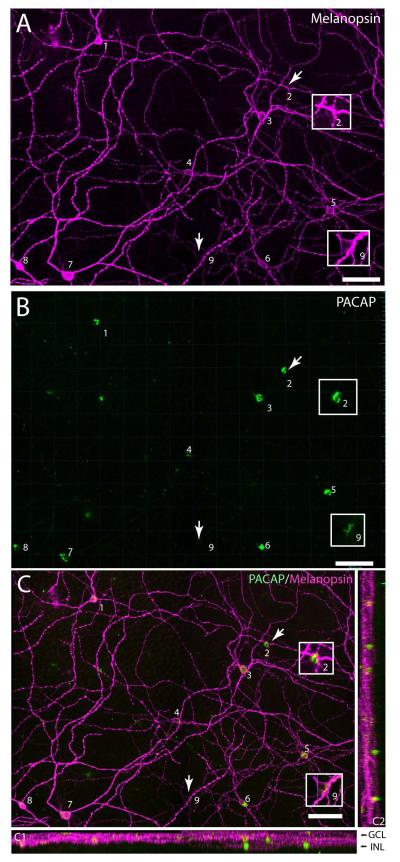
PACAP is found in melanopsin immunoreactive RGC’s of the macaque monkey. A. Melanopsin immunoreactivity(magenta) (visualized using the C-terminal directed anti-melanopsin antibody) shown in a flat-mount preparation of the Macaca mulatta (MM1) retina. The image represents a montage of 4 images each representing a Z-stack of 35 sections covering the depth required to ensure that both inner and outer stratifying melanopsin processes are visible. The melanopsin cells are numbered 1-9. Note the weak staining of two inner stratifying cells (2 and 9). B. PACAP(green) staining of the same piece of retina as shown in A with cells numbered as in A (1-9). Note the low level of PACAP found in cell 9.C.Extended view representing the same Z-stack of a total of 35 images of the cells shown in A-B presented in an XY (C), XZ (C1) and ZY (C2) plane to illustrate dendritic stratification. Melanopsin (magenta) and PACAP (green) co-stored in the same RGCs numbered 1-9. Scale bars: 80 μm. GCL; ganglion cell layer, INL; inner nuclear cell layer.
Retinal target areas in the brain innervated by PACAP
Since PACAP-IR was found in approximately 99 % of the melanopsin containing RGCs of the macaque retina, PACAP immunoreactivity when co-stored with CtB was used to demonstrate central retinal melanopsin projections in the macaque brain. We were, however, unable to evaluate whether all PACAP/melanopsin immunoreactive RGCs express detectable PACAP positive signals in their axon terminals in the brain. A similar approach has, however, been found useful in the rat brain (Hannibal and Fahrenkrug, 2004) and our findings in the monkey are comparable to the rat (Hannibal and Fahrenkrug, 2004). VIP, a neuropeptide which is densely expressed in ventrally located neurons of the retinorecipient part of the mammalian SCN, was used as a marker for the SCN (Moore, 1993). The CtB containing retinal nerve fibres show equal distribution and staining intensity in the two animals. PACAP-IR retina projections were demonstrated in the SCN, LGN and PrGC, the pretectum, the brachium of the superior colliculus (BSC) and the SC.
The suprachiasmatic nucleus
The monkey SCN was densely innervated by CtB immunoreactive fibers which occurred with a slight ipsilateral dominance (Fig. 4B, E, H and K) ascending into the ventral SCN from the optic chiasm. As previously reported in monkey (Moore, 1993), the mid part of the SCN received the most intensively stained part of the RHT projection (Fig. 4H) whereas the rostral (Fig. 4B) and caudal (Fig. 4K) SCN were less intensely innervated from the eye. Corresponding to the unilateral injections of CtB, retinal fibres innervating the ventro-lateral area of the rostral and mid SCN also displayed PACAP immunoreactivity (Fig. 4A, D and G;Fig. 5A-D). In the caudal part of the SCN retinal projections largely lacked PACAP immunoreactivity (Fig. 4J and K and Fig. 5 E-H). These PACAP negative fibres most likely represent retinal projections of a nonmelanopsin origin as found in several rodent species (Gooley et al., 2003;Morin et al., 2003;Sollars et al., 2003;Hannibal and Fahrenkrug, 2004). PACAP immunoreactive fibres lacking CtB were observed in the SCN in both animals illustrating the occurrence of innervation from the non-injected eye and most likely also representing a minor group of PACAP nerve fibres originating in the brain as found in rodents (Hannibal and Fahrenkrug, 2004) (see also Hannibal et al., 1997).
Figure 4.
Retinal innervation of the macaque rostral (A-C), rostral-mid (D-F), mid (G-I) and caudal (J-L) SCN. The SCN was identified by immunostaining for VIP (C, F, I, L, blue). Retinal projections were visualized by cholera toxin subunit B immunoreactivity (B, E, H, K, magenta) and the melanopsin projections were demonstrated by staining for PACAP (A, D, G, J, green) Boxed area in G and H are shown at higher magnification in Figure 5. Oc; optic chiasm. Scale bar; 100 μm.
Figure 5.
Retinal innervation of the macaque mid and caudal SCN. A-D. Higher magnification view of retinal projections representing the melanopsin ipRGCs in the mid SCN shown in boxed area in Figure 4G H, demonstrating co-localization between cholera toxin subunit B (B; CtB; magenta) and PACAP (A; green) immunoreactivity as visualized in C (yellow)and by white colour representing 100 % overlap in D (calculated using the co-localization plug-in in Fiji). Note the PACAP fibres not co-storing CtB may originate from the contralateral eye and to a minor extent from the brain. E-H. Same area of the caudal SCN shown in Figure 4J-K. Co-localization between PACAP (E; green) and CtB (F; magenta) was very sparse, as visualized in G (yellow) and by the few white dots representing 100 % overlap in H (calculated as in D). Oc; optic chiasm. Scale bars: A-D; 20 μm.
The lateral geniculate nucleus (LGN)
Retinal projections to the LGN in the macaque are shown in Figure 6. Distinct autofluorescence was found in cell bodies of the LGN in both animals using filters for the detection of both CtB and PACAP and verified using UV illumination. The autofluorescence appears as co-localization in different parts of the LGN but can be distinguished from the delicate nerve endings of the retinal projections (Fig.6). CtB projections were found in the rostro-caudal axis of the LGN. The retinal projections from the two eyes are segregated; crossed projections innervating layer I, IV and VI (Fig. 6B) and uncrossed retinal projections innervating layers II, III, and V are visible in both the MM1(Fig. 6A) and MF. PACAP immunoreactivity was located throughout the LGN (Fig. 6) representing both retinal and non-retinal innervation. PACAP-containing retinal fibres appeared to innervate both the parvocellular and the magnocellular part of the LGN whereas we found no innervations of the koniocellular layers. PACAP fibres lacking CtB immunoreactivity may represent innervation from the contralateral eye or may originate from other areas in the brain. PACAP immunoreactive fibres co-storing CtB were found in layer V,III and II on the ipsilateral side (Fig. 6C, D, F and I), and on the contralateral side in nerve fibres in layer VI, most intense in the lateral part (Fig. 6 O-P), and in layer IV and layer I (Fig. 6 K and N). The delicate retinal projections containing both CtB and PACAP immunoreactivity are shown at high magnification in layer III in Figure 6I and in layer VI in Figure 6P.
Figure 6.
PACAP containing retinal projections innervate the lateral geniculate nucleus(LGN) of the macaque monkey. A-B. Retinal projections visualized by CtB and PACAP immunostaining of the mid ipsilateral (A) and contralateral (B) LGN innervating layers II, III and V on the ipsilateral side and layers I, IV, and VI on the contralateral side (the images in A and B consist of 6×8 tiled images which were stitched together). C-H. High magnification images of the six sublayers on the ipsilateral side taken within the area indicated by the large box in A. Co-localization between PACAP and CtB was determined using the co-localization plug-in in Fiji software (see Materials and Methods) and is shown in white (exemplified by arrows in 6C-P). I.PACAP and CtB colocalization in the ventral lateral area of layer VI indicated by the small box in A shown at a higher magnification. J-O. Images of the six sublayers on the contralateral side taken within the area indicated by the large box in B.P.A higher magnification image from the ventral lateral area of layer VI indicated by the small box in B. Note that autofluorescent cell bodies (exemplified by arrowheads in 6C-P)found throughout the LGN appear as co-localization since they are seen with both filter settings. Scale bars: A-B,200 μm; C-H and J-O,50 μm; I and P, 25μm.
The pregeniculate complex (PrGC)
Retinal projections were found in the PrGC corresponding to the rostral part of the LGN complex with a similar distribution on the contra- and ipsilateral side (Fig. 7A). PACAP immunostaining was found in the same area, and a number of PACAP fibers co-stored CtB immunoreactivity (Fig. 7E and H). PACAP positive fibres not containing CtB were also found in this area of the PrGC as well as dorsal to these retinal projections (Fig.7 A-B). CtB immunoreactive nerve fibres not co-storing PACAP were also found in the PrGC (Fig.7 F-H). No PACAP immunoreactive cell bodies were found in the PrGC.
Figure 7.
PACAP containing retinal projections innervate the pregeniculate (PrGC) complex of the macaque monkey. A. Retinal projections visualized by CtB immunostaining of rostral contralateral PrGC. The image consists of 3×3 tiled wide field images that were stitched together. B. Same section as in A co-stained for PACAP showing strong immunoreactivity in the PrGC. C-E. Higher magnification images from the area indicated by the asterisks in A and B. F-H. Ultrahigh magnification of the area indicated by the squares in C-E.PACAP is found in retinal and non-retinal projections to the PrGC. Co-localization between CtB and PACAP containing retinal projections is shown in white in E and H (determined using the co-localization module in ImageJ, see Material and Methods). Scale bars: A-B; 100 μm, C-E; 50 μm, F-H; 20 μm.
The pretectum and superior colliculus (SC)
Retinal projections were found as bilateral projections to several pretectal nuclei as described previously (Gamlin, 2006). Dense innervation was observed in the pretectal olivary nucleus (PON) (Fig. 8) and in the nucleus of the optic tract (NOT) (Fig. 9), whereas the medial and posterior pretectal nuclei (PPN) receive sparse retinal innervation (not shown). Retinal projections were also found in the brachium of the superior colliculus (BSC) (Fig. 10) and within the SC (Fig. 11). In the PON, a minor part of the retinal projections co-stored PACAP immunoreactivity (Fig. 8E and H). However, PACAP seems also to occur in a small group of cell bodies scattered in the PON (Fig. 8B and D) giving rise to some of the non-retinal PACAP fibres found in the nucleus. In the NOT, which also received relatively dense innervation of CtB immunoreactive nerve fibres, the number of fibres also co-storing PACAP was limited and found mainly in delicate nerve fibre varicosities most likely passing the NOT towards the SC (Fig. 9E). As found in other pretectal nuclei, PACAP fibres of non-retinal origin also occur in the NOT (Fig. 9B and D). Single fibres immunoreactive for both CtB and PACAP could be found in the medial and in the posterior pretectal nucleus (not shown). In the BSC a relatively large contingent of retinal projections co-stored PACAP (Fig. 10). Again PACAP was found in delicate nerve fibre varicosities (Fig. 10E and H). Dense retinal innervation visualized by CtB immunoreactivity was found in the superficial layers of the SC with a significant contralateral dominance (Fig. 11). PACAP on the other hand, was found in nerve fibres in the superficial layer and in fibres and cell bodies of the deeper layers of the SC (Fig. 11C and D, I and L) and co-localization between CtB and PACAP was found in both the contra- and ipsilateral retinal projections (Fig. 11G, J and M).
Figure 8.
PACAP containing retinal projections representing the melanopsin ipRGCs innervating the olivary pretectal nucleus (PON).A. Retinal projections visualized by CtB immunostaining in the PON. The image consists of 3×3 tiled confocal images stitched together for better overview. B. Same section as in A co-stained for PACAP showing that PACAP was found in both retinal projections and in cell bodies located within the PON as seen at higher magnification (C-E) and ultrahigh (F-H) magnification representing the area indicated by the square in E. Co-localization between CtB and PACAP containing retinal projections is shown in white (determined using the colocalization module in ImageJ, see Material and Methods). Scale bars: A-B; 100 μm, C-E; 50 μm, F-H; 10 μm.
Figure 9.
PACAP containing retinal projections representing the melanopsin ipRGCs innervating the nucleus of the optic tract (NOT).A. Retinal projections visualized by CtB immunostaining in the NOT. The image consists of 4×4 tiled confocal images stitched together for better overview. B. Same section as in A co-stained for PACAP showing that PACAP was found in both retinal and non-retinal projections located within the NOT. C-E. High magnification images of the area indicated by the asterisks in A and B. Co-localization between CtB and PACAP containing retinal projections is shown in white in E (determined using the colocalization module in ImageJ, see Material and Methods). Scale bars: A-B; 200 μm, C-E; 20 μm.
Figure 10.
PACAP containing retinal projections representing the melanopsin ipRGCs innervating the brachium of the superior colliculus (BSC).A. Retinal projections visualized by CtB immunostaining in the BSC. The image consists of 3×3 tiled confocal images stitched together for better overview. B-C. Same section as in A co-stained for PACAP (B) and showing that PACAP was found in both retinal and non-retinal projections located within the BSC (C). D-F. Higher magnification images of the area indicated by the boxes in A-C.G-I. Ultrahigh magnification of the area indicated by the asterisks in D-F. Co-localization between CtB and PACAP containing retinal projections is shown in white in C, F, and I (determined using the colocalization module in ImageJ, see Material and Methods). Scale bars: A-C; 100 μm, D-F; 50 μm, G-I; 20 μm.
Figure 11.
PACAP containing retinal projections representing the melanopsin ipRGCs innervating the superior colliculus (SC). A-B. Retinal projections visualized by CtB immunostaining in the ipsi and contralateral SC. The image consists of 2×8 tiled confocal images stitched together for better overview. C-D. Same section as in A-B co-stained for PACAP showing that PACAP was found in cell bodies of deeper layers of the SC (indicated by arrows). High magnification demonstrates PACAP in retinal projections of both the ipsi-(E,F,G) and contralateral side (H-M). Co-localization between CtB and PACAP containing retinal projections is shown in white in G, J and M (determined using the colocalization module in ImageJ, see Material and Methods). Scale bars: A D; 500 μm, E-G; 50 μm, H-M; 20 μm.
Discussion
The present study uses a combination of immunohistochemistry for one of the RHT-neurotransmitters, PACAP, and the anterograde tracer CtB to visualize central projections of melanopsin ipRGCs in retinal target areas of the macaque brain. PACAP, as in other mammalian species, is found in 99% of melanopsin expressing RGC’s including both inner and outer stratifying populations. This was verified by using two well characterized anti-melanopsin antibodies directed against the N- and the C-terminal of the protein, respectively, and identical results were found using each of the antibodies in combination with the PACAP antibody. As in rodents, melanopsin ipRGCs project to areas involved in non-image forming processes such as the SCN, the PrGC which corresponds to the IGL in rodents, and the PON, the primary site for the regulation of the pupillary light reflex. Furthermore, this study confirms and extends a previous study demonstrating that ipRGCs project to structures in the brain involved in image-forming visual processing such as the LGN (Dacey et al., 2005) and the SC.
Visualization of central melanopsin projections has until now been conducted in rodent species. In mice, melanopsin projections to the brain have been demonstrated using two different genetic approaches; one in which the melanopsin gene was targeted using tau-lacZ driven by the melanopsin promoter in combination with CtB injections (Hattar et al., 2006) and one in which melanopsin projections were identified using a CRE-based melanopsin reporter mouse line (Ecker et al., 2010;Brown et al., 2010). Both methods reveal melanopsin projections in sleep-active area of the ventrolateral preoptic area, in the SCN and in the subparaventricular area, the lateral geniculate nucleus including the IGL and the ventral part of the complex. Melanopsin projections were also found in the pretectum, mainly in the PON and in the NOT, whereas single fibres could be found in the SC (Hattar et al., 2006). The Cre-based studies also demonstrate strong retinal projection to image-forming visual processing areas in the LGN and SC (Ecker et al., 2010;Brown et al., 2010). The slightly different results using these two approaches seems at least in part to be explained by different sensitivity of the two systems where the tau-lacZ labelled melanopsin ipRGCs primarily are of the M1 subtype of ipRGCs (Ecker et al., 2010;Brown et al., 2010). These cells express a higher level of melanopsin due to a specific isoform (short) of melanopsin. The Cre-based identified ipRGCs identify both M1 andalso M2 cells which exclusively express another (long) isoform of melanopsin (Pires et al., 2009). A third cell type, the M3 which contains both isoforms and is melanopsin immunoreactive, and a fourth and a fifth cell type, the M4 and M5 cells, that lack detectable melanopsin immunoreactivity and have weak intrinsic light responses, also are identified using this technique (Ecker et al, 2010).
We have used another approach in rat, hamster and mouse, to demonstrate melanopsin retinal fibre projections in the brain. By co-staining for PACAP and CtB we found very similar distribution of retinal projections in the albino rat, hamster and mouse except in the dorsal geniculate nucleus which contains few retinal projections (Hannibal and Fahrenkrug, 2004;Bergström et al., 2003;Hannibal and Fahrenkrug, 2006;Engelund et al., 2012) compared to the studies by Ecker (2010) and Brown (2010). These studies indicate that the combination of double immunostaining for CtB and PACAP can be a useful tool for the study of central melanopsin projections in the mammalian brain. The present study demonstrates the usefulness of this approach in the primate brain. By using two anti-melanopsin antibodies, each recognizing either the N- or the C-terminal part of melanopsin we first confirmed that PACAP is found in 99% of all melanopsin expressing RGCs in the monkey independent of whether they were inner or outer stratifying cells. We found only three melanopsin cells lacking PACAP immunoreactivity. The 0.6% of cells that labelled for melanopsin only represents a very small number and could be explained by too low a level of PACAP to be detected. We found that 95% of the PACAP cells co-store melanopsin. The 5% PACAP positive/melanopsin negative cells had cell bodies located in the GCL and were randomly distributed throughout the retina. Most likely these cells represent melanopsin RGCs with a level of melanopsin to low too be detected by the antisera we used in the current animal.
We then demonstrated the occurrence of retinal PACAP innervation in both non-image-forming and image-forming visual processing areas of the brain. In the ventral and mid SCN dense PACAP innervation corresponding to the retinal input was found, whereas the caudal part of the SCN received retinal innervation which seems to be of non-PACAP/melanopsin origin due to lack of PACAP immunoreactivity in these retinal nerve terminals. Lack of PACAP in CtB containing retinal projections was also found in a minor number of retinal nerve terminals in the mid SCN. It may be possible that these non-PACAP containing retinal fibres represent a low amount of PACAP in some retinal projections (since PACAP expression varies in the ipRGCs). However, it is more likely these CTB positive/PACAP negative nerve fibres represent non-melanopsin projecting retinal fibres as have been found in the rat and golden hamster where retrograde and anterograde tracer studies have described non-melanopsin RGCs projecting to the SCN (Gooley et al., 2003;Morin et al., 2003;Sollars et al., 2003;Hannibal and Fahrenkrug, 2004). This is different from the mouse where apparently almost all RGCs innervating the SCN express melanopsin (Baver et al., 2008).The role of these non-melanopsin projections remains to be established but may represent input from the classical ganglion cells. Recent studies in mice in which the melanopsin expressing RGCs are ablated demonstrate that the melanopsin containing ipRGCs are essential for photoentrainment, masking behaviour and the pupillary light reflex in mammals (Hatori et al., 2008;Guler et al., 2008) suggesting that these dorsally located retinal projections may participate in other retinal driven functions which remain to be determined.
The role of the IGL in rodent is well established and mediates both indirect photic information as well as non-photic information to the SCN (Harrington, 1997). Double immunohistochemistry for PACAP and CtB demonstrates that the most medial part of the rostral PrGC in the monkey is a target area of the PACAP/melanopsin projections. It is likely that this area of the PrGC may have a circadian function similar to the IGL of rodents (Harrington, 1997), i.e. sending indirect light information from the melanopsin containing ipRGCs to the monkey SCN. In a previous study, melanopsin expressing RGCs were identified based on retrograde tracing from the LGN (Dacey et al., 2005). The present study demonstrates PACAP containing retinal projections in all six layers of the LGN linking melanopsin expressing retinal projections (and PACAP) not only to non-image-forming functions but to higher visual perception as recently described in mice (Ecker et al., 2010;Brown et al., 2010).
The demonstration of PACAP in retinal projections to the PON supports a previous study of melanopsin projection to this part of the pretectum (Dacey et al., 2005) and provides additional anatomical evidence for a study in monkey which indicates a central role for melanopsin in the regulation and control of the pupillary light reflex (Gamlin et al., 2007). In both monkey and humans, melanopsin signalling seems to participate in the pupillary light reflex with a significant contribution giving rise to a characteristic component of the reflex (Gamlin et al., 2007). Sparse melanopsin projections have been found in the SC in mouse, rat and hamster (Hattar et al., 2006;Hannibal et al., 2004;Morin et al., 2003). The present study demonstrates PACAP/CtB projections in the SC, and double labelled projections were also found in the BSC indicating that PACAP/melanopsin ipRGCs project to the SC in monkey.
In conclusion, PACAP immunoreactive projections co-storing CtBrepresent ipRGC projections to visual targets in the macaque monkey. The present study supports and extends previous retrograde tracing studies demonstrating that melanopsin containing retinal projections reach areas in the brain involved in both non-image-forming and image-forming visual processing.
Acknowledgements
The Danish Biotechnology Center for Cellular Communication (JH),EY09380 (PG) and P30 EY03039 supported this work. We would also like to express our gratitude to Anita Hansen and Julie Hill for excellent technical assistance.
Footnotes
CONFLICT OF INTEREST STATEMENT The authors report no conflict of interest.
ROLES OF AUTHORS
Roles of authors:
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: JH, BBP, DD, PDG
Acquisition of data: JH, LK, CES, PDG
Analysis and interpretation of data: JH, BBP, DD, PDG
Drafting of the manuscript: JH, BBP, PDG
Critical revision of the manuscript for important intellectual content: JH, BBP, DD, PDG
Statistical analysis: JH
Obtained funding: JH, PDG
Administrative, technical, and material support: Anita Hansen and Julie Hill
Reference List
- Baver SB, Pickard GE, Sollars PJ, Pickard GE. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- Berghorn KA, Bonnett JH, Hoffman GE. cFos immunoreactivity is enhanced with biotin amplification. J Histochem Cytochem. 1994;42:1635–1642. doi: 10.1177/42.12.7983364. [DOI] [PubMed] [Google Scholar]
- Bergström AL, Hannibal J, Hindersson P, Fahrenkrug J. Light-induced phase shift in the Syrian hamster (Mesocricetus auratus)is attenuated by the PACAP receptor antagonist PACAP6-38 or PACAP immunoneutralization. Eur J Neurosci. 2003;9:2552–2562. doi: 10.1046/j.1460-9568.2003.03000.x. [DOI] [PubMed] [Google Scholar]
- Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelund A, Fahrenkrug J, Harrison A, Luuk H, Hannibal J. Altered pupillary light reflex in PACAP receptor 1-deficient mice. Brain Res. 2012;1453:17–25. doi: 10.1016/j.brainres.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Buhl T, Hannibal J. PreproPACAP-derived peptides occur in VIP-producing tumours and co-exist with VIP. Regul Pept. 1995;58:89–98. doi: 10.1016/0167-0115(95)00052-d. [DOI] [PubMed] [Google Scholar]
- Fu Y, Liao HW, Do MT, Yau KW. Non-image-forming ocular photoreception in vertebrates. Curr Opin Neurobiol. 2005;15:415–422. doi: 10.1016/j.conb.2005.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD. The pretectum: connections and oculomotor-related roles. Prog Brain Res. 2006;151:379–405. doi: 10.1016/S0079-6123(05)51012-4. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD. Pituitary adenylate cyclase activating peptide (PACAP) in the retinohypothalamic tract. A daytime regulator of the biological clock. J Neurosci. 1997;17:2637–2644. doi: 10.1523/JNEUROSCI.17-07-02637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Target areas innervated by PACAP immunoreactive retinal ganglion cells. Cell & Tissue Research. 2004;316:99–113. doi: 10.1007/s00441-004-0858-x. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Neuronal input pathways to the brain’s biological clock and their functional significance. Adv Anat Embryol Cell Biol. 2006;182:1–71. [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in PACAP containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22(RC191):1–7. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Ostergaard J, Georg B, Heegaard S, Larsen PJ, Fahrenkrug J. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci. 2004;45:4202–4209. doi: 10.1167/iovs.04-0313. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Moller M, Ottersen OP, Fahrenkrug J. PACAP and glutamate are co-stored in the retinohypothalamic tract. J Comp Neurol. 2000;418:147–155. [PubMed] [Google Scholar]
- Hannibal J, Vrang N, Card JP, Fahrenkrug J. Light dependent induction of c-Fos during subjective day and night in PACAP containing retinal ganglion cells of the retino-hypothalmic tract. J Biol Rhythms. 2001;16:457–470. doi: 10.1177/074873001129002132. [DOI] [PubMed] [Google Scholar]
- Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev. 1997;21:705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundahl CA, Fahrenkrug J, Hay-Schmidt A, Georg B, Faltoft B, Hannibal J. Circadian behaviour in neuroglobin deficient mice. PLoS ONE. 2012;7:e34462. doi: 10.1371/journal.pone.0034462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl F, Hannibal J, Fahrenkrug J. Photic induction of c-Fos in enkephalin neurons of the rat intergeniculate leaflet innervated by retinal PACAP fibres. Cell Tissue Res. 2007;329:491–502. doi: 10.1007/s00441-007-0422-6. [DOI] [PubMed] [Google Scholar]
- Jusuf PR, Lee SC, Hannibal J, Grunert U. Characterization and synaptic connectivity of melanopsin-containing ganglion cells in the primate retina. Eur J Neurosci. 2007;26:2906–2921. doi: 10.1111/j.1460-9568.2007.05924.x. [DOI] [PubMed] [Google Scholar]
- Moore RY. Organization of the primate circadian system. J Biol Rhythms. 1993;8(Suppl):S3–S9. [PubMed] [Google Scholar]
- Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J Comp Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cells projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Pires SS, Hughes S, Turton M, Melyan Z, Peirson SN, Zheng L, Kosmaoglou M, Bellingham J, Cheetham ME, Lucas RJ, Foster RG, Hankins MW, Halford S. Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina. J Neurosci. 2009;29:12332–12342. doi: 10.1523/JNEUROSCI.2036-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollars PJ, Smeraski CA, Kaufman JD, Ogilvie MD, Provencio I, Pickard GE. Melanopsin and non-melanopsin expressing retinal ganglion cells innervate the hypothalamic suprachiasmatic nucleus. Vis Neurosci. 2003;20:601–610. doi: 10.1017/s0952523803206027. [DOI] [PubMed] [Google Scholar]