Abstract
Secoisolariciresinol-diglycoside (SDG), a natural dietary lignan of flaxseeds now available in dietary supplements, is converted by intestinal bacteria to the mammalian lignans enterodiol and enterolactone. High levels of these lignans in blood and urine are associated with reduced risk of many chronic diseases. Our objective was to determine the bioavailability and pharmacokinetics of SDG in purified flaxseed extracts under dose-ranging and steady-state conditions, and to examine whether differences in secoisolariciresinol-diglycoside purity influence bioavailability. Pharmacokinetic studies were performed on healthy postmenopausal women after oral intake of 25, 50, 75, 86 and 172mg of secoisolariciresinol-diglycoside. Extracts differing in secoisolariciresinol-diglycoside purity were compared, and steady-state lignan concentrations measured after daily intake for one week. Blood and urine samples were collected at timed intervals and secoisolariciresinol, enterodiol and enterolactone concentrations measured by mass spectrometry. Secoisolariciresinol-diglycoside was efficiently hydrolyzed and converted to secoisolariciresinol. Serum concentrations increased rapidly after oral intake, peaking after 5-7h and disappearing with a plasma elimination half-life of 4.8h. Maximum serum concentrations of the biologically active metabolites, enterodiol and enterolactone were established after 12-24h and 24-36h, respectively, and the half-lives were 9.4h and 13.2h. Linear dose-responses were observed and secoisolariciresinol bioavailability correlated (r2=0.835) with cumulative lignan excretion. There were no significant differences in the pharmacokinetics of extracts differing in purity, and steady-state serum lignan concentrations were obtained after one-week of daily dosing. In conclusion, this study defines the pharmacokinetics of secoisolariciresinol-diglycoside and shows it is first hydrolyzed and then metabolized in a time-dependent sequence to secoisolariciresinol, enterodiol and ultimately enterolactone, and these metabolites are efficiently absorbed.
Keywords: Lignans, Secoisolariciresinol-diglycoside, Enterolactone, Pharmacokinetics, Metabolism, Humans
Background
Flaxseed is one of the richest dietary sources of lignans, containing up to 0.05 - 0.2% by weight of secoisolariciresinol-diglycoside (SDG) 1-6, a complex plant lignan first isolated from flaxseed in 1956 7 and a precursor of the intestinally derived mammalian lignans enterodiol and enterolactone 8-10. Urinary and serum enterolactone concentrations appear to be a useful surrogate marker of fiber intake 11-13.
We first showed that SDG is metabolized by intestinal bacteria in a series of steps that involve hydrolysis, dehydroxylation and demethylation to the intermediate enterodiol, and that this unique mammalian lignan is oxidized finally to enterolactone9, 10, 14. These observations were later confirmed by others 2, 4, 15. Enterolactone and enterodiol were so named by Setchell & Kirk after the characteristics of their structures became evident, namely possession of γ-butyrolactone and butanediol structures, respectively 9, 16, 17, and because physiologically both undergo enterohepatic recycling 1, 18. The history of these discoveries was reviewed elsewhere 17. There is a paucity of information on the pharmacokinetics and metabolism of SDG in humans 19-21, although its metabolic fate and disposition has been reported in several animal studies 22-25.
Following the original discovery of enterolactone and enterodiol in urine 8, 26 and noting the close similarity in chemical structure to many plant derived lignans shown to have anticancer activity 1, it was proposed that these novel mammalian lignans may be natural dietary anticancer agents 9. Numerous studies have since shown an association between high lignan concentrations in urine or blood, and low rates of many chronic diseases, including cancers that are common to the Western world 27, 28;29, 30-39. In contrast however, are a number of studies that fail to support associations between prostate, breast and esophageal cancers and lignans 40-43. Difficulties in elucidating the role of lignans in disease prevention is that most of these studies are epidemiological or have indirectly assessed lignan intake from diet recall or urinary and blood concentrations. Most have examined the role of flaxseed, a plant-based food that not only contains lignans but is also a rich source of n-3 fatty acids and fiber 44, so that discerning the contribution of lignans in any proposed health 45, 46 effect can be difficult.
Dietary supplements of SDG extracts of flaxseed are now commercially available and fundamental knowledge of the pharmacokinetics, bioavailability and metabolism of SDG is crucial to designing clinical studies to examine the effectiveness of this natural plant lignan. We describe the metabolism and pharmacokinetics of SDG in healthy postmenopausal women, focusing on its bioavailability and intestinal metabolism to the mammalian lignans, enterodiol and enterolactone. We have examined a range of dietary intakes of SDG under single-bolus oral intake and after consecutive daily intakes, and finally we have determined whether extracts differing in purity alter the metabolism and pharmacokinetics of SDG.
Subjects and Methods
Preparation of the SDG supplement
Secoisolariciresinol diglycoside was isolated from flaxseeds by a propriatory process (Lignan Research Inc., San Diego, California). Several different extracts of SDG were used in these studies, differing in purity. Study 1 used an extract that was 43% pure SDG based on our HPLC and electrospray ionization mass spectrometric (ESI-MS) methods described below. Study 2 used a commercially available flaxseed extract that contained 28.8% SDG (Brevail™, Lignan Research, San Diego, California), while Study 3 compared the pharmacokinetics of an extract of higher purity (74.4% pure SDG) with that of the 28.8% SDG commercial extract. These SDG extracts were prepared from commercially available flaxseeds and the amounts of SDG given were equivalent to that contained in 5 - 17 g of whole flaxseeds. The doses of SDG were chosen based upon the estimated range of usual dietary intakes of this lignan from flaxseed, or from diets high in fiber-rich foods.
Study design
Three interrelated studies were carried out to determine the pharmacokinetics of SDG in healthy postmenopausal women, defined as being at least two years from the time of last menses. Study subjects were not taking hormone replacement therapy, antibiotics, diuretics or any ‘over the counter’ medications likely to affect, gastrointestinal, liver or kidney function. Anyone with evidence of preexisting chronic liver and gastrointestinal diseases, or who had been administered antibiotics within the 3 months prior to the study, or who were vegetarians were excluded. Subjects were asked to abstain from eating flaxseed, or foods known to contain flaxseed for at least one week prior to, and during the studies.
The clinical arm of this study was executed at the Clinical Translational Research Center of Cincinnati Children's Hospital Medical Center, an NIH funded facility. The study protocol was approved by the human Institutional Review Board of the Cincinnati Children's Hospital Medical Center (Approval # 01-4-33), and written informed consent was obtained from all study subjects. These studies were initiated in 2003, which predated requirements for registration of clinical studies.
Study 1 - Dose-response study
This was a classical single-bolus oral administration randomized parallel design study comparing 86 mg and 172 mg of SDG contained in an flaxseed extract. Post-menopausal women (n=10) were randomized (n=5 per group) to one of the two different doses of a flaxseed extract containing 86 or 172 mg of SDG. For single-bolus oral administration, the SDG extract was encapsulated in No. 1-size gelatin capsules that were taken in the morning with a glass of water after an overnight fast. Blood samples were obtained by venipuncture, before (baseline) and then 2, 4, 6, 8, 12, 24, 30, 36, 48, and 60 h after swallowing the capsule. Blood was taken via an indwelling catheter for the first twelve hours and by repeated vein puncture for the later sample times. Urine was collected for 24h (in two pooled 12h collections) prior to swallowing the SDG extract, and thereafter, consecutive pooled 12h urine collections were obtained over the next 4 days. After completion of the single-bolus pharmacokinetic study on day 5 each subject began taking their assigned dose of SDG on a daily basis for 8-consecutive days to attain steady-state. On the morning, of the seventh day a 24h urine collection was started with the collection being made in 2 × 12h pools.
Study 2 - Effect of repeat SDG administration on steady-state lignan concentrations
This was a randomized double-blinded crossover study examining the effect of prolonged daily intakes of 25, 50 and 75 mg of SDG taken for 8-consecutive days to determine steady-state serum and urinary concentrations of SDG and its metabolites enterodiol and enterolactone with escalating doses of SDG. Healthy postmenopausal women (n=10, not enrolled in Study 1) were given in randomized fashion all 3 doses of SDG in a flaxseed extract that was 28.8% pure. The SDG was was encapsulated in No. 1-size gelatin capsules and taken orally with a glass of water after an overnight fast and before breakfast for 8-consecutive days. There was a minimum 7-day washout period between doses. A single blood sample was obtained by venipuncture before the first capsule was ingested (baseline), and then again on days 7 and 8, 4h after swallowing the SDG extract to determine steady-state peak serum concentrations of secoisolariciresinol, enterodiol and enterolactone. Pooled 24h urine samples were collected, in two pooled 12h collections, prior to taking the first capsule (baseline), and then again starting on day 7.
Study 3 – Comparison of SDG pharmacokinetics in extracts of differing purity
This was a randomized crossover-design pharmacokinetic study comparing single-bolus oral administration of SDG contained in two different flaxseeds extracts of different purity (74.4% and 28.8%) but delivering the same 50 mg dose of SDG. The object was to determine the extent to which the pharmacokinetics of SDG is influenced by other components in the extract. Healthy postmenopausal women (n=5) were given a single capsule of 50mg SDG of each flaxseed extract encapsulated in No. 1-size gelatin capsules and taken in the morning with a glass of water after an overnight fast. in a randomized crossover design with a one-month washout period between administrations. The pharmacokinetics of SDG were determined by the same single-bolus oral administration design performed in Study 1, adopting the same timings for blood and urine collections.
All blood samples collected from the 3 studies were centrifuged and the serum separated and immediately frozen at −20°C. The volumes of 12h urine pools were measured and 20 mL aliquots stored at −20°C until analyzed. Serum and urinary concentrations of secoisolariciresinol (SECO) and its principal metabolites, enterodiol and enterolactone were determined by GC-MS techniques developed in our laboratory and based on methodology reported previously 22, 47.
Data analysis, sample-size and power calculations
Lignan pharmacokinetics were determined from the serum concentration (ng/mL) vs time profiles for SECO, enterodiol and enterolactone employing a non-compartmental approach using WinNonlin 4.1 (Pharsight Corporation, Cary, NC) computer software as described previously 48. Urinary lignan excretion was expressed as mg/day by combining the urinary outputs for the 12h collections, and cumulative excretion for each lignan was determined over 96h. The fraction of the lignan excreted in urine was determined as the ratio of the cumulative amount of the sum of SECO, enterodiol and enterolactone excreted to the administered dose of SDG adjusted for the differences in the molecular weights due to the glucose moieties. Dose adjusted data for Cmax and AUCinf were calculated relative to the amount of SDG ingested. These studies were of a descriptive and observational nature. Power calculations were not performed to establish optimal sample-size because at the time of this study there was no prior published data on the pharmacokinetics of SDG to base calculations on. Rather, the sample-size was based on a compromise between what could feasibly be done in terms of the analytical load and having sufficient numbers of subjects to be able to observe dose-response effects. Comparison of the pharmacokinetic parameters among the different levels of SDG intakes was made using ANOVA and Students t-test.
Analytical methods
Determination of SDG content of flaxseed extracts by HPLC-ESI-MS analysis
The content of secoisolariciresinol (SECO) was measured by HPLC with electrospray ionization-mass spectrometry ESI-MS after enzymatic hydrolysis of glycoside moieties of the SDG extracts. Stable-isotopically labeled SECO, prepared by the late Professor DN Kirk, University of London by methodology described for stable-labeled enterolactone and enterodiol 16, 22 was used as an internal standard for quantification. Both SDG and SECO are detectable from their corresponding molecular ions thus providing a check on the completeness of enzymatic hydrolysis of the glycosides groups. Accurate amounts of each SDG sample extract were weighed, solubilized in distilled water, and the sample subjected to overnight hydrolysis by Helix pomatia digestive juice in buffer 6. This enzyme was previously shown to have a high β-glucosidase activity toward isoflavones 49 and similarly hydrolyzes lignan glucosides efficiently. The buffered enzyme solution was first prepared by mixing 50 mL of 0.5 M sodium acetate buffer, pH 4.5 and 1.0 mL of Helix pomatia digestive juice. This solution was passed through a C18 Bond Elut solid-phase cartridge that had been pre-charged by washing with 5 mL methanol followed by 5 mL water. This purified enzyme solution was added (5 mL) to each sample and incubated overnight at 37°C in a shaking water bath. After hydrolysis, 200 ng of [2H2]secoisolariciresinol was added to each sample. The hydrolyzed SDG, now in the form of secoisolariciresinol (SECO) was then extracted on a solid-phase C18 Bond Elut cartridge and recovered by elution with 5 mL methanol. This sample was taken to dryness and redissolved in buffer for analysis by HPLC with ESI-MS
HPLC analysis of SECO and SDG by ESI-MS
The following conditions were employed: the HPLC column was a Keystone Scientific 2.0 × 100 mm Betasil C8 column of 5μm particle size; flow rate was 0.2 mL/min; the sample-size loaded on-column was 20 μL. The mobile phase was composed of two solvents; A) 95% 10 mM ammonium acetate; 5% acetonitrile; B) 100 % acetonitrile. Chromatographic separation of SDG and SECO was achieved using the following gradient conditions: at time zero 100% A to 0% B, at 1 min 70% A to 30% B, at 16 min 25% A to 75% B, at 18 min 100%A to 100% B and ending at 20 min 100%A to 100% B. The column effluent was split 0.2 mL/min to the ESI probe. Electrospray ionization conditions were capillary 2.4 kV, sample cone 24 volts, source temperature100°C, desolvation gas 375°C, nebulizer flow 50 L/h, and desolvation gas flow 500 L/h. ESI was performed in negative ion mode monitoring the following ions with HPLC retention time as indicated: SECO 361.1 m/z 8.93-8.95 min, [2H2]SECO 363.1 m/z 8.91-8.93 min, and SDG 685.2 m/z 4.36-4.42 min. Quantification of the concentration of SECO in each extract was achieved by measuring the area response obtained for the peak for SECO relative to that of [2H2]SECO and by interpolation of calibration curves constructed of pure SECO. The calibration curves were linear over the range 0 – 1000 ng/mL and the intra-assay precision of the method as determined from triplicate analysis of the same samples was 1.6 – 8.2 % expressed as coefficient of variation. Highly pure samples of SDG were unavailable for construction of calibration curves and monitoring of the [M-H]- ion characteristic of SDG (m/z 685) serves only to verify completeness of enzymatic hydrolysis of the SDG to SECO. The concentration of SDG in these extracts was calculated from the measured concentration of SECO corrected by the ratio of the difference in the molecular weights of SECO (Mr = 362) and SDG (Mr = 686). From these data the % purity of SDG in these samples was accurately determined from the ratio of the measured/expected SDG concentration based on the sample-size taken for analysis.
Analysis of Secoisolariciresinol, Enterodiol and Enterolactone in serum and urine by mass spectrometry
The concentrations of lignans in serum and urine (0.5 mL) were measured by stable-isotope dilution mass spectrometry with selected ion monitoring after addition of stable-isotopically labeled internal standards for quantification 22. The methodology followed essentially the same approach we have reported for the measurement of isoflavones 48, 49.
Lignans were extracted from serum (0.5 mL) by solid phase extraction on a cartridge of octadecylsilane bonded silica cartridge (Bond Elut C18, Varian, Harbor City, CA), hydrolyzed enzymatically overnight at 37°C with a mixed β-glucuronidase/sulfatase preparation (Sigma, St Louis, MO). After hydrolysis the unconjugated lignans were isolated by solid-phase extraction on a C18 Bond Elut cartridge, and converted to tert-butlydimethylsilyl (t-BDMS) ethers (Regis, Morton Grove, IL) for analysis by GC-MS. For urine, samples of 0.5 mL volume were taken directly and the stable-labeled internal standards added. The samples were hydrolyzed overnight with the same mixed β-glucuronidase/sulfatase preparation and after hydrolysis the lignans were extracted by solid-phase extraction on a C18 Bond Elut cartridge. The urinary lignans were analyzed by HPLC with ESI-MS/MS detection without prior derivatization and under conditions of multiple reaction ion monitoring (MRM) as follows: enterolactone MS1 = m/z 297, MS2 = m/z 253, enterodiol MS1 = m/z 301, MS2 = m/z 253, [2H6]Enterolactone MS1 = m/z 303, MS2 = m/z 259, [2H6]Enterodiol MS1 = m/z 307, MS2 = m/z 258, Secoisolariciresinol MS1 = m/z 361, MS2 = m/z 165, and [2H2]Secoisolariciresinol MS1 = m/z 363 MS2 = m/z 167/165. The hydrolyzed urinary lignan extracts were re-dissolved in 100 μL of the mobile phase (95% 10 mol/L ammonium acetate, 5% acetonitrile). A sample-size of 20 μL was injected on column, and the mobile phase flow-rate was 0.2 mL/min. Separation of the individual lignans was achieved on a 100 × 2.0 mm Betasil (C8) reversed-phase HPLC column (Keystone Scientific, Bellefone PA). The column was eluted with a gradient of water/acetonitrile. The initial mobile phase was 95% 10mmol/L ammonium acetate, 5% acetonitrile decreasing to 70% 10mmol/L ammonium acetate, 30% acetonitrile over the first 1 min. This was further decreased to 25% 10mmol/L ammonium acetate in a constant gradient from 1 to 16 min, and then finally held isocratic with 25% 10mmol/L ammonium acetate, 75% acetonitrile for 2 min before returning to the original composition of 95% 10mmol/L ammonium acetate, 5% acetonitrile. ESI-MS/MS was performed on a Micromass Quattro LC. The desolvation temperature was 375°C, and the source temperature was 100°C. The sample cone was held at 40 volts, and the extractor at 2 volts. A collision energy of 24 eV was used and the collision cell pressure was 2.8×10-3 mbar. Data was collected in the negative ion mode. The lignan concentrations were established by comparing the lignan peak area ratio to that of the corresponding stable-labeled internal standards and interpolating these ratios against calibration plots constructed of known quantities of the pure lignans, secoisolariciresinol, enterodiol and enterolactone. The intra-assay precision of the method for serum lignans determined from replicate analysis of the same samples and expressed as coefficient of variation was 13%, 8%, and 16% for SECO, enterodiol and enterolactone, respectively. The intra-assay precision of the method for quantifying urinary lignans was 6%, 6%, and 7%, for SECO, enterodiol and enterolactone, respectively.
Results
Analysis of the Secoisolariciresinol-diglycoside extracts
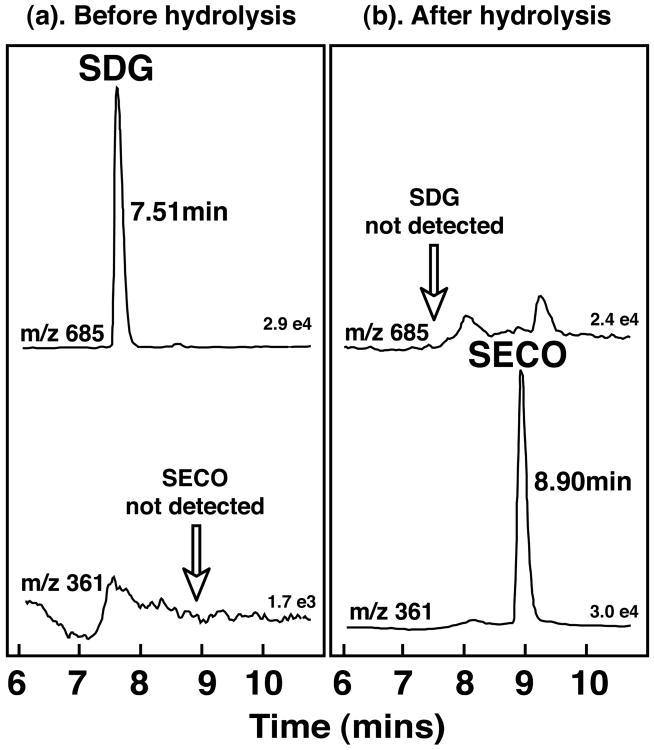
When aliquots of the Stock solutions of the SDG extracts were analyzed directly, without enzymatic hydrolysis, LC-MS analysis revealed only a single peak eluting at 7.51 min corresponding to the protonated molecular ion (m/z 685) of SDG, and there was no detectable peak for SECO (m/z 361). By contrast, following enzymatic hydrolysis with Helix pomatia SDG was no longer present and the mass spectrometric analysis revealed exclusively a peak corresponding to SECO eluting at retention time 8.9 min (Fig. 1). These studies established that under the enzymatic conditions employed, hydrolysis of SDG was complete. Using this approach the purity of the 3 different extracts of SDG used in the pharmacokinetic studies was determined to be 43% pure SDG, (Study 1), 28.8 % pure SDG (Studies 2 and 3), and 74.4% pure SDG (Study 3).
Figure 1.
Electrospray ionization (ESI)negative ion mass chromatograms for the ions m/z 685 and m/z 361 characteristic of secoisolariciresinol-diglycoside (SDG) and secoisolariciresinol (SECO) before and after enzymatic hydrolysis of the purified SDG extracts used in these pharmacokinetic studies. The chromatograms verify complete hydrolysis of SDG by the enzyme preparation.
Dose-Response studies of Secoisolariciresinol-diglycoside
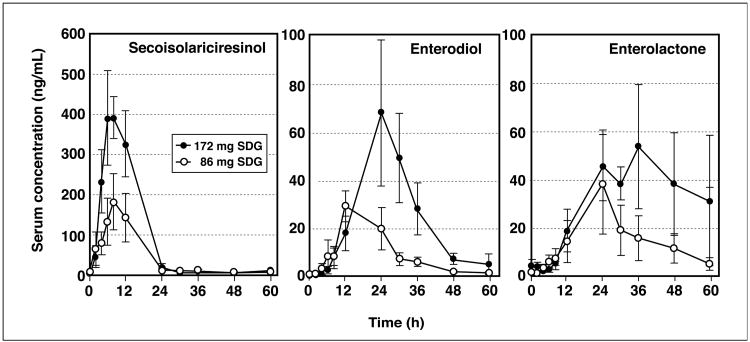
The shapes of the serum lignan appearance/disappearance concentration profiles were similar for all subjects administered the two different doses of SDG. Fig. 2 shows the group mean (±SEM) values plotted for serum SECO, enterodiol and enterolactone concentrations after administration of both doses of SDG. Despite some inter-individual variation in serum concentrations there was a significant dose-response effect (Table 1). The serum concentration profiles revealed a rapid absorption of SECO with average peak serum concentrations occurring 7.2 ± 1.0 h after ingestion of SDG (Table 1), and elimination of SECO from serum was in accord with first-order kinetics. There was no significant difference in the time to reach peak serum SECO concentrations (tmax) between the two doses of SDG. The mean (±SEM) elimination half-life (t1/2) for SECO was relatively rapid at 4.2 ± 0.3 h. The average volume of distribution (Vd/F) was high (111.5 ± 14.2 L), and the clearance rate rapid at 18.1 ± 1.7 L/h. The apparent bioavailability as measured from the AUCinf for serum SECO concentrations increased directly in proportion to the amount of SDG ingested and this was evident from the finding of no significant differences in the dose adjusted AUCinf and Cmax values (Table 1).
Figure 2.
Serum concentrations (mean ±SEM) of secoisolariciresinol, enterodiol, and enterolactone in healthy postmenopausal women (n = 5 per group) administered a single oral bolus dose of 86 mg or 172 mg of secoisolariciresinol-diglycoside (SDG).
Table 1.
Summary of the serum pharmacokinetics of secoisolariciresinol, enterodiol and enterolactone for healthy postmenopausal women determined from lignan concentrations after single-bolus oral intake of 86 mg (n=5 women), and 172 mg (n=5 women) of a 43% pure secoisolariciresinol-diglycoside (SDG) extract.
| SDG Intake (mg) | Cmax (ng/mL). | Tmax (h) | Half-life (h) | Vd/F (L) | Cl/F (L) | AUCinf (h*ng/mL) | AUCinf/D¥ (h*ng/mL/mg) | Cmax/Dose¥ (ng/mL/mg) |
|---|---|---|---|---|---|---|---|---|
| Secoisolariciresinol | ||||||||
| 86mg | 247 ± 50 | 6.8 ± 1.8 | 4.8 ± 0.5 | 132.5 ±25.2 | 19.2 ± 3.3 | 2675 ± 587 | 58.9 ± 13 | 2.9 ± 0.6 |
| 172mg | 544 ± 27* | 7.6 ± 1.3 | 3.7 ± 0.2+ | 90.5 ±14.6 | 17.1 ± 2.3 | 5609 ± 685# | 61.8 ± 7.5 | 3.2 ± 0.2 |
|
| ||||||||
| Mean(±SEM) | --- | 7.2 ± 1.0 | 4.2 ± 0.3 | 111.5 ± 14.2 | 18.1 ± 1.7 | --- | 60.4 ± 6.3 | 3.0 ± 0.3 |
|
| ||||||||
| Enterodiol | ||||||||
| 86mg | 35.3 ± 6 | 13.2 ± 2.9 | 10.2 ± 2.1 | --- | --- | 622 ±113 | --- | 0.4 ± 0.1 |
| 172mg | 75.6 ± 30 | 25.2 ± 1.2# | 7.7 ± 0.8 | --- | --- | 1949 ±704 | --- | 0.4 ± 0.2 |
|
| ||||||||
| Mean (±SEM) | --- | 19.2 ± 2.6 | 9.0 ± 1.2 | --- | --- | --- | --- | 0.4 ± 0.1 |
|
| ||||||||
| Enterolactone | ||||||||
| 86mg | 38.7 ± 18 | 27.6 ± 2.4 | 13.2 ± 4.2 | --- | --- | 1033 ±469 | --- | 0.5 ± 0.2 |
| 172mg | 49.1 ± 17 | 25.5 ± 5.1 | 9.6 ± 1.1 | --- | --- | 1303 ±259 | --- | 0.3 ± 0.1 |
|
| ||||||||
| Mean (±SEM) | --- | 26.7 ± 2.5 | 11.6 ± 2.4 | --- | --- | --- | --- | 0.4 ± 0.2 |
= Dose-adjusted based on level of SDG intake
p ≤0.001,
p=0.05,
p ≤0.01
Cmax = peak serum concentration, Tmax = time required to reach peak levels, Vd/F = volume of distribition normalized to the bioavailable fraction, CL/F = systemic clearance normalized to bioavailabale fraction, AUCinf = bioavailablity as apparent from area under the curve to infinity, D = dose.
Vd/F and Cl/F and AUCinf/D for enteriodiol and enterolactone not computed since dose of these lignans is unknown.
With both doses of SDG, the appearance of enterodiol and enterolactone in serum was delayed relative to SECO which is consistent with production of these metabolites in the distal intestine and colon, and in accord with the known precursor-product relationship between these lignans (Fig. 2). The average time to reach peak serum enterodiol and enterolactone concentrations was 19.2 ± 2.5 h and 26.7 ± 2.3 h, respectively. Due to the complex dynamics of enterodiol and enterolactone only limited information on the pharmacokinetics of these lignans can be deduced since the dose of enterodiol and enterolactone is unknown and dependent on the rate of input from intestinal metabolism of SDG. There was however a clear dose-response effect where the Cmax increased approximately in proportion to the intake of SDG, yielding no significant difference in the values for dose-adjusted Cmax (Cmax/D). For both enterodiol and enterolactone, the Cmax/D averaged 0.4 ± 0.1 and 0.7 ± 0.2 ng/mL/mg of SDG ingested, respectively. The mean elimination half-life of enterodiol and enterolactone was 9.0 and 11.6 h, respectively (Table 1).
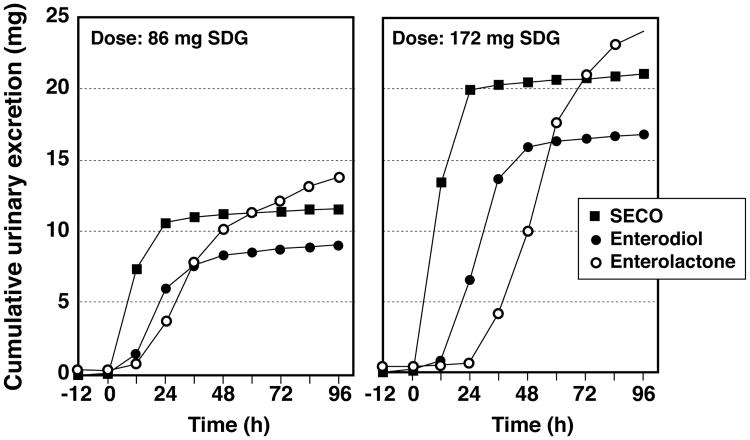
The mean cumulative excretion of SECO, enterodiol and enterolactone over the 5 days that urine was collected shown in Fig. 3. The urinary excretion profiles of these lignans were similar for both doses of SDG administered. SECO appeared in urine rapidly and reached almost maximal cumulative excretion within the first 24 h. Enterodiol appearance in urine was delayed with little appearing in the first 12 h after ingestion of SDG and near maximal cumulative excretion was attained after 48 h. The appearance of enterolactone in urine was still further delayed and even after 5-days complete excretion had not occurred, although it was estimated that about 90% cumulative excretion was attained by this time. Based on the amounts of SDG ingested, the mean % recovery of total lignans, the sum of SECO, enterodiol and enterolactone recovered in urine, was 74.6% and 68.2%, respectively for the two doses.
Figure 3.
Average 12-h cumulative urinary excretion (mg) over 5-days of secoisolariciresinol (SECO), enterodiol, and enterolactone in healthy postmenopausal women administered orally a flaxseed extract containing 86 mg and 172 mg of secoisolariciresinol-diglycoside (SDG). These doses deliver the equivalent of 45.4 mg and 90.8 mg of secoisolariciresinol after allowing for the molecular weight contribution of the diglycoside.
Influence of prolonged intake of SDG on steady-state lignan concentrations
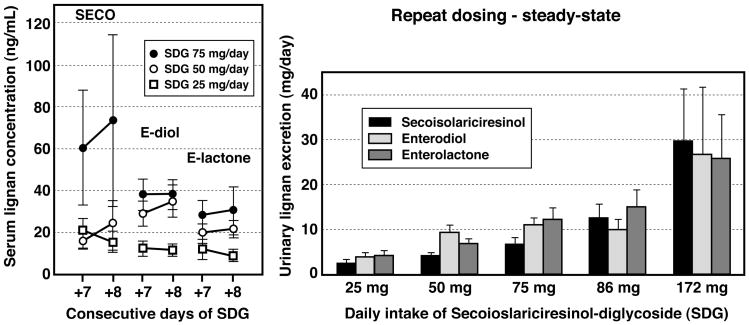
The mean (±SEM) serum lignan concentrations measured on two consecutive days (days +7 and +8) in the 10 healthy women who had consumed in a randomized crossover manner doses of 25, 50 and 75 mg of SDG for 8 consecutive days is shown in Fig. 4 (left panel). Even allowing for a large individual variability in serum lignan concentrations, a dose-response effect was evident for the mean steady-state serum concentrations of SECO, enterodiol and enterolactone. The serum lignan concetrations on days +7 and +8 were not statistically different indicating achievement of steady-state after one week of daily dosing. The urinary excretion of these lignans, determined from a single 24 h collection taken on day 7-8 for these three escalating doses of SDG is shown in Fig. 4 (right panel) combined with the steady-state data from Study 1, where higher doses of SDG (86 and 172 mg/day) were similarly administered. A positive linear dose-response relationship was evident over this wide-range of doses of SDG.
Figure 4.
Mean (±SEM) steady-state serum secoisolariciresinol, enterodiol and enterolactone concentrations (left panel) in healthy postmenopausal women given daily doses of 25 mg, 50 mg and 75 mg of SDG for 8-consecutive days measured on days 7 and 8. No statistically significant differences in the serum lignan concentrations were observed between the two days. Mean (±SEM) 24-h urinary lignan excretion (right panel) measured under steady-state in postmenopausal women after one-week of daily doses of 25 mg, 50 mg and 75 mg of SDG (28.8% purity extract) and 86 mg or 172 mg (43% purity extract). Data from Study 1 and Study 2 combined show positive linear dose-ranging effects of SDG on urinary lignan excretion.
Comparison of the pharmacokinetics of SDG in extracts differing in purity
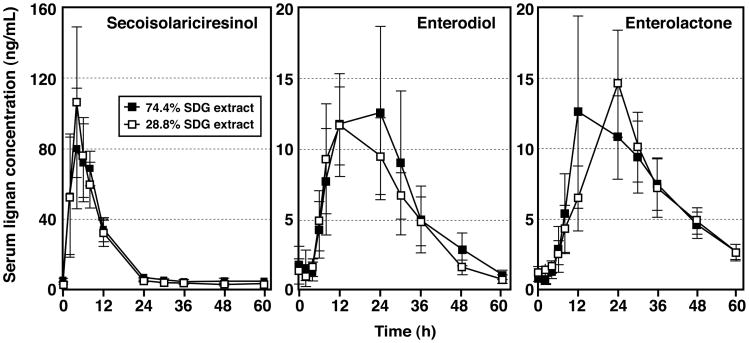
Results from the double-blinded randomized cross-over study of the serum pharmacokinetics following single oral-bolus administration of 50 mg of a 28.8% and 74.4% pure SDG extract performed in 10 healthy women are summarized in Table 2. This study was performed to determine whether other components in the flaxseeds extract influenced the bioavailability of SDG. Based upon the serum lignan concentration appearance/disapperance curves for SECO, enterodiol and enterolactone (Fig. 5) there were no statistically significant differences in the Cmax, tmax, AUCinf, or dose adjusted AUCinf and Cmax values between the two SDG extracts differing in purity, and the pharmacokinetics were consistent with the findings from Study 1 where higher doses of a 43% pure SDG extract was used.
Table 2.
Summary of the serum pharmacokinetics of secoisolariciresinol, enterodiol and enterolactone for healthy postmenopausal women (n=5) determined from serum lignan concentrations after a single-bolus oral intake of 50 mg of secoisolariciresinol-diglycoside (SDG) in a 28.8% pure and a 74.4% pure SDG extract given in a randomized double-blinded crossover design with a minimum one-week washout between.
| SDG Intake (50 mg) | Cmax (ng/mL). | Tmax (h) | Half-life (h) | Vd/F (L) | Cl/F (L) | AUCinf (h*ng/mL) | AUCinf/D¥ (h*ng/mL/mg) | Cmax/Dose¥ (ng/mL/mg) |
|---|---|---|---|---|---|---|---|---|
| Secoisolariciresinol | ||||||||
| 28.8% purity | 123 ±37 | 5.7 ±0.8 | 5.3 ±0.6 | 252 ±68 | 31 ±6 | 1065 ±252 | 40.3 ±9.5 | 4.6 ±1.4 |
| 74.4% purity | 126 ±30 | 5.6 ±1.2 | 5.5 ±0.1 | 190 ±14 | 24 ±2 | 1125 ±97 | 42.6 ±3.7 | 4.8 ±1.1 |
|
| ||||||||
| Mean (±SEM) | 124 ±23 | 5.6 ±0.7 | 5.4 ±0.3 | 224 ±37 | 28 ±3 | 1092 ±138 | 41.4 ±5.2 | 4.7 ±0.9 |
|
| ||||||||
| Enterodiol | ||||||||
| 28.8% purity | 17± 1.7 | 18.3 ±3.6 | 8.6 ±1.4 | --- | --- | 342 ±41 | --- | 0.6 ±0.1 |
| 74.4% purity | 19 ±5.0 | 16.0 ±3.3 | 11.2 ±1.6 | --- | --- | 404 ±97 | --- | 0.7 ±0.2 |
|
| ||||||||
| Mean (±SEM) | 18 ±2.3 | 17.3 ±2.4 | 9.8 ±1.1 | --- | --- | 370 ±47 | --- | 0.7 ±0.1 |
|
| ||||||||
| Enterolactone | ||||||||
| 28.8% purity | 16.8 ±3.7 | 24.0 ±0.0 | 13.9 ±1.6 | --- | --- | 487 ±90 | --- | 0.6 ±0.1 |
| 74.4% purity | 19.2 ±6.4 | 25.5 ±5.1 | 15.8 ±5.3 | --- | --- | 538 ±104 | --- | 0.7 ±0.2 |
|
| ||||||||
| Mean (±SEM) | 17.9 ±3.3 | 24.7 ±2.1 | 14.8 ±2.3 | --- | --- | 510 ±64 | --- | 0.7 ±0.1 |
= Dose-adjusted based on level of SDG intake
Cmax = peak serum concentration, Tmax = time required to reach peak levels, Vd/F = volume of distribution normalized to the bioavailable fraction, CL/F = systemic clearance normalized to bioavailable fraction, AUCinf = bioavailability as apparent from area under the curve to infinity D=dose.
Figure 5.
Serum concentrations (mean ± SEM) of secoisolariciresinol, enterodiol, and enterolactone in 10 healthy postmenopausal women following single-bolus oral administration of 50 mg of a 28.8% and 74.4% pure SDG extract given in a randomized double-blinded crossover design.
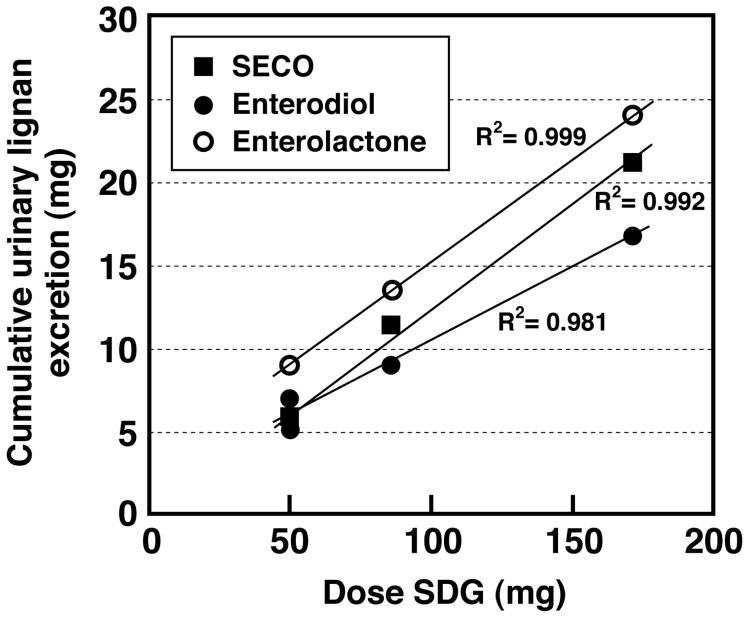
Furthermore, the cumulative urinary excretion profiles for SECO, enterodiol or enterolactone were similar for both SDG extracts, and consistent with those observed in Study 1 where a 43% purity SDG extract was ingested. Urinary excretion of SECO was almost complete after 24 h, while the delayed appearance of enterodiol meant that it was mostly excreted by 48h, and enterolactone was almost completely eliminated after 5-days following single oral dosing of both SDG extracts (data not shown). The average % recovery of total lignans relative to the 50 mg dose of SDG administered was 81.3 % and 74.3%, respectively for the 28.8 and 74.4% purity extracts. Combining all data from the single-bolus oral administration studies (Study 1 and Study 3), these findings indicate that independent of the purity of the extract used, there is a positive correlation between the cumulative 96 h urinary excretion of lignans and the dose of SDG ingested (r2>0.98) (Fig. 6). These results indicate that other components of flaxseed that may be present in the extract do not influence the metabolic fate, pharmacokinetics and metabolism of SDG.
Figure 6.
Positive linear correlation between mean cumulative 5-day urinary lignan excretion and dose of SDG administered as a single-bolus oral dose to healthy postmenopausal women. Data from Study 1 (43% pure SDG) and Study 3 (28.8% and 74.4% pure SDG) are combined and plotted.
Correlations between serum and urinary lignans
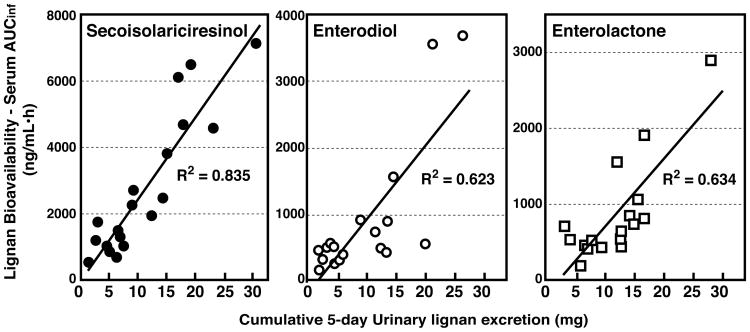
Since the single-oral bolus pharmacokinetics of SDG were similar in Studies 1 and 3 and lignan pharmacokinetics were unaffected by the relative purity of the extracts ingested, data were collectively analyzed to examine the relationship between serum and urinary lignans for all 20 women (Table 3). Linear positive correlations were observed between the bioavailability of SECO (r2= 0.835), enterodiol (r2= 0.623) and enterolactone (r2= 0.634) and the cumulative 5-day urinary excretion of these lignans (Fig. 7).
Table 3.
Summary of serum pharmacokinetics for Secoisolariciresinol, Enterodiol, and Enterolactone expressed as means ± SEM for 20 healthy postmenopausal women determined from single-bolus oral administration of purified secoisolariciresinol-diglycoside (SDG) combining all data from Study 1 and Study 3.
| Summary Table | Secoisolariciresinol | Enterodiol | Enterolactone |
|---|---|---|---|
| Tmax (h) | 6.4 ± 0.6 | 18.2 ± 1.7 | 25.7 ± 1.5 |
| Half-life (h) | 4.8 ± 0.3 | 9.4 ± 0.8 | 13.2 ± 1.5 |
| Vd/F (L) | 170.4 ± 23.7 | --- | --- |
| Cl/F (L) | 23.2 ± 2.1 | --- | --- |
| AUC inf/Dose SDG (hr*ng/mL/mg) | 50.4 ± 4.5 | 15.7 ± 2.1 | 19.2 ± 2.8 |
| Cmax/Dose SDG (ng/mL/mg) | 5.2 ± 0.5 | 0.6 ± 0.07 | 0.5 ± 0.1 |
Cmax = peak serum concentration, Tmax = time required to reach peak levels, Vd/F = volume of distribution normalized to the bioavailable fraction, CL/F = systemic clearance normalized to bioavailable fraction, AUCinf = bioavailability as apparent from area under the curve to infinity.
Figure 7.
Correlations for secoisolariciresinol, enterodiol and enterolactone between the bioavailability (as measured from the AUCinf of the plasma concentration curves following single-bolus oral administration of SDG) and the cumulative 5-day urinary excretion (mg) of each lignan. Plots combine data from the two single-bolus oral administration pharmacokinetic studies (Study 1 and Study 3).
Discussion
The difficulty in interpreting nutritional and clinical studies of flaxseed is that flax contains many nutrients that could contribute to the reported biological effects 45, 46. Studies of purified SDG extracts, which are now commercially available as supplements, provide one means of overcoming this limitation and of teasing out the potential importance of the lignan component of flaxseed. However, there have been few studies on the bioavailability or metabolic fate of SDG, or on the dynamics of its conversion to enterodiol and enterolactone in humans 19-21. A number of studies have examined the effects of feeding flaxseeds to humans on urinary 19, 50-53 and plasma 19, 54 enterodiol and enterolactone concentrations and there is a wealth of literature related to typical concentrations in people on habitual diets 13, 55-63. Few have included measurements of SECO and there is only limited or incomplete information on the dynamics of SDG metabolism. One study described the metabolism of SDG in raw ground flaxseed fed to adults but blood collections were made for only 24 h after feeding 19, an insufficient time for the accurate determination of the pharmacokinetics of enterodiol and enterolactone based on their delayed appearance in serum due to the fact that these lignans are produced by intestinal bacteria in the distal region of the intentianl tract 1, 17. The only other pharmacokinetic study was a single dose study of SDG andministered to 12 healthy volunteers and only enterodiol and enterolactone was measured20. We have described the first multi-dosing study of the serum and urinary pharmacokinetics and metabolic fate of SDG in healthy postmenopausal women, focussing on dose-response effects and effects of using SDG extracts of flaxseed differing purity. These studies were performed using the classical single-bolus oral administration study design of serum appearance/disappearance kinetics of this lignan, and the dynamics of its hydrolysis to SECO and its rate of conversion to the specific metabolites enterodiol and enterolactone was also determined.
Data from Study 1 in which doses of 86 mg and 172 mg of SDG were administered as a single oral-bolus dose, when combined with the data from Study 3 of two different 50 mg doses yielded consistent dose-response information over a relatively wide range of dietary SDG intakes. The SDG extracts used in these studies differed in purity because of changes in the manufacturing processes employed over the course of these studies, but nevertheless our data show that irrespective of extract purity, the pharmacokinetics, bioavailability and metabolism of SDG is similar and not affected by the presence of other components in the flaxseed. Assessing the exact purity of SDG was difficult because of the lack of a highly pure synthetic standard of SDG for analytical calibration. Stable-isotope dilution analysis was therefore used to determine the actual purity of the SDG extracts administered, by determining the concentrations of the aglycon, SECO, released after enzymatic hydrolysis. Chemically synthesized SECO and its stable-labeled analog were used to quantify SECO, an approach that has been used in the compilation of several databases for the lignan content of foods 64, 65. Enzymatic hydrolysis of whole and ground flaxseeds has previously been shown to yield lower values for the SDG content than if acid or alkaline hydrolysis is used and it has been suggested that enzymatic hydrolysis fails to completely hydrolyze SDG 5. However, Helix pomatia digestive juice contains a high activity of β-glucosidase, and we have shown that it completely hydrolyzes glycosides of lignans (Figure 1), and also isoflavones 49. Hydrolysis efficiency can be readily monitored by ESI-MS 3, 66, 67, because both SDG and SECO can be simultaneously detected with very high sensitivity by monitoring specific ions for these compounds. By this approach, under the enzymatic conditions employed, all SDG was converted to SECO and no SDG remained (Figure 1). SECO has been shown to exist in oligomers 68, and other chemical forms may exist within the seed that are resistant to enzymatic hydrolysis but could be released by more rigorous and destructive hydrolytic methods. This fraction of SDG would not be expected to be bioavailable since hydrolysis would not occur in the intestinal tract.
Following single-bolus oral administration of SDG, the appearance of SECO in serum was rapid with peak concentrations occurring after 5 - 7 hours independent of the dose ingested or the extent of purity of the extract. This is reflective of rapid hydrolysis of the diglycoside moiety in the intestinal tract. The enzymes responsible for this hydrolytic cleavage of the sugar groups are presumed to reside on the brush border membrane of the proximal intestine and to be analogous to those involved in the hydrolysis of isoflavone glucosides 49, 69. The delay in attaining peak serum enterodiol and enterolactone concentrations, 19.2 ± 2.6 h and 26.7 ± 2.5 h, respectively is consistent with the timing of the bacterial conversion of SECO, which takes place by the action of bacteria colonizing the distal intestine and colon. This delayed appearance of enterodiol and enterolactone in plasma is consistent with a previous study of 12 healthy young adults 20. The serum profiles of the three lignans were qualitatively similar (Figures 2 and 5) and positive dose-response relationships were observed between the intake of SDG and the pharmacokinetics. There was some inter-individual variation in serum and urinary concentrations as noted in other studies of adults consuming their usual diets 60, 70. The urinary excretion of SECO, enterodiol and enterolactone paralleled the serum kinetics with the most of the SECO being excreted within the first 24 h of ingestion of SDG, consistent with the rapid 4.2 h terminal elimination half-life of SECO from serum (Table 1). Enterodiol and enterolactone were mostly eliminated in urine after 2 and 5 days respectively, although at the highest intake of SDG it was evident that even after 5-days enterolactone was still being excreted. The bioavailability of SECO as measured from the AUCinf of the serum profiles correlated positively with the cumulative 5-day excretion (Figure 7). A similar relationship was observed for enterodiol and enterolactone. The latter data are consistent with the positive correlation previously reported between enterolactone concentrations in serum and spot urines 60. The fractional recovery of lignans in urine measured by the sum of SECO, enterodiol and enterolactone, ranged from 60 – 80 %, which is significantly higher than reported for the fractional recovery of isoflavones from soy 48, 71, 72. Assuming the approximate intake of SDG was 30 mg then the recovery of these lignans in feces, would have accounted for about 3 mg, or 10% of the ingested dose. Based on our pharmacokinetic studies it is apparent that renal clearance of isoflavones and elimination in the urine is the predominant route by which lignans are removed from the body.
Based on the pharmacokinetics displayed in these classical single-bolus oral administration studies it can be calculated that steady-state concentrations of lignans would be attained after 5-7 days of daily administration. This was confirmed in the data from Study 2 where the different doses of SDG (25, 50, 75 mg) were maintained for 8-consecutive days and serum measurements of SECO enterodiol and enterolactone made on days 7 and 8 (Figure 4). Although there was some inter-individual variation among the subjects within the dosing groups, there was no significant difference in the serum concentrations for any of the lignans between the two days and this was the case for all doses of SDG taken. These findings indicate steady-state had been attained after one week of intake of SDG, a conclusion similarly made after feeding ground flaxseed for 8 consecutive days 19, 51, 52. Based on our pharmacokinetic data a dietary intake of 50 mg purified SDG would appear to provide similar urinary enterolactone excretion rates to those observed after feeding about 5- 10 g flaxseed. Circulating enterolactone concentrations observed in our study for intakes of 50 mg of SDG are similar to those associated with reduced risk for breast cancer 73 and cardiovascular disease 27.
Finally, our studies demonstrate that the pharmacokinetics and metabolism of SDG was not influenced by the presence of differing levels of impurities in the extracts. Many of these other components were characterized by the manufacturer to include caffeic, ferulic, cinnamic and coumaric acids, pinoresinol-diglycoside and other products of secondary plant metabolism. Given that there is generally considerable variability in the purity of commercial dietary supplements of natural plant extracts, as has been previously shown for soy isoflavone supplements 74-76, the issue of whether the these other components could alter the pharmacokinetics of the active ingredient is important to evaluate. Our studies show that the pharmacokinetic behavior and metabolism of SDG was similar over a range of extracts differing in SDG purity from 28.8% to 74.4%. This also indicates that, even if other secondary plant compounds are present, SDG is by far the major contributor to enterodiol and enterolactone production.
In conclusion, our studies define accurately the time-course for the dynamics of lignan formation from SDG and provide fundamental data on the pharmacokinetics of lignans in healthy adults. Such data will facilitate the design of future clinical studies aimed at evaluating the nutritional benefits of SDG, as opposed to flaxseeds, by utilizing purified SDG extracts.
Acknowledgments
Support: This work was supported by an unrestricted industry contract from Barleans Organic Oils, LLC, Ferndale, WA 98248, and the Clinical Translational Research Center of Cincinnati Children's Hospital Medical Center utilized for subject visits was supported by Grant 8 UL1 TR000077-04 from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health.
References
- 1.Setchell KDR, Adlercreutz H. In: Role of the Gut Flora in Toxicity and Cancer. Rowland IR, editor. Academic Press; London, UK: 1988. pp. 315–334. [Google Scholar]
- 2.Thompson LU, Robb P, Serraino M, Cheung F. Nutr Cancer. 1991;16:43–52. doi: 10.1080/01635589109514139. [DOI] [PubMed] [Google Scholar]
- 3.Obermeyer WR, Musser SM, Betz JM, Casey RE, Pohland AE, Page SW. Proc Soc Exp Biol Med. 1995;208:6–12. doi: 10.3181/00379727-208-43824. [DOI] [PubMed] [Google Scholar]
- 4.Thompson LU, Rickard SE, Cheung F, Kenaschuk EO, Obermeyer WR. Nutr Cancer. 1997;27:26–30. doi: 10.1080/01635589709514497. [DOI] [PubMed] [Google Scholar]
- 5.Westcott ND, Muir AD, Northrup SF. Analysis of flax lignans after enzymatic and chemical extraction. Fargo, North Dakota: 1998. [Google Scholar]
- 6.Setchell KDR, Childress C, Zimmer-Nechemias L, Cai J. Journal of Medicinal Food. 1999;2:193–198. doi: 10.1089/jmf.1999.2.193. [DOI] [PubMed] [Google Scholar]
- 7.Bakke JE, Klosterman HJ. North Dakota Academy of Science. 1956;10:18–22. [Google Scholar]
- 8.Setchell KDR, Lawson AM, Mitchell FL, Adlercreutz H, Kirk DN, Axelson M. Nature. 1980;287:740–742. doi: 10.1038/287740a0. [DOI] [PubMed] [Google Scholar]
- 9.Setchell KDR, Lawson AM, Borriello SP, Harkness R, Gordon H, Morgan DM, Kirk DN, Adlercreatz H, Anderson LC, Axelson M. Lancet. 1981;2:4–7. doi: 10.1016/s0140-6736(81)90250-6. [DOI] [PubMed] [Google Scholar]
- 10.Axelson M, Sjovall J, Gustafsson BE, Setchell KDR. Nature. 1982;298:659–660. doi: 10.1038/298659a0. [DOI] [PubMed] [Google Scholar]
- 11.Adlercreutz H, Hockerstedt K, Bannwart C, Hamalainen E, Fotsis T, Bloigu S. In: Progress in Cancer Research and Therapy. Bresciani F, King R, Lippman M, Raynaud JP, editors. Raven Press Ltd.; New York: 1988. pp. 409–412. [Google Scholar]
- 12.Hutchins AM, Lampe JW, Martini MC, Campbell DR, Slavin JL. J Am Diet Assoc. 1995;95:769–774. doi: 10.1016/S0002-8223(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 13.Lampe JW. J Nutr. 2003;133(3):956S–964S. doi: 10.1093/jn/133.3.956S. [DOI] [PubMed] [Google Scholar]
- 14.Borriello SP, Setchell KDR, Axelson M, Lawson AM. J Appl Bacteriol. 1985;58:37–43. doi: 10.1111/j.1365-2672.1985.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 15.Bowey E, Adlercreutz H, Rowland I. Food Chem Toxicol. 2003;41:631–636. doi: 10.1016/s0278-6915(02)00324-1. [DOI] [PubMed] [Google Scholar]
- 16.Cooley G, Farrant RD, Kirk DN, Patel S, Wynn S, Buckingham MJ, Hawkes GE, Hursthouse MD, Galas AMR, Lawson AM, Setchell KDR. Journal Chemical Society Perkin Transactions. 1884;11:489–497. [Google Scholar]
- 17.Setchell KDR. In: Flaxseed in human nutrition. Cunnane S, Thompson L, editors. AOCS Press; Champaign, IL: 1995. pp. 82–98. [Google Scholar]
- 18.Axelson M, Setchell KDR. FEBS Lett. 1981;123:337–342. doi: 10.1016/0014-5793(81)80322-5. [DOI] [PubMed] [Google Scholar]
- 19.Nesbitt PD, Lam Y, Thompson LU. Am J Clin Nutr. 1999;69:549–555. doi: 10.1093/ajcn/69.3.549. [DOI] [PubMed] [Google Scholar]
- 20.Kuijsten A, Arts IC, Vree TB, Hollman PC. J Nutr. 2005;135:795–801. doi: 10.1093/jn/135.4.795. [DOI] [PubMed] [Google Scholar]
- 21.Kuijsten A, Arts IC, van't Veer P, Hollman PC. J Nutr. 2005;135:2812–2816. doi: 10.1093/jn/135.12.2812. [DOI] [PubMed] [Google Scholar]
- 22.Setchell KDR, Lawson AM, McLaughlin LM, Patel S, Kirk DN, Axelson M. Biomedical Mass Spectrometry. 1983;10:227–235. doi: 10.1002/bms.1200100321. [DOI] [PubMed] [Google Scholar]
- 23.Saarinen NM, Smeds A, Makela SI, Ammala J, Hakala K, Pihlava JM, Ryhanen EL, Sjoholm R, Santti R. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:311–319. doi: 10.1016/s1570-0232(02)00339-2. [DOI] [PubMed] [Google Scholar]
- 24.Dean B, Chang S, Doss GA, King C, Thomas PE. Archives of biochemistry and biophysics. 2004;429:244–251. doi: 10.1016/j.abb.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 25.Murray T, Kang J, Astheimer L, Price WE. J Agric Food Chem. 2007;55:4907–4912. doi: 10.1021/jf070266q. [DOI] [PubMed] [Google Scholar]
- 26.Stitch SR, Toumba JK, Groen MB, Funke CW, Leemhuis J, Vink J, Woods GF. Nature. 1980;287:738–740. doi: 10.1038/287738a0. [DOI] [PubMed] [Google Scholar]
- 27.Vanharanta M, Voutilainen S, Lakka TA, Van der Lee M, Adlercreutz H, Salonen JT. Lancet. 1999;354:2112–2115. doi: 10.1016/S0140-6736(99)05031-X. [DOI] [PubMed] [Google Scholar]
- 28.van der Schouw YT, Pijpe A, Lebrun CE, Bots ML, Peeters PH, van Staveren WA, Lamberts SW, Grobbee DE. Arterioscler Thromb Vasc Biol. 2002;22:1316–1322. doi: 10.1161/01.atv.0000027176.83618.1a. [DOI] [PubMed] [Google Scholar]
- 29.de Kleijn MJ, van der Schouw YT, Wilson PW, Grobbee DE, Jacques PF. J Nutr. 2002;132:276–282. doi: 10.1093/jn/132.2.276. [DOI] [PubMed] [Google Scholar]
- 30.Dai Q, Franke AA, Jin F, Shu XO, Hebert JR, Custer LJ, Cheng J, Gao YT, Zheng W. Cancer Epidemiol Biomarkers Prev. 2002;11:815–821. [PubMed] [Google Scholar]
- 31.Kim MK, Chung BC, Yu VY, Nam JH, Lee HC, Huh KB, Lim SK. Clin Endocrinol (Oxf) 2002;56:321–328. doi: 10.1046/j.1365-2265.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 32.Vanharanta M, Voutilainen S, Nurmi T, Kaikkonen J, Roberts LJ, Morrow JD, Adlercreutz H, Salonen JT. Atherosclerosis. 2002;160:465–469. doi: 10.1016/s0021-9150(01)00603-7. [DOI] [PubMed] [Google Scholar]
- 33.Wang LQ. Journal of Chromatography B. 2002;777:289–309. doi: 10.1016/s1570-0232(02)00281-7. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Thompson LU. Breast Cancer Res Treat. 2003;80:163–170. doi: 10.1023/A:1024513815374. [DOI] [PubMed] [Google Scholar]
- 35.Horn-Ross PL, John EM, Canchola AJ, Stewart SL, Lee MM. J Natl Cancer Inst. 2003;95:1158–1164. doi: 10.1093/jnci/djg015. [DOI] [PubMed] [Google Scholar]
- 36.McCann SE, Muti P, Vito D, Edge SB, Trevisan M, Freudenheim JL. Int J Cancer. 2004;111:440–443. doi: 10.1002/ijc.20262. [DOI] [PubMed] [Google Scholar]
- 37.Linseisen J, Piller R, Hermann S, Chang-Claude J. Int J Cancer. 2004;110:284–290. doi: 10.1002/ijc.20119. [DOI] [PubMed] [Google Scholar]
- 38.Vij U, Kumar A. Natl Med J India. 2004;17:22–26. [PubMed] [Google Scholar]
- 39.Boccardo F, Lunardi G, Guglielmini P, Parodi M, Murialdo R, Schettini G, Rubagotti A. Eur J Cancer. 2004;40:84–89. doi: 10.1016/s0959-8049(03)00576-8. [DOI] [PubMed] [Google Scholar]
- 40.Kilkkinen A, Virtamo J, Virtanen MJ, Adlercreutz H, Albanes D, Pietinen P. Cancer Epidemiol Biomarkers Prev. 2003;12:1209–1212. [PubMed] [Google Scholar]
- 41.Kilkkinen A, Virtamo J, Vartiainen E, Sankila R, Virtanen MJ, Adlercreutz H, Pietinen P. Int J Cancer. 2004;108:277–280. doi: 10.1002/ijc.11519. [DOI] [PubMed] [Google Scholar]
- 42.Stattin P, Adlercreutz H, Tenkanen L, Jellum E, Lumme S, Hallmans G, Harvei S, Teppo L, Stumpf K, Luostarinen T, Lehtinen M, Dillner J, Hakama M. Int J Cancer. 2002;99:124–129. doi: 10.1002/ijc.10313. [DOI] [PubMed] [Google Scholar]
- 43.Lin Y, Wolk A, Hakansson N, Lagergren J, Lu Y. Cancer Epidemiol Biomarkers Prev. 2013;22:308–312. doi: 10.1158/1055-9965.EPI-12-1138. [DOI] [PubMed] [Google Scholar]
- 44.Bhatty RS. In: Flaxseed in Human Nutrition. Cunnane SC, Thompson LU, editors. AOCS Press; Champaign, Illinois: 1995. pp. 22–42. [Google Scholar]
- 45.Cunnane SC, Thompson LU. Flaxseed in human nutrition. AOCS Press; Champaign, IL: 1995. [Google Scholar]
- 46.Cunnane SC, Hamadeh MJ, Liede AC, Thompson LU, Wolever TM, Jenkins DJ. Am J Clin Nutr. 1995;61:62–68. doi: 10.1093/ajcn/61.1.62. [DOI] [PubMed] [Google Scholar]
- 47.Axelson M, Setchell KDR. FEBS Letters. 1980;122:49–53. doi: 10.1016/0014-5793(80)80399-1. [DOI] [PubMed] [Google Scholar]
- 48.Setchell KDR, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. Journal of Nutrition. 2003;133:1027–1035. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 49.Setchell KDR, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. American Journal of Clinical Nutrition. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 50.Shultz T, Bonorden W, Seaman W. Nutr Res. 1991;11:1089–1100. [Google Scholar]
- 51.Phipps WR, Martini MC, Lampe JW, Slavin JL, Kurzer MS. J Clin Endocrinol Metab. 1993;77:1215–1219. doi: 10.1210/jcem.77.5.8077314. [DOI] [PubMed] [Google Scholar]
- 52.Lampe JW, Martini MC, Kurzer MS, Adlercreutz H, Slavin JL. Am J Clin Nutr. 1994;60:122–128. doi: 10.1093/ajcn/60.1.122. [DOI] [PubMed] [Google Scholar]
- 53.Lampe JW, Gustafson DR, Hutchins AM, Martini MC, Li S, Wahala K, Grandits GA, Potter JD, Slavin JL. Cancer Epidemiol Biomarkers Prev. 1999;8:699–707. [PubMed] [Google Scholar]
- 54.Tarpila S, Aro A, Salminen I, Tarpila A, Kleemola P, Akkila J, Adlercreutz H. Eur J Clin Nutr. 2002;56:157–165. doi: 10.1038/sj.ejcn.1601298. [DOI] [PubMed] [Google Scholar]
- 55.Adlercreutz H, Fotsis T, Bannwart C, Wahala K, Brunow G, Hase T. Clin Chim Acta. 1991;199:263–278. doi: 10.1016/0009-8981(91)90120-2. [DOI] [PubMed] [Google Scholar]
- 56.Kilkkinen A, Stumpf K, Pietinen P, Valsta LM, Tapanainen H, Adlercreutz H. Am J Clin Nutr. 2001;73:1094–1100. doi: 10.1093/ajcn/73.6.1094. [DOI] [PubMed] [Google Scholar]
- 57.Horner NK, Kristal AR, Prunty J, Skor HE, Potter JD, Lampe JW. Cancer Epidemiol Biomarkers Prev. 2002;11:121–126. [PubMed] [Google Scholar]
- 58.Jacobs DR, Jr, Pereira MA, Stumpf K, Pins JJ, Adlercreutz H. Br J Nutr. 2002;88:111–116. doi: 10.1079/BJNBJN2002601. [DOI] [PubMed] [Google Scholar]
- 59.Nurmi T, Voutilainen S, Nyyssonen K, Adlercreutz H, Salonen JT. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;798:101–110. doi: 10.1016/j.jchromb.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 60.Stumpf K, Adlercreutz H. Clin Chem. 2003;49:178–181. doi: 10.1373/49.1.178. [DOI] [PubMed] [Google Scholar]
- 61.Milder IE, Kuijsten A, Arts IC, Feskens EJ, Kampman E, Hollman PC, Van't Veer P. J Nutr. 2007;137:1266–1271. doi: 10.1093/jn/137.5.1266. [DOI] [PubMed] [Google Scholar]
- 62.Tetens I, Turrini A, Tapanainen H, Christensen T, Lampe JW, Fagt S, Hakansson N, Lundquist A, Hallund J, Valsta LM. Food & nutrition research. 2013;57 doi: 10.3402/fnr.v57i0.19805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu W, Tanabe M, Harada KH, Koizumi A. Environmental health and preventive medicine. 2013 doi: 10.1007/s12199-013-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazur W, Fotsis T, Wahala K, Ojala S, Salakka A, Adlercreutz H. Anal Biochem. 1996;233:169–180. doi: 10.1006/abio.1996.0025. [DOI] [PubMed] [Google Scholar]
- 65.Liggins J, Grimwood R, Bingham SA. Anal Biochem. 2000;287:102–109. doi: 10.1006/abio.2000.4811. [DOI] [PubMed] [Google Scholar]
- 66.Bambagiotti-Alberti M, Coran SA, Ghiara C, Giannellini V, Raffaelli A. Rapid Commun Mass Spectrom. 1994;8:595–598. doi: 10.1002/rcm.1290080805. [DOI] [PubMed] [Google Scholar]
- 67.Milder IE, Arts IC, Venema DP, Lasaroms JJ, Wahala K, Hollman PC. J Agric Food Chem. 2004;52:4643–4651. doi: 10.1021/jf0497556. [DOI] [PubMed] [Google Scholar]
- 68.Frank J, Eliasson C, Leroy-Nivard D, Budek A, Lundh T, Vessby B, Aman P, Kamal-Eldin A. Br J Nutr. 2004;92:169–176. doi: 10.1079/BJN20041154. [DOI] [PubMed] [Google Scholar]
- 69.Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G. FEBS Letters. 1998;436:71–75. doi: 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 70.Hausner H, Johnsen NF, Hallund J, Tetens I. J Nutr. 2004;134:1197–1200. doi: 10.1093/jn/134.5.1197. [DOI] [PubMed] [Google Scholar]
- 71.Kurzer MS, Lampe JW, Martini MC, Adlercreutz H. Cancer Epidemiol Biomarkers Prev. 1995;4:353–358. [PubMed] [Google Scholar]
- 72.Setchell KDR, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NB, Wolfe B, Brashear WT, Desai P, Oldfield MF, Botting NP, Cassidy A. American Journal of Clinical Nutrition. 2003;77:411–419. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 73.Pietinen P, Stumpf K, Mannisto S, Kataja V, Uusitupa M, Adlercreutz H. Cancer Epidemiol Biomarkers Prev. 2001;10:339–344. [PubMed] [Google Scholar]
- 74.Setchell KDR, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirscher AS, Cassidy A, Heubi J. Journal of Nutrition. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 75.Nurmi T, Mazur W, Heinonen S, Kokkonen J, Adlercreutz H. J Pharm Biomed Anal. 2002;28:1–11. doi: 10.1016/s0731-7085(01)00612-4. [DOI] [PubMed] [Google Scholar]
- 76.Penalvo JL, Heinonen SM, Nurmi T, Deyama T, Nishibe S, Adlercreutz H. J Agric Food Chem. 2004;52:4133–4138. doi: 10.1021/jf0497509. [DOI] [PubMed] [Google Scholar]