Abstract
Background
Experts have called for the inclusion of values clarification (VC) exercises in decision aids (DA) as a means of improving their effectiveness, but little research has examined the effects of such exercises.
Objective
To determine whether adding a VC exercise to a DA on heart disease prevention improves decision making outcomes.
Design
Randomized trial.
Setting
UNC Decision Support Laboratory.
Patients
Adults ages 40–80 with no history of cardiovascular disease.
Intervention
A web-based heart disease prevention DA with or without a VC exercise.
Measurements
Pre and post-intervention decisional conflict and intent to reduce CHD risk. Post-intervention self-efficacy and perceived values concordance.
Results
We enrolled 137 participants (62 in DA; 75 in VC) with moderate decisional conflict (DA 2.4; VC 2.5) and no baseline differences among groups. After the interventions, we found no clinically or statistically significant differences between groups in decisional conflict (DA 1.8; VC 1.9; absolute difference VC-DA 0.1, 95% CI −0.1 to 0.3), intent to reduce CHD risk (DA 98%; VC 100%; absolute differences VC-DA: 2%, 95% CI −0.02% to 5%), perceived values concordance (DA 95%, VC 92%; absolute difference VC-DA −3%, 95% CI −11 to +5%), or self efficacy for risk reduction (DA 97%, VC 92%; absolute difference VC-DA −5%, 95% CI −13 to +3%). However, VC tended to change some decisions about risk reduction strategies.
Limitations
Use of a hypothetical scenario. Ceiling effects for some outcomes.
Conclusions
Adding a VC intervention to a DA did not further improve decision making outcomes in a population of highly educated and motivated adults responding to scenario-based questions. Work is needed to determine the effects of VC on more diverse populations and more distal outcomes.
Introduction
Decision aids have been widely recommended as an adjunct to decision making for healthcare decisions with clinical equipoise.1,2 In this setting, decision aids help individuals learn about healthcare options, clarify their values, and make decisions about their health. The benefits of decision aids, which have been enumerated in several systematic evidence reviews,3,4 include improved patient knowledge, improved expectations about the benefits and harms associated with a decision, reduced conflict from feeling uninformed, increased participation in decision making, reduced indecision, and reduced conflict from unclear values.
With preliminary evidence for effectiveness of decision aids in clarifying values, the International Panel for Decision Aid Standards5 recommended that every decision aid include some form of values clarification. Values clarification (VC) is a process by which people form and communicate the relative desirability of decision options and their features. Most decision aids to date allow individuals to implicitly consider their values by learning about the pros and cons of decision options.6 However, values clarification can be done in more explicit ways. Examples include ranking and rating different features of decision options to facilitate a decision (ranking and rating), and viewing others engaged in decision making and identifying one’s own similarity to the values of individuals making those choices (social matching). The effect of adding such explicit values clarification exercises to decision aids is unclear with evidence limited to a few studies, which used different methods and achieved mixed results.4,6
We previously developed a decision aid on heart disease prevention called Heart to Heart.7 This decision aid encouraged CHD prevention and facilitated a choice among equally effective options for CHD risk reduction. In testing, this decision aid was effective in increasing intent for CHD risk reduction.8 Like most decision aids, however, it did not include an explicit values clarification exercise.
An explicit values clarification exercise might be a useful addition to a CHD prevention decision aid because pilot work suggests that individuals value the attributes of CHD prevention options differently 9 and that a majority of individuals have difficulty determining the best way to reduce their CHD risk.8 Therefore, in this study, we sought to determine whether adding an explicit values clarification exercise to our decision aid on heart disease prevention improved decision making outcomes, including decisional conflict, intent for screening, perceived value concordance, and self-efficacy.
Methods
We performed a randomized trial examining the effect of adding a values clarification exercise to a decision aid on heart disease prevention. An overview of the study design is shown in Figure 1. The study was conducted in the UNC Decision Support Laboratory and was approved by UNC’s Institutional Review Board with each participant providing informed consent prior to participation.
Figure 1.

Study Design
Study participants
For our study, we recruited a convenience sample of men and women from a registry of participants interested in decision support testing. Individuals in this registry had previously responded to mass information emails, television commercials, print media, and word of mouth with their interest and are routinely invited to opt into studies for which they are eligible.
For this study, we sent emailed or mailed invitations to registry participants who were ages 45–80, had no prior history of cardiovascular disease, and had no prior participation in studies about CHD prevention (n~300). We then called registry participants who indicated initial interest to confirm their eligibility. During these phone calls, we asked individuals their age and gender and included individuals (men 45 and older and women age 55 and older) who were likely to be at moderate to high risk of heart disease when considering risk factor information that we provided as part of a hypothetical scenario in our study (described below).
Hypothetical Scenario
To ensure that each participant was at moderate to high risk and had treatment options for the primary prevention of CHD to consider, we provided all participants with a hypothetical scenario. This scenario asked participants to imagine a visit with their doctor in which they were told they had elevated blood pressure (160/99 mmHg) and high cholesterol (total 230 mg/dl and HDL of 35 mg/dl), making them at overall moderate (6–10%) to high risk (>10%) based on population estimates for people of their own age decade and gender.10 Participants then combined this hypothetical information with their own age, gender, smoking status, and diabetes status to navigate the decision aid (and, if assigned, values clarification exercise).
Randomization
We used a computerized random-number generator to assign participants to our decision aid alone (DA) or our decision aid in combination with an explicit values clarification exercise (DA + VC). Random assignments were sealed in security envelopes until after baseline survey administration. The research assistant then opened the seal on the security envelope, determined the assignment, and helped the subject access the appropriate intervention.
The Interventions
We had all participants view the Heart to Heart decision aid (available at www.med-decisions.com). This decision aid helps individuals with no prior history of heart disease make decisions about how to lower their future likelihood of CHD events. It calculates an individual’s overall risk of CHD events in the next 10 years using a continuous Framingham risk equation11 and presents this risk in both numerical and graphical formats. The decision aid then shows patients a list of up to four evidence-based treatment options for risk reduction (aspirin, blood pressure medicine, cholesterol medicine, and/or smoking cessation), a list of pros and cons of each option (including the benefit for heart disease, benefit for other medical conditions, side effects, degree of difficulty for most people, and cost), a presentation of the change in risk resulting from initiation of options either singly or in combination, and encouragement to consider their values and make a decision. Like most decision aids, the Heart to Heart decision aid helps patients implicitly consider their values (see Figure 2) by providing information about the pros and cons of various risk reducing options (including their risk reduction singly or in combination). It additionally prompts individuals to make a decision.
Figure 2.
Implicit Values Clarification Embedded within Heart to Heart Decision Aid
Figure 2a. Calculations of Risk Reductions Possible by Intervening with Various Combinations of Treatment
Figure 2b. Summary of Treatment Options and their Pros/Cons
Figure 2c. Encouragement to Consider Values and Make a Decision
The explicit values clarification exercise that we added to the Heart to Heart decision aid for participants in our second intervention arm was a ranking and rating exercise. We chose a ranking and rating exercise because ranking and rating are simple, familiar tasks with results that can be summarized and reflected back to individuals to allow additional consideration of values. Values in our ranking and rating exercise were decisional attributes common to each of the treatment options presented in the decision aid (benefit for other medical conditions, side effects, degree of difficulty for most people, cost, and effect on others). These attributes were a subset of decisional attributes identified as relevant to participants in formative research9 and were chosen for their differences across treatment options.
During the values clarification exercise (see Figure 3), participants first ranked the decisional attributes according to their individual importance to their decision making. They then rated whether each attribute related to their personal decision options; rating proceeded in the order of the previously ranked decisional attributes. At the end of the exercise, participants viewed a summary table of their answers allowing additional consideration of their values.
Figure 3.

Overview of Explicit Values Clarification Exercise Added to Heart to Heart Decision Aid
Figure 3a. Ranking of Importance of Factors Related to Decision.
Figure 3b. Rating Relevance of Decision Factor for Personally Relevant Treatment Options*
* Rating proceeded in the order of importance of previously ranked decision factors.
Figure 3c. Summary of Participant’s Responses to Ranking and Rating Exercise
* The more hearts to the left or the more in a row, the more favorable the option.
Delivery of the Surveys and Interventions
At the beginning of the study session, all participants completed a baseline questionnaire to assess their demographic information, attitudes toward heart disease prevention, decisional conflict, and intent to reduce their CHD risk. They were then randomized to the DA or DA + VC arms. Participants in both groups viewed the decision aid, entering risk factor information and navigating interactively through decision aid content. All participants were then asked to make a choice among treatment options, including the option of doing nothing, to lower their risk of heart disease. Participants in the DA + VC arm then worked through the ranking and rating values clarification exercise and again indicated their choice for lowering their CHD risk. All participants completed a final questionnaire assessing their decisional conflict, intent to reduce their CHD risk, their self-efficacy for risk reduction, and their perception that they made a decision consistent with their values. All participants received $25 for their participation.
Decision Making Outcome Measures
The primary outcome for our study was decisional conflict. We additionally measured several other secondary outcomes: intent for CHD risk reduction, perceived values concordance, and self-efficacy for risk reduction. Finally, we measured three post-hoc outcomes: intent for specific treatments post-intervention; changes in intent for specific treatments post (compared with pre) intervention; and total number of treatments intended after the intervention.
We measured decisional conflict at baseline and post-intervention using the Decisional Conflict Scale, a well-validated 16-item scale examining several features of conflicted decision making, including feeling uncertain, uninformed, unclear about one’s values, unsupported, or ineffective in making choices.12,13 The Decisional Conflict Scale discriminates well among persons who plan to immediately make and implement a decision and those who will delay both decision-making and action (alpha 0.81, test-retest reliability 0.81). The 3-item values subscale has been widely used by experts to measure the effect of decision aids on values and includes the following questions: 1) “I am clear which benefits matter most to me,” 2) “I am clear about which risks and side effects matter most to me,” and 3) “I am clear about which is most important to me (the benefits or the risks/side effects). For both the full Decisional Conflict Scale and the values subscale, we summed and averaged responses. Scores range from 1 to 5 with scores less than 2 indicating intent to immediately make and implement a decision.
We used a single question at baseline and post-intervention (and additionally between the DA and VC components in the DA + VC group) to assess participants’ intent to change behavior: “Are you planning to lower your chances of heart disease?” We counted a yes answer to this question as intent to lower CHD risk.
We measured participants’ perceived values concordance (i.e. their perception that their decision was consistent with their values) post-intervention by agreement with a single statement: “My decision was consistent with my personal values.” Participants provided answers on a 5-point Likert scale dichotomized to strongly agree/agree versus all others.
We assessed self-efficacy for risk reduction at baseline and post-intervention with the single statement, “I am confident that I can carry out my plan to lower my chances of heart disease.” Again, participants provided answers on a 5-point Likert scale with answers dichotomized to strongly agree/agree versus others.
We measured intent for specific treatments using a follow-up question to our general intent question listed above. All participants who agreed that they intended to lower their CHD risk checked boxes indicating their intended method(s) for lowering their CHD risk among a list of 6 possible methods (aspirin, blood pressure medicine, cholesterol medicine, smoking cessation, diet, and exercise), four of which were the focus of our intervention because of they are supported by evidence of the highest design and quality.
Process Measures
Because the addition of a values clarification exercise might affect tool use and patients’ understanding and use of other information provided in the decision aid, we measured several process measures. These included total time spent with the tool (measured electronically by the computer), and agreement with simple declarative statements that Heart to Heart was easy to use, easy to understand, helped personalize information, and helped decide what was important. For those in the DA+VC arm, we also asked how easy the processes of ranking and rating were, whether they used the summary table to make a decision, and whether the summary table showed how they felt.
Statistical Analysis
To assess the success of randomization, we compared the baseline characteristics of subjects who received the DA with those who received the DA + VC. To assess the effects of the explicit values clarification intervention, we then compared DA and DA + VC groups with respect to our primary outcome, post-intervention decisional conflict, using both t-tests and Wilcoxon rank-sums. Because these tests produced similar results, we report t-tests and present means and confidence intervals for ease of interpretation. We assessed the effect of the explicit values clarification intervention on our secondary outcomes (intent to be screened, perceived values concordance, and self-efficacy for CHD risk reduction) using Pearson’s chi square tests. We then compared DA and DA + VC groups with respect to post-intervention intent for specific treatment and post-intervention changes (compared to baseline) in intent for specific treatment, using Pearson chi square tests. We additionally performed regression analyses (linear regression for continuous outcomes and logistic regression for dichotomous outcomes) to compare study arms with respect to our primary and some secondary outcomes, after adjusting for small (but potentially meaningful) differences in baseline values. Finally, we compared the number of intended treatments post intervention using the Cochrane-Armitage Trend Test. Wherever possible, we report absolute differences with 95% confidence intervals to allow estimation of both the effect size and the precision of our results. All analyses were conducted using SAS 9.2 Statistical Software (Cary, NC).
Sample Size Calculation
We calculated a sample size to detect a mean difference of 0.40 in the decisional conflict scale. We chose this difference because we felt it would be clinically meaningful and because it would be consistent with the difference in the decisional conflict scale and its values subscale between those who received decision support and those who didn’t in previous decision aid studies for preventive interventions.12 Using this difference, a DA decisional conflict score of 2.0 for the control group (which we estimated from our pilot work and existing literature14), a standard deviation of 0.6, a two-sided alpha of 0.025, and a power of 90%, we calculated a sample size of 64 in each group (128 total) to detect a 0.4 point difference, using a Wilcoxon Rank-Sum test. A sample size of 128 would allow us approximately 80% power to detect a 14% or greater difference in secondary dichotomous outcomes (intent to reduce CHD risk, perceived values concordance, and self efficacy for risk reduction).
When a study of a CHD prevention decision aid15 came to our attention during the study period, we recalculated a sample size using decisional conflict parameters from that study (baseline decisional conflict 2.3, average difference in decisional conflict pre-post decision aid 0.3) and found a similar sample size. Thus, we continued recruitment as planned.
Results
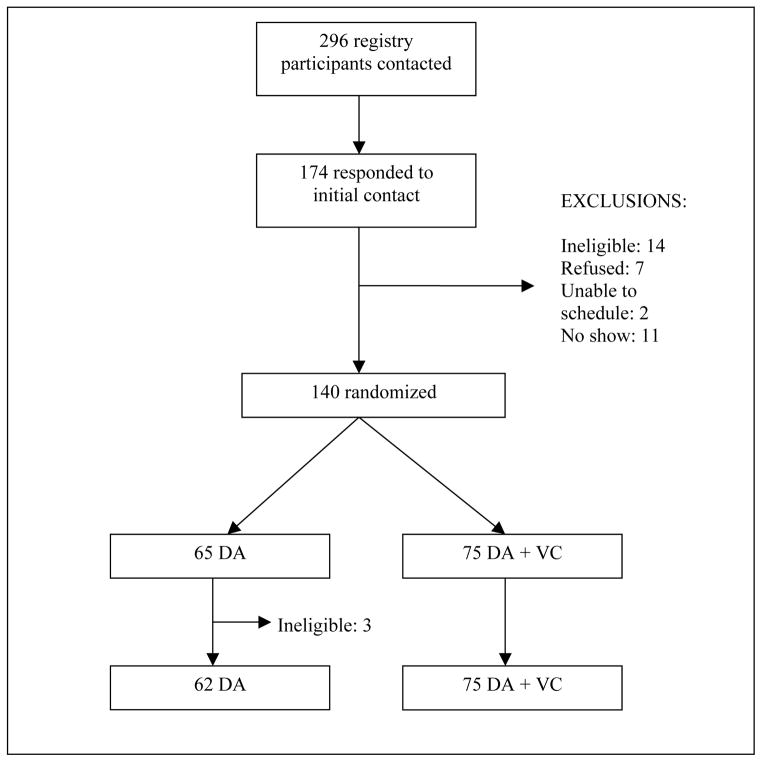
We contacted 296 decision registry participants about participation in our study (see Figure 4). 174 responded with interest. Of those, 160 were eligible. Seven refused participation, 2 could not be scheduled for a research session, and 11 who were scheduled did not present for their session. 140 individuals were randomized (65 to the DA group and 75 to the DA + VC group) and 137 ultimately completed the study.
Figure 4.
Recruitment Flow Diagram
Participant characteristics are shown in Table 1. Mean age was 52 and 78% were female. Most were white, had at least some college education, and favored shared or patient-led decision making. Using a combination of personal and study provided risk factor information, most had a 10-year risk of CHD events of 10%. There were no appreciable baseline differences by study assignment.
Table 1.
Participant Characteristics (n=137)
| Characteristic | DA | DA + VC |
|---|---|---|
|
| ||
| Mean age | 52.4 | 52.5 |
|
| ||
| Female (%) | 74% | 81% |
|
| ||
| Race: | ||
| White (%) | 79% | 77% |
| Black (%) | 21% | 17% |
|
| ||
| Education: | ||
| At least some college (%) | 93% | 95% |
|
| ||
| Smoking (%) | 11% | 8% |
|
| ||
| Diabetes (%) | 8% | 5% |
|
| ||
| Accurate perception of CHD risk* (%) | 25% | 32% |
|
| ||
| Knowledgeable about the best options for CHD risk reduction† (%) | 77% | 79% |
|
| ||
| Preferred participation in decision making about CHD (%): | ||
| I decide | 2% | 11% |
| Share decision | 96% | 85% |
| MD decide | 2% | 4% |
|
| ||
| Decisional Conflict (mean)‡: | ||
| Overall | 2.4 | 2.5 |
| Values subscale | 2.5 | 2.6 |
|
| ||
| Intent to reduce CHD risk (%) | ||
| Any plan | 97% | 97% |
| Best plan† | 68% | 60% |
Estimation requires consideration of both personal and hypothetical information contributing to CHD risk
Includes best evidence plans promoted by our decision aid, including hypertension med, cholesterol med, smoking cessation, and aspirin.
Reported on 1–5 scale; to convert to 0–100 scale, subtract 1 and multiply by 25.
The Effect of the Intervention on Primary and Secondary Outcomes
Adding a ranking and rating exercise to a decision aid on heart disease prevention had no apparent effect on our primary outcomes in this study (see Table 2). Although both of our interventions reduced decisional conflict (DA −0.6; DA + VC −0.6), we found no clinically or statistically significant differences between groups in decisional conflict post-intervention (DA 1.8; DA+VC 1.9; absolute difference VC-DA 0.1, 95% CI −0.1 to 0.3; adjusted difference −0.01, p 0.95). Intent to reduce CHD risk was high at baseline and did not differ by group post intervention (DA 98%; DA+VC 100%; absolute differences VC-DA: 2%, 95% CI −0.02% to 5%). Additionally, a majority of individuals in both the DA and DA+VC groups reported decisions consistent with their values post-intervention (DA 95%; DA+VC 92%; absolute difference −3%, 95% CI −11% to +5%) and self-efficacy was similar between groups post-intervention (DA 97%; DA+VC 92%; absolute difference VC-DA: −5%, 95% CI −13 to +3%).
Table 2.
The Effect of Values Clarification on the Decision: Differences in Post-Decision Aid Outcomes by Study Group
| DA | DA + VC | Absolute Difference: VC – DA (95% CI) | Adjusted Absolute Difference, p value | |
|---|---|---|---|---|
|
| ||||
| Primary Outcome | ||||
|
| ||||
| Decisional conflict*: | ||||
| Overall | 1.8 | 1.9 | +0.1 (−0.1, +0.3) | −0.01, p= 0.95 |
| Values subscale | 1.8 | 1.9 | +1.8 (−0.2 to +0.4) | −0.04, p= 0.73 |
|
| ||||
| Secondary Outcomes | ||||
|
| ||||
| Intent to reduce CHD risk | ||||
| Any plan | 98% | 100% | +2% (−0.02% to +5%) | -- |
| Best plan† | 71% | 65% | −6% (−10% to 21%) | ‡, p=0.82 |
|
| ||||
| Decision c/w with values | 95% | 92% | −3% (−11% to +5%) | -- |
|
| ||||
| Self-efficacy to lower at least one CHD risk factor | 97% | 92% | −5% (−13% to +3%) | -- |
reported on 1–5 scale; to convert to 0–100 scale, subtract 1 and multiply by 25.
Includes best evidence plans promoted by our decision aid, including hypertension med, cholesterol med, smoking cessation, and aspirin.
adjusted absolute difference can not be estimated for categorical variables
The Effect of the Decision Aid on Post-Hoc Outcomes
Tables 3 shows the proportion of individuals intending to start specific risk reducing treatments at various points in time. There were no significant differences in the proportion of individuals planning to start specific risk reducing treatments post intervention (post-DA in the DA group and post-VC in the DA+VC group). Table 4, however, shows that there was a trend toward more participants in the DA+VC group changing their decisions about cholesterol (absolute difference +7%, 95% CI −5% to +21%) and blood pressure medications (absolute difference +9%, 95% CI −3 to +21%). There were no apparent differences between groups in the proportion of participants changing decisions about other CHD risk factors. There were also no differences in the proportion of individuals planning various numbers of treatments post-intervention (p 0.56 for trend).
Table 3.
Planned Interventions at Various Timepoints Relative to Administration of the Decision Aid
| Planned Interventions | DA | VC | Absolute Difference (VC-DA), 95% CI | Adjusted Absolute Difference (VC-DA), p value | |||
|---|---|---|---|---|---|---|---|
| Baseline | Post DA | Baseline | Post DA | Post VC | |||
| Smoking, if smoker (n=13) | 91% | 82% | 88% | 75% | 75% | −7% (−45% to +31%) | *, p= 0.76 |
| BP medication (n=137) | 71% | 73% | 71% | 72% | 72% | −1% (−15% to +14%) | *, p= 0.95 |
| Cholesterol medication (n=137) | 68% | 71% | 60% | 69% | 65% | −6% (−21% to +10%) | *, p= 0.82 |
| Aspirin, if can take aspirin (n=125) | 67% | 82% | 80% | 56% | 86% | +4% (−8% to +17%) | *, p= 0.70 |
adjusted absolute difference can not be estimated for categorical variables
Table 4.
Proportion of Participants Changing Intent For Specific Treatment After the Intervention
| Changed Intent | DA (n=62) | DA + VC (n=75) | Absolute Difference (95% CI) |
|---|---|---|---|
|
| |||
| % changing intent about: | |||
| Smoking, if a smoker (n=19) | 9% | 38% | +29% (−9% to +66%) |
| BP medication (n=137) | 11% | 20% | +9% (−3% to +21%) |
| Cholesterol medication (n=137) | 16% | 24% | +7% (−5% to +21%) |
| Aspirin, if can take aspirin (n=125) | 27% | 22% | −5% (−20% to +10%) |
| Any change | 90% | 94% | +4% (−4% to +13%) |
The Effect of the Interventions on Process Measures
The addition of a ranking and rating exercise to a decision aid on heart disease prevention increased the mean number of minutes spent with the tool from 5 minutes (range 1 to 12 minutes) to 11 minutes (range 4 to 21 minutes). It did not, however, increase participants’ perceptions that the tool helped decide what was important (DA 87%; DA + VC 75%). A majority of participants in the DA+VC group reported the ranking and rating was easy to do (76% and 80% respectively) and the summary table showed how they felt (78%), however, only 62% reported they used the summary table to make a decision. Importantly, the ranking and rating exercise did not change participants’ perceptions that the decision aid was easy to use (DA 100%; DA+VC 95%), easy to understand (DA 97%; DA+VC 95%), personalized information for them (DA 95%; DA+VC 96%).
Discussion
In a hypothetical lab-based study, adding a ranking and rating exercise to a heart disease prevention decision aid did not improve participants’ decisional conflict, intent to reduce their CHD risk, sense of values concordance, or self-efficacy for risk reduction. It also did not improve process measures for the decision. Both our DA and our DA + VC interventions reduced decisional conflict and resulted in perceptions of decisions consistent with personal values and self-efficacy for CHD risk reduction. Neither had an effect on intent to reduce CHD risk, which was high at baseline and remained essentially unchanged in this study.
These findings raise questions about the additional benefits of an explicit values clarification exercise as part of a decision aid (compared with implicit consideration of values), but by no means provide a definitive answer. Explicit values clarification exercises come in many forms and relatively few have been tested as adjuncts to decision aids. 4,6,16–19 Additionally, ranking and rating exercises, such as the one in this study, may be conceptualized in several ways that might affect their efficacy. Inclusion of additional or different decisional attributes, a different process for ranking and rating, or a different summary of the values clarification exercise might have produced different results.
Measurement of different outcomes might additionally affect conclusions. We chose the decisional conflict scale as our primary outcome because it is widely used and its values subscale directly measures an individuals clarity about which benefits, risks, and side effects of a decision matter most, and which are more important (the benefits or the risks and side effects). Using this scale, the baseline decisional conflict in our study was moderate (DA 2.4; DA +VC 2.5) and, when converted to the newer 0–100 scale (by subtracting 1 and multiplying by 25: DA 35; DA + VC 37.5), similar to the decisional conflict reported in the usual care group of many other decision aid studies on topics ranging from surgery for ischemic heart disease to colorectal cancer screening.4 Using this scale, we showed improvements (−0.6 units on a 1–5 scale; −14 on a 0–100 scale) in both the DA and DA+ VC arms, which exceed the mean difference in decisional conflict (−6.12, 95% CI −8.62 to −3.63 on a 0–100 scale) in randomized studies comparing the effects of decision aids and usual care.4 We, however, found no differences between intervention arms. It is unclear whether this lack of effect is because there really is no difference in decisional conflict, because the decisional conflict scale can’t be meaningfully lowered more than we did by our decision aid alone (i.e. there is a floor effect), or because the decisional conflict scale itself is insufficient to capture the differential benefits of an explicit values clarification exercise (i.e. values clarification involves tradeoffs other than those among benefits, risks, and side effects; e.g. cost, level of difficulty). These latter possibilities are empiric questions. Some existing evidence argues against a floor effect for the decisional conflict scale because studies of decision aids for breast cancer treatment, anti-thrombotic therapy for atrial fibrillation, and blood transfusion prior to heart surgery have achieved appreciably lower decisional conflict scores (ranging from 10 to 17 on a 0–100 scale) than the post-intervention decisional conflict scores in our study (DA 21; DA +VC 22.8 on a 0–100 scale).4 It is possible, however, that the achievable decisional conflict may be different for CHD prevention decisions and more testing is necessary. The ability of the values subscale of the decisional conflict scale to capture relevant values could be tested simply through cognitive interviewing. Until such issues are resolved, other outcomes should also be explored to measure the benefit of explicit values clarification.
What outcome might best capture the effects of an explicit values clarification exercises is unclear. In our study, we attempted to explore several outcomes we felt might be potentially useful: the perception that decisions are consistent with personal values, self-efficacy to carry through on one’s decision, and intent to adhere to one’s chosen decision. Unfortunately, we were limited by ceiling effects (probably due to use of a hypothetical scenario and our choice of study population) and these outcomes will need to be examined further in future studies. Outcomes proposed (or used) by other decision aid researchers (e.g. the variance in behavioral intent explained by values, and the percentage agreement or correlation between values and behavioral intent)4,16,20,21 should also be further explored; however, care must be taken to ensure that all relevant values are accounted for.
Whatever values clarification exercise or measure is employed, future studies of explicit values clarification exercises should learn from the limitations of our work. First, we recruited a non-clinical population and used hypothetical scenarios to ensure our decision-makers had adequate CHD risk and, thus, adequate opportunity for tradeoffs among CHD risk reducing options. This may have produced limited investment in the decision making task and have artificially elevated self-efficacy. Studying actual clinical populations making CHD prevention decisions would provide more realistic estimations of the benefit of explicit values clarification exercises for such a decision. Second, we recruited our population from a decision aid registry. While this allowed for ease of recruitment, it meant that we sampled a highly educated and motivated population and encountered ceiling effects for some of our outcomes of interest (e.g. intent to reduce CHD risk and self-efficacy). Studying a more diverse population (by recruiting a clinical population or oversampling individuals with lower motivation and self-efficacy) would provide a better opportunity to define the effects of explicit values clarification exercises. Finally, our sample size was too small to test for interactions in intervention effect. It is possible that the efficacy of values clarification exercise varies by characteristics such as literacy level and familiarity with decisions, thus, future work should plan for larger sample sizes and subgroup analyses to explore this possibility.
Limitations aside, we feel our study raises important questions for values clarification research. Future studies should explore different values clarification exercises and different outcomes in more diverse populations.
Acknowledgments
The authors would like to thank Betsy Greer at the UNC Sheps Center for Health Services Research for help with patient recruitment.
Grant support:
Financial support for this study was provided in part by a grant from the American Heart Association (grant number 0530164N), the National Heart Lung and Blood Institute (grant number 1 K23 HL074375), and the National Cancer Institute (grant number K05 CA129166). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Footnotes
This article was presented at the annual meeting of the Society of Medical Decision Making in October 2008 and the American Heart Association Research Symposium in November 2008.
Reprint request: sls593@med.unc.edu
References
- 1.O’Connor AM, Legare F, Stacey D. Risk communication in practice: the contribution of decision aids. BMJ. 2003;327(7417):736–40. doi: 10.1136/bmj.327.7417.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheridan SL, Harris RP, Woolf SH. Shared decision making about screening and chemoprevention. a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26(1):56–66. doi: 10.1016/j.amepre.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor AM, Stacey D, Entwistle V, Llewellyn-Thomas H, Rovner D, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2003;(2):CD001431. doi: 10.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009;(3):CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Patient Decision Aid Standards Collaboration. [Accessed 10 March 2009];IPDAS Collaboration Background Document [Web Page] 2005 Feb 17; Available at http://www.ipdas.ohri.ca/IPDAS_Background.pdf.
- 7.Pignone M, Sheridan SL, Lee YZ, Kuo J, Phillips C, Mulrow C, et al. Heart to Heart: a computerized decision aid for assessment of coronary heart disease risk and the impact of risk-reduction interventions for primary prevention. Prev Cardiol. 2004;7(1):26–33. doi: 10.1111/j.1520-037x.2004.2417.x. [DOI] [PubMed] [Google Scholar]
- 8.Sheridan SL, Shadle J, Simpson RJ, Jr, Pignone MP. The impact of a decision aid about heart disease prevention on patients’ discussions with their doctor and their plans for prevention: a pilot randomized trial. BMC Health Serv Res. 2006;6:121. doi: 10.1186/1472-6963-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheridan SL, Behrend L, Vu MB, Meier A, Griffith JM, Pignone MP. Individuals’ responses to global CHD risk: a focus group study. Patient Educ Couns. 2009;76(2):233–9. doi: 10.1016/j.pec.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasternak RC, Abrams J, Greenland P, Smaha LA, Wilson PW, Houston-Miller N. 34th Bethesda Conference: Task force #1--Identification of coronary heart disease risk: is there a detection gap? J Am Coll Cardiol. 2003;41(11):1863–74. doi: 10.1016/s0735-1097(03)00358-9. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor AM. [Accessed 30 May 2007];User Manual - Decisional Conflict Scale [Web Page] 1999 Available at http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_DecConflict2006.pdf.
- 13.O’Connor AM. [Accessed 11 March 2009];User Manual - Decisional Conflict Scale [Web Page] 2005 Available at http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf.
- 14.Davison BJ, Kirk P, Degner LF, Hassard TH. Information and patient participation in screening for prostate cancer. Patient Educ Couns. 1999;37(3):255–63. doi: 10.1016/s0738-3991(98)00123-2. [DOI] [PubMed] [Google Scholar]
- 15.Lalonde L, O’Connor AM, Drake E, Duguay P, Lowensteyn I, Grover SA. Development and preliminary testing of a patient decision aid to assist pharmaceutical care in the prevention of cardiovascular disease. Pharmacotherapy. 2004;24(7):909–22. doi: 10.1592/phco.24.9.909.36104. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor AM, Wells GA, Tugwell P, Laupacis A, Elmslie T, Drake E. The effects of an ‘explicit’ values clarification exercise in a woman’s decision aid regarding postmenopausal hormone therapy. Health Expect. 1999;2(1):21–32. doi: 10.1046/j.1369-6513.1999.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes-Rovner M, Kroll J, Rovner DR, et al. Patient decision support intervention: increased consistency with decision analytic models. Med Care. 1999;37(3):270–84. doi: 10.1097/00005650-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery AA, Fahey T, Peters TJ. A factorial randomised controlled trial of decision analysis and an information video plus leaflet for newly diagnosed hypertensive patients. Br J Gen Pract. 2003;53(491):446–53. [PMC free article] [PubMed] [Google Scholar]
- 19.Driscoll DL, Rupert DJ, Golin CE, et al. Promoting prostate-specific antigen informed decision-making. Evaluating two community-level interventions. Am J Prev Med. 2008;35(2):87–94. doi: 10.1016/j.amepre.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Rothert ML, Holmes-Rovner M, Rovner D, et al. An educational intervention as decision support for menopausal women. Res Nurs Health. 1997;20(5):377–87. doi: 10.1002/(sici)1098-240x(199710)20:5<377::aid-nur2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Dodin S, Legare F, Daudelin G, Tetroe J, O’Connor A. Making a decision about hormone replacement therapy. A randomized controlled trial. Can Fam Physician. 2001;47:1586–93. [PMC free article] [PubMed] [Google Scholar]