Abstract
Anorexia nervosa (AN) is a psychiatric illness characterized by restricted eating and an intense fear of gaining weight. Most individuals with AN are females, diagnosed first during adolescence, 40% to 80% of whom exhibit excessive exercise, and an equally high number with a history of anxiety disorder. We sought to determine the cellular basis for individual differences in AN vulnerability by using an animal model, activity-based anorexia (ABA), that is induced by combining food restriction (FR) with access to a running wheel that allows voluntary exercise. Previously, we showed that by the 4th day of FR, the ABA group of adolescent female rats exhibit > 500% greater levels of non-synaptic α4βδ−GABAARs at the plasma membrane of hippocampal CA1 pyramidal cell spines, relative to the levels found in age-matched controls that are not FR and without wheel access. Here, we show that the ABA group exhibits individual differences in body weight loss, with some losing nearly 30%, while others lose only 15%. The individual differences in weight loss are ascribable to individual differences in wheel activity that both precedes and concurs with days of FR. Moreover, the increase in activity during FR correlates strongly and negatively with α4βδ−GABAAR levels (R= - 0.9, p<0.01). This negative correlation is evident within 2 days of FR, before body weight loss approaches life-threatening levels for any individual. These findings suggest that increased shunting inhibition by α4βδ−GABAARs in spines of CA1 pyramidal neurons may participate in the protection against the ABA-inducing environmental factors of severe weight loss by suppressing excitability of the CA1 pyramidal neurons which, in turn, is related indirectly to suppression of excessive exercise. The data also indicate that, although exercise has many health benefits, it can be maladaptive to individuals with low levels of α4βδ−GABAARs in the CA1, particularly when combined with FR.
1. INTRODUCTION
Anorexia nervosa (AN) is a psychiatric illness characterized by restricted eating and an intense fear of gaining weight, even when the patient is severely under-weight. AN has one of the highest mortality rates among mental illnesses (10-20%) (Sullivan, 1995, Birmingham et al., 2005, Bulik et al., 2007), even surpassing depression. There are no accepted pharmacological treatments for AN (Powers and Bruty, 2009, Aigner et al., 2011, Barbarich-Marsteller et al., 2012), as its etiology remains unclear. However, the epidemiology of AN provides clues about the biological basis of the disease. No less than 40% and as many as 80% of individuals with AN exhibit excessive exercise (Davis et al., 1999, Hebebrand et al., 2003) that often precedes the formal diagnosis (Davis et al., 1997). Equally many also have a history of anxiety disorders (Kaye et al., 2004, Dellava et al., 2010, Thornton et al., 2011). The first onset of AN is most commonly at puberty, with 90 to 95% of the cases occurring among females (DSM-5) (APA, 2013), indicating that anorexic behavior during this pivotal, final stage of brain development may be associated with ovarian hormone surges of puberty that perturb anxiety regulation. Still, it is perplexing why only 0.9% of the female population is diagnosed with AN during their lifetime (Hudson et al., 2007), when nearly all females experiment with dieting during adolescence (Lucas et al., 1991). We sought to determine the cellular basis for the individual differences in AN vulnerability by using an animal model, activity-based anorexia (ABA).
The rodent ABA model captures two hallmarks of AN. One is voluntary excessive exercise, which is evoked by imposition of food restriction. The other is voluntary food restriction, as the food restricted animals paradoxically begin to choose exercise over feeding, even during the period of food access. When the ABA-inducing environment is imposed upon adolescent female rats, this combination of behaviors leads to severe body weight loss and mortality, unless removed from the ABA-inducing environment by around the fifth day (Routtenberg and Kuznesof, 1967, Barbarich-Marsteller et al., 2013, Chowdhury et al., 2013, Gutierrez, 2013). Adolescent female rats placed in an ABA-inducing environment for four days exhibit a 500% greater level of non-synaptic α4βδ−GABAA receptors (α4βδ−GABAARs) at dendritic spines of CA1 pyramidal cells, relative to controls (Aoki et al., 2012). Since the hippocampus plays an important role in anxiety regulation (Huttunen and Myers, 1986, Kataoka et al., 1991, Talaenko, 1993), this increase would be expected to reduce excitability of CA1 pyramidal cells and the animal’s anxiety level. However, this rise could alternatively have exacerbated the stress-induced anxiety through desensitization of these receptors in CA1 by allopregnanolone, since allopregnanolone occurs naturally at puberty onset (Shen et al., 2007, Shen et al., 2010), thereby promoting excessive exercise. This study aimed to determine whether the rise of α4βδ−GABAARs is causal to the animal’s hyperactivity and to also explore the possibility that α4βδ−GABAARs increased as a result of hyperactivity. In order to understand the relationship between hyperactivity and α4 subunit expression, we analyzed the relationship between α4βδ−GABAAR expression and wheel activity and also examined brains of animals at an earlier time point of ABA induction, when only half of the animals have begun to exhibit the food restriction-evoked hyperactivity. Results indicate that heightened levels of α4βδ−GABAARs are not evoked by hyperactivity but instead, found within brains of animals with the minimal levels of food restriction-evoked hyperactivity. This relationship is consistent with the interpretation that α4βδ−GABAAR expression is a biomarker for a mechanism conferring protection against food restriction-evoked excessive exercise and weight loss.
2. MATERIALS AND METHODS
2.1 Materials
The goat antibody directed against the α4 subunit of GABAA receptors (GABAARs) was purchased from Santa Cruz Biotechnology (Dallas Texas, catalog #SC-7355). This antibody recognizes a single 67kD band by Western blotting (Sanna et al., 2003, Griffiths and Lovick, 2005a, Griffiths and Lovick, 2005b, Shen et al., 2007). Preadsorption of the antibody with a synthetic peptide corresponding to the antigenic site (catalog #SC-7355p from Santa Cruz) greatly reduces immunoreactivity, as was confirmed previously by light and electron microscopy (Aoki et al., 2012, Sabaliauskas et al., 2012) and by Western blotting (Sanna et al., 2003). Application of the antibody onto the hippocampus of female mice with genetic deletion of the α4 subunit also yields much lower levels of immunolabeling, compared to the hippocampus of wildtype age-matched female mice (Sabaliauskas et al., 2012). Genetic deletion of the α4 subunits reduces the membranous expression of δ subunits at the CA1 spines, confirming that α4 and δ subunits are partners in native GABAA receptors at CA1 spines and that the immunocytochemical detections of α4 (and δ subunits) in the CA1 pyramidal cells reflect the presence of α4βδ-GABAARs (Sabaliauskas et al., 2012). Specificity of both the membranous and cytoplasmic pools of α4-labeling was ascertained through the exhaustive controls described above (Aoki et al., 2012, Sabaliauskas et al., 2012).
The secondary antibody was rabbit anti-goat IgG, conjugated to 0.8 nm colloidal gold (catalog #25220 from Electron Microscopic Sciences, Hatfield, PA). The silver-intensification kit used to enhance 0.8 nm colloidal gold particles was purchased from KPL (Kirkegaard & Perry Laboratories, Inc., Gaithersburg Maryland).
Epoxy resin, grids, fixatives and most electron microscopic supplies were purchased from Electron Microscopic Sciences, while chemicals, such as bovine serum albumin, buffers and salts were purchased from Sigma Chem (St. Louis, MO).
2.2. ABA induction and behavioral controls
This study describes data obtained from two sets of data: those data obtained from animals that underwent four days of ABA induction and euthanized on P44 and another set of data from animals that underwent two days of ABA induction and were euthanized on P42. The electron microscopic immunocytochemical data from the P44 set of tissue were described earlier (Aoki et al., 2012) but were re-analyzed in this study, for further analysis of the relationship to individual animal’s wheel running activity. The ABA induction and methods for electron microscopic data collection from brains of animals euthanized on P42 were never presented previously and therefore, are described in detail here.
ABA induction of the P42 group of animals was as described earlier for the P44 group of animals (Aoki et al., 2012), except that the animals were euthanized two days earlier. Sprague-Dawley female rats were purchased as a group of 8 to 12 from Taconic Farms and delivered to the New York State Psychiatric Institute’s animal facility on postnatal day 21 (P21). Upon arrival, animals were individually housed in a reverse 12 hour dark: 12 hour light cycle in the absence of males. From P36 through P42, body weight, food intake, and wheel activity (where applicable) were measured daily within 20 minutes prior to the dark cycle. On P36, each group of 8 to 12 rats that were delivered together were divided into 4 treatment groups: 1) Control (CON, N=8): 24 hour per day food access with no wheel access; 2) Exercise (EX, N=7): 24 hour per day food and wheel access; 3) Food-restricted (FR, N=8): 1 hour per day food access with no wheel access; 4) Activity-based anorexia (ABA, N=8): 1 hour per day food access and 24 hour per day wheel access. Animals with access to a running wheel (EX and ABA) were housed in a standard home cage with an activity wheel attached (Med Associates, Inc., St. Albans, VT). Baseline wheel activity with 24 hour per day access to food was recorded for the EX and ABA groups on P37-P39; activity was quantified based on the number of wheel rotations per day, which was converted to km of running per day. On P40, restricted food access began for FR and ABA groups, with animals receiving unlimited access to food for 1 hour per day at the onset of the dark cycle. On P42, animals of all four groups were anesthetized, and then euthanized by transcardial perfusion with fixatives, to collect brain tissue. All procedures were in accordance with the protocols approved by the Institutional Animal Care and Use Committees of the New York State Psychiatric Institute, Columbia University and New York University and adhered to the NIH Guide for the Care and Use of Laboratory Animals (8th Edition, 2011). The animals’ estrous cycles were not monitored, because the cycles become disrupted by food restriction (Dixon et al., 2003) and are not yet fully developed at this age (Hodes and Shors, 2005).
2.3. Brain tissue preparation
Brain tissue was fixed by transcardial perfusion with fixatives within 2 to 4 h prior to the light-to-dark transition. Prior to perfusion, all animals were deeply anesthetized using urethane (34%; 0.65 – 0.85 cc/185 g body weight, i.p.). Animals were transcardially perfused with 50 ml of saline containing heparin (4000 U/liter) at a flow rate of 50 ml/min. This was followed, without interruption, by perfusion with 500 ml of 4% paraformaldehyde, buffered with 0.1M phosphate buffer (pH 7.4), at a flow rate of 50 ml/min over a 9 min period.
Within 30 min following transcardial perfusion, the entire brain was extracted from the skull. The left hemisphere was post-fixed for up to 9 hours in the same fixative solution used for perfusion. Coronal brain sections containing the hippocampal formation were prepared using a vibratome, set to a thickness of 40 to 60 μm. These sections were stored at 4°C, suspended in a buffer solution consisting of saline (0.9% w/v NaCl), buffered with 0.01M phosphate buffer (pH 7.4) and containing 0.05% w/v sodium azide to prevent bacterial growth (PBS-azide).
2.4. Immunocytochemistry
Immunocytochemistry was performed to detect α4 subunits of GABAARs within the hippocampus of the rats, as was described previously (Aoki et al., 2012, Wable et al., in press). The vibratome sections from all animals were processed strictly in parallel so as to ensure that the conditions for tissue processing were equalized with respect to exposure time to immunoreagents, ambient temperature, concentrations of the reagents, and chemical quality. The entire ICC procedure was repeated three times to verify that the immunolabeling pattern remained consistent across experimental runs.
The anti-α4 subunit antibody solution was diluted to 2 ng/ml (1:100), using PBS-Azide with 1% bovine serum albumin (PBS-BSA-Azide) as the diluent buffer. Incubation of sections in the primary antibody solution was for 3 days at room temperature under constant agitation. The secondary antibody used to detect binding of the primary antibody was donkey anti-goat IgG, conjugated to 0.8 nm colloidal gold, diluted to be 1:100. Incubation in this secondary antibody was overnight at room temperature and under constant agitation. Tissue was post-fixed for 10 min at room temperature, using 2% glutaraldehyde in PBS (pH 7.4) as the fixative. In order to prepare these sections for silver-intensification, which enlarges the 0.8 nm colloidal gold particles to sizes large enough to be detectable under the electron microscope, sections were immersed for 2 min in citrate buffer (pH 7.4, 0.1 M), then immersed in the silver-intensification solution for 12 min at room temperature. The silver-intensification step was terminated by rinsing for 1 min in the citrate buffer, then in PBS. These sections were processed through the osmium-free steps of fixation so as to avoid loss of silver-intensified gold particles (SIGs) by oxidation. The osmium-free steps involved exposure to tannic acid, iridium tetrabromide, and uranyl acetate, as was described previously (Shen et al., 2010). Ultrathin sections were prepared from vibratome section surfaces and oriented tangentially to the vibratome section surface so as to maximize capture of surface-most portions of vibratome sections, where exposure to the immunoreagents would be maximal.
2.5. Electron microscopic quantification
All parts of the quantitative analysis, including image acquisition, were conducted with the experimenter blind to the animals’ environmental treatments.
Dendritic spines of the CA1 hippocampal pyramidal cells were subjected to quantitative electron microscopic analyses. Dendritic spine profiles within ultrathin sections spanning stratum radiatum of the CA1 field of the dorsal hippocampus were identified as oval profiles, approximately 0.5 to 1 μm in diameter at the greatest diameter, free of microtubules, vesicles or mitochondria, with a characteristic narrowing of the profile to less than 0.25 μm (the spine neck), and with a thick postsynaptic density (PSD) along the portion of the plasma membrane that is apposed to an axon terminal containing numerous vesicles. So as to maximize immunodetection, care was taken to sample portions of the vibratome section that were within 1 μm deep from the surface, where exposure to the immunoreagents would be maximal. Vibratome section surface, as opposed to cracks in tissue created during the steps subsequent to incubation with antibodies, was identified by the characteristic serrated surface formed by the vibratome blade as it cuts through brain tissue. We evaluated all morphologically identifiable spinous profiles strictly in the order that they were encountered along the surface of vibratome sections so as to avoid bias in the sampling procedure. Analysis was terminated at the point of encountering the 200th spine profile.
Although most labeled spines contained only one SIG particle, we sought to capture any potential change in the level of α4-immunoreactivity that could be evoked by the environmental condition. To this end, two counting procedures were performed. One was to measure the number of SIG particles associated with spines, so as to assess differences in levels of immunoreactivity. The number of SIG particles associated with spines was assessed for a group of 10 spines, analyzed strictly in the order of encounter. This assessment was repeated 20 times per animal (i.e., total of 200 spines per animal), to obtain a mean value of SIG occurring per 10 spines. The other counting procedure was to assess the proportion of dendritic spine profiles immunolabeled for the α4 subunit, using spines (rather than SIG particles) as units of counting and disregarding the level of immunoreactivity per spine. In other words, any single spine profile was categorized as labeled, so long as it contained 1 or more SIG particles. For the assessment of the proportion of spines labeled, we counted the number of spines labeled for every group of 10 spines, strictly in the order that we encountered them. This assessment of the proportion of spine profiles labeled was repeated 20 times per animal, to obtain a mean value of 20 assessments, representing the analysis of 200 spine profiles per animal.
Occasionally, a single spine profile from the ABA tissue contained more than 1 SIG particle (Fig. 1C and D). To assess the level of immunoreactivity at spines, the number of SIG particles per group of 10 spine profiles was determined. This procedure was also repeated 20 times, to obtain a mean value representing the analysis of 200 spine profiles.
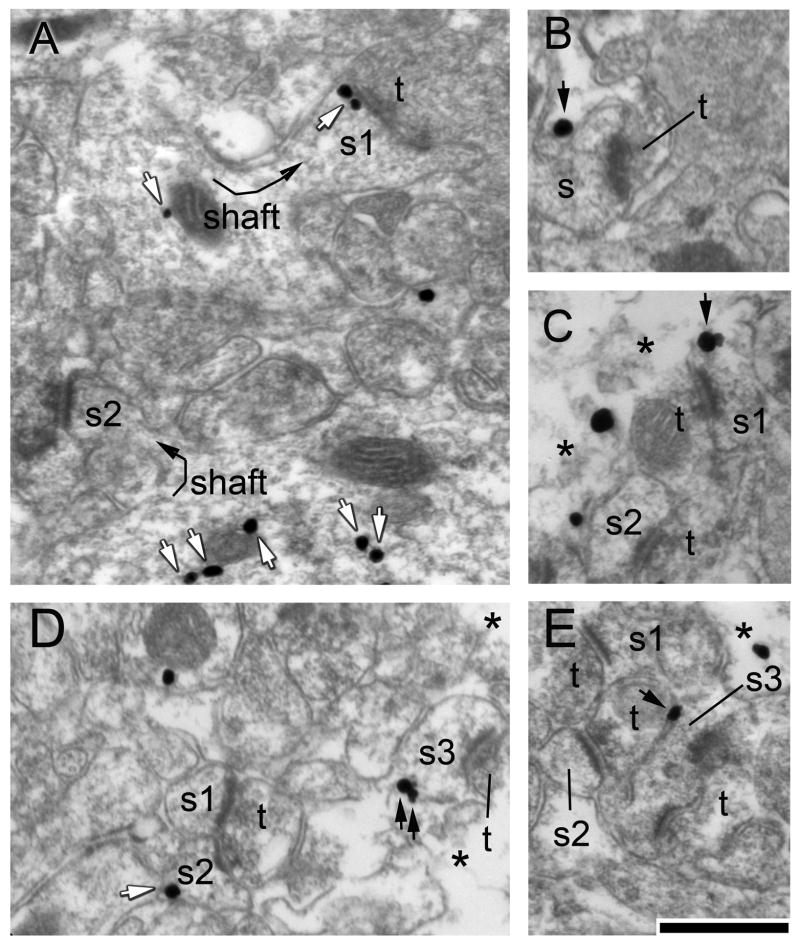
Figure 1. GABAAR α4 subunits in the hippocampal CA1 at P42 occur along the extracellular surface of the plasma membrane and intracellularly.
Panel A. Spine s1 protruding from a dendritic shaft (curved arrow) exhibits a thick postsynaptic density (PSD), indicating that it is postsynaptic to a terminal (t) forming an excitatory synapse. This spine exhibits two silver-intensified gold particles (SIG) along the cytoplasmic surface of the PSD and one near a mitochondrion within the parent dendritic shaft (sh). Another dendritic shaft in the near vicinity contains five SIG particles but no SIG particle within the spine (s2) emanating from the shaft (curved arrow). White arrows in this and other panels highlight SIG particles that occur intracellularly. Panel B. The spine that is postsynaptic to the terminal exhibits an SIG particle along the extracellular surface of the plasma membrane (black arrow). Panel C. SIG immunolabeling was analyzed along the extreme surface of the vibratome section, as is indicated by the broken membranes and disappearance of the neuropil at the transition from the section surface to the pure EPON zone (asterisks here and in other panels). Spine s1 exhibits a single cluster of SIG particles that lies directly over the plasma membrane, while spine s2 exhibits no SIG that is within 10 nm from the extracellular surface. The asterisk in this and other panels depict the transition from tissue to vibratome section surface. Panel D. Two spines, s1 and s2, are postsynaptic to a terminal, of which s2 exhibits an SIG particle along the intracellular surface of the plasma membrane. A third spine, s3, exhibits two SIG particles along its plasma membrane. Panel E. Of the three spines, s3 is the only one that exhibits an SIG particle that resides directly over the plasma membrane. The calibration bar in panel E indicates 500 nm and applies to all panels.
Receptor subunits occurring at sites removed from the plasma membrane are not functional, but they can represent the reserve pool that is recruited rapidly to the plasma membrane. Therefore, a subset of immunolabeled spine profiles that exhibit membranous labeling and specifically along the extracellular surface (as the epitope recognized by the antibody was within the extracellular domain (Sabaliauskas et al., 2012)) were sub-categorized as ‘Spines Labeled Membranously’ among the total number of spines that were labeled either at the plasma membrane or in the cytoplasm (Fig. 1).
Similarly, the SIG particles occurring specifically at the plasma membrane were counted in a subcategory distinct from those occurring cytoplasmically or along the intracellular surface of the plasma membrane. SIG particles were categorized as intracellular when they were displaced from the extracellular surface of the plasma membrane by greater than 20 nm (equal to the thickness of a unit membrane) at a direct magnification of 40,000x. Examples of SIG particles categorized to be occurring intracellularly are shown in Fig. 1A and D (white arrows).
In summary, six types of quantifications were performed for tissue from each animal, generating the following mean values: the proportion of spine profiles labeled at the extracellular surface of the plasma membrane (‘membranous spine labeling’); the proportion of spine profiles labeled intracellularly (‘intracellular spine labeling’); the proportion of spine profiles labeled anywhere (‘total spine labeling’ = membranous or intracellular); the number of SIG particles localized to the extracellular surface of the plasma membrane of spinous profiles (‘membranous SIG labeling’); the number of SIG particles occurring intracellularly (‘intracellular SIG labeling’); and the number of SIG particles occurring either at the plasma membrane or intracellularly of spinous profiles (‘total SIG labeling’). The mean values of these six types of spine labeling were obtained for each animal.
2.6. Quantification of ABA-induced physiological changes
ABA induces body weight loss as well as hyperactivity (Aoki et al., 2012). ABA vulnerability was measured using the following measurements of body weight changes: (i) Grams of body weight loss during the days of food restriction. For the animals that were euthanized on P42, this value corresponded to body weight loss from P40 to P42, corresponding to experimental days 4 and 5, relative to baseline at the beginning of experimental day 3 (P40), where baseline equaled the body weight measured after three full days of access to the wheel; and (ii) Percent of body weight loss, relative to baseline. For animals that were euthanized on P44, this value corresponded to body weight loss in grams and percent, from P40 to P44, corresponding to experimental days 4, 5, 6 and 7, relative to baseline at the beginning of experimental day 3 (P40).
In order to assess individual differences in wheel activity among the ABA group of animals, the following three measurements were made: (i) Wheel activity during the 2 days preceding FR (“Pre FR”, corresponding to experimental days 2 and 3 and ages P38 to P40). (ii) Wheel activity during the 2 days of food restriction (“Post FR”), which, for the P42 group, corresponded to experimental days 4 and 5 and which, for the P44 group, corresponded to experimental days 4, 5, 6 and 7. For the P44 group, we also analyzed wheel activity during the first 2 days of food restriction (experimental days 4 and 5, corresponding to ages P40 to P42) together and separately from the last 2 days of food restriction (experimental days 6 and 7, corresponding to ages P42 to P44). (iii) Food restriction-induced increase in wheel activity (“Post Minus Pre FR”); (iv) Total wheel activity (“Pre plus Post FR”). Wheel activity was measured in units of km.
2.7. Statistical analyses
In order to test the hypothesis that α4 expression within spines contributes to a mechanism that suppresses food restriction-evoked hyperactivity or body weight loss, regression analyses were run, using the software Statistica 64 to identify predictors of variables. Multiple regression analysis was performed to determine the relative contributions made by multiple predictors for a variable. An unpaired t-test was used to determine the significance of difference of mean values of two independent groups. ANOVA, followed by Fisher’s post hoc analysis, was used to determine the significance of the differences among the means of three groups. Tukey’s post hoc test was run when comparing the significance of the difference of the mean values among four or more groups. For all tests, p<0.05 was defined as significant. All of these tests were preceded by a test for normality, using the Kolmogorov-Smirnov and Lilliefors test for normality and the Shapiro-Wilk’s W test. All variables were found to be normally distributed. 3.
3. RESULTS
3.1. Ultrastructural localization of GABAAR α4 subunits within stratum radiatum of the hippocampal CA1 at P42 reveals specific labeling at dendritic spines receiving excitatory synapses
The hippocampal CA1 of P42 animals were analyzed for the ultrastructural localization of GABAAR α4-immunoreactivity, as was conducted earlier for the P44 brain tissue (Aoki et al., 2012). Silver-intensified gold particles (SIG) reflecting immunoreactivity to the α4 subunit of GABAARs were readily detectable as irregularly shaped electron-dense clusters exceeding 20 nm in diameter. Control experiments (See Methods section 2.1) revealed that SIG particles occurring at both the cytoplasmic and the plasma membrane domains reflect specific labeling.
Within stratum radiatum of the CA1, these SIG particles occurred abundantly in the cytoplasm of dendritic shafts. It has been established that native GABAARs containing α4 subunits consist of two α4’s in a pentamer with one δ subunit, instead of a γ subunit (Araujo et al., 1998). The γ subunit is required for interaction with the GABAAR anchoring protein, gephyrin (Huang and Scheiffele, 2008, Tretter et al., 2008). Accordingly, immunoreactivity to α4 subunits of GABAARs in the stratum radiatum occurred at membranous sites removed from symmetric, presumably inhibitory synapses (Nusser et al., 1998, Wei et al., 2003). One consequence of the non-synaptic anchoring of α4-containing GABAARs is that these receptors can become localized to dendritic spines, which are target sites for excitatory synaptic inputs (Shen et al., 2007, Aoki et al., 2012). As was observed in these previous studies, immunoreactivity for the α4 subunit within the CA1 stratum radiatum of this cohort of 8 ABA and 7 CON rats occurred along the extracellular surface of the plasma membrane of dendritic spines, adjacent to portions exhibiting the postsynaptic densities (PSDs) where glutamate receptors are anchored (Racz and Weinberg, 2013) (Fig 1). This spatial relationship to excitatory synapses enables shunting inhibition through activation of α4-containing GABAARs by ambient GABA that diffuses beyond inhibitory synapses (Shen et al., 2010). In order to determine the prevalence of α4-containing GABAARs at sites enabling shunting inhibition of excitatory synaptic inputs, the ultrastructural analysis focused on quantifying the prevalence of GABAAR α4-immunoreactivity at dendritic spines.
3.2. Correlation between α4-immunoreactivity at P44 and hyperactivity evoked by food restriction
We previously reported that the ABA animals, as a group, exhibited > 500% greater α4-immunoreactivity in CA1 spines, relative to the values in the CA1 spines of CONs, while food restriction alone and exercise alone evoked modest increases that did not reach statistical significance (Aoki et al., 2012).
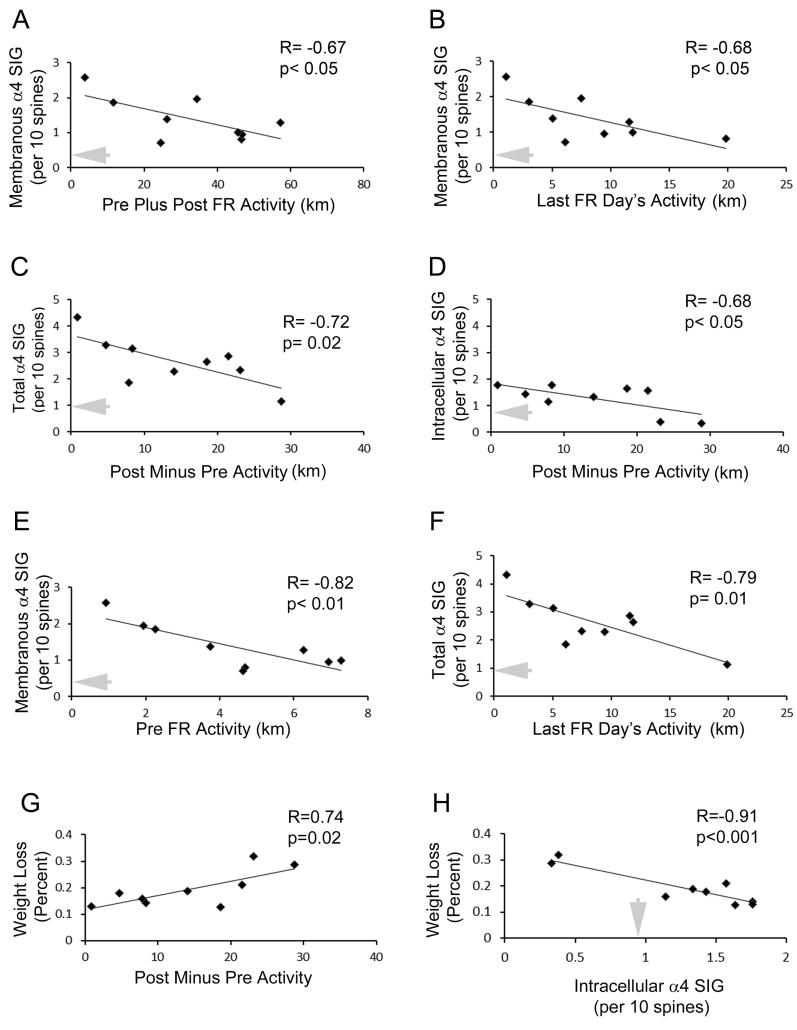
Closer examination of the 9 ABA animals’ food restriction-evoked increases in activity during the last 2 days of the 4-day food restriction period revealed strong correlation with total (i.e., membranous and intracellular) α4-immunoreactivity in spines (R= - 0.72, p=0.02) (Fig. 2C). This correlation was negative, meaning that animals exhibiting the greatest increase in wheel activity were the ones that exhibited the lowest levels of α4-immunoreactivity. The correlation between wheel running and α4 immunoreactivity increased during the food-restriction period, since an even stronger correlation was found between the extent of activity on the last day and total spine labeling (membranous plus cytoplasmic), using the number of SIG particles (R= - 0.79, p=0.01; Fig. 2F) as well as proportion of spines labeled (R= - 0.73, p=0.02) as the units for counting. This strengthened correlation of α4 immunoreactivity to the last day of activity may have reflected a change in the running phenotype observed for 3 animals out the 9 ABA animals: these 3 animals had been exhibiting extreme hyperactivity up to P42 (i.e., during the first 2 days of food restriction), but reduced activity to moderate levels during the last 2 days of food restriction (P42 to P44). These 3 animals exhibited intermediate levels of α4-immunoreactivity on P44, like those of animals that had exhibited only moderate levels of running throughout the entire food-restriction period (the green intermediate group in Fig. 5, for which two activity profiles are shown in the right panel). This pattern suggests that some animals were able to attain resiliency to ABA-induction (suppression of extreme hyperactivity and thereby less body weight loss) through up-regulation of α4βδ−GABAAR. Conversely, those animals that could not respond to the ABA-inducing environment with an up-regulation of α4βδ-GABAAR were the most vulnerable to ABA-induction, as assessed by their extreme wheel hyperactivity and severe body weight loss. The latter two variables – wheel running and weight loss - were significantly correlated, as might be expected (R= 0.74, p=0.022) (Fig. 2G).
Figure 2. α4-immunoreactivity in the hippocampus of ABA animals at P44 correlates negatively with wheel activity.
Panel A. Membranous SIG correlates with the extent of rats’ activity during the entire experimental period, spanning from the days before and through food restriction. The correlation is negative - those animals that exhibit the greatest elevation in α4 immunoreactivity run the least. Panel B. Membranous SIG also correlates with the last day of food restriction. Panel C. Total SIG level (membranous or intracellular) correlates negatively with the hyperactivity evoked by food restriction during the latter two days of the food restriction. Panel D. The intracellular portion of the SIG correlates with the food restriction-evoked hyperactivity during the last two out of the four days of food restriction. Panel E. The membranous portion of SIG counts correlates with wheel activity before food restriction was imposed. Panel F. Total SIG level correlates negatively and most strongly with hyperactivity during the last day of food restriction. Panel G. Weight loss correlates strongly and positively with the increase in wheel running activity that is evoked by food restriction. Panel H. Weight loss also correlates strongly but negatively with the intracellular level of α4 expression. Those individuals that fail to up-regulate α4 levels intracellularly lose the most amount of body weight. In all panels, the grey arrow indicates the average level of α4 measured within or on the plasma membrane of CA1 dendritic spines of the P44 CON group. The y-axes of the three graphs in panels C, D and F are drawn to the same scale so as to facilitate comparison of values.
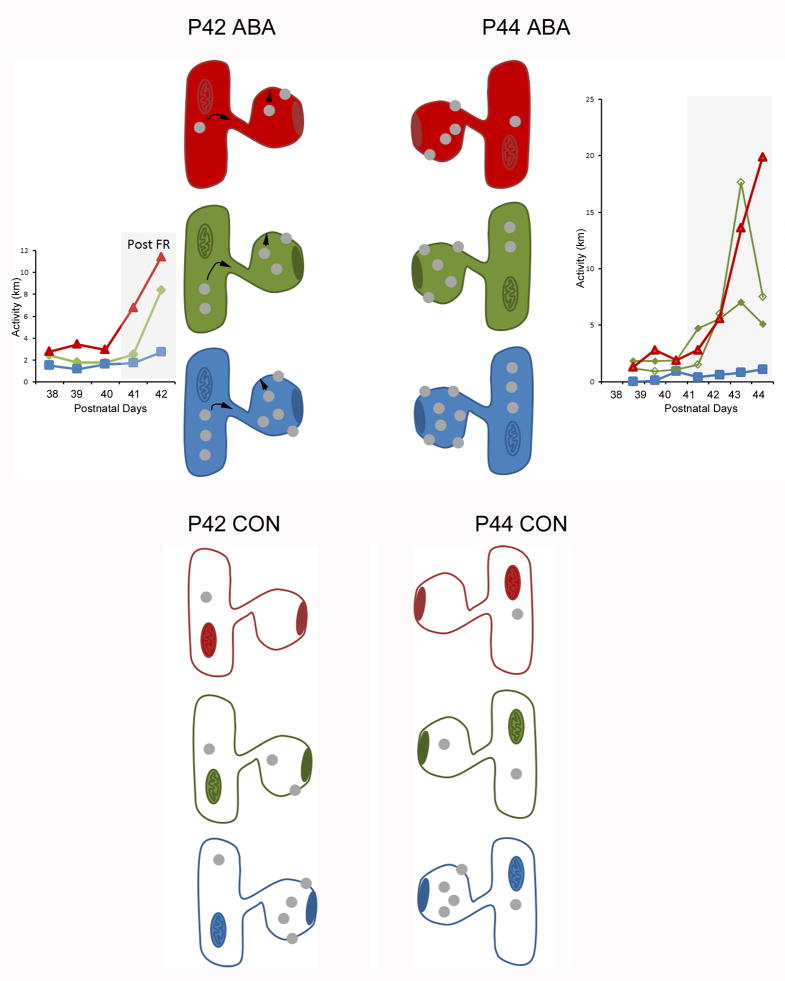
Figure 5. Summary of results.
This schematic summarizes the α4-immunoreactivity levels observed at the plasma membrane and intracellularly within dendritic spines of the CA1 pyramidal cells across two stages of ABA induction, P42 and P44. The spine head shows a PSD, where excitatory axons target. The shafts to which the spines are connected are depicted by the presence of a mitochondrion.
The pattern of immunoreactivity correlated negatively with the animal’s activity on the wheel. The spines filled with red, green and blue colors depict the α4-immunoreactivity observed within brains of animals that exhibited the greatest (red lines in graphs), intermediate (green lines in graphs) and lowest (blue lines in graphs) activity on the wheel, respectively, following ABA induction. Each grey dot represents the level roughly equal to 0.5 SIG particle per 10 spines. The activities (in km per day) are actual values recorded from 6 representative animals. ABA induced de novo synthesis of α4 subunits but the increased expression among the P42 group was not yet statistically significantly different from those of CON animals (depicted with a colorless cytoplasm) that never received food restriction or the wheel access. α4-immunoreactivity within spines of the P42 dendrites is in flux, as is indicated by the black arrows. By P44, following four days of food restriction, there was a significant increase in the level of α4, relative to age-matched controls. Based on the consistent correlation between activity and α4-immunoreactivity, it is likely that those individuals that exhibited the least activity (the Blue group) already expressed higher levels of α4 before ABA induction and were able to respond to food restriction with the greatest increase in α4 expression. The response in α4-immunoreactivity was more rapid for the intracellular domain than for the membrane domain. This spatio-temporal pattern is depicted by individual differences within the cytoplasmic domain at P42, followed by an increase in the membranous domain by P44.
The α4 labeling specifically at the membrane contributed to the negative correlation between wheel running activity and α4 expression, since the membranous SIG labeling correlated with the total distance that animals ran during the 7 days, starting from the pre-food restriction wheel-acclimation through four days of food restriction, up to P44 (R= - 0.67, p<0.05; Fig. 2A). Membranous SIG labeling also correlated with the distance run during the last (i.e., fourth, P44) day of food restriction (R=0.68, p<0.05) (Fig. 2B).
3.3. Correlation between α4-immunoreactivity at P44 and activity preceding food restriction
Having observed that SIG labeling correlated with the total distance run, starting from the pre-food restriction period to the end of the four days of food restriction (Fig. 2A), we investigated the contribution made by the pre-food restriction period to the SIG labeling. Correlation analyses indicated that individual differences in running were already present prior to food restriction and that the two days preceding food restriction actually exhibited the strongest correlation with membranous labeling at P44, measured five days later (R= - 0.82, p<0.01 for membranous SIG counts, Fig. 2E; R= - 0.81 and p=0.008 for the proportion of spines labeled at the plasma membrane). A positive correlation was found between the activity preceding food restriction with the average activity during the four days of food restriction (R= 0.77, p=0.015) and also weakly with the activity on the last day of food restriction (R=0.61, p=0.08).
Multiple regression analyses were run to quantify the contribution of the various measures of activity to predicting membranous SIG. When preFR activity and activity on the last day were used as the regressors, together they contributed 71.5 % of the variance (predictability) in the membranous SIG counts. While preFR activity alone contributed 66.1 % (semi-partial correlation sR = -0.69, change in F=13.6, p=0.008) of the 71.5% variance of membranous SIG, the activity on the last day contributed an additional 5.4 % (sR = -0.399, change in F=1.13, p=0.328). When preFR activity and an average of the four post-FR activity were used as the regressors, preFR activity contributed 66.1 % of the variance of membranous SIGs (sR= - 0.66, change in F=13.6, p=0.008), while postFR activity contributed no additional prediction (sR= - 0.005, change in F=0, p=0.99). An interpretation consistent with these observations is that a factor regulating activity during the pre-food restriction period regulates activity during the post-food restriction period as well as the membranous α4 expression. The activity on the last day of food restriction performed better as a predictor for the membranous SIG counts (R= - 0.67, p<0.05) than the average activity over all four days of food restriction (R=-0.63, p=0.07) or the running during the first two days of food restriction (R=-.54, p=0.14), suggesting that it takes about about four days for the pre-FR regulating factor to affect activity during the food restriction period. In order to test this possibility, we analyzed the behavior and brains of animals that underwent only two days of food restriction, from P40 to P42.
3.4. Weight loss among the ABA group compared to the FR group at P42
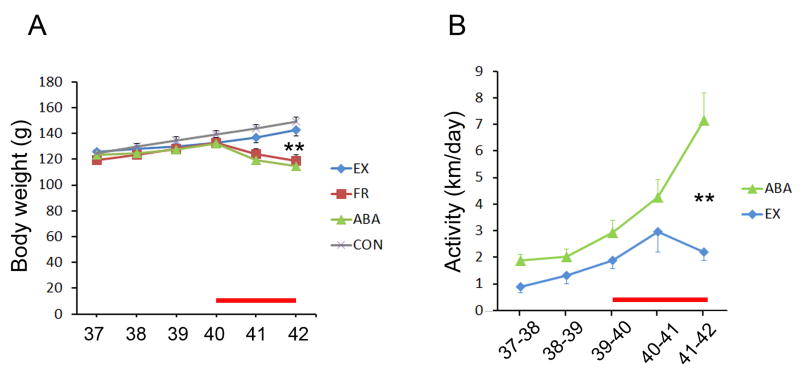
As expected, all 8 rats of the ABA group and all 8 rats of the FR group exhibited weight loss during the 2 days of food restriction, relative to the baseline body weight just prior to the start of food restriction (Fig. 3A). The extent of weight loss among the ABA group varied greatly among individuals, ranging from 8.8% to 18.0% (12.4 g to 23.0 g). In comparison, weight loss among FR animals that underwent food restriction without access to the wheel was less variable (6.6% to 11.5%) and the EX group that received access to the wheel without food restriction showed no weight loss. Comparison of the mean weight of all four groups at P42 revealed a highly significant difference of the ABA group, relative to the CON or the EX groups (MS=91.027, df=27, p=0.001 for both comparisons, by Tukey’s post hoc analysis), but no difference from the FR group (p=0.7). This difference in body weight loss between the ABA and CON group was already evident by P41 (MS=70.425, df=27; p<0.001) although the difference between ABA and EX groups’ mean weight had not yet attained significance at P41 (p=0.07).
Figure 3. Body weight and exercise of ABA animals, relative to age-matched controls.
Panel A. The body weight was measured daily and compared for the 8 ABA, 7 EX, 8 FR, and 8 CON animals. Panel B. The daily activity of 8 ABA and 7 EX animals was measured as the distance run on the wheel. Animals were acclimated to the wheel starting the age of postnatal day 37. The red line along the x-axis in panels A and B indicate the dates of food restriction for the ABA and FR groups. For both graphs, the values represent mean ± SEM. ** indicates p<0.001, comparing ABA to CON in panel A and ABA to EX in panel B.
3.5. Food restriction-induced hyperactivity among the ABA group compared to the EX group at P42
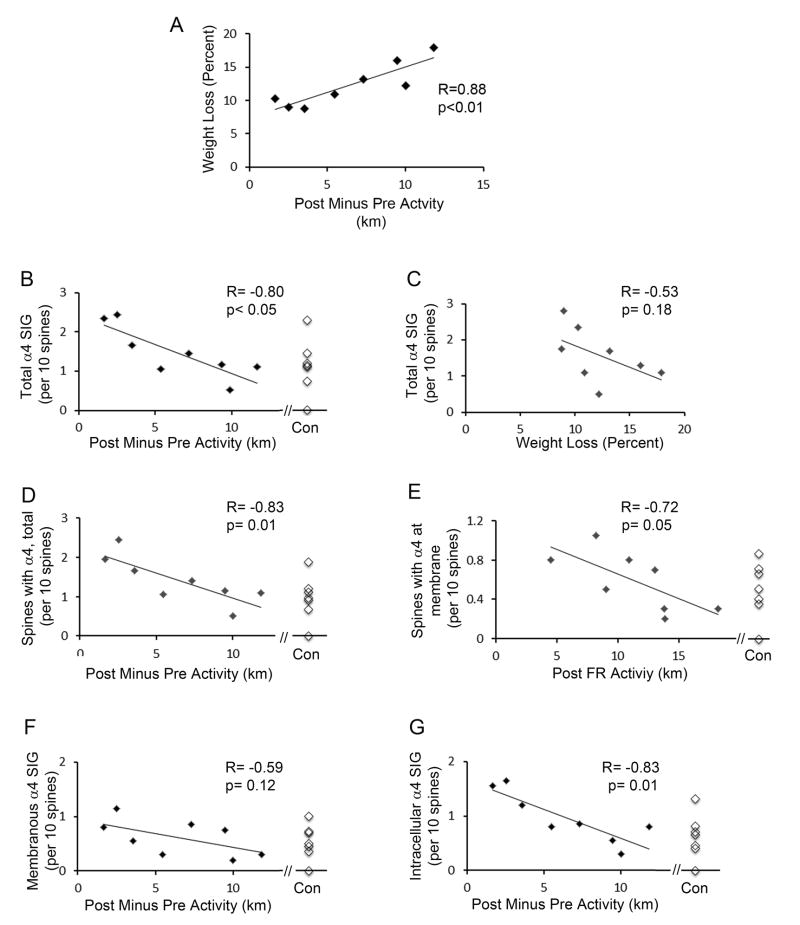
Fifteen animals (7 EX and 8 ABA) were given access to the wheel from P37 to P42. All animals increased voluntary wheel activity over the five-day period, as was observed earlier (Sherwin, 1998, Gutierrez, 2013). Each animal’s wheel activity was measured as km per day, then averaged for the two groups (Fig. 3B). This measurement revealed a statistically significant difference in activity between the two groups by P42 (p<0.001, unpaired t-test, t-value = 4.46). We compared the two groups’ activity during the two days before FR (P38 to P40), the two days during food restriction (P40 to P42), the difference between before- and after food restriction (Post Minus Pre FR), and total activity (Pre Plus Post FR). As expected, the 8 rats of the ABA group exhibited a strong food restriction-evoked change in activity (Post Minus Pre FR activity) relative to the EX group that continued to have 24 h food access (6.5 ± 1.3 km for ABA; 2.9 ± 0.8 km for the EX; p<0.05 by unpaired t-test). However, the extent of wheel activity was highly variable among the ABA group, with the distance run by 2 out of the 8 of the rats being less than the mean level of activity by the EX group, while 3 other rats ran more than 3-fold of the EX group’s mean. Regression analysis of the ABA group revealed that the extent of body weight loss correlated strongly with the increase in activity evoked by food restriction (R=0.88; p<0.005; Fig. 4A).
Figure 4. α4-immunoreactivity in the hippocampus of ABA animals at P42 is negatively correlated to wheel-activity.
The food restriction-evoked increase in wheel activity was quantified as the distance run during the 2 days after food restriction began minus the distance run during the 2 days preceding food restriction. All animals exercised more after food restriction. Panel A. The extent of increase in activity after food restriction correlates strongly with the body weight lost following food restriction. Each data point in Panel A and all other panels represent the values of one animal’s food restriction-evoked hyperactivity during the 2 days of food restriction, correlated with the percent of body weight lost during the same 2 days. Panel B. α4-immunoreactivity within spines was quantified by counting all silver-intensified immunogold particles (SIG) that occur along the extracellular surface of the plasma membrane and intracellularly (i.e., Total count) for every group of 10 spines encountered. Repeated measures of this SIG count per 10 spines correlates negatively with the hyperactivity that is evoked by food restriction. Each point represents data from one animal. Here and in other panels, α4 immunoreactivity of tissue from 7 CON animals are shown for comparison with the values of ABA animals. Panel C. Total SIG count correlates less strongly with the percent of weight lost during the 2 days of food restriction. Panel D. The proportion of spines exhibiting α4-immunoreactivity for every group of 10 spines encountered also correlates strongly with the hyperactivity evoked by food restriction. Panel E: The counts of SIG particles that occur specifically at the extracellular surface of spines’ plasma membranes correlates with the total level of activity during the 2 days that follows food restriction. Panel F. SIG at the spine membrane correlates only weakly with the extent of hyperactivity after food restriction. Panel G. SIG levels that reside in the cytoplasm correlate very strongly with the food restriction-evoked hyperactivity.
3.6. α4 immunoreactivity resulting from two days of food restriction at P42 in the ABA group, relative to the CON group
Having confirmed that two days of food restriction is sufficient to evoke an increase in voluntary running and greater weight loss than by food restriction alone, quantitative comparison of α4 levels in hippocampal CA1 spines was conducted across the CON and ABA groups of the P42 group. In contrast to the P44 ABA group, which exhibited a >500% greater level of α4 at the plasma membrane, relative to the values measured from the CA1 spines of age-matched CON animals (p<0.001) (Aoki et al., 2012), the P42 ABA group exhibited no significant group difference in α4-immunmoreactivity at spines, relative to the P42 CON group, using all six methods of quantification: (i) proportion of spines labeled membranously; (ii) proportion of spines labeled intracellularly or (iii) proportion of spines labeled intracellularly or membranously (i.e., total spine labeling); (iv) SIG labeled membranously; (v) SIG labeled intracellularly; and (vi) total (membranous plus intracellular) SIG labeling. For example, the α4-immunolabeling at the plasma membrane of ABA tissue was greater than that of the CON by 20% but this difference was not statistically significant (p=0.6 by unpaired t-test). Similarly, intracellular α4-immunolabeling within spines of the ABA group was greater by 41% than the levels from the CON group, but this difference also was not statistically significant (p=0.3, unpaired t-test).
3.7. Correlation between α4-immunoreactivity and ABA hyperactivity at P42
The lack of statistically significant differences between the CON and ABA group of animals at P42 is due to the large individual differences in the individuals’ responses to the ABA-inducing environment. In order to determine whether individual differences in α4-immunoreactivity of the ABA group of animals at P42 correlated to individual differences in weight loss and/or wheel activity, regression analyses were conducted within the ABA group. Of the six ways in which to quantify α4-immunoreactivity, strong correlation was observed for the total (cytoplasmic plus membranous) α4 SIG counts relative to the increase in wheel activity evoked by food restriction (Post Minus Pre FR activity) (R= - 0.80, p=0.02; Fig. 4B). Importantly, the correlation was negative, as was observed at P44, since those animals with the least activity exhibited the highest levels of α4-immunoreactivity, while those animals with the highest activity levels exhibited lowest levels of α4-immunoreactivity. The proportion of spines labeled for α4 (cytoplasmic plus membranous, i.e., ‘Total’) also correlated strongly and negatively with the increase in wheel activity during the food restriction period (R= - 0.83, p=0.01; Fig. 4D). In contrast, the total SIG counts within spines correlated only weakly with percent (R= - 0.53, p=0.18; Fig. 4C) or gram of body weight loss (R= - 0.47, p=.23), although still negatively. This weaker correlation of α4-immunoreactivity with body weight change indicated that α4-immunoreactivity is related to body weight loss indirectly, via the correlation between body weight loss and the increase in wheel activity (Fig 4A).
Of the two subcellular compartments where SIG could reside, the correlation to food restriction-evoked increase in wheel activity was much stronger for the intracellular compartment (R= -0.83, p=0.01; Fig. 4G), which was the same compartment that exhibited greater difference, relative to the CON group. In comparison, correlation to food restriction-evoked increase in wheel activity was weaker for the membranous compartment (R= - 0.59, p=0.12; Fig. 4F), the same compartment that exhibited less difference, relative to the CON group. Immunolabeling specifically at the plasma membrane correlated with wheel activity during two days of food restriction (R= - 0.72, p=0.05; Fig. 4E), but less with the increase of wheel activity that was evoked by food restriction (R=0.59, p=0.12) (Fig. 4F).
None of the six methods of α4 quantification revealed significant correlation with rats’ wheel activity prior to food restriction. The α4 quantification that revealed relatively stronger correlation was with the proportion of spines labeled at the membrane (R= - 0.41; p=0.31). The rats’ wheel activity prior to food restriction correlated the least with the intracellular SIG labeling (R= - 0.01; p=0.99).
These correlations at P42 contrasted sharply with the P44 data. Unlike the P44 data, which revealed strong correlation of membranous labeling to the extent of running during pre-FR, the P42 data revealed no or weak correlation of membranous labeling to pre-FR wheel activity (R= - 0.39, p=0.35 for the membranous SIG counts; R= - 0.41, p=0.31 for the proportion of spines with membranous labeling). In parallel, there was no correlation between running activity preceding food restriction with the activity following food restriction in the P42 group (R=0.45, p=0.26). This difference in the correlations across the two stages of food restriction suggests, again, that it takes more than two days but less than four days for the unknown factor to re-establish individual differences in the α4 expression level together with activity level.
3.8. Comparison of the Intracellular labeling at P42 and P44
The intracellular α4-labeling reflects the dynamic pool that is readily available for turnover of α4βδ−GABAAR – i.e., insertion of receptors into the plasma membrane or of their endocytosis from the plasma membrane. At P42, the level of intracellular SIG labeling of the ABA group of animals consisted of 60 ± 1% of total SIG counts, while at P44, the level of intracellular SIG labeling for the ABA group was significantly lower (37 ± 4%) (p<0.05 by unpaired t-test, comparing P42 versus P44 ABA groups). Since the overall level of α4 (intracellular plus membranous) increased five-fold from P42 to P44, the change in ratio of the intracellular versus membranous α4 from P42 to P44 is likely to reflect an increased rate of exocytosis, relative to the endocytosis, of the α4βδ−GABAAR during the latter days of food restriction.
At P42, the intracellular SIG of the ABA group contributed strongly to the negative correlation in the total SIG labeling associated with dendritic spines, relative to the food restriction-evoked increase in wheel running activity (Fig. 4G), suggesting a close co-regulation of wheel running activity and de novo synthesis of α4βδ−GABAAR. By comparison, the membranous SIG labeling at P42 of the ABA group correlated less with the food restriction-evoked increase in activity (Fig 4F), indicating a looser co-regulation of activity with exocytosis of the α4βδ−GABAAR.
By P44, the intracellular (Fig. 2D) and membranous SIG labeling (Fig. 2A and 2B) contributed similarly to the correlation of increased wheel running activity evoked by food restriction (Fig. 2C), suggesting that both the de novo synthesis and exocytosis of α4βδ−GABAAR were co-regulated with activity during the latter days of food restriction. In contrast to P42, when the intracellular counts correlated weakly with the percent of body weight lost (R= -0.54, p=0.16), at P44, these two variables correlated strongly and negatively (R= - 0.9, p<0.001) (Fig. 2H), indicating a strengthened co-regulation of de novo synthesis of α4βδ−GABAAR and activity and hence of protection from body weight loss.
4. DISCUSSION
Physical exercise has many health benefits (van Praag, 2008), including anxiolysis (Duman et al., 2008, Herring et al., 2010, Schoenfeld et al., 2013), improvement of cognition and of mood (Deslandes et al., 2009). These benefits are likely to be linked to cellular changes in the hippocampus, including angiogenesis (Van der Borght et al., 2009), neurogenesis and the release of neurotrophins (Neeper et al., 1996, Kitamura et al., 2003), but are also influenced by stressors (Stranahan et al., 2006, Hare et al., 2012, Onksen et al., 2012) that induce neurogenesis-dependent anxiety (Fuss et al., 2010, Onksen et al., 2012). For humans, dieting is one stressor that is often combined with exercise. Another human condition in which stress frequently co-exists with exercise is AN (Davis et al., 1997, Davis et al., 1999, Hebebrand et al., 2003). Our findings using an animal model of AN identified non-synaptic α4βδ−GABAARs in the hippocampal CA1 as a biomarker associated with individual differences in vulnerability to food restriction-evoked excessive exercise.
4.1. Contrasting responses at the plasma membrane versus intracellularly
Within brains of animals that responded to ABA induction with increased levels of α4-immunoreactivity, the elevation occurred both intracellularly, i.e., within the cytoplasm, and at the plasma membrane of hippocampal CA1 spines. There was no apparent depletion of the intracellular pool, suggesting that trafficking of α4βδ−GABAARs from dendritic shafts to the spine cytoplasm occurs concurrently with trafficking from spine cytoplasm to the spine plasma membrane. The intracellular receptors may still support GABAergic modulation as a readily available pool for exocytosis, precluding the requirement for de novo synthesis of proteins or mRNAs. The strong correlation observed between the intracellular α4 and food restriction-evoked hyperactivity at P42 (Table 1) suggests that the increased de novo synthesis or reduction in the proteolysis of α4βδ−GABAARs begins immediately and in direct response to food restriction. The correlation between the membranous α4 and activity emerged with a delay, from P42 to P44 (compare Fig. 4E to Fig. 2B and Tables 1A versus 1B), suggesting that trafficking of α4βδ−GABAARs from the cytoplasm to the spine plasma membrane has a slower response rate. These observations indicate that it would be worthwhile studying the membranous versus intracellular distributions of α4βδ−GABAAR in the hippocampus of ABA animals following pharmacological manipulations that directly interfere with α4βδ−GABAAR trafficking.
Table 1.
A Correlation analyses of wheel running and body weight to α4 immunoreactivity at P44, following 4 days of food restriction
| P44 Ante Mortem DATA | SIG Particles | Proportion of Spines | ||||
|---|---|---|---|---|---|---|
| Intracellular | Membranous | Total (I+M) | Intracellular | Membranous | Total (I+M) | |
| Days 2 + 3 (Pre-FR) | ○ | ** | ○ | ○ | ** | ○ |
| Days 4 + 5 (Early FR) | ○ | ○ | ○ | ○ | ○ | ○ |
| Days 6 + 7 (Late FR) | ○ | ○ | * | ○ | ○ | = |
| Day 7 (Last day of FR) | ○ | = | ** | ○ | ○ | * |
| Days 4 + 5 + 6 + 7 (All FR Days) | ○ | ○ | ○ | ○ | ○ | ○ |
| Total (Days 2 + 3 + 4 + 5 + 6 + 7) | ○ | = | ○ | ○ | ○ | ○ |
| FR-evoked increase, Early ((4+5)-(2+3)) | ○ | ○ | ○ | ○ | ○ | ○ |
| FR-evoked increase, Late ((6+7)-(2+3)) | = | ○ | * | ○ | ○ | ○ |
| Weight Lost by end of Day 7 | *** | ○ | ○ | ** | ○ | ○ |
| B Correlation analyses of wheel running and body weight to α4 immunoreactivity at P42, following 2 days of food restriction | ||||||
| P42 Ante Mortem DATA | SIG Particles | Proportion of Spines | ||||
| Intracellular | Membranous | Total (I+M) | Intracellular | Membranous | Total (I+M) | |
| Days 2 + 3 (Pre-FR) | ○ | ○ | ○ | ○ | ○ | ○ |
| Days 4 + 5 (FR) | * | ○ | * | * | = | ** |
| Total (Days 2 + 3 + 4 + 5) | ○ | ○ | ○ | ○ | ○ | ○ |
| FR-evoked increase ((4+5)-(2+3)) | ** | ○ | ** | ** | ○ | ** |
| Weight Lost by end of Day 5 | ○ | ○ | ○ | ○ | ○ | ○ |
Tables 1A and 1B summarize the results from correlation analyses. Table 1A: N=9 for the animals euthanized on P44, after 4 days of food restriction. Table 1B: N=8 for animals euthanized on P42, after 2 days of food restriction.
indicates p>0.05, R<0.70.
indicates p≤0.05, R=0.7.
indicates p<0.05, R>0.7
indicates p≤0.01, R≥0.8
indicates <0.005, R≥0.90
4.2. Individual differences in α4-immunoreactivity correlate strongly and negatively with hyperactivity
Those animals that exhibited the greatest resilience to ABA induction, as characterized by their minimal body weight loss, also exhibited minimal levels of hyperactivity in response to food restriction. These same resilient animals exhibited the highest levels of α4-immunoreactivity at spines. High level of membranous α4βδ−GABAAR expression in the hippocampus would be expected to be anxiolytic (Huttunen and Myers, 1986, Kataoka et al., 1991, Talaenko, 1993), as it allows for stronger tonic inhibition of hippocampal pyramidal cells (Shen et al., 2007) (Fig. 6). α4-immunoreactivity correlated less with body weight loss at P42 (compare Tables 1A and 1B). A scenario consistent with these observations is that α4βδ−GABAARs, which become elevated in the hippocampus of a subpopulation of individuals but with a delay of about 2 days, participate in mechanisms suppressing hyperactivity and it is this suppression of hyperactivity that prevents body weight loss to levels that approach mortality.
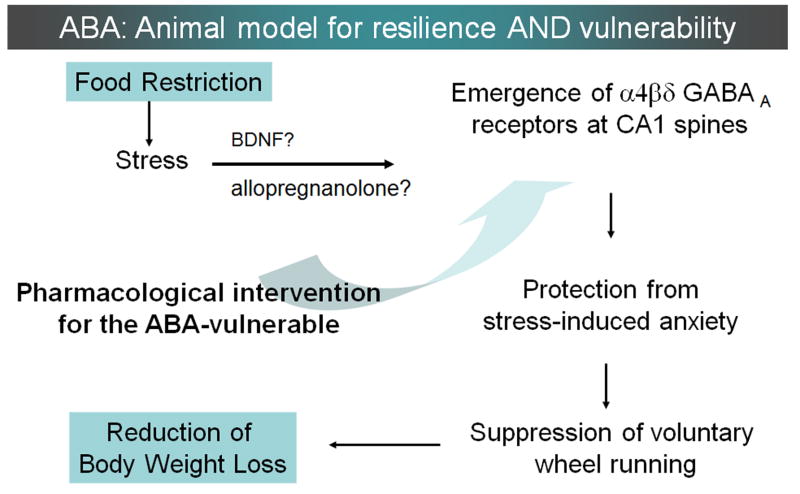
Figure 6. The hypothesized sequence of events that link up-regulation of α4βδ-GABAARs to suppression of food restriction-evoked hyperactivity.
It has long been recognized that food restriction evokes a paradoxical increase of wheel running upon rodents (Routtenberg and Kuzneof, 1967; Barbarich-Marsteller et al., 2013; Gutierrez, 2013) and multiple plausible explanations have been offered as to why food-restricted rodents run (reviewed in Gutierrez, 2013). Some of the explanations are that running is a motivated foraging behavior, manifestation of an excessively activated reward system, or is a thermoregulatory behavior. The present study supports a complementary view – namely, that wheel activity is a manifestation of heightened anxiety. This idea is supported by the following findings: (1) wheel running correlates with α4βδ-GABAARs expression at CA1 spines (data presented in this paper) and GABAergic input upon CA1 pyramidal cells (Chowdhury et al., 2013); (2) Increased expression of α4βδ-GABAARs at CA1 spines reduces excitability of CA1 pyramidal cells (Shen et al., 2007; Shen et al., 2010); and (3) reduction of hippocampal excitability is anxiolytic (Huttunen and Myers, 1986; Kataoka et al., 1991; Talaenko, 1993). This view is further supported by our preliminary observation, indicating that hyperactivity correlates positively with anxiety traits, as measured by the behavior of mice on the elevated plus maze after a day of food restriction (Gauri Wable, Jung-yun Min and Chiye Aoki, unpublished observations). This schematic also summarizes the possible mechanisms leading to the emergence of α4βδ-GABAARs at spines of the CA1 pyramidal cells. One likely possibility is that food restriction causes BDNF release (Lee et al., 2002) which, in turn, augments the synthesis of α4 and δ subunits (Roberts et al., 2006). Stress associated with food restriction may also lead to the elevation of allopregnanolone, another agent that potently increases the expression of α4 and δ subunits of GABAARs (Smith et al., 1998, Smith et al., 2007). This working hypothesis can be tested by determining whether ABA vulnerability can be reduced through experimental manipulations that up-regulate α4 and δ subunit expressions.
A strong correlation of α4-immunoreactivity to activity was already present by P42, when the ABA group’s mean level of α4 still overlapped with those of the CON group. This indicates that individual differences in α4βδ−GABAAR levels at P42 are not likely to have arisen from wheel activity but reflect individuals’ trait differences. The data further suggest that those CON animals with high levels of α4βδ−GABAARs were likely to be the individuals that would have responded to food restriction with minimal hyperactivity, while those CON animals with low levels of α4βδ−GABAARs are likely to be the ones that would have exhibited excessive food restriction-evoked activity. On the other hand, at least 3 among the 8 P44-ABA rats converted from being hyperactive to moderately active during the latter 2 of the 4 days of food restriction and exhibited α4 at levels matching the consistently moderate runners’ (Fig 5). This suggests that protection from ABA-induction could be attained within 2 days of exposure to food restriction, through α4βδ-GABAAR up-regulation (Fig. 6). The strongly negative correlation between the intracellular α4 and weight loss at P44 suggests that the increase in de novo synthesis of α4βδ−GABAAR supported the animal’s survival through a mechanism suppressing wheel running activity.
4.3. Membranous α4 at P44 correlates with ‘basal’ wheel activity but is supported by the intracellular pool
The measure of activity that significantly predicted membranous levels of α4-subnits at P44 was the degree of activity during the days that preceded food restriction, i.e., the ‘basal’ level of wheel running activity. This further supports the idea that there may have been a homeostatic set point for the membranous expression of α4βδ−GABAARs that existed before food restriction. The membranous α4 at the end of P42 did not correlate at all with activity during the days that preceded food restriction, perhaps because the homeostatic set-point became perturbed during the most dynamic phase of P40 to P42, and was re-established by P44. The P40-42 period may have been particularly dynamic, due to the addition of food restriction as a new source of stress, in addition to a pre-existing stressor, such as housing in isolation (Stranahan et al., 2006).
The strong correlation observed between ‘basal’ wheel activity and membranous α4 at P44 suggests that this homeostatic set-point regulates the animal’s propensity for hyperactivity when faced with chronic stress, a parameter that is more generalizable than just food restriction. It follows that individual differences in activity reflect individual differences in trait anxiety that exist before food restriction. We are not the first to suggest that individual differences in basal activity reflect the animal’s trait anxiety. Mouse strains with high trait anxiety exhibit stronger food restriction-evoked hyperactivity than the strains that are without notable trait anxiety (Gelegen et al., 2008). Our unpublished work also indicates a positive correlation between animals’ food restriction-evoked hyperactivity and anxiety, as measured by the elevated-plus maze after a day of food restriction (Wable and Aoki, ms in preparation).
4.4. Putative molecular cascades that lead to the rise of α4βδ−GABAARs
Unlike the α1βγ−GABAARs, trafficking of α4βδ−GABAARs to the plasma membrane is not regulated by its own ligand, GABA. Instead, trafficking of α4βδ−GABAARs to the plasma membrane is regulated by network activity (Joshi and Kapur, 2009). BDNF is one molecule released in response to neural activity (Lu, 2003, Tan et al., 2008, Xu et al., 2010) that also regulates the synthesis (Roberts et al., 2006) and trafficking of α4βδ−GABAARs from the cytoplasm to the plasma membrane (Joshi and Kapur, 2009). BDNF is released from excitatory neurons but not from GABAergic interneurons (Gorba and Wahle, 1999). The elevation of α4βδ−GABAARs occurred near PSDs of excitatory synapses on spines, where the BDNF receptor, trkB resides (Aoki et al., 2000, Spencer-Segal et al., 2011). This spinous location, rather than at GABAergic synapses on dendritic shafts and cell bodies, is optimal for the BDNF-dependent trafficking of α4βδ−GABAARs to the spine plasma membrane.
Exercise evokes the release of BDNF and other neurotrophins (Neeper et al., 1996, Gomez-Pinilla et al., 2002, Stranahan et al., 2009). This is why we originally expected animals with the strongest hyperactivity to be the ones with the highest levels of α4. Contrary to this expectation, the correlation between activity and α4 was negative. BDNF is also released in response to the stress caused by food restriction (Lee et al., 2002) and other stressors (Bath et al., 2012, Bath et al., 2013). The extent of stress experienced by individuals is likely to have been variable, since body weight loss varied widely. Indeed, body weight loss did correlate extremely strongly with the intracellular α4βδ-GABAAR expression by P44. BDNF release could still be a permissive factor influencing α4βδ-GABAAR levels (Fig. 6), but the quantity of BDNF released is not likely to be determined by the degree of wheel running activity or body weight loss. The quantity of BDNF released could be the basis for individual differences in ABA-vulnerability and trait (i.e., not food restriction-evoked) anxiety. In support of the putative role by BDNF in both the food restriction-evoked activity and trait anxiety, it was shown that C57BL6J mice, which were characterized as ABA-resilient, also exhibited increased BDNF-mRNA in the hippocampus following food restriction, while the A/J strains, known for their high trait anxiety, did not exhibit up-regulation of BDNF-mRNA in the hippocampus and became more hyperactive following food restriction (Gelegen et al., 2008).
Withdrawal from allopregnanolone or from its precursor, progesterone, such as by ovariectomy, also induces up-regulation of α4 (Smith et al., 1998). Since starvation depletes the animal’s body of progesterone (Riddle et al., 2013), this could be an alternative or additional molecule that stimulated up-regulation of α4βδ-GABAARs within the CA1 of ABA-induced adolescent females (Fig. 6). Although the rise of α4βδ−GABAAR specifically in the hippocampal CA1 of females can increase stress-induced anxiety, this requires the acute release of allopregnanolone (Shen et al., 2007). The rise of α4βδ−GABAARs is likely to have been more purely anxiolytic among food-restricted adolescent female ABA rats, due to starvation-induced depletion of progesterone and allopregnanolone.
4.5. Clinical implications
Benzodiazepines are not as effective in the treatment of AN as one might predict, based on AN’s strong co-morbidity with anxiety disorders (Kaye et al., 2009). This may be because α4βδ-GABAAR need to be targeted but escape modulation by benzodiazepines, as conferred by the GABAARs’ subunit composition (Seeburg et al., 1990). If so, drugs designed to boost α4βδ−GABAAR activity may be more effective in suppressing the excessive exercise that hinders recovery from AN (Fig. 6). Although exercise has many health benefits, the prescription of exercise may need to be considered carefully, in combination with an individual’s stress level, developmental stage and body weight.
The highlights of our study are as follows.
Food restriction (FR) upon adolescent female rats evokes robust wheel running.
Individual differences in activity exist pre-FR and correlate with post-FR activity.
α4-GABAAR levels at hippocampal spines are increased by FR.
α4 levels in CA1 spines are higher in animals with minimal pre- & post-FR activity.
α4 in CA1 spines are strongly correlated to weight loss (R=-0.9).
Acknowledgments
We are grateful to Barry Cohen for helpful discussions on statistical analyses. We thank Anna Rita Colacino Ficarrotta, Kei Tateyama, Jia-Yi Wang and Alisa Liu for their technical assistance. This study was supported by the following grants: The Klarman Foundation Grant Program in Eating Disorders Research to CA and NBM; National Institutes for Health grants R21MH091445-01 to CA and NBM, R01NS066019-01A1 to CA, R01NS047557-07A1 to CA, NEI Core grant EY13079 to CA, R25GM097634-01 to CA, UL1 TR000038 from the National Center for the Advancement of Translational Science (NCATS) to TGC; NIMH Training Program in Systems and Integrative Neuroscience T32 MH019524 to GW; NYU’s Research Challenge Fund to CA; NYU Dean’s Undergraduate Research Fund to Alisa Liu land J-Y Wang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aigner M, Treasure J, Kaye W, Kasper S Disorders WTFOE. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of eating disorders. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2011;12:400–443. doi: 10.3109/15622975.2011.602720. [DOI] [PubMed] [Google Scholar]
- Aoki C, Sabaliauskas N, Chowdhury T, Min JY, Colacino AR, Laurino K, Barbarich-Marsteller NC. Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA(A) receptor alpha4 and delta subunits at the plasma membrane of hippocampal CA1 spines. Synapse. 2012;66:391–407. doi: 10.1002/syn.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Wu K, Elste A, Len G, Lin S, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. Journal of neuroscience research. 2000;59:454–463. doi: 10.1002/(SICI)1097-4547(20000201)59:3<454::AID-JNR21>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- APA APA. Diagnostic and Statistical Manual of Mental Disorders DSM-5. Washington, DC: 2013. [DOI] [PubMed] [Google Scholar]
- Araujo F, Ruano D, Vitorica J. Absence of association between delta and gamma2 subunits in native GABA(A) receptors from rat brain. European journal of pharmacology. 1998;347:347–353. doi: 10.1016/s0014-2999(98)00122-8. [DOI] [PubMed] [Google Scholar]
- Barbarich-Marsteller NC, Laurino K, Colacino AR. Pharmacological treatments for anorexia nervosa. NOVA Science Publishers; 2012. [Google Scholar]
- Barbarich-Marsteller NC, Underwood MD, Foltin RW, Meyers MM, Walsh BT, Barrett JS, Marsteller DA. Identifying novel phenotypes of vulnerability and resistance to activity-based anorexia in adolescent female rats. The International journal of eating disorders. 2013 doi: 10.1002/eat.22149. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, Lee FS. Variant brainderived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biological psychiatry. 2012;72:499–504. doi: 10.1016/j.biopsych.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Schilit A, Lee FS. Stress effects on BDNF expression: Effects of age, sex, and form of stress. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- Birmingham CL, Su J, Hlynsky JA, Goldner EM, Gao M. The mortality rate from anorexia nervosa. The International journal of eating disorders. 2005;38:143–146. doi: 10.1002/eat.20164. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Slof-Op’t Landt MC, van Furth EF, Sullivan PF. The genetics of anorexia nervosa. Annu Rev Nutr. 2007;27:263–275. doi: 10.1146/annurev.nutr.27.061406.093713. [DOI] [PubMed] [Google Scholar]
- Chowdhury T, Wable G, Sabaliauskas N, Aoki C. Adolescent female C57BL/6 mice with vulnerability to activity-based anorexia exhibit weak inhibitory input onto hippocampal CA1 pyramidal cells. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.03.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Katzman DK, Kaptein S, Kirsh C, Brewer H, Kalmbach K, Olmsted MP, Woodside DB, Kaplan AS. The prevalence of high-level exercise in the eating disorders: etiological implications. Comprehensive psychiatry. 1997;38:321–326. doi: 10.1016/s0010-440x(97)90927-5. [DOI] [PubMed] [Google Scholar]
- Davis C, Katzman DK, Kirsh C. Compulsive physical activity in adolescents with anorexia nervosa: a psychobehavioral spiral of pathology. J Nerv Ment Dis. 1999;187:336–342. doi: 10.1097/00005053-199906000-00002. [DOI] [PubMed] [Google Scholar]
- Dellava JE, Thornton LM, Hamer RM, Strober M, Plotnicov K, Klump KL, Brandt H, Crawford S, Fichter MM, Halmi KA, Jones I, Johnson C, Kaplan AS, Lavia M, Mitchell J, Rotondo A, Treasure J, Woodside DB, Berrettini WH, Kaye WH, Bulik CM. Childhood anxiety associated with low BMI in women with anorexia nervosa. Behaviour research and therapy. 2010;48:60–67. doi: 10.1016/j.brat.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes A, Moraes H, Ferreira C, Veiga H, Silveira H, Mouta R, Pompeu FA, Coutinho ES, Laks J. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009;59:191–198. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- Dixon DP, Ackert AM, Eckel LA. Development of, and recovery from, activity-based anorexia in female rats. Physiology & behavior. 2003;80:273–279. doi: 10.1016/j.physbeh.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain research. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PloS one. 2010;5 doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelegen C, van den Heuvel J, Collier DA, Campbell IC, Oppelaar H, Hessel E, Kas MJ. Dopaminergic and brain-derived neurotrophic factor signalling in inbred mice exposed to a restricted feeding schedule. Genes, brain, and behavior. 2008;7:552–559. doi: 10.1111/j.1601-183X.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNFmediated mechanism that promotes neuroplasticity. Journal of neurophysiology. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gorba T, Wahle P. Expression of TrkB and TrkC but not BDNF mRNA in neurochemically identified interneurons in rat visual cortex in vivo and in organotypic cultures. The European journal of neuroscience. 1999;11:1179–1190. doi: 10.1046/j.1460-9568.1999.00551.x. [DOI] [PubMed] [Google Scholar]
- Griffiths J, Lovick T. Withdrawal from progesterone increases expression of alpha4, beta1, and delta GABA(A) receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. The Journal of comparative neurology. 2005a;486:89–97. doi: 10.1002/cne.20540. [DOI] [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express alpha4, beta1 and delta GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. 2005b;136:457–466. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Gutierrez E. A rat in the labyrinth of anorexia nervosa: contributions of the activity-based anorexia rodent model to the understanding of anorexia nervosa. The International journal of eating disorders. 2013;46:289–301. doi: 10.1002/eat.22095. [DOI] [PubMed] [Google Scholar]
- Hare BD, D’Onfro KC, Hammack SE, Falls WA. Prior stress interferes with the anxiolytic effect of exercise in C57BL/6J mice. Behavioral neuroscience. 2012;126:850–856. doi: 10.1037/a0030617. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Exner C, Hebebrand K, Holtkamp C, Casper RC, Remschmidt H, Herpertz-Dahlmann B, Klingenspor M. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiology & behavior. 2003;79:25–37. doi: 10.1016/s0031-9384(03)00102-1. [DOI] [PubMed] [Google Scholar]
- Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Archives of internal medicine. 2010;170:321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Hormones and behavior. 2005;48:163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Scheiffele P. GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development. Current opinion in neurobiology. 2008;18:77–83. doi: 10.1016/j.conb.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen P, Myers RD. Tetrahydro-beta-carboline micro-injected into the hippocampus induces an anxiety-like state in the rat. Pharmacology, biochemistry, and behavior. 1986;24:1733–1738. doi: 10.1016/0091-3057(86)90513-7. [DOI] [PubMed] [Google Scholar]
- Joshi S, Kapur J. Slow intracellular accumulation of GABA(A) receptor delta subunit is modulated by brain-derived neurotrophic factor. Neuroscience. 2009;164:507–519. doi: 10.1016/j.neuroscience.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y, Shibata K, Miyazaki A, Inoue Y, Tominaga K, Koizumi S, Ueki S, Niwa M. Involvement of the dorsal hippocampus in mediation of the antianxiety action of tandospirone, a 5-hydroxytryptamine1A agonistic anxiolytic. Neuropharmacology. 1991;30:475–480. doi: 10.1016/0028-3908(91)90009-z. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. The American journal of psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature reviews Neuroscience. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neuroscience research. 2003;47:55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of neurochemistry. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learning & memory. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas AR, Beard CM, O’Fallon WM, Kurland LT. 50-year trends in the incidence of anorexia nervosa in Rochester, Minn.: a population-based study. The American journal of psychiatry. 1991;148:917–922. doi: 10.1176/ajp.148.7.917. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brainderived neurotrophic factor and nerve growth factor in rat brain. Brain research. 1996;726:49–56. [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onksen JL, Briand LA, Galante RJ, Pack AI, Blendy JA. Running-induced anxiety is dependent on increases in hippocampal neurogenesis. Genes, brain, and behavior. 2012;11:529–538. doi: 10.1111/j.1601-183X.2012.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers PS, Bruty H. Pharmacotherapy for eating disorders and obesity. Child Adolesc Psychiatr Clin N Am. 2009;18:175–187. doi: 10.1016/j.chc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. Microdomains in forebrain spines: an ultrastructural perspective. Molecular neurobiology. 2013;47:77–89. doi: 10.1007/s12035-012-8345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MC, McKenna MC, Yoon YJ, Pattwell SS, Santos PM, Casey BJ, Glatt CE. Caloric Restriction Enhances Fear Extinction Learning in Mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. The Journal of biological chemistry. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. Journal of comparative and physiological psychology. 1967;64:414–421. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Homanics GE, Smith SS, Aoki C. Knockout of the gamma-aminobutyric acid receptor subunit alpha4 reduces functional delta-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain research. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Spiga S, Follesa P, Biggio G. Changes in GABA(A) receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7770–7777. doi: 10.1523/JNEUROSCI.5352-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH, Wisden W, Verdoorn TA, Pritchett DB, Werner P, Herb A, Luddens H, Sprengel R, Sakmann B. The GABAA receptor family: molecular and functional diversity. Cold Spring Harbor symposia on quantitative biology. 1990;55:29–40. doi: 10.1101/sqb.1990.055.01.006. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nature neuroscience. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin CM. Voluntary wheel running: a review and novel interpretation. Animal behaviour. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3alpha-OH-5alpha-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor alpha4 subunit in association with increased anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of phosphorylated TrkB receptor in the mouse hippocampal formation depends on sex and estrous cycle stage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6780–6790. doi: 10.1523/JNEUROSCI.0910-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature neuroscience. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. Mortality in anorexia nervosa. The American journal of psychiatry. 1995;152:1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- Talaenko AN. The Neurochemical profiles of the dorsal hippocampus and the antiaversive effects of anxiolytics in different models of anxiety in rats. J Nerv Ment Dis. 1993;43:621–626. [PubMed] [Google Scholar]
- Tan J, Widjaja S, Xu J, Shepherd RK. Cochlear implants stimulate activity-dependent CREB pathway in the deaf auditory cortex: implications for molecular plasticity induced by neural prosthetic devices. Cerebral cortex. 2008;18:1799–1813. doi: 10.1093/cercor/bhm206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LM, Dellava JE, Root TL, Lichtenstein P, Bulik CM. Anorexia nervosa and generalized anxiety disorder: further explorations of the relation between anxiety and body mass index. Journal of anxiety disorders. 2011;25:727–730. doi: 10.1016/j.janxdis.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, Van der Zee EA, Meerlo P. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19:928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular medicine. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- Wable GS, Barbarich-Marsteller NC, Chowdhury TG, Sabaliauskas NA, Farb CR, Aoki C. Excitatory synapses on dendritic shafts of the caudal basal amygdala exhibit elevated levels of GABAA receptor alpha4 subunits following the induction of activity-based anorexia Synapse. doi: 10.1002/syn.21690. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Normal hearing is required for the emergence of long-lasting inhibitory potentiation in cortex. Journal of Neuroscience. 2010;30:331–341. doi: 10.1523/JNEUROSCI.4554-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]