Abstract
The PubMed and ScienceDirect bibliographic databases were searched for the period of 1998–2009 to evaluate the impact of climatic and environmental determinants on food- and waterborne diseases. The authors assessed 1,642 short and concise sentences (key facts), which were extracted from 722 relevant articles and stored in a climate change knowledge base. Key facts pertaining to temperature, precipitation, water, and food for 6 selected pathogens were scrutinized, evaluated, and compiled according to exposure pathways. These key facts (corresponding to approximately 50,000 words) were mapped to 275 terminology terms identified in the literature, which generated 6,341 connections. These relationships were plotted on semantic network maps to examine the interconnections between variables. The risk of campylobacteriosis is associated with mean weekly temperatures, although this link is shown more strongly in the literature relating to salmonellosis. Irregular and severe rain events are associated with Cryptosporidium sp. outbreaks, while noncholera Vibrio sp. displays increased growth rates in coastal waters during hot summers. In contrast, for Norovirus and Listeria sp. the association with climatic variables was relatively weak, but much stronger for food determinants. Electronic data mining to assess the impact of climate change on food- and waterborne diseases assured a methodical appraisal of the field. This climate change knowledge base can support national climate change vulnerability, impact, and adaptation assessments and facilitate the management of future threats from infectious diseases. In the light of diminishing resources for public health this approach can help balance different climate change adaptation options.
Keywords: Campylobacter sp., climate change, climate variability, Cryptosporidium sp., environment, food, food- and waterborne diseases, Listeria sp., Norovirus, ontology, precipitation, rain, reservoir, Salmonella sp., Vibrio sp.
INTRODUCTION
Environmental determinants are drivers of food- and waterborne diseases. They include climatic variables which have changed significantly as a result of global climate change and are projected to shift even more in the future (Intergovernmental Panel on Climate Change, 2007; United Nations Framework Convention on Climate Change, 2007). As a consequence, these shifts could alter the exposure pathways of food- and waterborne diseases (Boxall et al., 2009; Fawell and Nieuwenhuijsen, 2003). They exert their adverse health outcomes through indirect exposure routes, which are responsive to climatic conditions (Semenza and Menne, 2009). Climatic conditions can influence the fate and transport of pathogens, as well as their viability, stability, and reproduction rates in the environment.
Elevated ambient temperatures augment the replication cycles of most food- and waterborne pathogens. Temperature measurements are among the best climatic data available to date, with an extensive monitoring network throughout the world. Global average temperatures in 2007 increased over preindustrial times by 1°C, but this increase has been more pronounced in Europe, with a 1.2°C rise. This is mainly due to Europe's large land mass, which warms faster than the oceans. This disproportionate increase in annual average temperatures in Europe is projected to reach 1.0–5.5°C (using 1961–1990 as a baseline for 2080–2100 for high emission and medium emission scenarios; Christensen et al., 2007). High-temperature extreme events, such as heat waves, doubled in average length in Western Europe between 1880 and 2005, whereas the frequency of hot days has almost tripled (Della-Marta et al., 2007). Projections indicate that Europe will continue to experience an increase in the frequency, intensity, and duration of extreme high temperatures events (Schär et al., 2004).
The most important constituent of the hydrological cycle is precipitation, an important driver of many waterborne pathogens. Europe has experienced a general precipitation increase of 6–8% over the last century, but regional differences are significant, such as an increase in precipitation in the north versus a drying in the south. Precipitation is more difficult to model than temperature, and thus models differ considerably. For intermediate projections, Northern Europe is expected to experience a 5–20% increase, whereas Southern Europe is projected to experience a decrease of between 5 and 30%. In fact, droughts are projected to become longer lasting and more severe, particularly in drought-prone areas. Nonetheless, the intensity of extreme rain events has increased over the past 50 years even in areas that have experienced a decrease in precipitation, such as the Mediterranean. This is a trend that is projected to continue (Sillmann and Roeckner, 2008).
Projected climate changes such as these will directly affect the exposure pathways of food-and waterborne diseases, warranting particular attention by public health practitioners. Moreover, changes in these weather events might indirectly influence recreational activities, consumption habits, or adaptation behaviors.
To evaluate the impact of a changing climate on food- and waterborne diseases we took advantage of an electronic knowledge base established for this purpose (Semenza et al., 2011). Initially an ontology was constructed of the common vocabulary and terms used in peer-reviewed literature. An ontology can define and categorize, with different levels of formality, the meaning of terms and the relationships between them (Gruber, 1993; Musen, 1992). Information was then extracted and synthesized from selected articles, organized and structured according to the predefined specifications of the ontology, and entered into an electronic knowledge base. Advanced data mining techniques were employed to systematically obtain detailed information about any aspect of climate change and food- and waterborne diseases. In contrast to traditional reviews, this approach searched, extracted, and evaluated data from the knowledge base. Such a methodical and thorough strategy can deliver comprehensive information stratified by geospatial or thematic aspects.
METHODS
The literature review made use of a climate change knowledge base of 1,642 short and concise sentences (key facts) extracted from 722 peer-reviewed articles (years 1998–2009) on climate change and food- and waterborne pathogens (Semenza et al., 2011). Key facts were tagged with predefined terms (275 terms from the terminology of the climate change ontology) that were organized according to thematic (pathogens, food, water, climate/environment, reservoirs) and spatial (continent, region, country) aspects and extensively networked, thereby generating 6,341 data points. The quality of the individual studies was rated based on the GRADE approach and is described in detail in Semenza et al. (2011). The qualitative climate change impact assessment described subsequently took into account the study design and the directness of causal inference and relied on high-quality papers to assess the impact of climate change on food- and waterborne diseases. Indirect evidence was not incorporated into the impact assessment. In contrast to traditional literature reviews, this electronic approach systematically mined the knowledge base (approximately 50,000 words for the 1,642 key facts), scrutinized the evidence (terms), and compiled the findings according to exposure pathways.
Semantic network maps were generated to visualize connections between these terms (Figure 1; Heer et al., 2005; Visualization Lab, 2010). Epidemiological data (Figures 2 and 3) were extracted from the European Center for Disease Prevention and Control (ECDC) Annual Epidemiological Report on Communicable Diseases in Europe (ECDC, 2010a).
FIGURE 1.
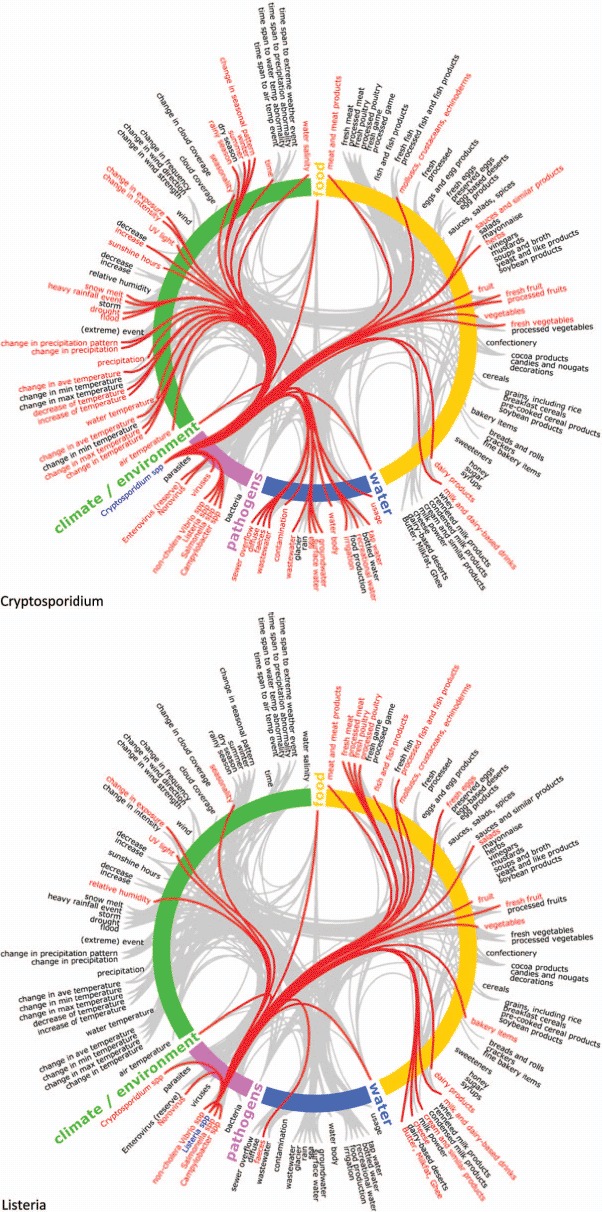
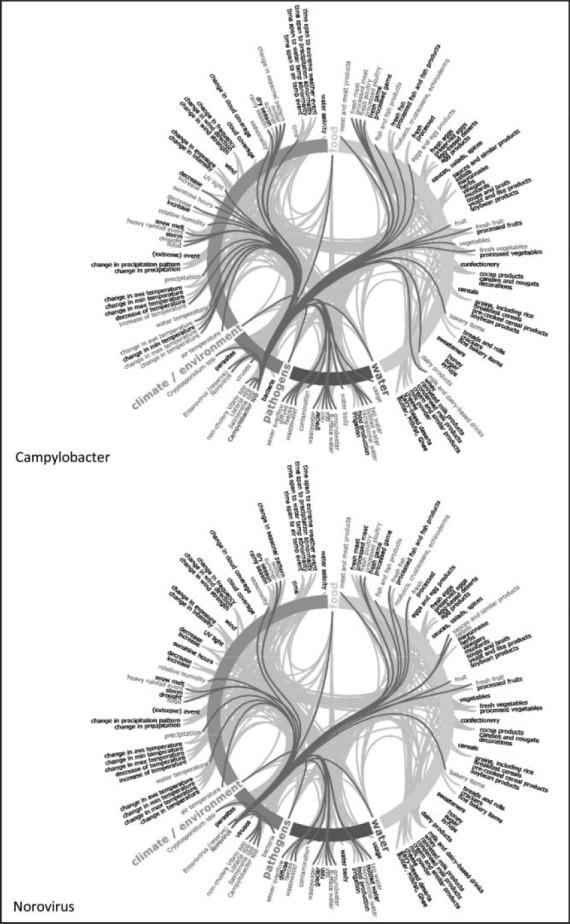
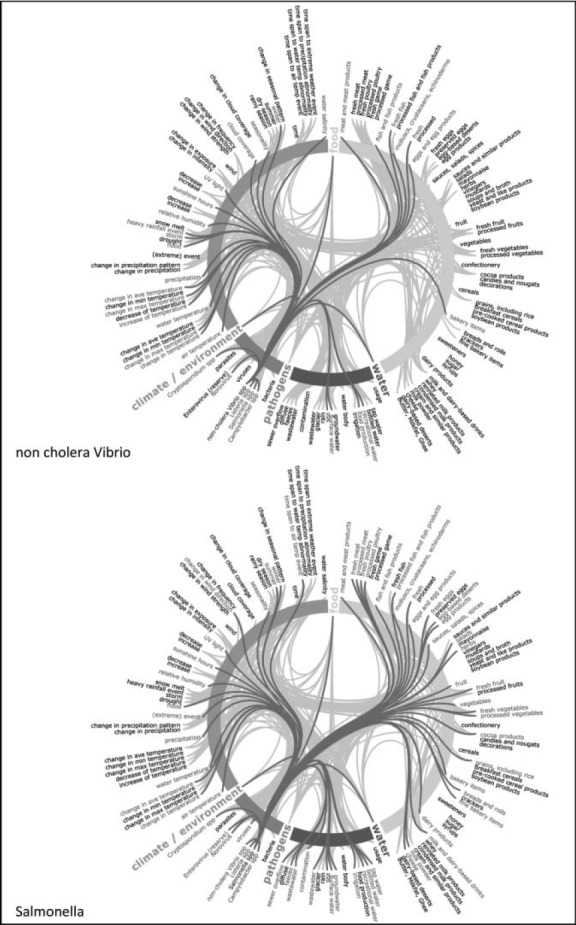
Semantic network maps of thematic attributes for Cryptosporidium sp. and Listeria sp. Climate change knowledgebase for food- and water-borne diseases, 1998–2009. Note: Maps to be read clockwise: thematic aspects are arranged concentrically and colour coded (food = yellow; water = blue; pathogens = pink; climate/environment = green). Cryptosporidium and Listeria pathogens on the bottom left of the circle are coloured blue and initiate connections with other terms, represented with red stings linking to red terms. Gray strings in the background represent the network of all connections not activated in this view. The list of 275 terms in the ontology has been reduced for this map to app. 140 terms due to space restrictions. The 4th level of the hierarchy was deleted for this map but the link was retained by moving it to its ancestor items at the third level (Color figure available online).
FIGURE 2.
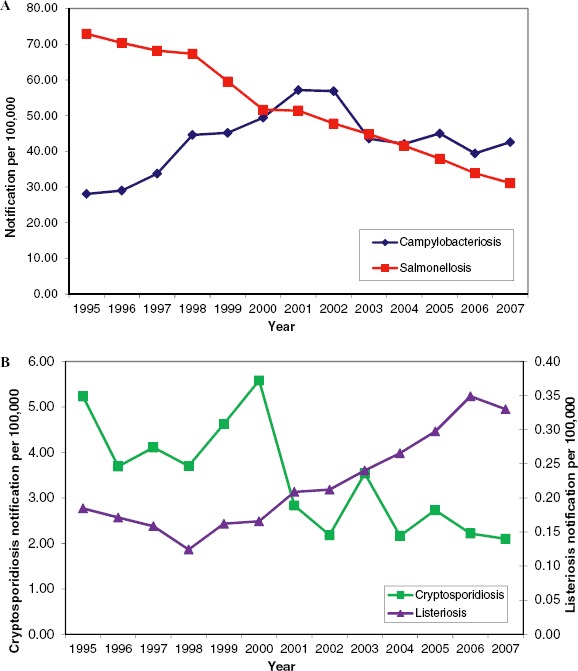
Annual notifications of campylobacteriosis, cryptosporidiosis, listeriosis, and salmonellosis in the EU and EEA/EFTA countries from 1995 to 2007. These data reflect incomplete reporting by member states. The 2007 data for campylobacteriosis were reported from 25 EU member states, plus Iceland, Lichtenstein, and Norway (Greece and Portugal did not report). Salmonellosis was reported by all EU countries plus Iceland, Liechtenstein and Norway. Cryptosporidiosis notifications are based on 10 of the 19 countries providing data (9 countries reported zero cases). Listeriosis was reported by 29 countries, with the exception of Portugal. Please note the different scales on the y axes in Figure 4B (Color figure available online).
FIGURE 3.
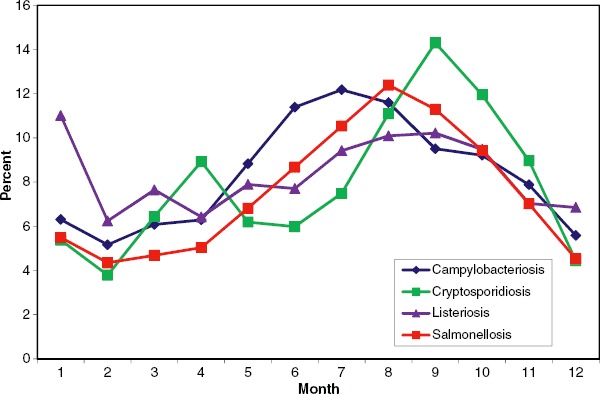
Seasonal distribution of campylobacteriosis, cryptosporidiosis, listeriosis, and salmonellosis in the EU and EEA/EFTA countries, in 2007. Source: Country reports. Campylobacteriosis: Austria, Belgium, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Luxembourg, Malta, Netherlands, Poland, Slovakia, Slovenia, Spain, Sweden, United Kingdom, Iceland, and Norway. Latvia reported zero cases. Cryptosporidiosis: Belgium, Bulgaria, Finland, Germany, Ireland, Luxembourg, Malta, Slovenia, Spain, Sweden, United Kingdom. Cyprus, Czech Republic, Estonia, Finland, Hungary, Latvia, Lithuania, Poland, and Slovakia reported zero cases. Listeriosis: Austria, Belgium, Bulgaria, Cyprus, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Luxembourg, Netherlands, Poland, Slovakia, Slovenia, Spain, Sweden, United Kingdom, and Norway. Malta and Iceland reported zero cases. Salmonellosis: Austria, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, Germany, Greece, Hungary, Ireland, Italy, Latvia, Luxembourg, Netherlands, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom, Iceland, and Norway (Color figure available online).
The absolute number of terms for food, water, reservoir, climate/environment, temperature, and precipitation were displayed on radar diagrams (star plot or spider charts), a graphical method of plotting multivariate data on a two-dimensional chart, on multiple axes (one per pathogen) originating from the same pole (Figure 4). They visually illustrate the absolute number of terms and their relative importance to the other pathogens. Radar diagrams help to identify groups of pathogens by exposure pathway (e.g., precipitation) or potential outliers (Cryptosporidium sp.).
FIGURE 4.
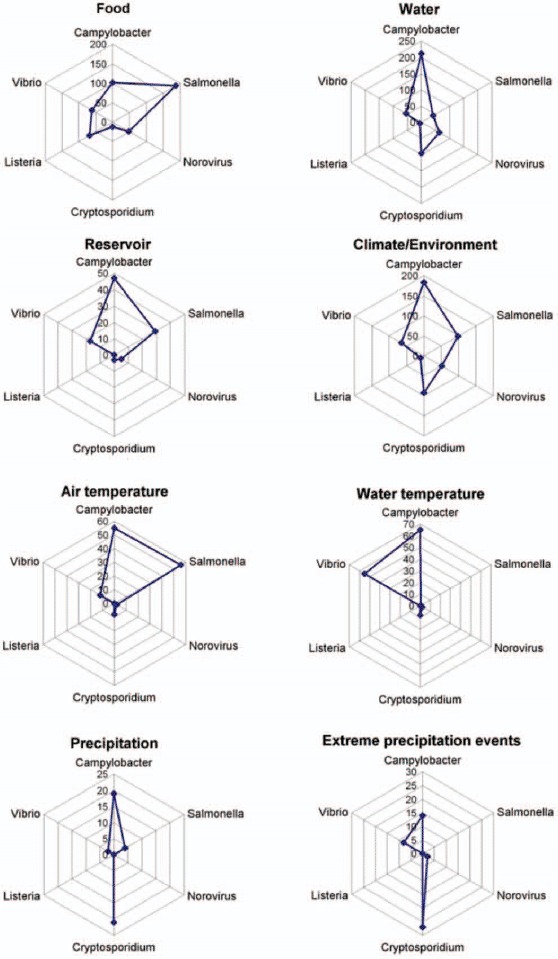
Radar diagram of thematic aspects for food, water, climate/environment, reservoir, air temperature, water temperature, precipitation, and heavy rainfall event, by pathogen from the climate change knowledgebase for food- and waterborne diseases, 1998–2009. Axes (spokes) with different scales. A radar diagram is a graphical method of plotting multivariate data on a two-dimensional chart, on multiple axes originating from the same pole (Color figure available online).
RESULTS
The thematic attributes and their interconnections were extracted from the knowledge base for the following pathogens: Campylobacter sp., Cryptosporidium sp., Listeria sp., Norovirus, Salmonella sp., and noncholera Vibrio sp. The connections were visualized using strings on semantic network maps (see Figure 1 and the Appendix). The links represent documented relationships between 275 terms from the ontology and the pathogens. The connectivity varies considerably by pathogen. For example, in the case of Listeria sp., the preponderance of connections is weighted toward food determinants, but for Cryptosporidium sp. water determinants are more significant. Furthermore, the semantic network maps exemplify the richness of data available for Salmonella sp. and illustrate the comparative scarcity of data for noncholera Vibrio sp. (Appendix). The maps also reveal discrepancies in network density between two well-established food-borne pathogens such as Campylobacter sp. and Norovirus.
The complex relationships between determinants and pathogens are summarized in Table 1. This does not evaluate the association, positive or negative, but presents an overview of the presence or absence of data to date. A positive (relative risk > 1) or negative (relative risk < 1) association between determinants and pathogens is symbolized by ⇔ lack of association (relative risk = 1) is indicated by  , and lack of data is represented by a question mark (?). The nature of these relationships is critically appraised and evaluated for each pathogen separately in the qualitative impact assessments described subsequently. For this impact assessment, peer-reviewed articles were graded by the experts. The reviews were grouped according to high, moderate, low, or very low data quality (Table 2). The majority of reviews of Norovirus articles (51%) and almost half of Campylobacter sp. reviews (47%) ranked the quality as high, in contrast to about a third of the reviews for Salmonella sp. (34%), Cryptosporidium sp. (30%), Listeria sp. (30%), and noncholera Vibrio sp. (28%). With regard to study design, between one quarter and one half were meta-analyses or reviews and only a small fraction represented randomized controlled trials (Table 2). The following review is based directly on the 1,642 key facts in the knowledge base, rather than on the articles from the literature search. Through electronic queries high-quality data were extracted from the key facts and organized according to exposure pathways.
, and lack of data is represented by a question mark (?). The nature of these relationships is critically appraised and evaluated for each pathogen separately in the qualitative impact assessments described subsequently. For this impact assessment, peer-reviewed articles were graded by the experts. The reviews were grouped according to high, moderate, low, or very low data quality (Table 2). The majority of reviews of Norovirus articles (51%) and almost half of Campylobacter sp. reviews (47%) ranked the quality as high, in contrast to about a third of the reviews for Salmonella sp. (34%), Cryptosporidium sp. (30%), Listeria sp. (30%), and noncholera Vibrio sp. (28%). With regard to study design, between one quarter and one half were meta-analyses or reviews and only a small fraction represented randomized controlled trials (Table 2). The following review is based directly on the 1,642 key facts in the knowledge base, rather than on the articles from the literature search. Through electronic queries high-quality data were extracted from the key facts and organized according to exposure pathways.
TABLE 1.
Selected pathogens with environmental/climatic variables (and factors associated with climate) from the climate change knowledge base for food- and waterborne diseases, 1998–2009
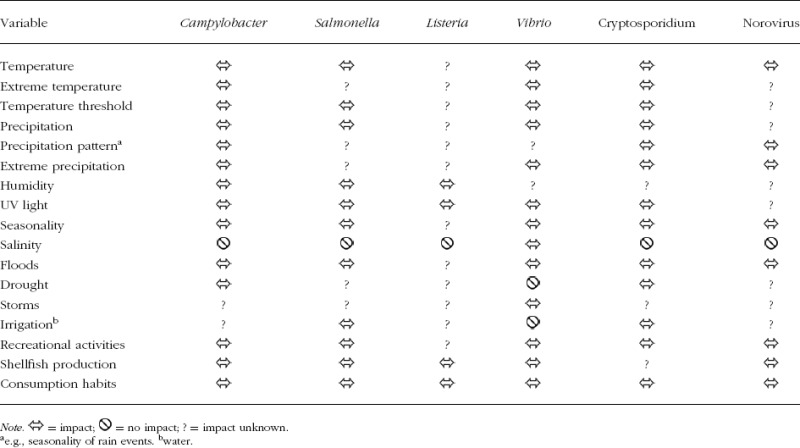 |
TABLE 2.
Reviews of articles by predefined categories: data quality, study design and data source, causal inference from the climate change knowledge base for food- and waterborne diseases, 1998–2009
| Reviews | Campylobacter | Salmonella | Listeria | Vibrio | Cryptosporidium | Norovirus |
|---|---|---|---|---|---|---|
| Data quality | ||||||
| High | 81 | 65 | 17 | 16 | 27 | 48 |
| Moderate | 85 | 98 | 34 | 33 | 53 | 33 |
| Low | 2 | 7 | 3 | 6 | 7 | 3 |
| Very low | __ | 1 | __ | __ | __ | 1 |
| Not classified | 4 | 20 | 2 | 3 | 3 | 9 |
| Study design | ||||||
| Meta-analyses | 19 | 8 | 2 | 3 | 11 | 15 |
| Review | 40 | 40 | 11 | 22 | 36 | 8 |
| Randomized controlled trial | 2 | 1 | __ | __ | 2 | 2 |
| Nonrandomized intervention study | __ | __ | __ | 1 | __ | __ |
| Cohort study | 9 | 6 | 1 | __ | 1 | 6 |
| Case-control study | 11 | 14 | 2 | __ | 3 | 2 |
| Cross-sectional (survey) | 3 | 2 | 1 | __ | 1 | 4 |
| Ecological study | 13 | 10 | 1 | 10 | 2 | 2 |
| Case study | 31 | 15 | 2 | 4 | 12 | 11 |
| Expert opinion | __ | 2 | __ | 1 | __ | 1 |
| In vivo experiment | 10 | 13 | 2 | 1 | 2 | 3 |
| In vitro experiment | 4 | 14 | 18 | __ | 3 | 1 |
| Molecular evidence | 2 | 1 | 2 | __ | 2 | 9 |
| Outbreak | 11 | 21 | 2 | 1 | 4 | 6 |
| Data source | __ | __ | __ | __ | __ | 4 |
| Other | 15 | 24 | 10 | 15 | 7 | 11 |
| Not classified | 2 | 20 | 2 | __ | 4 | 9 |
| Casual inference | ||||||
| Direct | 84 | 56 | 16 | 22 | 22 | 40 |
| Moderate | 57 | 58 | 10 | 19 | 39 | 31 |
| Indirect | 29 | 57 | 28 | 17 | 26 | 15 |
| Not classified | 2 | 20 | 2 | __ | 3 | 8 |
Note. The numbers represent reviews conducted by independent reviewers and not the number of peer reviewed articles; categories may overlap (e.g., multiple pathogens per study).
Campylobacter
Epidemiology
In humans, campylobacteriosis is caused by thermophilic Campylobacter sp., with C. jejuni being the most common species. Symptoms include watery, sometimes bloody diarrhea, abdominal pain, fever, headache, and nausea. In 2007, Campylobacter sp. was the most commonly reported gastrointestinal bacterial pathogen in humans in the European Union (Figure 2A) and experienced a 14% increase compared with 2006 (ECDC, 2010a). The absolute number of 203,798 confirmed cases is probably a substantial underestimate of the true disease incidence in Europe, due to significant underreporting and also because there is considerable variation in monitoring, microbiological testing, and reporting procedures between EU member states.
Seasonality
Campylobacteriosis displays strong seasonality in Europe, with a peak between June and August, although transmission occurs in all seasons (Figure 3). We found that 37 articles contained information on seasonality, 15 of which were rated as high quality and 22 of which were of moderate quality. We found that 23 publications directly addressed seasonality, whereas 10 articles linked seasonality and Campylobacter sp. indirectly.
An important aspect of seasonality is temperature. Within a temperature range of 8–20°C, incremental increases in air temperature are associated with rises in the incidence of campylobacteriosis; the steepest increase in incidence in humans occurs at average temperatures between 8 and 13°C, with a smaller increase at temperatures above 13°C (Patrick et al., 2004). Environmental measurements of Campylobacter in raw sewage display a seasonal pattern related to campylobacteriosis incidence, with a peak in occurrences in the summer (Jones, 2001).
Temperature Determinants
The radar diagram in Figure 4 plots the number of terms for specific environmental determinants for all sixpathogens investigated. It illustrates that air and water temperature determinants of Campylobacter sp. have been extensively studied. A total of 54 publications related to this topic are stored in the knowledge base.
Campylobacteriosis incidence was linked to mean temperatures, although the strength of association was not consistent in all studies (Bi et al., 2008; Fleury et al., 2006; Kovats et al., 2005). High ambient temperatures and relatively low humidity were associated with an incidence increase, but showed a time lag of 2–3 weeks (Kovats et al., 2005; Patrick et al., 2004). Other predictors of the variance in campylobacteriosis incidence included precipitation one month prior to water sampling, hours of sunlight (with a 5-week lag), and relative humidity (with a 5-week lag), which accounted for 44% of the variance (Patrick et al., 2004; Vereen et al., 2007). Environmental sampling of Campylobacter sp. from lakes, streams, and recreational water bodies indicated that recovery rates are higher in fall and winter, but lower in spring and summer (Carter et al., 1987; Obiri-Danso and Jones, 1999). In contrast, Greek and Polish reports indicated that there is no significant relationship between Campylobacter sp. concentrations and surface water temperatures (Abulreesh et al., 2006). Although Campylobacter sp. survived well at 4°C in water/milk or in food, it was nevertheless very susceptible to environmental stresses such as drying, freezing, high temperatures, or UV exposure (Appendix I; Blaser, 2000; Jones, 2001).
Food Determinants
The ontological knowledge base contains 102 terms linking Campylobacter sp. with food (Figure 4). The principal reservoir of Campylobacter sp. is the alimentary tract of domesticated and wild animals, including poultry, cattle, pigs, and sheep. Thus, food such as meat, raw milk, and fresh vegetables can easily be contaminated and fecal-oral transmission can be the source of human outbreaks (Altekruse et al., 1999; Kapperud et al., 2003). Indeed, 70% of the Campylobacter outbreaks in England and Wales between 1995 and 1999 were transmitted through food (Frost et al., 2002). The recently increasing incidence of Campylobacter enteritis was strongly correlated with the increased consumption of chicken (Baird-Parker, 1994). Commercially raised poultry was almost always contaminated with C. Jejuni (Blaser, 2000) and this transmission route may become more important in the future (Danis et al., 2009). An average temperature above 8°C showed a large increase in the prevalence of Campylobacter in broiler flocks (Patrick et al., 2004). Therefore, even a small increase in temperature as an effect of climate change may increase the risk of human infection if cooling throughout the food chain is not commensurately addressed. Rising temperatures could increase the risk of domestically acquired diseases, as temperatures facilitating the growth of Campylobacter sp. on food (>30°C) would be reached more often (Doyle and Roman, 1981). A 1°C rise in temperature has been found to correspond to a 5% increase in reported Campylobacter infections in England and a 4.5% increase in Canada, with a threshold temperature of 0°C (Bi et al., 2008).
The successful adaptation to threats posed by climate change is possible, as shown by the decrease of food-borne disease in England and Wales between 1981 and 2006. This was achieved by reducing pathogen levels in major food groups and improving food hygiene domestically and institutionally (Lake et al., 2009).
Water Determinants
A total of 87 articles analyzed the association between water and Campylobacter sp. Exposure routes such as contaminated drinking or recreational water have been associated with campylobacteriosis. The knowledge base contains 188 entries that pertain to water (Figure 4). Fifteen of the 415 terms for Campylobacter sp. refer specifically to precipitation, with 9 for extreme precipitation (Figure 4). Outbreaks of campylobacteriosis are often triggered by heavy rain events, especially if these are preceded by periods of drought. During these extreme events, manure runoff or sewage overflow can contaminate watersheds, groundwater, or water treatment plants and distribution systems (Clark et al., 2003; Rechenburg and Kistemann, 2009; Semenza et al., 1998).
Precipitation in early spring can trigger campylobacteriosis outbreaks (Pebody et al., 1997; World Health Organization, 2008), which could in part explain the seasonal increase in sporadic cases during May, as illustrated in Figure 3. Campylobacter sp. outbreaks occur more often in rural areas, where households often tend to be supplied by private water sources, which tend to be more susceptible to contamination during extreme weather events (Hearnden et al., 2003; Pebody et al., 1997). In Sweden, a positive association was found between Campylobacter incidence and average pipeline length per person for populations not connected to public water supplies (Nygard et al., 2004).
Behavioral factors, extended periods of sunshine, and higher air temperatures may also be responsible for the disease incidence. The peak in cases between June and August could be partly due to exposure from recreational water use in swimming pools during the main outdoor bathing season (Dwight et al., 2007; Smith et al., 2006)
With the predicted increase in heavy rainfall events, the risk of surface and groundwater contamination is expected to rise (Figure 4). In 1997, contaminated water was estimated to be responsible for 0.2–0.5% of the Campylobacter infections in the Netherlands (Medema et al., 1997). Therefore, the requirement for water treatment plants to produce hygienically safe drinking water remains a high priority. This is supported by the finding that outbreaks linked to public water supplies have declined in England and Wales since 2000, whereas those linked to private water supplies increased in the same period (Smith et al., 2006). Climate change may increase the use of rainwater during times of drought in certain localities. If the harvesting of rainwater increases, Campylobacter sp. in untreated roof runoff water may contribute to an increased risk of animal and human disease (Palmer et al., 1983 Savill et al., 2001).
In summary, campylobacteriosis is the highest reported food- and waterborne disease in Europe and exhibits strong seasonality. The pathogen has been associated with a number of meteorological variables and specific weather events, which indicates that campylobacteriosis peaks may shift as a result of climate change in the future.
Salmonella
Epidemiology
Infections with Salmonella species other than S. typhi and S. paratyphi are designated as salmonellosis. Generally, clinical symptoms such as fever, diarrhea, abdominal pain, nausea, and vomiting may develop 12–36 hr (range = 6–72 hr) after ingestion. They tend to be self-limiting and persist from several hours to a few days. In 2007, a total of 155,566 salmonellosis cases were confirmed by all EU and European Environmental Agency (EEA)/European Free Trade Association (EFTA) countries, which translates into an overall notification rate of 34 per 100,000 individuals (ECDC, 2010a). There has been a continuous decline in salmonellosis cases over the last decade (Figure 2A), probably due to improved food hygiene and other public health measures. However, the same reporting limitations as discussed for campylobacteriosis are applicable.
Seasonality
Salmonellosis in Europe is linked to seasonality, with a peak in late summer (Figure 3). Climatic variables are important determinants since Salmonella sp. are susceptible to sunlight and drying out, but can proliferate faster at higher temperatures (McMichael et al., 2006). Above a 6°C threshold, the risk of Salmonella infection increased in several European countries (van Pelt et al., 2004). Several studies showed a clear tendency for salmonellosis to increase as the weather warms up and reaches peak temperatures in late summer (D'Souza et al., 2004; Fleury et al., 2006; Kovats et al., 2004).
Temperature Determinants
The effect of temperature on Salmonella is addressed in 27 articles. For Salmonella sp., the knowledge base reveals 56 terms relating to air temperature (Figure 4). Ambient seasonal temperature increases are suspected drivers of 30% of reported salmonellosis cases. This trend became particularly apparent when a time lag was applied between average temperature and salmonellosis cases, although the time span of this lag varied among studies. For example, a positive association between the mean temperature of the previous month and the number of salmonellosis notifications in the present month was found (D'Souza et al., 2004), whereas other studies found the greatest effect of temperature between two days and up to five weeks before the onset of illness (Iturriza Gomara et al., 2008; Naumova et al., 2007).
One time series analysis suggests that for every degree increase in weekly temperature above the threshold, the log relative risk of salmonellosis (threshold −10°C) increased by 1.2% (Fleury et al., 2006). Other studies indicate a rise in salmonellosis for each 1°C increase in weekly temperature for ambient temperatures above about 5°C by 5–10% up to 13.1% for S. enteritidis, whereas in the laboratory the rate of multiplication was directly related to temperature within a range of 7.5–37°C (Baird-Parker, 1994; Kovats et al., 2004).
Food Determinants
Salmonellosis is a common food-borne disease. Overall, the ontological knowledge base lists 188 terms relating to food and Salmonella sp. There is a correlation between Salmonella sp. shedding in animals and human salmonellosis in summer and early fall (Baird-Parker, 1994; Callaway et al., 2008). A rather obvious cause is the multiplication of Salmonella sp. in food at warmer temperatures. A short time lag between a rise in temperature and an incidence increase indicated that inappropriate food preparation and storage before consumption were key factors. In contrast, longer term lags indicate contamination during the food production process (Lake et al., 2009).
Water Determinants
The ontological knowledge base contains 41 terms for water (Figure 4). Seasonal detection frequencies for Salmonella sp. in water environments were related to monthly maximum precipitation in summer and fall following fecal contamination events (Craig et al., 2003; Martinez-Urtaza et al., 2004). Floods caused by heavy rainfall events may disrupt water treatment and sewage systems and contribute to increased exposure to Salmonella sp. and other pathogens (Kovats et al., 2000b).
Another important climate factor was humidity, as this affects the growth and culturability of Salmonella sp (Lesne et al., 2000; Shi et al., 2007). On the other hand, the need for increased irrigation with potentially contaminated water in dry months poses a risk for transmission of Salmonella sp., as studies on vegetables have shown (Okafo et al., 2003).
In summary, temperature has a clear impact on salmonellosis and food poisoning notifications, representing improper food storage and handling at the time of eating (Table 1). Nevertheless, as Figure 2A illustrates, salmonellosis has continued to decline throughout Europe over the last decade, in part due to control measures. Thus, apart from climatic factors, carefully designed health promotion and food safety policies should be able to mitigate the probable negative impacts on public health.
Cryptosporidium
Epidemiology
Cryptosporidiosis in humans is caused by the protozoan parasites Cryptosporidium parvum and C. hominis. The disease is characterized by abdominal cramps, loss of appetite, nausea, vomiting, and watery diarrhea that spontaneously resolves over a couple of weeks in otherwise healthy patients. In 2007, 10 EU and EEA/EFTA countries reported a total of 6,253 confirmed cases, whereas nine countries reported zero cases (Figure 2B; ECDC, 2010a). The overall notification rate was 2.4 cases per 100,000 individuals, with the caveat that there were differences in reporting between countries.
Seasonality
The knowledge base contained 19 articles linking Cryptosporidium sp. with seasonality. There is a pronounced seasonality in the data, with an early autumn peak and a smaller spring peak (Figure 3; Hunter, 2003; Smith et al., 2006). Disaggregation of data reveals different peaks in different countries. For example, Ireland reported an increase in spring and Spain reported a peak in summer (Semenza and Nichols, 2007). The seasonality of cryptosporidiosis has changed over the years within England and Wales; the spring peak has substantially decreased since 2001 and the autumn peak has increased. The reasons for this decrease are the improved drinking water regulations combined with significant investment in drinking water treatment. Improved water treatment such as the filtration of previously unfiltered water was a main factor in risk reduction (Lake et al., 2007; Semenza and Nichols, 2007; Sopwith et al., 2005).
The seasonality of sporadic cases may be explained by the seasonal contamination of surface waters in early spring. During the period from April to July, cryptosporidiosis is strongly associated with maximum river flow and a positive correlation between rainfall, the peak of the rainfall event, oocyst concentrations in river water and human diseases was documented (Kistemann et al., 2002). During the winter, less Cryptosporidium oocysts were detectable in surface waters (Hörman et al., 2004). From August to November secondary Cryptosporidium infections (as opposed to the primary or index case who introduces the disease) are more common, implicating human sewage as the main cause of infection (Lake et al., 2005). Combined sewer overflow can lead to heavy contamination of the environment and water catchments, for example, through badly protected wells (Kistemann et al., 2003; Kistemann et al., 2002). Another explanation may be holiday travel and swimming pool use, but evidence for this hypothesis is lacking (Lake et al., 2007; Semenza and Nichols, 2007).
Water Determinants
Cryptosporium sp. is a waterborne disease. This is also reflected by the literature: the knowledge base contains 56 publications on Cryptosporidium sp. and water. There are 96 terms relating to water and 21 for precipitation, but only 12 terms for food (Figure 4). Heavy rainfall (27 terms) has been associated with the contamination of water supplies and outbreaks of cryptosporidiosis (Kovats et al., 1999; Kovats et al., 2000b). The concentration of Cryptosporidium oocysts in river water increased significantly during rainfall events (Kistemann et al., 2002; Rose et al., 2001). The effect of rainfall on parasite concentrations was due in part to surface runoff, together with the resuspension of river bottom and storm drain sediment (Atherholt et al., 1998). Outbreaks associated with surface water contamination linked to humans have been caused as a result of sewage discharge and runoff, which occurred during heavy rainfall events in the United States, United Kingdom, and Canada (Rose et al., 2002). Heavy precipitation can result in the persistence of oocysts in the water distribution system and the infiltration of drinking water reservoirs from springs and lakes (Casman et al., 2001). Outbreaks associated with contaminated groundwater were due to springs and wells, which were not properly protected from sewage and runoff or wells located adjacent to rivers and streams (Rose et al., 2002).
Temperature Determinants
Temperature is one of the most critical processes governing the viability of oocysts in the environment. The knowledge base lists eight publications relating to temperature effects. At temperatures between 1 and 15°C Cryptosporidium oocysts could maintain high levels of infectivity for periods of at least 24 weeks (King and Monis, 2007) and in water the persistence of oocysts was not affected by temperatures <30°C (Nasser et al., 2003). At above 37°C oocysts were extremely susceptible; with temperatures >40°C, rapid inactivation occurred at rates greater than 3 logs per day for oocysts deposited in the feces of beef and dairy cattle (King and Monis, 2007). However, warmer temperatures may also have increased the survival of oocysts in areas prone to soil subsurface freezing or lake ice covers, resulting in substantial numbers remaining infective after the winter period, where previously they may have been inactivated (King and Monis, 2007). Once oocysts are excreted into the terrestrial environment and released from feces their survival is greatly limited due to desiccation. This may vary greatly, with increased rates of inactivation expected in more arid environments. Oocysts above the soil matrix may be extremely vulnerable to desiccation, whereas those within it may be protected (King and Monis, 2007).
Climate/Environment Determinants
The link between climatic determinants and Cryptosporidium sp. is a subject in which there has been considerable research undertaken. The knowledge base lists 93 key climatic and environmental facts for Cryptosporidium sp. (Figure 4). Climatic factors, the incidence of infection in animal and human populations, as well as the excretion of oocysts in certain watersheds all influence the occurrence of cryptosporidiosis (Rose et al., 2002). Climatic factors likely to affect the transmission and survival of Cryptosporidium sp. are temperature and changes in precipitation. An increase in the frequency and intensity of extreme precipitation events could increase the risk of transmission of cryptosporidiosis, whereas higher winter precipitation is unlikely to have an impact on cases of cryptosporidiosis. Cryptosporidium oocysts are inactivated during the winter, as they are susceptible to freezing and thawing cycles; nevertheless, with less freezing, oocysts may increasingly survive through the winter (King and Monis, 2007).
In the future, more intense precipitation events may increase the saturation of soil profiles and mobilize infectious oocysts more often and in combination with urbanization and deforestation of the landscape, significantly increasing the risk that Cryptosporidium oocysts pose (King and Monis, 2007). Precipitation can flush pathogens into waterways and lead to more rapid stream velocities, which increases the risk of cryptosporidiosis epidemics (Casman et al., 2001). Another effect of precipitation changes could be an increase in the amount of river effluent reaching the sea, contaminating coastal areas. Finally, increased flooding could result in flooded sewage treatment plants and an increased risk of cryptosporidiosis outbreaks (EEA, 2007).
In summary, climatic variables are a major determinant in the transmission of Cryptosporidium sp. (Table 1). A large number of studies have examined the role of surface water, tap water, and heavy rainfall events in disease transmission. A rise in precipitation is predicted to lead to an increase in cryptosporidiosis, although the strength of the relationship varies by climate category (Jagai et al., 2009; Rose et al., 2002).
Listeria
Epidemiology
Listeriosis in humans is caused by the bacterium Listeria monocytogenes and is characterized by meningitis and sepsis, 3–70 days (M = 3 weeks) after infection. In 2007, there were 1,635 reported cases across 29 European countries, the majority (56%) of which were reported in individuals over 65 years of age (ECDC, 2010a). Although the notification rate (0.35 per 100,000 individuals) is significantly lower than for the diseases discussed previously, the rate has been increasing over the years (Figure 2B). Listeriosis seems to be increasing in industrialized countries worldwide (Buzby and Roberts, 1997).
Seasonality
The seasonal trend shown by the aggregated EU data is weak compared with the other pathogens (Figure 3), but for France a seasonal effect with an increase in summer temperatures has been observed (Goulet et al., 2006). As Listeria sp. are ubiquitous in the environment and grow in a wide temperature range, they are present year round and can be detected worldwide regardless of climatic zone. Thus, it is unlikely that changes in season will directly affect the occurrence of Listeria sp. in their habitat. However, indirect transmission pathways may be affected.
Temperature Determinants
Listeria sp. grow within a wide temperature range and only few publications were found linking this bacteria to climatic determinants. The database contains 7 climate-relevant publications, but no terms for air temperature or water temperature were found for Listeria sp.
Food Determinants
The knowledge base has 69 terms related to food and L. monocytogenes, compared with only 6 on water, and 10 on climate/environment (Figure 4). There are 39 publications generating these 69 terms. Food products can become contaminated with Listeria sp. during processing and preparation, and then domestically acquired (Hall et al., 2002). For example, the potential for food contamination with L. monocytogenes could increase in the future. Breakdowns within the cooling chain during (extreme) warm-weather events may increase the risk of infection. For example, the storage of cold-smoked trout at 10°C, instead of the recommended 0–3°C, for 17 days resulted in multiplication and high numbers of L. monocytogenes being present in the product (Miettinen et al., 1999). Raw milk products, uncooked refrigerated processed meats, and ready-to-eat meat products have been reported as vehicles for the disease (European Food Safety Authority, 2007). Studies, particularly from Northern Europe, have shown ready-to-eat smoked and cold-salted fish products to be contaminated with L. monocytogenes (Miettinen et al., 1999; Rorvik et al., 2000).
In summary, this comprehensive analysis of climatic determinants for Listeria sp. revealed a lack of associations as well as a number of data gaps. No information was available about temperature thresholds, extreme precipitation events, or temperature limits. Data was also lacking on seasonality, floods, droughts, storms, irrigation, or recreational activities. Despite the lack of information, it is unlikely that climate change will directly impact the incidence of listeriosis in Europe, although it could lead to more cases through indirect pathways (Table 1).
Norovirus
Epidemiology
Noroviruses are single-stranded RNA viruses without an envelope, and belong to the Caliciviridae family. The viruses cause acute gastroenteritis in humans and account for about one third and one half of gastroenteritis in children and adults, respectively. Symptoms include projectile vomiting, watery nonbloody diarrhea with abdominal cramps, nausea, myalgia, malaise, and headaches, occasionally also with a low-grade fever. Norovirus is not a reportable disease in the EU and therefore only scattered data are available. Since 2003, the percentage of individual cases reported in Germany increased consistently, which was assumed to be a result of better diagnostics combined with a real increase in the number of cases (Koch, 2004).
Seasonality
The database contained 23 articles reflecting seasonality and Norovirus infections. In Germany, Norovirus infections occurred throughout the year, with a seasonal rise from October to March and a typical peak in November to January (Bradt et al., 2005). The reasons for this seasonality are not yet clear, but it is assumed that low temperatures, low humidity, and low insulation enhance the survival of Norovirus. Additionally, people tend to spend more time indoors during the winter, and they also tend to have weaker immune systems during this season. In Lower Saxony, Germany, sporadic cases in 2005 occurred predominantly in the interseasonal phase (spring and summer; Bradt et al., 2005). The seasonal winter peak of Europe and North America is not mirrored by a southern hemisphere winter/spring peak in Australia, as the peak occurs there in December (Marshall et al., 1999). Thus, climate does not seem to be the main determinant, but rather a contributor alongside a number of other factors, such as industrial food production and global shipping/transport. However, there is a significant lack of information about the role of human factors influencing Norovirus infections.
Food Determinants
The knowledge base contained 26 articles referring to the association between food and Norovirus infections, corresponding to 61 terms (Figure 4). The sources of food-borne Norovirus infections included meals with multiple ingredients (40.2%), produce (16.5%), seafood (13.0%), and baked goods (8.7%; Greig and Ravel, 2009). Meat was also a potential route for indirect zoonotic transmission (e.g., raw pork; Mattison et al., 2007). Animals often acquired the pathogens via contaminated fodder or water (Tauxe, 1997). Virus shedding by infected animals can last for two months (van der Poel et al., 2003). In principle, any food can become contaminated if handled by an infected person or if rinsed with contaminated water.
Globalization of the food chain has been linked to the increasing levels of food-borne Norovirus infections, exemplified by an outbreak in Sweden caused by raspberries from China (Hjertqvist et al., 2006; Hui, 2006). However, further research is needed regarding the transmission of Norovirus via contaminated food and especially the exposure of animals to the pathogen and their role as a reservoir.
Water Determinants
The knowledge base contained 35 articles linking Norovirus and water determinants. There are 55 terms on water and Norovirus in the knowledge base, but none on precipitation (Figure 4). Waterborne Norovirus outbreaks are not common, but can be caused by contamination of drinking or recreational water (Carrique-Mas et al., 2003; Nygard et al., 2003). Cross-connections during the repair of public water pipelines or back-siphonage within household installations have also been documented (Merbecks et al., 2004; Taylor et al., 1981). Outbreaks linked to private water supplies occurred more often and had a much higher overall incidence rate than outbreaks linked to public water supplies (Blackburn et al., 2004; Smith et al., 2006). Contaminated public water supplies caused higher attack rates since more people used these supplies (Hui, 2006). As documented in Finland, it was possible for Norovirus to survive for four months during periods of low temperatures and ice-covered surface waters; in this instance, an initially food-borne Norovirus strain caused a subsequent waterborne outbreak 70 km downstream (Kukkula et al., 1999).
Detection rates of Norovirus in surface water samples showed a winter peak, related to winter peaks in sewage and caused by the feces of infected humans. The sewage treatment plants’ effluent concentrations of Norovirus are reduced at least 2-log in contrast to concentrations within raw sewage (Katayama et al., 2008; Pusch et al., 2005). Outbreaks that can be traced to swimming pools showed a seasonal peak in summer, as would be expected (Lawson et al., 1991; Smith et al., 2006).
Climatic Determinants
Twenty-seven publications linking Norovirus and climatic determinants were reviewed. There were also food-borne outbreaks linked to climate and weather events, as shown in Paris in 2002, where heavy rainfall and floods resulted in wastewater overflow that contaminated shellfish farming sites (Doyle et al., 2004). The occurrence of Norovirus in Mediterranean shellfish was also related to heavy rainfall events. Additionally, it was related to peaks in diarrhea incidence because the coastal water is sewage-contaminated (Miossec et al., 2000). Among terrestrial animals acting as a potential reservoir, direct seasonal relationships of Norovirus contamination have not been proven, but the indirect effects of weather events on the quality of water consumed outside can be assumed (Wang et al., 2006). The predicted increase of heavy rainfall events under climate change scenarios could lead to an increase in Norovirus infections because floods are known to be linked to Norovirus outbreaks (Anderson et al., 2006).
In summary, the link established between climatic variables and Norovirus is weak in part due to the relative paucity of published data (Table 1). For example, no data are published on temperature extremes or thresholds or on the after-effects of storms, droughts, or rain events.
Vibrio (Noncholera)
Epidemiology
The genus Vibrio (including V. vulnificus and V. parahaemolyticus as well as V. cholerae And Vibrio sp.) are motile, curved rods and inhabit estuarine waters, brackish waters, or coastal areas. Open wounds can be infected with Vibrio sp. if they come into contact with contaminated water. This may lead to necrotization and cause septicemia. Elevated mean temperatures can increase growth opportunities in fresh- and saltwater bodies. Vibrio sp. are indigenous to the Mediterranean, the Baltic, and the North Sea and can multiply in saline water, with increased growth rates at temperatures over 20°C (Randa et al., 2004). Human Vibrio infections are not reportable by law in the EU.
Seasonality
The knowledge base contained five articles linking Vibrio sp. and seasonality. Strong seasonal fluctuations in concentrations of Vibrio sp. can be observed which correlate with a seasonal pattern of infections (Hemmer et al., 2007). High numbers of the bacteria can be observed during times of warm water temperatures and zooplankton blooms (Lipp et al., 2002). Maximum concentrations were predominantly found in late summer and early autumn and demonstrate a direct link to salinity and water temperatures. Approximately 78% of all infections occur between May and October (Daniels et al., 2000a; Daniels et al., 2000b).
Water Determinants
The knowledge base contains 53 key water facts relating to noncholera Vibrio sp. (Figure 4). Vibrio sp. spread with rising water temperatures and exploit prolonged periods of advantageous environmental conditions (Pedersen and Larsen, 1997). The clear seasonal trend was related to water temperatures from 12 to 15°C, up to an optimum of 26°C (Randa et al., 2004). In Israel, infections were significantly correlated with high temperatures (r = .62, p < .0001) during the preceding 25–30 days (Paz et al., 2007). It was also noted by Paz et al. (2007) that in hot climates the minimum temperature was the most important factor contributing to the growth of Vibrio sp.
Around the Baltic Sea, notified V. vulnificus infections occurred during hot summer months and increased with water temperatures above 20°C (Hemmer et al., 2007). During the hot summer of 1994, 11 infections with V. vulnificus occurred, compared witha baseline of 3–4 cases. Simultaneously, an increase in rainbow trout mortality caused by several bacteria was observed. The incidence of fish vibriosis was closely related to the water temperature in the aquaculture.
Temperature Determinants
There were only 12 terms for Vibrio sp. and air temperature, but 55 terms for water temperature. Information about the impact of air temperature was abstracted from 5 articles. The direct relationship between temperature and infections was noticed in 2006: during the summer, elevated ambient temperatures throughout the moderate climate regions of northwestern Europe resulted in elevated water temperatures. Three Swedish, two German, and 15 Danish patients developed infections following contact with warm bathing water in the Baltic and the North Sea (Andersen, 2006; Andersson and Ekdahl, 2006; Frank et al., 2006). Bathing during unusually warm summer weather in Northern Europe poses a considerable risk to individuals with open wounds suffering from pre-existing chronic conditions or those who are immunocompromised.
In summary, there is evidence of a strong link between rising summer (water) temperatures, prolonged summer seasons, and noncholera Vibrio sp. infections, but the increase in the disease burden is expected to be modest due to low present incidence rates (Table 1). An increase in absolute infection numbers can be assumed for the future. The Baltic Sea in particular provides an environment in which only small changes of the actual conditions (e.g., temperature) result in increased Vibrio sp. populations.
CONCLUSION
This review describes the association between environmental determinants and food- and waterborne diseases. Specifically, individual weather events/measurements have been linked to these pathogens, rather than general climate change phenomena. The strength of data for specific climatic variables is apparent, but also the lack of explicit climate change examples. Thus, the unpredictable nature of climate change and its links to the spread of communicable diseases creates the need for knowledge mapping, which this climate change knowledge base attempts to provide. A tool kit was developed by the ECDC (2010b) to aid EU Member States to assess and manage changes in the risk of infectious diseases posed by climate change. This ECDC handbook provides decision algorithms to weigh the probability of an outbreak linked to climate change versus the severity of consequences to society (or risk groups). This knowledge base can support these decision-making processes.
In summary, a novel approach to cataloguing, quantifying, and assessing the present state of knowledge in the field of climate change and food-and waterborne diseases has been presented. This comprehensive analysis revealed a number of data gaps for selected pathogens and climatic determinants (Table 1). To attribute eventual changes in disease incidence to climate change, sophisticated surveillance systems and tools should be put in place to monitor the environmental precursors of pandemics. Relying on predictive models and monitoring for early warning can guide climate change adaptation and protect the health of the public.
ACKNOWLEDGMENTS
The authors would like to thank Ana-Belen Escriva for her help with the literature review, and Sandra Alves and Andrea Buksarova for their help with the manuscript. Giorgio Semenza, Laszlo Balkanyi, Andreas Jansen, and Helen Hanimann provided constructive feedback on the manuscript.
APPENDIX I
Semantic network maps of thematic attributes by food and waterborne pathogens. Climate change knowledgebase for food- and water-borne diseases, 1998–2009
REFERENCES
- Abulreesh H.H., Paget T.A., Goulder R. Campylobacter in waterfowl and aquatic environments: Incidence and methods of detection. Environ. Sci. Technol. 2006;40:7122–7131. doi: 10.1021/es060327l. [DOI] [PubMed] [Google Scholar]
- Altekruse S.F., Stern N.J., Fields P.I., Swerdlow D.L. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 1999;5:28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P.H. Infections with seawater bacteria. EPI-NEWS. 2006;26–32:1. [Google Scholar]
- Anderson P., Brownstein J., Confalonieri U., Causey D., Chan N., Ebi K.L., et al. Climate change futures: Health, ecological and economic dimensions. Boston, MA: The Center for Health and the Global Environment, Harvard Medical School; 2006. [Google Scholar]
- Andersson Y., Ekdahl K. Wound infections due to Vibrio cholerae in Sweden after swimming in the Baltic Sea, summer 2006. Euro. Surveill. 2006;11(8):E060803.2. doi: 10.2807/esw.11.31.03013-en. [DOI] [PubMed] [Google Scholar]
- Atherholt T.B., LeChevallier M.W., Norton W.D., Rosen J.S. The effect of rainfall on giardia and crypto. J. Amer. Water Works Assoc. 1998;90:66–80. [Google Scholar]
- Baird-Parker A.C. Foods and microbiological risks. Microbiology. 1994;140:687–695. doi: 10.1099/00221287-140-4-687. [DOI] [PubMed] [Google Scholar]
- Bi P., Cameron A.S., Zhang Y., Parton K.A. Weather and notified Campylobacter infections in temperate and sub-tropical regions of Australia: An ecological study. J. Infect. 2008;57:317–323. doi: 10.1016/j.jinf.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Blackburn B.G., Craun G.F., Yoder J.S., Hill V., Calderon R.L., Chen N., et al. Surveillance for waterborne disease outbreaks associated with drinking water, United States, 2001–2002. MMWR Surveill. Summ. 2004;53(8):23–45. [PubMed] [Google Scholar]
- Blaser M.J. Campylobacter jejuni and related species. In: Mandell G.L., Bennett J.E., Dolin R., editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, PA: Churchill Livingstone; 2000. pp. 2276–2285. [Google Scholar]
- Boxall A.B., Hardy A., Beulke S., Boucard T., Burgin L., Falloon P.D., et al. Impacts of climate change on indirect human exposure to pathogens and chemicals from agriculture. Environ. Health Perspect. 2009;117:508–514. doi: 10.1289/ehp.0800084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradt K., Monazahian M., Baillot A., Heckler R. Norovirus infections [Norovirus – Erkrankungen]: Annual Report of the Public Health Authority of the Federal State of Lower Saxony, Germany (NGLA), 23–25. 2005.
- Buzby J.C., Roberts T. Economic costs and trade impacts of microbial foodborne illness. World Health Stat. Q. 1997;50:57–66. [PubMed] [Google Scholar]
- Callaway T.R., Edrington T.S., Anderson R.C., Byrd J.A., Nisbet D.J. Gastrointestinal microbial ecology and the safety of our food supply as related to Salmonella. J. Anim. Sci. 2008;86(14 Suppl):E163–172. doi: 10.2527/jas.2007-0457. [DOI] [PubMed] [Google Scholar]
- Carrique-Mas J., Andersson Y., Petersen B., Hedlund K.O., Sjogren N., Giesecke J. A Norwalk-like virus waterborne community outbreak in a Swedish village during peak holiday season. Epidemiol. Infect. 2003;131(1):737–744. doi: 10.1017/s0950268803008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A.M., Pacha R.E., Clark G.W., Williams E.A. Seasonal occurrence of Campylobacter spp. in surface waters and their correlation with standard indicator bacteria. Appl. Environ. Microbiol. 1987;53:523–526. doi: 10.1128/aem.53.3.523-526.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casman E., Fischhoff B., Small M., Dowlatabadi H., Rose J., Morgan M.G. Climate change and cryptosporidiosis: A qualitative analysis. Climatic Change. 2001;50:219–249. [Google Scholar]
- Christensen J.H., Hewitson B., et al. Climate Change 2007: The physical science basis. New York, NY: Cambridge University Press; 2007. Regional climate projections. [Google Scholar]
- Clark C.G., Price L., Ahmed R., Woodward D.L., Melito P.L., Rodgers F.G., et al. Characterization of waterborne outbreak-associated Campylobacter jejuni, Walkerton, Ontario. Emerg. Infect. Dis. 2003;9:1232–1241. doi: 10.3201/eid0910.020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig D.L., Fallowfield H.J., Cromar N.J. Effectiveness of guideline faecal indicator organism values in estimation of exposure risk at recreational coastal sites. Water Sci. Technol. 2003;47:191–198. [PubMed] [Google Scholar]
- D'Souza R.M., Becker N.G., Hall G., Moodie K.B. Does ambient temperature affect foodborne disease? Epidemiology. 2004;15:86–92. doi: 10.1097/01.ede.0000101021.03453.3e. [DOI] [PubMed] [Google Scholar]
- Daniels N.A., MacKinnon L., Bishop R., Altekruse S., Ray B., Hammond R.M., et al. Vibrio parahaemolyticus infections in the United States, 1973–1998. J. Inf. Dis. 2000b;181:1661–1666. doi: 10.1086/315459. [DOI] [PubMed] [Google Scholar]
- Daniels N.A., Ray B., Easton A., Marano N., Kahn E., McShan A.L., II, Del Rosario L., et al. Emergence of a new Vibrio parahaemolyticus serotype in raw oysters: A prevention quandary. JAMA. 2000a;284:1541–1545. doi: 10.1001/jama.284.12.1541. [DOI] [PubMed] [Google Scholar]
- Danis K., Di Renzi M., O'Neill W., Smyth B., McKeown P., Foley B., Tohani V., Devine M. Risk factors for sporadic Campylobacter infection: an all-Ireland case-control study. Euro. Surveill. 2009;14(7) [PubMed] [Google Scholar]
- Della-Marta P.M., Haylock M.R., Luterbacher J., Wanner J. Doubled length of western European summer heat waves since 1880. J. Geophys. Research. 2007;112:D15103. [Google Scholar]
- Doyle A., Barataud D., Gallay A., Thiolet J.M., Le Guyaguer S., Kohli E., et al. Norovirus foodborne outbreaks associated with the consumption of oysters from the Etang de Thau, France, December 2002. Euro. Surveill. 2004;9(3):24–26. doi: 10.2807/esm.09.03.00451-en. [DOI] [PubMed] [Google Scholar]
- Doyle M.P., Roman D.J. Growth and survival of Campylobacter fetus susp. jejuni as a function of temperature and pH. J. Food Protect. 1981;44:596–601. doi: 10.4315/0362-028X-44.8.596. [DOI] [PubMed] [Google Scholar]
- Dwight R.H., Brinks M.V., Sharavanakumar G., Semenza J.C. Beach attendance and bathing rates for Southern California beaches. Ocean and Coastal Management. 2007;50:847–858. [Google Scholar]
- European Center for Disease Prevention and Control. Annual epidemiological report on communicable diseases in Europe 2009. Stockholm, Sweden: ECDC; 2010a. [Google Scholar]
- European Center for Disease Prevention and Control. Handbook for national vulnerability, impact and adaptation assessments on climate change and communicable diseases. 2010b. Retrieved from http://ecdc.europa.eu/en/publications/Publications/1003_TED_handbook_climatechange.pdf.
- European Environmental Agency. Climate change and water adaptation issues. 2007. EEA Technical Report No 2.
- European Food Safety Authority. The community summary report on trends and sources of zoonoses, zoonotic agents, antimicrobial resistance and foodborne outbreaks in the European Union in 2006. EFSA Journal. 2007. p. 130. Retrieved from http://www.efsa.europa.eu/en/efsajournal/pub/130r.htm.
- Fawell J., Nieuwenhuijsen M.J. Contaminants in drinking water. Br Med Bull. 2003;68:199–208. doi: 10.1093/bmb/ldg027. [DOI] [PubMed] [Google Scholar]
- Fleury M., Charron D.F., Holt J.D., Allen O.B., Maarouf A.R. A time series analysis of the relationship of ambient temperature and common bacterial enteric infections in two Canadian provinces. Int. J. Biometeorol. 2006;50:385–391. doi: 10.1007/s00484-006-0028-9. [DOI] [PubMed] [Google Scholar]
- Frank C., Littman M., Alpers K., Hallauer J. Vibrio vulnificus wound infections after contact with the Baltic Sea, Germany. Euro. Surveill. 2006;11(8):E060817.1. doi: 10.2807/esw.11.33.03024-en. [DOI] [PubMed] [Google Scholar]
- Frost J.A., Gillespie I.A., O'Brien S.J. Public health implications of Campylobacter outbreaks in England and Wales, 1995–9: Epidemiological and microbiological investigations. Epidemiol. Infect. 2002;128:111–118. doi: 10.1017/s0950268802006799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet V., Jacquet C., Martin P., Vaillant V., Laurent E., de Valk H. Surveillance of human listeriosis in France, 2001–2003. Euro. Surveill. 2006;11(6):79–81. [PubMed] [Google Scholar]
- Greig J.D., Ravel A. Analysis of foodborne outbreak data reported internationally for source attribution. Int. J. Food Microbiol. 2009;130:77–87. doi: 10.1016/j.ijfoodmicro.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Gruber T.R. A translation approach to portable ontologies. Knowledge Acquisition. 1993;5:199–220. [Google Scholar]
- Hall G.V., D'Souza R.M., Kirk M.D. Foodborne disease in the new millennium: out of the frying pan and into the fire? Med. J. Aust. 2002;177:614–618. doi: 10.5694/j.1326-5377.2002.tb04984.x. [DOI] [PubMed] [Google Scholar]
- Hearnden M., Skelly C., Eyles R., Weinstein P. The regionality of campylobacteriosis seasonality in New Zealand. Int. J. Environ. Health Res. 2003;13:337–348. doi: 10.1080/09603120310001616128. [DOI] [PubMed] [Google Scholar]
- Heer J., Card S.K., Landay J.A. Prefuse: A toolkit for interactive information visualization. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. 2005. pp. 421–430.
- Hemmer C.J., Frimmel S., Kinzelback R., Gurtler L., Reisinger E.C. Global warming: Trailblazer for tropical infections in Germany? Dtsch Med Wochenschr. 2007;132:2583–9. doi: 10.1055/s-2007-993101. [DOI] [PubMed] [Google Scholar]
- Hjertqvist M., Johansson A., Svensson N., Abom P.E., Magnusson C., Olsson M., et al. Four outbreaks of Norovirus gastroenteritis after consuming raspberries, Sweden, June–August 2006. Euro. Surveill. 2006;11(9):E060907, 1. doi: 10.2807/esw.11.36.03038-en. [DOI] [PubMed] [Google Scholar]
- Hörman A., Rimhanen-Finne R., Maunula L., von Bonsdorff C.H., Torvela N., Heikinheimo A., et al. Campylobacter spp., Giardia spp., Cryptosporidium spp., noroviruses, and indicator organisms in surface water in south-western Finland, 2000–2001. Appl. Environ. Microbiol. 2004;70:87–95. doi: 10.1128/AEM.70.1.87-95.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E.K. Reasons for the increase in emerging and re-emerging viral infectious diseases. Microbes and Infection. 2006;8:905–916. doi: 10.1016/j.micinf.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P.R. Climate change and waterborne and vector-borne disease. J. Appl. Microbiol. 2003;94(Suppl.):37S–46S. doi: 10.1046/j.1365-2672.94.s1.5.x. [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change. Fourth assessment report: Climate changesynthesis report. 2007. Retrieved from http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr_spm.pdf.
- Iturriza Gomara M., Simpson R., Perault A.M., Redpath C., Lorgelly P., Joshi D., et al. Structured surveillance of infantile gastroenteritis in East Anglia, UK: Incidence of infection with common viral gastroenteric pathogens. Epidemiol. Infect. 2008;136(1):23–33. doi: 10.1017/S0950268807008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagai J.S., Castronovo D.A., Monchak J., Naumova E.N. Seasonality of cryptosporidiosis: A meta-analysis approach. Environ. Res. 2009;109:465–478. doi: 10.1016/j.envres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. Campylobacters in water, sewage and the environment. Symp. Ser. Soc. Appl. Microbiol. 2001;30:68S–79S. doi: 10.1046/j.1365-2672.2001.01355.x. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Espeland G., Wahl E., Walde A., Herikstad H., Gustavsen S., et al. Factors associated with increased and decreased risk of Campylobacter infection: A prospective case-control study in Norway. Am. J. Epidemiol. 2003;158:234–242. doi: 10.1093/aje/kwg139. [DOI] [PubMed] [Google Scholar]
- Katayama H., Haramoto E., Oguma K., Yamashita H., Tajima A., Nakajima H., et al. One year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res. 2008;42:1441–1448. doi: 10.1016/j.watres.2007.10.029. [DOI] [PubMed] [Google Scholar]
- King B.J., Monis P.T. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology. 2007;134:309–323. doi: 10.1017/S0031182006001491. [DOI] [PubMed] [Google Scholar]
- Kistemann T., Classen T., Exner M. Epidemiologically confirmed: the first waterborne outbreak of Giardiasis in Germany. Bbr. 2003;7:40–46. [Google Scholar]
- Kistemann T., Claşen T., Koch C., Dangendorf F., Fischeder R., Gebel J., et al. Microbial load of drinking water reservoir tributaries during extreme rainfall and runoff. Appl. Environ. Microbiol. 2002;68:2188–2197. doi: 10.1128/AEM.68.5.2188-2197.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch J. Seasonal distribution of norovirus cases in Germany: 2001–2004. RKI Epidemiologisches Bulletin. 2004.
- Kovats R.S., Edwards S.J., Charron D., Cowden J., D'Souza R.M., et al. Climate variability and Campylobacter infection: an international study. Int. J. Biometeorol. 2005;49:207–214. doi: 10.1007/s00484-004-0241-3. [DOI] [PubMed] [Google Scholar]
- Kovats R.S., Edwards S.J., Hajat S., Armstrong B.G., Ebi K.L., Menne B., et al. The effect of temperature on food poisoning: A time series analysis of salmonellosis in ten European countries. Epidemiol. Infect. 2004;132:443–453. doi: 10.1017/s0950268804001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats R.S., Haines A., Stanwell-Smith R., Martens P., Menne B., Bertollini R. Climate change and human health in Europe. BMJ. 1999;318:1682–1685. doi: 10.1136/bmj.318.7199.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats R.S., Menne B., McMichael A.J., Bertollini R., Soskolne C. WHO Regional Publication, European Series, No. 88. Geneva, Switzerland: World Health Organization; 2000b. Climate change and stratospheric ozone depletion: Early effects on our health in Europe. [PubMed] [Google Scholar]
- Kovats R.S., Menne B., McMichael A.J., Corvalan C., Bertollini R. Climate change and human health: Impact and adaption. Geneva, Switzerland: World Health Organization; 2000a. [Google Scholar]
- Kukkula M., Maunula L., Silvennoinen E., von Bonsdorff C.H. Outbreak of viral gastroenteritis due to drinking water contaminated by Norwalk-like viruses. J. Inf. Dis. 1999;180:1771–1776. doi: 10.1086/315145. [DOI] [PubMed] [Google Scholar]
- Lake I.R., Bentham G., Kovats R.S., Nichols G.L. Effects of weather and river flow on cryptosporidiosis. J. Water Health. 2005;3:469–474. doi: 10.2166/wh.2005.048. [DOI] [PubMed] [Google Scholar]
- Lake I.R., Gillespie I.A., Bentham G., Nichols G.L., Lane C., Adak G.K., Threlfall E.J. A re-evaluation of the impact of temperature and climate change on foodborne illness. Epidemiol. Infect. 2009;137:1–10. doi: 10.1017/S0950268809002477. [DOI] [PubMed] [Google Scholar]
- Lake I.R., Nichols G., Bentham G., Harrison F.C.D., Hunter P.R., Kovats R.S. Cryptosporidiosis decline after regulation, England and Wales, 1989–2005. Emerg. Infect. Dis. 2007;13:623–625. doi: 10.3201/eid1304.060890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson H.W., Braun M.M., Glass R.I., Stine S.E., Monroe S.S., Atrash H.K., et al. Waterborne outbreak of Norwalk virus gastroenteritis at a south-west US resort: Role of geological formations in contamination of well water. Lancet. 1991;337(8751):1200–1204. doi: 10.1016/0140-6736(91)92868-3. [DOI] [PubMed] [Google Scholar]
- Lesne J., Berthet S., Binard S., Rouxel A., Humbert F. Changes in culturability and virulence of Salmonella typhimurium during long-term starvation under desiccating conditions. International Journal of Food Microbiology. 2000;60:195–203. doi: 10.1016/s0168-1605(00)00311-1. [DOI] [PubMed] [Google Scholar]
- Lipp E.K., Huq A., Colwell R.R. Effects of global climate on infectious disease: The cholera model. Clin. Microbiol. Rev. 2002;15:757–770. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J.A., Yuen L., Catton M.G., Wright P.J. Summer vomiting disease? Med. J. Aust. 1999;171:686–687. doi: 10.5694/j.1326-5377.1999.tb123860.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Urtaza J., Saco M., de Novoa J., Perez-Pineiro P., Peiteado J., Lozano-Leon A., et al. Influence of environmental factors and human activity on the presence of Salmonella serovars in a marine environment. Appl. Environ. Microbiol. 2004;70:2089–2097. doi: 10.1128/AEM.70.4.2089-2097.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison K., Shukla A., Cook A., Pollari F., Friendship R., Kelton D., et al. Human Noroviruses in swine and cattle. Emerg. Infect. Dis. 2007;13:1184–1188. doi: 10.3201/eid1308.070005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A.J., Woodruff R.E., Hales S. Climate change and human health: Present and future risks. Lancet. 2006;367:859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- Medema G.J., van Asperen I.A., Havelaar A.H. Assessment of the exposure of swimmers to microbiological contaminants in fresh waters. Water Sci. Technol. 1997;35:157–63. [Google Scholar]
- Merbecks S.-S., Beier D., Gruschwitz A., Müller L., Partisch M. Increase of norovirus cases due to drinking water contamination. RKI Epidemiologisches Bulletin. 2004.
- Miettinen M.K., Siitonen A., Heiskanen P., Haajanen H., Bjorkroth K.J., Korkeala H.J. Molecular epidemiology of an outbreak of febrile gastroenteritis caused by Listeria monocytogenes in cold-smoked rainbow trout. J. Clin. Microbiol. 1999;37:2358–2360. doi: 10.1128/jcm.37.7.2358-2360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec L., Le Guyader F., Haugarreau L., Pommepuy M. Magnitude of rainfall on viral contamination of the marine environment during gastroenteritis epidemics in human coastal population. Rev. Epidemiol. Sante Publique. 2000;48(Suppl 2):S62–S71. [PubMed] [Google Scholar]
- Musen M.A. Dimensions of knowledge sharing and reuse. Computers and Biomedical Research. 1992;25:435–467. doi: 10.1016/0010-4809(92)90003-s. [DOI] [PubMed] [Google Scholar]
- Nasser A.M., Zaruk N., Tenenbaum L., Netzan Y. Comparative survival of Cryptosporidium, coxsackievirus A9 and Escherichia coli in stream, brackish and sea waters. Water Sci. Technol. 2003;47(3):91–96. [PubMed] [Google Scholar]
- Naumova E.N., Jagai J.S., Matyas B., DeMaria A., Jr., MacNeill I.B., Griffiths J.K. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol. Infect. 2007;135:281–292. doi: 10.1017/S0950268806006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard K., Andersson Y., Rottingen J.A., Svensson A., Lindback J., Kistemann T., Giesecke J. Association between environmental risk factors and Campylobacter infections in Sweden. Epidemiol. Infect. 2004;132:317–325. doi: 10.1017/s0950268803001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard K., Torven M., Ancker C., Knauth S.B., Hedlund K.O., Giesecke J., et al. Emerging genotype (GGIIb) of Norovirus in drinking water, Sweden. Emerg. Infect. Dis. 2003;9:1548–1552. doi: 10.3201/eid0912.030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiri-Danso K., Jones K. Distribution and seasonality of microbial indicators and thermophilic Campylobacters in two freshwater bathing sites on the River Lune in north-west England. J. Appl. Microbiol. 1999;87:822–832. doi: 10.1046/j.1365-2672.1999.00924.x. [DOI] [PubMed] [Google Scholar]
- Okafo C.N., Umoh V.J., Galadima M. Occurrence of pathogens on vegetables harvested from soils irrigated with contaminated streams. Sci. Total Environ. 2003;311:49–56. doi: 10.1016/S0048-9697(03)00057-3. [DOI] [PubMed] [Google Scholar]
- Palmer S.R., Gully P.R., White J.M., Pearson A.D., Suckling W.G., Jones D.M., et al. Waterborne outbreak of Campylobacter gastroenteritis. Lancet. 1983;1(8319):287–290. doi: 10.1016/s0140-6736(83)91698-7. [DOI] [PubMed] [Google Scholar]
- Patrick M.E., Christiansen L.E., Waino M., Ethelberg S., Madsen H., Wegener H.C. Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Appl. Environ. Microbiol. 2004;70:7474–7480. doi: 10.1128/AEM.70.12.7474-7480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz S., Bisharat N., Paz E., Kidar O., Cohen D. Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ. Res. 2007;103:390–396. doi: 10.1016/j.envres.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Pebody R.G., Ryan M.J., Wall P.G. Outbreaks of Campylobacter infection: Rare events for a common pathogen. Commun. Dis. Rep. CDR Rev. 1997;7(3):R33–R37. [PubMed] [Google Scholar]
- Pedersen K.D., Larsen J.L. Vibrio damsela associated with diseased fish in Denmark. Appl. Environ. Microbiol. 1997;63:3711–3715. doi: 10.1128/aem.63.9.3711-3715.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch D., Oh D.Y., Wolf S., Dumke R., Schroter-Bobsin U., Hohne M., et al. Detection of enteric viruses and bacterial indicators in German environmental waters. Arch. Virol. 2005;150:929–947. doi: 10.1007/s00705-004-0467-8. [DOI] [PubMed] [Google Scholar]
- Randa M.A., Polz M.F., Lim E. Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitavie PCR. Appl. Environ. Microbiol. 2004;70:5469–5476. doi: 10.1128/AEM.70.9.5469-5476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechenburg A., Kistemann T. Sewage effluent as a source of Campylobacter sp. in a surface water catchment. Int. J. Environ. Health Res. 2009;19:239–249. doi: 10.1080/09603120802460376. [DOI] [PubMed] [Google Scholar]
- Rorvik L.M., Aase B., Alvestad T., Caugant D.A. Molecular epidemiological survey of Listeria monocytogenes in seafoods and seafood-processing plants. Appl. Environ. Microbiol. 2000;66:4779–4784. doi: 10.1128/aem.66.11.4779-4784.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J.B., Epstein P.R., Lipp E.K., Sherman B.H., Bernard S.M., Patz J.A. Climate variability and change in the United States: potential impacts on water and foodborne diseases caused by microbiologic agents. Environ. Health Perspect. 2001;109(Suppl 2):211–221. doi: 10.1289/ehp.01109s2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J.B., Huffman D.E., Gennaccaro A. Risk and control of waterborne cryptosporidiosis. FEMS Microbiol. Rev. 2002;26:113–123. doi: 10.1111/j.1574-6976.2002.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Savill M.G., Hudson J.A., Ball A., Klena J.D., Scholes P., Whyte R.J., McCormick R.E., Jankovic D. Enumeration of Campylobacter in New Zealand recreational and drinking waters. J. Appl. Microbiol. 2001;91:38–46. doi: 10.1046/j.1365-2672.2001.01337.x. [DOI] [PubMed] [Google Scholar]
- Schär C., Vidale P.L., Lüthi D., Frei C., Häberli C., Liniger M.A., Appenzeller C. The role of increasing temperature variability in European summer heatwaves. Nature. 2004;427(6972):332–336. doi: 10.1038/nature02300. [DOI] [PubMed] [Google Scholar]
- Semenza J.C., Höser C., Herbst S., Rechenburg A., Suk J.E., Frechen T., Kistemann T. Knowledge mapping for climate change and food and waterborne diseases. Crit. Rev. Environ. Sci. Tech. 2012;42:378–411. doi: 10.1080/10643389.2010.518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza J.C., Menne B. Climate change and infectious diseases in Europe. Lancet ID. 2009;9:365–375. doi: 10.1016/S1473-3099(09)70104-5. [DOI] [PubMed] [Google Scholar]
- Semenza J.C., Nichols G. Cryptosporidiosis surveillance and waterborne outbreaks in Europe. Euro.Surveill. 2007;12(5):1–9. doi: 10.2807/esm.12.05.00711-en. [DOI] [PubMed] [Google Scholar]
- Semenza J.C., Roberts L., Henderson A., Bogan J., Rubin C.H. Water distribution system and diarrheal disease transmission: A case study in Uzbekistan. American Journal of Tropical Medicine and Hygiene. 1998;59:941–946. doi: 10.4269/ajtmh.1998.59.941. [DOI] [PubMed] [Google Scholar]
- Shi X., Namvar A., Kostrzynska M., Hora R., Warriner K. Persistence and growth of different Salmonella serovars on pre- and post-harvest tomatoes. J. Food Protect. 2007;70:2725–2731. doi: 10.4315/0362-028x-70.12.2725. [DOI] [PubMed] [Google Scholar]
- Sillmann J., Roeckner E. Indices of extreme events in projections of anthropogenic climate change. Climatic Change. 2008;86:83–104. [Google Scholar]
- Smith A., Reacher M., Smerdon W., Adak G.K., Nichols G., Chalmers R.M. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992–2003. Epidemiol. Infect. 2006;134:1141–1149. doi: 10.1017/S0950268806006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopwith W., Osborn K., Chalmers R., Regan M. The changing epidemiology of cryptosporidiosis in North-West England. Epidemiol. Infect. 2005;133:785–793. doi: 10.1017/S0950268805004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauxe R.T. Emerging foodborne diseases: An evolving public health challenge. Emerg. Infect. Dis. 1997;3:425–434. doi: 10.3201/eid0304.970403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.W., Gary G.W., Jr., Greenberg H.B. Norwalk-related viral gastroenteritis due to contaminated drinking water. Am. J. Epidemiol. 1981;114:584–592. doi: 10.1093/oxfordjournals.aje.a113224. [DOI] [PubMed] [Google Scholar]
- United Nations Framework Convention on Climate Change. Article 1. 2007. Retrieved from http://unfccc.int/2860.php.
- van der Poel W.H., van der Heide R., Verschoor F., Gelderblom H., Vinje J., Koopmans M.P. Epidemiology of Norwalk-like virus infections in cattle in the Netherlands. Vet. Microbiol. 2003;92:297–309. doi: 10.1016/s0378-1135(02)00421-2. [DOI] [PubMed] [Google Scholar]
- van Pelt W., Mevius D., Stoelhorst H.G., Kovats S., van de Giessen A.W., Wannet W., et al. A large increase in Salmonella infections in 2003 in the Netherlands: Hot summer or side effect of the avian influenza outbreak? Euro. Surveill. 2004;9(7):17–19. [PubMed] [Google Scholar]
- Vereen E., Jr., Lowrance R.R., Cole D.J., Lipp E.K. Distribution and ecology of Campylobacters in coastal plain streams (Georgia, United States of America) Appl. Environ. Microbiol. 2007;73:1395–1403. doi: 10.1128/AEM.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visualization Lab. Flare: Sample dependency graph. 2010. Retrieved from http://flare.prefuse.org/apps/dependency_graph.
- Wang Q.H., Souza M., Funk J.A., Zhang W., Saif L.J. Prevalence of noroviruses and sapoviruses in swine of various ages determined by reverse transcription-PCR and microwell hybridization assays. J. Clin. Microbiol. 2006;44:2057–2062. doi: 10.1128/JCM.02634-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Protecting health in Europe from climate change. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]


