Summary
To improve long-term outcomes for Burkitt leukaemia/lymphoma (BL) or aggressive lymphomas in adults, we assessed the benefit of adding rituximab and filgrastim support to a dose-dense modified chemotherapy regimen from the Cancer and Leukemia Group B (CALGB) 9251 trial. One hundred and five patients (aged 19–79 years) were enrolled; 27% were >60 years old; 47% had high or high-intermediate risk by International Prognostic Index (IPI) criteria. Common severe toxicities included stomatitis/upper gastrointestinal toxicity (69%), renal insufficiency (10%), neurological events (25%) and pulmonary events (18%). Seven died from treatment-related causes (1 central nervous system bleed, 4 infections, 2 respiratory failure); 5 were > 60 years old. Results in this adult population are encouraging as complete response (CR) was observed in 83% and 4-year event-free (EFS) and overall survivals (OS) were 74% and 78%, respectively. Results compare favourably to our prior chemotherapy alone study (CALGB 9251) but despite this, high-risk patients still had worse outcomes. In conclusion, short duration, intensive chemo-immunotherapy is feasible and should be considered in adults with BL as it results in high remission rates and durable remissions.
Keywords: Burkitt Leukaemia, Burkitt Lymphoma, Chemo-immunotherapy, rituximab
INTRODUCTION
Burkitt leukaemia/lymphoma (BL) is a rapidly progressive B-cell malignancy that often presents in extranodal sites or as an acute leukaemia. Characteristically, the monomorphic medium-sized Burkitt cells bear a translocation of MYC, but this is not specific and the gold standard for diagnosis, i.e., the distinction between BL and other aggressive B-cell lymphomas, continues to evolve (Swerdlow et al, 2008). Typical Burkitt tumour cell immunophenotype entails expression of moderate to strong levels of immunoglobulin (Ig)M with light chain restriction plus CD19, CD20, CD22, CD10, BCL6, and CD38, but rarely BCL2 and never TdT. Greater than 90% of the cells express the proliferation antigen, Ki67. Cytogenetically, most cases of BL have MYC translocation from band 8q24 to the IGH chain region, 14q32 or, less commonly to the lambda (IGL, 22q11) or kappa (IGK, 2p12) locus (Simon et al, 1998). During the time this study was conducted (2002–2011), definitions differed from today’s more precise characterization, and cases with features intermediate between Burkitt and diffuse large B-cell lymphoma were often termed ‘Burkitt-like’ in the older terminology. More recently, this group has been subsumed in the current nomenclature by ‘B cell lymphoma unclassifiable with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma’. Thus, this report presents the data for all treated patients, but also the subgroup of 58 with central pathology review and confirmation of Burkitt disease using our current definitions (Leoncini et al, 2008).
Despite high initial response rates, cures for adults with BL were uncommon when standard diffuse large B-cell or acute lymphoblastic leukaemia regimens were used. When laboratory evidence demonstrated that Burkitt cells had a high proliferative rate and were highly sensitive to alkylating agents and antimetabolites, regimens with fractionated cyclophosphamide, high-dose methotrexate and high-dose cytarabine were developed that improved outcomes (Hoelzer et al, 1996; Magrath et al, 1996). In the Cancer and Leukemia Group B (CALGB) 9251 study, we evaluated a dose dense, intensive chemotherapy regimen resulting in a high overall survival (OS) rate of 52% (43–60%). However, outcomes were clearly worse for older patients and those classified as high risk by the International Prognostic Index (IPI) (Rizzieri et al, 2004; The International Non-Hodgkin’s Lymphoma Prognostic Factors Project 1993). The strong expression of the surface antigen CD20 in BL led to its use in BL with encouraging results in small studies (Thomas, et al, 2006; Maruyama et al, 2010; Mohamedbhai et al, 2010). Additionally, the expected incidence and duration of severe neutropenia observed in CALGB 9251 suggested that primary prophylaxis with myeloid growth factor support might prove beneficial. Thus, CALGB study 10002 (Alliance) was designed as a phase 2 study for patients with Burkitt or Burkitt-like leukaemia/ lymphoma (Harris et al, 1999) to determine the response rate, event-free survival (EFS), and OS of adults receiving rituximab with short duration, high intensity chemotherapy with filgrastim support.
PATIENTS AND METHODS
Eligible patients were those ≥16 years of age, previously untreated with a diagnosis of Burkitt or ‘Burkitt-like’ leukaemia or lymphoma per the definitions used at the time of study conduct (Diebold et al, 2001) and who were not known to be human immunodeficiency virus (HIV) positive. Patients were enrolled based on pathology diagnosis by their local haematopathologist, though confirmatory material was requested for central pathology review. Liver and kidney function <1.5 times the upper limit of normal (ULN) was required, unless the abnormal function was felt, in the investigator’s opinion, to be due to the disease. Local institutional review boards at participating institutions approved the study, and all patients provided written informed consent. This study was listed on clinicaltrials.gov as NCT00039130.
Treatment
The treatment regimen is outlined in Table I. Patients could not have received any therapy for their disease prior to enrollment and initiation of therapy on this study. Following a week of cytoreduction (cycle 1), patients received alternating cycles of multiagent therapy with filgrastim every 3 weeks for 6 more cycles, given over 19 weeks. Delays were allowed until the absolute neutrophil count had recovered to ≥ 1.0 × 109/l, platelet count ≥75 × 109/l, and the patient had been off growth factor for more than 2 days. In addition, the patient must have recovered from therapy-induced mucositis. Known large effusions were expected to be drained prior to the administration of methotrexate and this agent was held in any cycle in which the creatinine clearance was less than 50 ml/min. Predefined dose reduction algorithms were utilized for hepatic dysfunction (vincristine, etoposide, doxorubicin, cyclophosphamide), central nervous system toxicity (doxorubicin), peripheral nervous system toxicity (vincristine) and cerebellar toxicity (cytarabine). All patients were screened for hepatitis B and those positive were closely monitored for reactivation. Unless there was clinical concern for central nervous system (CNS) involvement, a lumbar puncture (LP) was not performed until the start of cycle 2 (day 8), and then one dose of triple intrathecal therapy was given with each of cycles 2–7. Patients proven to have CNS disease continued to receive systemic therapy with the addition of triple intrathecal therapy twice weekly until the cerebrospinal fluid was clear, then monthly for 4 treatments, followed by cranial radiation with 2400 cGy in 12 fractions. Those with gonadal disease received 2600 cGy to the testes during systemic therapy. Rituximab was first administered using stepped-up dosing in cycle 2, then at standard dosing, once per cycle for courses 3–7 (Table I).
Table I. CALGB 10002 treatment schema.
| Cycle 1 | Dose-Schedule based on actual weight | Days Given |
|---|---|---|
| Cyclophosphamide | 200 mg/m2/day | 1–5 |
| Prednisone | 60 mg/m2/day oral | 1–7 |
| Allopurinol | 300 mg/day oral | 1–14 |
| Cycles 2, 4, and 6 | Cycle length 21 days | |
| Ifosfamide | 800 mg/m2/day over 1 h with Mesna | 1–5 |
| Dexamethasone | 10 mg/m2/day | 1–5 |
| Methotrexate | 150 mg/m2 load, then 1.35 g/m2 over 23.5 h | 1 |
| Leucovorin* | 25 mg/m2 36 h after initiation of methotrexate, then 10 mg/m2 every 6 h until level <0.05 µM | 2 |
| Vincristine | 2 mg push | 1 |
| Cytarabine | 1000 mg/m2/day over 2 h | 4–5 |
| Etoposide | 80 mg/m2/day over 1 h | 4–5 |
| Filgrastim | 5 µg/kg/day | 7, until ANC> 0.5 × 109/l |
| Rituximab | ** | 8, 10 and 12 of cycle 2 only |
| Rituximab | ** | 8 of cycle 4 and 6 |
| Intrathecal therapy | *** | 1 |
| Cycles 3, 5 and 7 | Cycle length 21 days | |
| Cyclophosphamide | 200 mg/m2/day | 1–5 |
| Dexamethasone* | 10 mg/m2/day | 1–5 |
| Methotrexate | 150 mg/m2 load, then 1.35 g/m2 over 23.5 h | 1 |
| Leucovorin** | 50 mg/m2 36 h after initiation of methotrexate, then 10 mg/m2 every 6 h until level <0.05 µM | 2 |
| Vincristine | 2 mg push | 1 |
| Doxorubicin | 25 mg/m2/day | 4–5 |
| Filgrastim | 5 µg/kg/day | 7, until ANC> 0.5 × 109/l |
| Rituximab | ** | 8 |
| Intrathecal therapy | *** | 1 |
intravenous or oral
Rituximab administered in cycle 2 at a dose of 50 mg/m2 on day 8 and 375 mg/m2/day on days 10 and 12. For cycles 3–7, rituximab was given at 375 mg/m2 only on day 8 of each cycle.
| *** Intrathecal therapy | Methotrexate 15 mg, Cytarabine 40 mg, Hydrocortisone 50 mg; Patients with central nervous system disease received additional intrathecal therapy twice weekly until clear of malignant cells, then once weekly for 4 weeks, then radiotherapy was initiated |
| **** Methotrexate | Dose held for creatinine clearance < 50ml/min. |
CALGB, Cancer and Leukemia Group B; ANC, absolute neutrophil count
Evaluation and Response Criteria
Toxicity was monitored in all patients using the CALGB-expanded National Cancer Institute Common Toxicity Criteria, version 2.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcmanual_v4_10-4-99.pdf) and was monitored throughout therapy as well as during follow-up if late toxicities were noted. Radiographic scans of the chest, abdomen and pelvis as well as other known areas of disease in patients with lymphoma were required after every 2 courses of therapy, as were bone marrow examinations in patients with marrow involvement. Response criterion followed the standard at the time, which mirrors the updated criterion by Cheson et al, (1997), however this is less stringent than current criterion for lymphoma response, which requires nodal masses in aggressive lymphomas to have functional evaluation with positron emission tomography (PET) imaging for response to be assessed. While this was commonly done for patients on this study, it was not mandated as early in the study gallium scans were used in some cases instead.
Statistical Methods
This phase II study was powered for 100 evaluable patients with the expectation that about 85% of the subjects would be under 60 years of age and this stratum was used to test the null hypothesis that the complete response (CR) rate with this treatment is ≤ 60% in those < 60 years old versus the alternative hypothesis that the CR rate is ≥ 80%, with type I and type II error rates of approximately 0.08 and 0.1, respectively. The response rates for those ≥ 60 years old were calculated and presented descriptively. Formal disease status evaluation was planned for every 3 months in the first 2 years, every 6 months for 3 more years and annually for 5 more. Endpoints were censored at the time of last clinical evaluation for disease-free and event-free status. OS was measured from study entry to death from any cause or censored on the date last known alive. Treatment failure was defined as progressive disease, death from any cause, or removal from protocol therapy without response. Survival function estimates were computed using the product-limit method and survival distributions were compared using the log-rank test (Kalbfleisch & Prentice, 1980). The database was updated for this analysis on October 30, 2013.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center (Durham, NC). As part of the quality assurance program of the CALGB (ALLIANCE), members of the audit Committee visited all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumour response and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 29 patients of the 105 patients under this study.
RESULTS
Patient characteristics
The study was activated on 15 May 2002 and closed to accrual on 29 September 2009, with 105 patients enrolled. The median follow-up time for the 80 survivors was 6.4 years with a range of 2.4–10.3 years. Using the WHO classification at the time the study was enacted (Diebold et al, 2001), 89 had classic Burkitt and 16 had Burkitt-like leukaemia/lymphoma according to the local haematopathologist’s diagnosis. Using the definition of >25% marrow involvement or any peripheral blood Burkitt cells to define leukaemia, there were 29 (28%) patients with leukaemia and 76 (72%) with lymphoma. Ninety-seven patients had MYC analysed and 79 were positive by either local or central pathology testing. Ten of the lymphoma patients and 6 of the leukaemia patients had Burkitt-like histology. Material for central pathology review was obtained for 104 (99%) with 99 (94%) having sufficient material to render a diagnosis. Using the definitions employed at the time the protocol was initiated (Diebold et al, 2001), 58 patients were confirmed as BL, 20 as probable Burkitt lymphoma; 21 were felt on central review to be a different high risk, aggressive lymphoma such as ‘double hit’ or ALL. Using current definitions (Leoncini et al 2008), the 58 confirmed as BL remained so, though 16 were felt to likely be Burkitt but with insufficient material for complete central confirmation of pathology, and 25 were other high-risk subtypes.
Table II summarizes the pretreatment characteristics and known risk factors for all patients. Additionally, 14 (14%) presented with CNS disease. There were major differences between the two age cohorts with more males in the younger group (80% vs 39%; p<0.0001) and there was a greater percentage of higher IPI risk patients in the ≥60 cohort (p<0.0001).
Table II. Pretreatment characteristics for all 105 patients enrolled on CALGB 10002 and for comparison 133 patients enrolled on the previous study CALGB 9251.
The current study included: 15% Burkitt-like disease, 28% with leukaemia and 14% with central nervous system disease at diagnosis, compared with 22%, 38% and 8%, respectively, in CALGB 9251.
| Characteristic | <60 years old |
≥60 years old |
p-value (comparing ages) |
CALGB 10002 Total |
CALGB 9251 (Rizzieri et al 2004) |
P value (comparing studies) |
|---|---|---|---|---|---|---|
| Patients (n) | 77 | 28 | 105 | 133 | ||
| Median age, years (range) | 36 (19–59) | 64 (60–79) | 43 (19–79) | 48 (17–78) | 0.411 | |
| Age ≥ 60 years | 28 (27%) | 32 (24%) | 0.769 | |||
| Males, n (%) | 62 (80%) | 11 (39%) | < 0.0001 | 73 (69.5%) | 92 (69%) | 0.954 |
| Race | 0.92 | 0.450 | ||||
| White | 68 (88%) | 25 (89%) | 93 (89%) | 118 (89%) | ||
| Hispanic | 4 (5%) | 1 (4%) | 5 (5%) | 3 (1%) | ||
| Black | 4 (5%) | 1 (4%) | 5 (5%) | 10 (8%) | ||
| Asian | 1 (1%) | 1 (1%) | 2 (2%) | 2 (1%) | ||
| Missing | 1(1%) | |||||
| B Symptoms | 38 (49%) | 12 (43%) | 0.66 | 50 (48%) | 72 (55%) | 0.289 |
| Performance Status (CALGB) ≥ 2 | 17 (22%) | 8 (29%) | 0.61 | 25 (24%) | 40 (30%) | 0.281 |
| ≥ 2 Extra-Nodal Sites | 46 (60%) | 13 (46%) | 0.27 | 59 (56%) | 51 (38%) | 0.006 |
| Elevated LDH | 52 (67%) | 22 (79%) | 0.34 | 74 (70%) | 116 (87%) | 0.001 |
| Lymphoma Stage 3 or 4 | 35 (45%) | 16 (57%) | 0.38 | 51 (49%) | 114 (86%) | <0.0001 |
| IPI Risk Group | < 0.0001 | 0.005 | ||||
| Low | 31 (40%) | 0 (0%) | 31 (30%) | 15 (11%) | ||
| Low-Intermediate | 17 (22%) | 8 (29%) | 25 (24%) | 46 (35%) | ||
| High-Intermediate | 18 (23%) | 11 (39%) | 29 (28%) | 44 (33%) | ||
| High | 11 (14%) | 9 (32%) | 20 (19%) | 28 (21%) |
CALGB, Cancer and Leukemia Group B; LDH, lactate dehydrogenase; IPI, International Prognostic Index
Treatment Delivery and Toxicity
Overall, 81 patients (77%) completed at least 6 of the 7 planned cycles of therapy, with the median time between cycles of 3 weeks. Adverse non-fatal events or patient withdrawal accounted for 16 patients (15%) not completing all cycles. There were 9 patients who ended treatment due to death. Five were treatment-related and 4 died of progressive disease (2 actively being treated and 2 who withdrew early and later progressed). Two additional patients died of treatment-related complications after all therapy was completed: 1 died 2 months after all therapy completed and 1 withdrew due to toxicities after 3 cycles and died 2 months later, though neither had progressive disease at the time of death. Thus 7 deaths were felt to be directly related to the therapy. Two deaths were in the <60-year-old cohort (1 infection and 1 pulmonary failure) and 5 in the ≥60-year-old group (3 infection, 1 CNS bleeding event and 1 pulmonary failure). Among the ≥60-year-old cohort of 28 patients, 11 (39%) completed all 7 cycles as compared with 83% of those under 60 years of age; the older patients had higher rates of ending therapy for adverse events, withdrawal, or early death compared to the younger cohort (57% vs 12%). Only two (1.9%) patients overall did not complete therapy due to early progression – one in each age cohort. Three enrolled patients were withdrawn early because one was determined to have a different lymphoma, one was HIV-positive and one underwent an allogeneic transplantation as soon as a CR was achieved.
Data were available from all patients to assess toxicity. The most common clinically significant toxicities are listed in Table III. Grade 4 neutropenia still occurred in most patients. Severe (≥grade 3) febrile neutropenia or documented bacterial infection occurred at least once in 98 patients (93%). Mucositis or stomatitis was common (69% of patients had grade 3+), and 30% had grade 3+ nausea, vomiting or diarrhoea. Renal insufficiency was seen in 10% of patients; 8% had tumour lysis syndrome, but none was life-threatening. Nineteen patients (18%) had grade 3+ pulmonary adverse events from a variety of causes, though primarily described as dyspnea/hypoxia, upper respiratory toxicity (not otherwise specified), pneumonitis or pleural effusions. Motor or sensory neuropathies or confusion were reported in 25% of patients: grade 3 sensory in 8 patients, grade 3 motor in 4 patients, and grade 3 confusion in 4 patients with 1 grade 4. While isolated cases of seizures, behavioral changes and mood disorders were also encountered, no late onset leucoencephalopathy was reported.
Table III. Maximum toxicities reported per patient for entire treatment course.
| Patients, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Grade 3 (severe) |
Grade 4 (life-threatening) |
Grade 3–5 | *p-value | ||||
| Toxicity | <60 years | ≥60 years | <60 years | ≥60 years | <60 years | ≥60 years | |
| Infection or febrile neutropenia | 58 (75) | 19 (68) | 13 (17) | 4 (14) | 72 (94) | 26 (93) | 1.000 |
| Mucositis, stomatitis, oesophagitis | 40 (52) | 15 (54) | 10 (13) | 7 (25) | 50 (65) | 22 (79) | 0.24 |
| Gastrointestinal (non-mucous membrane associated) | 20 (26) | 8 (29) | 2 (3) | 2 (7) | 22 (29) | 10 (36) | 0.48 |
| Hepatobiliary | 27 (35) | 6 (21) | 1 (1) | 1 (4) | 28 (36) | 7 (25) | 0.35 |
| Renal | 4 (5) | 5 (18) | 1 (1) | 1 (4) | 5 (6) | 6 (21) | 0.06 |
| Pulmonary | 7 (9) | 3 (11) | 6 (8) | 1 (4) | 14 (18) | 5 (18) | 1.00 |
| Cardiac/circulatory | 6 (8) | 4 (14) | 5 (6) | 4 (14) | 11 (14) | 8 (29) | 0.15 |
| Metabolic | 32(42) | 14 (50) | 11 (14) | 7 (25) | 43 (56) | 21 (75) | 0.11 |
| Dermatological | 9 (12) | 5 (18) | 1 (1) | 0 | 10 (13) | 5 (19) | 0.54 |
| Neurological | 14 (18) | 11(39) | 1 (1) | 0 | 15 (19) | 11 (39) | 0.045 |
| Haemorrhage | 13 (17) | 4 (14) | 0 | 0 | 13 (17) | 5 (18) | 1.00 |
- Infection includes all infections under the category of infection, including febrile neutropenia.
- Mucositis, stomatitis, oesophagitis includes just these events.
- Gastrointestinal includes all other AEs under the gastrointestinal category, excluding mucositis, stomatitis and oesophagitis.
P-value for the differences in grade 3–5 toxicities between age cohorts
Response and Survival
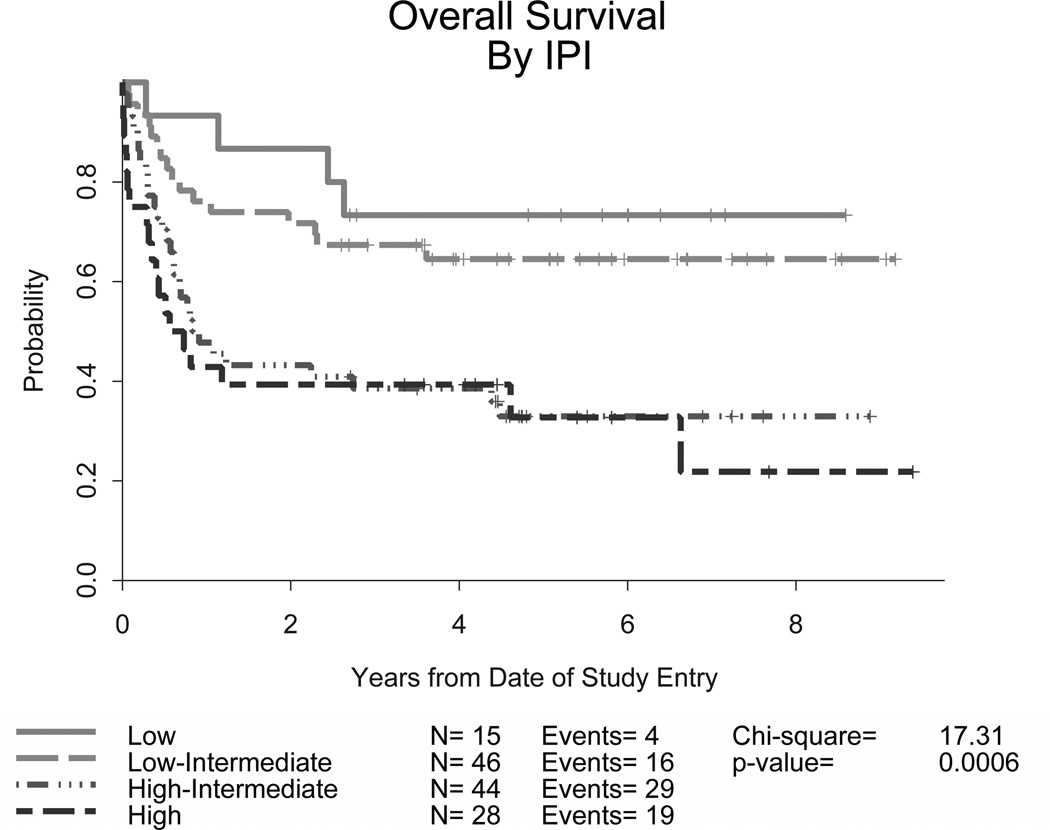
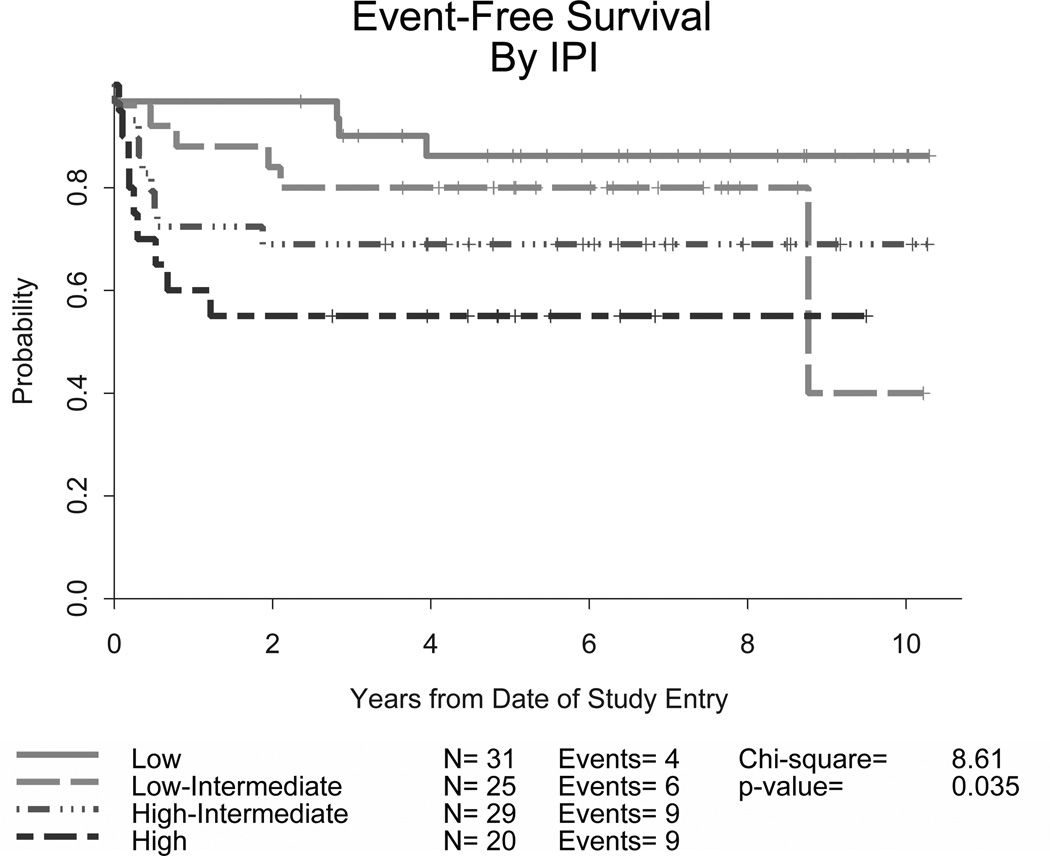
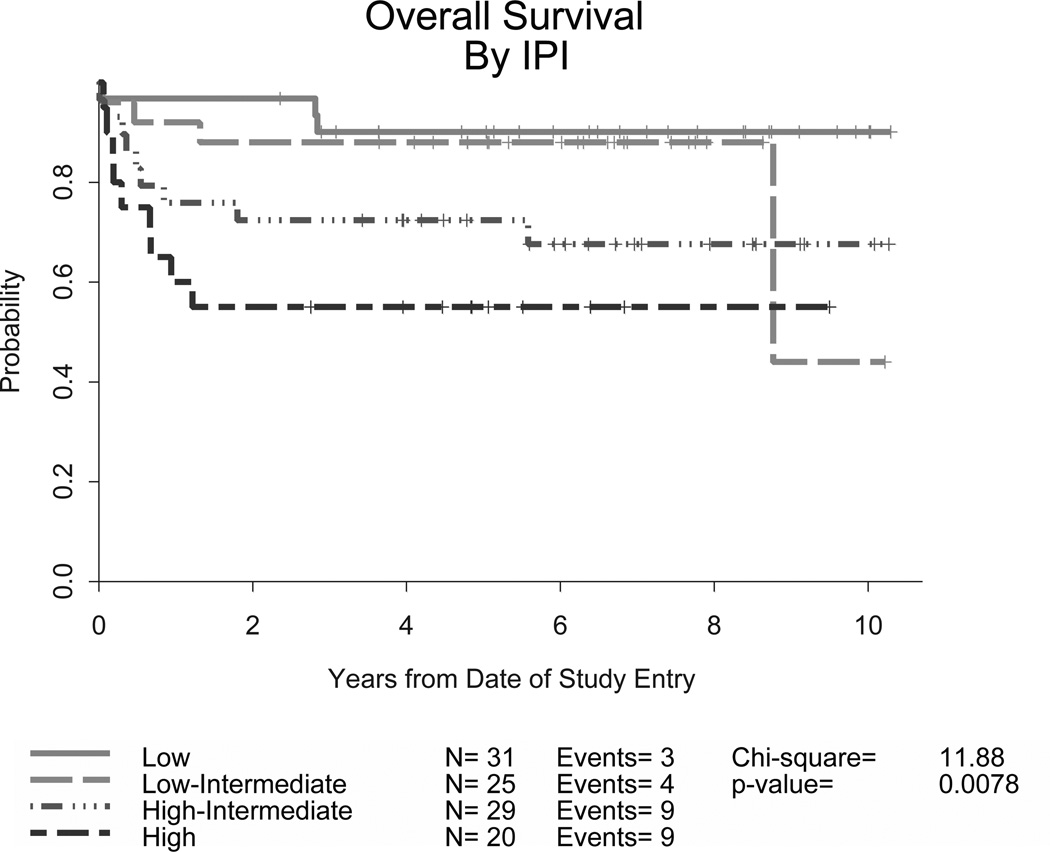
The overall CR rate was 83% (87/105) (95% confidence interval [CI] 74–90%) and there was no significant difference in the CR rate for younger adults (86%, 95% CI, 76–93%) versus those aged 60 years or older (75%. 95% CI, 55–89%). Currently, 77 (73%) patients remain in remission on long-term follow up (81% of those under 60 and 54% of those ≥60 years old, p =0.002). At 2 years, the EFS was 78% (95% CI, 69 – 85%) and OS was 80% (95% CI, 71 – 86%). Ten patients (10%) progressed after attaining a remission and subsequently received various therapies; 7 died due to disease with a median post-relapse survival of 1 year. Outcomes were better for the younger cohort of patients (Table IV). CNS relapses were noted in only 4 patients, 2 with low/intermediate and 2 with high IPI risk scores; none of these were in the group of 14 patients with CNS disease at study entry. Information on Ki-67 expression was available for 72 patients: 4 were <90% and 7 were equal to 90%. In this small group of lower expressing patients, there was no clear difference in remission rates or outcomes when compared to the higher expressing group. Overall, the survival curves plateaued approximately 2 years after completing treatment, with few relapses following this time point. Though outcomes were encouraging for all groups, response rates and survival endpoints differed significantly according to IPI risk criteria (p<0.0001), with higher risk patients having worse EFS and OS (Table IV and Figures 1A and 1B). Nevertheless, over half of these high risk patients were long term survivors. The 4-year EFS and OS for 31 patients with low IPI scores were 86% and 90%, respectively.
Table IV. Response evaluation by age group and for all patients on CALGB studies 10002 and 9251; and by IPI category.
| < 60 years | ≥ 60 years | CALGB 10002 | CALGB 9251 | |
| Patients (n) | 77 | 28 | 105 | 133 |
| Complete Response (95% CI) | 86% (76, 93%) | 75% (55, 89%) | 83% (74, 90%) | 69% (61, 77%) |
| Current Status of All Patients: | ||||
| Continuous Remission | 62 (80%) | 15 (54%) | 77 (73%) | 58 (44%) |
| Treatment-related death | 2 (3%) | 5 (18%) | 7 (7%) | 15 (11%) |
| Died from Progressive disease | 9 (12%) | 7 (25%) | 16 (15%) | 54 (41%) |
| Died from another cause | 4 (5%) | 1 (3%) | 5 (5%) | 6 (5%) |
| 2-year probability EFS (95% CI) | 0.87 (0.77, 0.93) | 0.54 (0.34, 0.70) | 0.78 (0.69, 0.85) | 0.49 (0.40, 0.57) |
| 4-year probability EFS (95% CI) | 0.82 (0.71, 0.89) | 0.54 (0.34, 0.70) | 0.74 (0.65, 0.81) | 0.46 (0.38, 0.55) |
| 2-year probability OS (95% CI) | 0.87 (0.77, 0.93) | 0.61 (0.40, 0.76) | 0.80 (0.71, 0.86) | 0.57 (0.48, 0.65) |
| 4-year probability OS (95% CI) | 0.84 (0.74, 0.91) | 0.61 (0.40, 0.76) | 0.78 (0.69, 0.85) | 0.52 (0.43, 0.60) |
| Hazard Ratio | 3.0 (1.4, 6.3) | |||
| CALGB 10002 | CALGB 9251 | |||
| IPI Category | 4-year probability EFS (95% CI) |
4-year probability OS (95% CI) |
4-year probability EFS (95% CI) |
4-year probability OS (95% CI) |
| Low | 0.86 (0.67,0.95) | 0.90 (0.72,0.97) | 0.67 (0.38,0.85) | 0.73 (0.44,0.89) |
| Low-Intermediate | 0.80 (0.58,0.91) | 0.88 (0.67,0.96) | 0.56 (0.41,0.69) | 0.65 (0.49,0.77) |
| High-Intermediate | 0.69 (0.49,0.82) | 0.72 (0.52,0.85) | 0.36 (0.22,0.50) | 0.39 (0.24,0.52) |
| High IPI | 0.55 (0.31,0.73) | 0.55 (0.31,0.73) | 0.35 (0.19,0.53) | 0.39 (0.22,0.57) |
CALGB, Cancer and Leukemia Group B; IPI, International Prognostic Index; EFS, event-free survival; OS, overall survival; 95% CI, 95% confidence interval.
Figure 1. Event-free (A) and overall survival (B) for all patients stratified by IPI criteria in CALGB 10002 and overall survival for all patients stratified by IPI criteria in CALGB 9251 (C).
Though developed for diffuse large B-cell lymphoma, the IPI was found to predict outcomes for our patients with BL as well. The addition of rituximab appears to improve outcomes compared to the prior regimen (CALGB 9251) without. CALGB, Cancer and Leukemia Group B; IPI, International Prognostic Index
In focusing on the 58 patients with material submitted and confirmed to be BL using current criterion, the 2-year EFS and OS was 79% (95% CI, 66–88%) and 81% (95% CI, 68–89%) respectively. The 25 with other high risk lymphoma based on central pathology review had a slightly lower 2-year EFS and OS (64%; [95% CI, 42–79%] for both).
Comparison with prior ‘chemotherapy only’ results
These data compare favourably with our prior study for a similar adult patient population, CALGB 9251 (Rizzieri et al, 2004). It is important to note in this retrospective comparison that our prior study involved more patients with an elevated lactate dehydrogenase (LDH) or advanced stage disease and thus a slightly higher overall IPI risk grouping (Table II). With the current study’s addition of growth factor support and immunotherapy, similar rates of treatment-related mortality (TRM) were noted (9% compared to 13%). Response rates, EFS and OS improved when comparing across IPI risk groups (Table IV and Figure 1C).
A number of Cox proportional hazards models were fit to determine the best model. Inclusion of the individual factors in the IPI resulted in a better model than the summary risk category. The risk factors included in the model were age as a continuous variable and categorical variables coded 0,1 indicating more than 1 extra-nodal site, advanced stage of disease, performance status greater than 1, elevated LDH and study. After adjustment for these factors, the hazard ratio was 0.38, indicating a marked reduction in risk using the current CALGB 10002 regimen (p=0.0001; data not shown).
DISCUSSION
Burkitt lymphoma responds well to intensive chemotherapy that includes high doses of antimetabolites and alkylating agents delivered in a dose dense fashion (Magrath et al, 1996; Murphy et al, 1986; Schwenn et al, 1991; Hann et al, 1990; McMaster et al, 1991; Larson et al, 1995;). However, relapses still occur and treatment-related toxicities have made this approach infeasible for many older patients. Applying the principles of chemo-immunotherapy is attractive in this disease given the high expression of CD20 and improved outcomes seen in other lymphomas and a single agent ‘window study’ in Burkitt lymphoma (Meinhardt et al, 2010). Thus, this study evaluated three modifications to our prior regimen – the addition of rituximab, primary prophylaxis with filgrastim and the elimination of prophylactic cranial radiation. Results are encouraging, with a high rate of study completion, high remission rates and encouraging long term survival. We found that the IPI was highly predictive of long-term outcome in this cohort of BL patients. Of note, those under 60 years of age trended to a higher remission rate than those 60 years of age or older, though this was not statistically significant. What was significant was the increased durability of remission in the younger vs. older population (81% vs. 54%), possibly indicating a different biology in Burkitts disease in older patients, as is noted in other hematopoietic malignancies (Rao et al, 2009) or the importance of the higher proportion of subjects in the younger age group completing the entire dose dense therapy protocol.
These data should be interpreted in light of our evolving understanding of BL and other high risk, aggressive non-Hodgkin lymphomas. As the diagnosis has always been made from a constellation of morphology, immunophenotyping and cytogenetics, discordance between haematopathologists has been a well-recognized concern (Rizzieri et al, 2004). Our evolving understanding of the illness has led the most current WHO classification schema (Leoncini et al 2008) to be more restrictive in the diagnosis of BL, while deleting the ‘Burkitt-like’ designation in use at the time this study was implemented. The WHO recognizes that still there are cases in which diagnosis of BL versus other aggressive lymphomas is controversial and in these cases the aggressive non-Burkitt lymphomas (including, but not limited to, ‘double’ or ‘triple hit’ lymphomas) are typically treated as BL, though optimal treatment remains to be determined (Leoncini et al 2008). Our study includes such patients based on the current definitions in use. However, a separate analysis of the 58 subjects with material submitted and confirmed to be BL with the current classification system revealed outcomes similar to the whole group of 105 patients, while those with other high risk diseases seem to fare a bit worse, though the subgroups are small.
Common antimetabolite-containing regimens, such as CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, high-dose cytarabine), yield good results in this disease; however, most experience has been reported with children or young adults (Magrath et al, 1996; Mead et al, 2002). Notably, lowering the dose of methotrexate to 3 g/m2 was associated with poor results for intermediate and high risk patients (2-year EFS 49%) (Mead et al, 2008). While there has been a retrospective analysis of adding rituximab to a CODOX-M/IVAC type backbone that was discouraging (Barnes et al, 2011), prospective chemo-immunotherapy studies have recently been completed and report the addition of rituximab to a modified CODOX-M/IVAC backbone has very encouraging results (Corazzelli et al, 2012; Evens et al, 2013). Further, Dunleavy et al (2011) reported preliminary data on the use of ‘DA-EPOCH-R’ (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab) in patients with MYC-positive diffuse large cell or Burkitt lymphoma, noting 97% EFS. The use of rituximab and younger patient age were both associated with improved outcomes in these studies, and also seen in our report. The populations in the above studies were similar to ours, though the overall small size of the studies did not allow breakdown by IPI categorization, as we have, to allow a more direct comparison. Kasamon et al (2013) recently published a small report focused on adults and the use of high dose, alkylator and rituximab-based therapy for higher risk patients. This group noted that induction followed by high dose cyclophosphamide therapy (but not using stem cell support), followed by maintenance, resulted in a 3-year overall survival of 57%, comparable to our high risk group, however TRM was high (24%) (Kasamon et al, 2013). Ferreri et al (2012) reported the results of a similar dose intense, short course, chemoimmunotherapy induction followed by high dose alkylator-based therapy (requiring stem cell support for many) focusing on adults with Burkitts disease associated with HIV virus. Again the chemo-immunotherapy combination proved tolerable with high rates of response and 11 of 15 remaining progression-free at 2 years follow-up (Ferreri et al, 2012). The Northern Italian Leukaemia Group recently published results for adult Burkitt patients using a similar backbone of chemoimmunotherapy as our current study, noting a high TRM of 18% but similar long term outcome with 67% 3-year OS, with marked differences based on IPI status, as we have also shown (Intermesoli et al, 2012). Similarly, Hoelzer et al (2012) presented preliminary results of a similar approach in a cohort of 363 adults, in which they noted that chemoimmunotherapy was well tolerated and resulted in high response rates although, commensurate with our data, results in high risk IPI patients remained less encouraging.
In order to assess the added benefit of the combination of immunotherapy and chemotherapy in the adult population, we compared the results from CALGB 10002 with our prior study, CALGB 9251, which used a nearly identical chemotherapeutic approach but without the use of the monoclonal antibody. CALGB 9251 also used more intensive CNS prophylaxis than now appears necessary as well as less cytarabine. While the treatment groups were similar in these 2 studies, there was a trend to more low risk patients in the current study (30% vs. 11%). Although the TRM was similar, the current study resulted in a higher proportion of patients completing at least 6 cycles of therapy (77% vs. 65%) and fewer patients progressing while on study (2% vs. 14%). Improved outcomes with the CALGB 10002 regimen were also noted when comparing within individual IPI risk categories (Table IV and Figure 1). In a multivariate analysis adjusting for risk factors, treatment on the current CALGB 10002 chemo-immunotherapy regimen improved survival compared with the prior regimen of CALGB 9251 (hazard ratio 0.38, p=0.0001), supporting the use of chemo-immunotherapy for BL in adults. However, only a randomized, prospective study can truly validate this conclusion. There is still room for improvement in older patients, who experience a higher rate of adverse events, and in those who are high risk by IPI criteria. Newer therapies targeting cell surface antigens other than CD20 or dysregulated B-cell receptor or intracellular pathways have been effective in other haematological malignancies. Bruton’s tyrosine kinase inhibition in CLL (Ponader et al, 2012; Herman et al, 2011), CD22 targeting with an immunoconjugate, such as inotuzumab ozogamicin (Advani et al, 2010), CD19 targeting chimeric antigen receptors or T-cell engaging bi-specific antibodies, such as blinatumomab (Topp et al, 2011), are encouraging in their early data and merit exploration in these patients.
Acknowledgements
The research for CALGB 10002 (Alliance) was supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Christiana Care Health Services, Inc. CCOP, Wilmington, DE, Stephen Grubbs, M.D., supported by CA45418
Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, M.D., Ph.D., supported by CA32291
Duke University Medical Center, Durham, NC, Jeffrey Crawford, M.D., supported by CA47577
Monter Cancer Center of North Shore - LIJ Health Systems, Lake Success, NY, Daniel Budman, MD, supported by CA35279
Rhode Island Hospital, Providence, RI, William Sikov, M.D., supported by CA08025
Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, M.D., supported by CA59518
The Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, M.D., supported by CA77658
University of California at San Diego, San Diego, CA, Barbara A. Parker, M.D., supported by CA11789
University of Chicago, Chicago, IL, Hedy L. Kindler, M.D., supported by CA41287
University of Illinois MBCCOP, Chicago, IL, David J. Peace, M.D., supported by CA74811
University of Iowa, Iowa City, IA, Daniel A. Vaena, M.D., supported by CA47642
University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, M.D., supported by CA31983
University of Minnesota, Minneapolis, MN, Bruce A. Peterson, M.D., supported by CA16450
University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, M.D., supported by CA47559
University of Vermont, Burlington, VT, Steven M. Grunberg, M.D., supported by CA77406
Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, M.D., supported by CA03927
Footnotes
Prior Presentations: Selected as an Oral Presentation for ASH 2010
Author Contribution:
DR- Patient Care, Data Acquisition, Data Analysis, Manuscript Preparation.
JJ- Data Analysis, Manuscript Preparation.
JB- Protocol Design, Patient Care, Data Acquisition, Data Analysis, Manuscript Preparation, Funding.
GL- Data Analysis, Data Acquisition, Manuscript Preparation.
KB- Patient Care, Data Analysis, Manuscript Preparation.
RL- Protocol Design, Patient Care, Data Acquisition, Data Analysis, Manuscript Preparation, Funding.
EH- Data Acquisition, Data Analysis. Manuscript Preparation
BC- Protocol Design, Manuscript Preparation.
BP- Patient care, Data Acquisition, Data Analysis, Manuscript Preparation
TS- Patient care, Data Acquisition, Data Analysis, Manuscript Preparation
SN- Patient care, Data Acquisition, Data Analysis, Manuscript Preparation
Conflicts of Interest: The authors report there are no reported conflicts of interest related to this study
References
- Advani A, Coiffier B, Czuczman MS, Dreyling M, Foran J, Gine E, Gisselbrecht C, Ketterer N, Nasta S, Rohatiner A, Schmidt-Wolf IG, Schuler M, Sierra J, Smith MR, Verhoef G, Winter JN, Boni J, Vandendries E, Shapiro M, Gayad L. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study. Journal of Clinical Oncology. 2010;28:2085–2093. doi: 10.1200/JCO.2009.25.1900. [DOI] [PubMed] [Google Scholar]
- Barnes JA, Lacasce AS, Feng Y, Toomey CE, Neuberg D, Michaelson JS, Hochberg EP, Abramson JS. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt's lymphoma: a retrospective analysis. Annals of Onocology. 2011;22:1859–1864. doi: 10.1093/annonc/mdq677. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid M, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. Revised response criteria for malignant lymphoma. Journal of Clinical Oncology. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Corazzelli G, Frigeri F, Russo F, Frairia C, Arcamone M, Esposito G, De Chiara A, Morelli E, Capobianco G, Becchimanzi C, Volzone F, Saggese M, Marcacci G, De Filippi R, Vitolo U, Pinto A. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and 'unclassifiable' highly aggressive B-cell lymphoma. British Journal of Haematology. 2012;156:234–244. doi: 10.1111/j.1365-2141.2011.08947.x. [DOI] [PubMed] [Google Scholar]
- Diebold J, Jaffe ES, Raphael M, Warnke RA. World health Organization Classification of Tumours. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. pp. 181–184. [Google Scholar]
- Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, Widemann B, Staudt LM, Jaffe ES, Little RF, Wilson WH. Low-intensity therapy in adults with Burkitt’s lymphoma. New England Journal of Medicine. 2013;369:1915–1925. doi: 10.1056/NEJMoa1308392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evens AM, Carson KR, Kolesar J, Nabhan C, Helenowski I, Islam N, Jovanovic B, Barr PM, Caimi PF, Gregory Sa, Gordon LI. A multicenter phase II study incorporating high-dose rituximab and liposomal doxorubicin into the CODOX-M/IVAC regimen for untreated Burkitt's lymphoma. Annals of Oncology. 2013;24:3076–3081. doi: 10.1093/annonc/mdt414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri AJ, Bruno Ventre M, Donadoni G, Cattaneo C, Fumagalli L, Foppoli M, Mappa S, Govi S, DiNocola M, Rossi G, Tirelli U, Caligaris-Cappio F, Spina M, Re A. Safety and activity of a new intensive short-term chemoimmunotherapy in HIV-positive patients with Burkitt lymphoma. British Journal of Haematology. 2012;159:252–255. doi: 10.1111/bjh.12020. [DOI] [PubMed] [Google Scholar]
- Hann IM, Eden OB, Barnes J, Pinkerton CR. MACHO chemotherapy for Stage IV B-cell lymphoma and B-cell acute lymphoblastic leukemia of childhood. British Journal Haematology. 1990;76:359–364. doi: 10.1111/j.1365-2141.1990.tb06368.x. [DOI] [PubMed] [Google Scholar]
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. Journal of Clinical Oncology. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, Flynn J, Jones J, Blum KA, Buggy JJ, Hamdy A, Johnson AJ, Byrd JC. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer D, Ludwig W, Thiel E, Gassmann W, Löffler H, Fonatsch C, Rieder H, Heil G, Heinze B, Arnold R, Hossfeld D, Büchner T, Koch P, Freund M, Hiddemann W, Maschmeyer G, Heyll A, Aul C, Faak T, Kuse R, Ittel TH, Gramatzki M, Diedrich H, Kolbe K, Fuhr HG, Fischer K, Schadeck-Gressel C, Weiss A, Strohscheer I, Metzner B, Fabry U, Gökbuget N, Völkers B, Messerer D, Uberla K. Improved outcome in adult B cell acute lymphoblastic leukemia. Blood. 1996;87:495–508. [PubMed] [Google Scholar]
- Hoelzer D, Walewski J, Döhner H, Schmid M, Hiddemann W, Baumann A, Serve H, Dührsen U, Hottman A, Thiel E, Dengler J, Kneba M, Schuler M, Schmidt-Wolf I, Beck J, Hertenstein B, Reichle A, Domanska-Czys K, Fietkau R, Horst H, Rieder H, Schwartz S, Burmeister T, Goekbuget N. Substantially Improved Outcome of Adult Burkitt Non-Hodgkin Lymphoma and Leukemia Patients with Rituximab and a Short-Intensive Chemotherapy; Report of a Large Prospective Multicenter Trial. Blood (ASH Annual Meeting abstracts. 2012;120:667a. [Google Scholar]
- Intermesoli T, Rambaldi A, Rossi G, Delaini F, Romani C, Pogliani EM, Pagani C, Angelucci E, Terruzzi E, Levis A, Cassibba V, Mattei D, Gianfaldoni G, Scattolin AM, Di Bona E, Oldani E, Parolini M, Gökbuget N, Bassan R. High cure rates in Burkitt lymphoma and leukemia: A Northeren Italy Leukemia Group study of the German short intensive rituximabchemotherapy program. Haematologica. 2013;98:1718–1725. doi: 10.3324/haematol.2013.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbfleisch J, Prentice R. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons, Inc.; 1980. [Google Scholar]
- Kasamon YL, Brodsky RA, Borowitz MJ, Ambinder RF, Crilley PA, Cho SY, Tsai HL, Smith BD, Gladstone DE, Carraway HE, Huff CA, Matsui WH, Bolaños-Meade J, Jones RJ, Swinnen LJ. Brief intensive therapy for older adults with newly diagnosed Burkitt or atypical Burkitt lymphoma/leukemia. Leukemia and Lymphoma. 2013;54:483–490. doi: 10.3109/10428194.2012.715346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, Duggan D, Davey FR, Sobol RE, Frankel SR. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85:2025–2037. [PubMed] [Google Scholar]
- Leoncini L, Raphael M, Stein H, Harris NL, Jaffe ES, Kluin PM. World health Organization Classification of Tumours. In: Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 262–264. [Google Scholar]
- Magrath I, Adde M, Shad A, Venzon D, Seibel N, Gootenberg J, Neely J, Arndt C, Nieder M, Jaffe E, Wittes RA, Horak ID. Adults and children with small non cleaved cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. Journal of Clinical Oncology. 1996;14:925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- Maruyama D, Watanabe T, Maeshima AM, Nomoto J, Taniguchi H, Azuma T, Mori M, Munakata W, Kim SW, Kobayashi Y, Matsuno Y, Tobinai K. Modified cyclophosphamide, vincristine, doxorubicin, and methotrexate (CODOX-M) / ifosfamide, etoposide, and cytarabine (IVAC) therapy with or without rituximab in Japanese adult patients with Burkitt lymphoma(BL) and B cell lymphoma, unclassifiable, with features intermediated between diffuse large B cell lymphoma and BL. International Journal of Hematology. 2010;92:732–743. doi: 10.1007/s12185-010-0728-0. [DOI] [PubMed] [Google Scholar]
- McMaster ML, Greer JP, Greco FA, Stein RS, Cousar JB, Flexner JM, Hainsworth JD. Effective treatment of small non-cleaved cell lymphoma with high-intensity brief-duration chemotherapy. Journal of Clinical Oncology. 1991;9:941–946. doi: 10.1200/JCO.1991.9.6.941. [DOI] [PubMed] [Google Scholar]
- Mead GM, Sydes MR, Walewski J, Grigg A, Hatton CS, Pescosta N, Guarnaccia C, Lewis MS, McKendrick J, Stenning SP, Wright D. An International evaluation of CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of the United Kingdom Lymphoma Group LY06 study. Annals of Oncology. 2002;13:1264–1274. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- Mead GM, Barrans SL, Qian W, Walewski J, Radford JA, Wolf M, Clawson SM, Stenning SP, Yule CL, Jack AS. A prospective clinicopathologic study of dose modified CODOX-M/ IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria. Blood. 2008;112:2248–2260. doi: 10.1182/blood-2008-03-145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A, Burkhardt B, Zimmermann M, Borkhardt A, Kontny U, Klingebiel T, Berthold F, Janka-Schuab G, Klein C, Kabickova E, Klapper W, Attarbaschi A, Schrappe M, Reiter A. Phase 2 window study on rituximab in newly diagnosed pediatric mature B cell NHL lymphoma and Burkitt Leukemia. Journal of Clinical Oncology. 2010;28:3115–3121. doi: 10.1200/JCO.2009.26.6791. [DOI] [PubMed] [Google Scholar]
- Mohamedbhai SG, Sibson K, Marafioti T, Kayani I, Lowry L, Goldstone AH, Linch DC, Ardeshna KM. Rituximab in combination with CODOX-M/IVAC: a retrospective analysis of 23 cases of non-HIV related B cell non-Hodgkin lymphoma with proliferation index >95% British Journal of Haematology. 2011;152:175–181. doi: 10.1111/j.1365-2141.2010.08447.x. [DOI] [PubMed] [Google Scholar]
- Murphy SB, Bowman WP, Abromowitch M, Mirro J, Ochs J, Rivera G, Pui CH, Fariclough D, Berard CW. Results of treatment of advanced-stage Burkitt lymphoma and B-cell acute lymphoblastic leukemia with high-dose fractionated cyclophosphamide and coordinated high-dose methotrexate and cytarabine. Journal of Clinical Oncology. 1986;4:1732–1739. doi: 10.1200/JCO.1986.4.12.1732. [DOI] [PubMed] [Google Scholar]
- Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O’Brien S, Chiorazzi N, Burger JA. Bruton's tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Valk PJM, Metzeler KH, Acharya CR, Tuchman SA, Stevenson MM, Rizzieri DA, Delwel R, Buske C, Bohlander SK, Potti A, Löwenberg B. Age-specific Differences in Oncogenic Pathway Dysregulation and Anthracycline sensitivity in Patients with Acute Myeloid Leukemia. Journal of Clinical Oncology. 2009;27:5580–5586. doi: 10.1200/JCO.2009.22.2547. [DOI] [PubMed] [Google Scholar]
- Rizzieri DA, Johnson J, Niedzwiecki D, Lee EJ, Vardiman JW, Powell BL, Barcos M, Bloomfield CD, Schiffer CA, Peterson BA, Canellos GP, Larson RA. Intensive Chemotherapy with and without Cranial Radiation for Burkitt Leukemia and Lymphoma Final Results of Cancer and Leukemia Group B Study 9251. Cancer. 2004;100:1438–1448. doi: 10.1002/cncr.20143. [DOI] [PubMed] [Google Scholar]
- Schwenn MR, Blattner SR, Lynch E, Weinstein HJ. HiCOM: A two-month intensive chemotherapy regimen for children with Stage III and IV Burkitt lymphoma and B-cell acute lymphoblastic leukemia. Journal of Clinical Oncology. 1991;9:133–138. doi: 10.1200/JCO.1991.9.1.133. [DOI] [PubMed] [Google Scholar]
- Simon R, Durrleman S, Hoppe RT, Bonadonna G, Bloomfield CD, Rudders RA, Cheson BD, Berard CW. The non-Hodgkin’s lymphoma Pathologic classification project: long-term follow up of 1,153 patients with non-Hodgkin’s lymphoma. Annals of Internal Medicine. 1988;109:939–945. doi: 10.7326/0003-4819-109-12-939. [DOI] [PubMed] [Google Scholar]
- The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. New England Journal of Medicine. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Faderl S, O’Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, Giles FJ, Verstovsek S, Wierda WE, Pierce SA, Shan J, Brandt M, Hagemeister FB, Keating MJ, Cabanillas F, Kantarjian H. Chemoimmunotherapy with Hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-Like lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, Stelljes M, Schaich M, Degenhard E, Köhne-Volland R, Brüggemann M, Ottmann O, Pfeifer H, Burmeister T, Nagorsen D, Schmidt M, Lutterbuese R, Reinhardt C, Baeuerle PA, Kneba M, Einsese H, Riethmüller G, Hoelzer D, Zugmaier G, Bargou RC. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. Journal of Clinical Oncology. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]