Abstract
Bacillus subtilis responds to a sudden decrease in temperature by transiently inducing the expression of the des gene encoding for a lipid desaturase, Δ5-Des, which introduces a double bond into the acyl chain of preexisting membrane phospholipids. This Δ5-Des-mediated membrane remodeling is controlled by the cold-sensor DesK. After cooling, DesK activates the response regulator DesR, which induces transcription of des. We show that inhibition of fatty acid synthesis by the addition of cerulenin, a potent and specific inhibitor of the type II fatty acid synthase, results in increased levels of short-chain fatty acids (FA) in membrane phospholipids that lead to inhibition of the transmembrane-input thermal control of DesK. Furthermore, reduction of phospholipid synthesis by conditional inactivation of the PlsC acyltransferase causes significantly elevated incorporation of long-chain FA and constitutive upregulation of the des gene. Thus, we provide in vivo evidence that the thickness of the hydrophobic core of the lipid bilayer serves as one of the stimulus sensed by the membrane spanning region of DesK.
Keywords: Bacillus subtilis, cerulenin, thermosensor, unsaturated fatty acids
Introduction
When bacteria are exposed to temperatures below those of their normal growth conditions, the lipids of their membrane become rigidified, leading to a suboptimal functioning of cellular activities (Mansilla and de Mendoza 2005; Mansilla et al. 2008). These organisms can acclimate to such new conditions decreasing the transition temperature of their membrane lipids, this is, the temperature at which membrane lipid bilayers undergo a reversible change of state from a liquid-crystalline (disordered) to a gel (ordered) array of the fatty acyl chains. In most bacteria, the role of introducing acyl chain disorder is fulfilled by unsaturated fatty acids (UFA), which have much lower transition temperatures than saturated fatty acids (Cronan and Gelmann 1973). Desaturation of the acyl chains of membrane phospholipids results in an increase in the membrane lipid bilayer fluidity, with restoration of normal cell function at the lower temperature.
Cold shock imposes severe constraints on the biophysical properties of Bacillus subtilis cytoplasmic membrane (Mansilla et al. 2004). In laboratory settings, a sudden temperature downshift, from 37 to 25 or 20°C, is used to trigger in B. subtilis a transiently transcriptional induction of the des gene coding for their sole lipid desaturase, Δ5-Des. This enzyme introduces double bonds in Δ5 positions of the acyl chain of preexisting membrane phospholipids (Aguilar et al. 1998; Altabe et al. 2003). This short-term membrane adaptation requires a canonical two-component regulatory system comprising the histidine kinase DesK and the response regulator DesR (Aguilar et al. 2001) (see Fig. S1). Upon cooling, DesK phosphorylates DesR, which stimulates the expression of Δ5-Des (Albanesi et al. 2004; Cybulski et al. 2004). By introducing a double bond into saturated lipids, Δ5-Des induces a kink in the fatty acids (FA) that increases membrane disorder, offsetting the fluidity decrease that otherwise accompanies cooling. This DesK-dependent desaturation of membrane phospholipids enhances survival of B. subtilis at low temperatures (Weber et al. 2001). Although the structure of full-length DesK has not yet been solved, structural studies of the catalytic core of DesK highlights the plasticity of the central Dimerization and Histidine phosphotransfer domain and suggest an important role of the transmembrane (TM) sensor domain in catalysis regulation, either by modifying the mobility of the ATP-binding domains for autokinase activity or by modulating binding of DesR to sustain the phosphotransferase and phosphatase activity (Albanesi et al. 2009). A model in which the TM domain of DesK promotes these structural changes through conformational signals transmitted by the membrane-connecting two-helical coiled-coil was postulated (Albanesi et al. 2009).
DesK is a multipass TM sensor and its activation upon a decrease in the ambient temperature appears intimately related to a decrease in the order of the acyl chain of membrane phospholipids (Cybulski et al. 2002). However, the mechanism that allows DesK to discriminate the lipid environment to promote membrane remodeling upon a drop in environmental temperature remains fragmentarily understood. Reconstitution of full-length DesK into proteoliposomes showed that, whereas the structure of the lipid head group does not affect thermosensing, the length of the acyl chains, that determine the thickness of the hydrophobic core of the lipid bilayer, exerts a profound regulatory effect on kinase domain activation at low temperatures (Martín and de Mendoza 2013). Thus, a likely hypothesis is that at low temperature, the membrane becomes thicker due to an increase in the lipid order and this change in bilayer thickness could be sensed by the TM surface of DesK, favoring its autokinase activity. However, this hypothesis is challenged by the fact that the reconstitution experiments were performed in phosphatidylcholine (PC) vesicles containing straight-chain monounsaturated FA of different chain length (Martín and de Mendoza 2013). Nevertheless, PC is absent in B. subtilis, which instead contains phosphatidylethanolamine and phosphatidylglycerol with acyl chains mainly composed of branched-chain FA.
In this paper, we have investigated the mechanism by which the antibiotic cerulenin, a specific inhibitor of FA synthesis (Fig. 1), abolishes the cold-induced UFA production. We found that the key change lies in production of an excess of FA of short chain length which are incorporated into membrane phospholipids. This is sufficient to alter the lipid–protein interaction required for activation of DesK at low temperature. Furthermore, artificially increasing the synthesis and incorporation of long-chain FA into B. subtilis membranes results in constitutive expression of des at high temperature. Our results strongly suggest that the thickness of the bilayer is an important parameter regulating the signaling state of DesK associated to its native plasma membrane. These findings accord with previous in vitro studies aimed at understanding how the compositional and functional diversity of the surrounding membrane modulates DesK sensor function.
Figure 1.
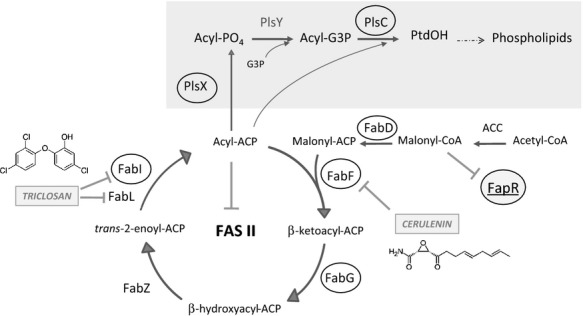
Pathway of lipid synthesis in Bacillus subtilis. Elongation of fatty acids (FA) is catalyzed by the type II fatty acid synthase (FASII) via a repeated cycle of condensation, reduction, dehydration, and a second reduction of carbon–carbon bonds, giving rise to acyl-acyl carrier protein (acyl-ACP) with two additional methylene groups at the end of each cycle. Generation of malonyl-CoA by acetyl-CoA carboxylase (ACC) is required to start the cycle of chain elongation by the complex. Phospholipid synthesis (shaded) initiates by the action of PlsX which converts acyl-ACPs to acyl-PO4. Then PlsY transfers the acyl moiety to the 1 position of glycerol-3-P (G3P) to form acyl-G3P. Acylation of the two position to form phosphatidic acid (PtdOH) is catalyzed by PlsC. Fungal toxin cerulenin inhibits the elongation condensing enzyme FabF, precluding not only FA but also phospholipid synthesis. The last step of the elongation cycle, catalyzed by enoyl-ACP reductases (FabI and FabL) is inhibited by triclosan. Expression of the genes coding for enzymes surrounded by ellipses is repressed by FapR, whose activity is, in turn, antagonized by malonyl-CoA.
Materials and Methods
Bacterial strains and growth conditions
Bacterial strains and plasmids used in the present study are listed in Table 1. Escherichia coli and B. subtilis strains were routinely grown in Luria Bertani (LB) broth at 37°C (Sambrook et al. 1989). Spizizen salts (Spizizen 1958), supplemented with 0.5% glucose, 0.01% each tryptophan and phenylalanine, and trace elements (Harwood and Cuttings 1990) were used as the minimal medium for B. subtilis. This medium was designated MM. Antibiotics were added to media at the following concentrations: erythromycin (Erm) 0.5 μg mL−1; lincomycin (Lm) 12.5 μg mL−1; chloramphenicol (Cm) 5 μg mL−1; kanamycin (Km) 5 μg mL−1; ampicilin (Amp): 100 μg mL−1, spectinomycin (Sp): 100 μg mL−1. For the experiments involving desKC and plsC expression under the control of the inducible promoters PxylA and Pspac, 0.01% xylose and 1 mmol/L Isopropyl β-D-1-thiogalactopyranoside (IPTG) were added, respectively.
Table 1.
Bacterial strains and plasmids.
| Strain or plasmid | Relevant characteristics1 | Source or reference |
|---|---|---|
| Bacillus subtilis strains | ||
| JH642 | trpC2 pheA1 | Laboratory stock |
| AKP3 | JH642 amyE::Pdes–lacZ; Cmr | Aguilar et al. (2001) |
| CM21 | AKP3 desKR::Km, thrC::PxylA-desR; Kmr Spr Cmr | Albanesi et al. (2004) |
| GS77 | JH642 fabF1 (FabFI108F) cerulenin-resistant | Schujman et al. (1998) |
| BLUP87 | GS77 amyE::Pdes–lacZ; Cmr | This study |
| BLUP34 | JH642 plsC::PspacOid-plsC; Cmr | This study |
| BLUP102 | JH642 plsC::PspacOid-plsC; Spr | This study |
| BLUP103 | BLUP102 amyE::Pdes-lacZ; Cmr Spr | This study |
| Escherichia coli strain | ||
| DH5α | supE44 thi-1 ΔlacU169 (Φ80lacZΔM15) endA1 recA1hsdR17gyrA96 relA1trp-6 cysT329::lac inmpI(209) | Laboratory stock |
| Plasmids | ||
| pCR-BluntII-TOPO | pCR-Blunt II-TOPO E. coli cloning vector; Kmr | Invitrogen |
| pJM116 | Integrative vector to construct transcriptional fusions to lacZ; integrates at the amyE locus of B. subtilis; Cmr | Perego (1993) |
| pHPKS | B. subtilis replicative vector of low copy number, Ermr Lmr | Johansson and Hederstedt (1999) |
| pCm::Sp | Antibiotic switching vector; Ampr, Spr | Steinmetz and Richter (1994) |
| pDH88 | Integrative plasmid containing the IPTG-induciblePspac promoter; Cmr | Henner (1990) |
| pMUTIN4 | Integrative plasmid containing the IPTG-inducible Pspac-oid promoter; Ermr Lmr | Vagner et al. (1998) |
| pAR11 | Contains Pdes cloned into the EcoRI–BamHI sites of pJM116 | Aguilar et al. (2001) |
| pCM9 | PxylA-desKC cloned into pHPKS | Albanesi et al. (2004) |
| pLUP30 | PspacOid of pMUTIN4 cloned into the EcoRI–HindIII sites of pDH88 | This study |
| pLUP32 | 5´ end of the plsC gene cloned into the HindIII–SphI sites of pLUP30 | This study |
| pLUP124 | PxylA-desK cloned into pHPKS | This study |
Cmr, Spr, Kmr, Ermr, Lmr, and Ampr denote resistance to chloramphenicol, spectinomycin, kanamycin, erythromycin, lincomycin, and ampicillin, respectively.
Genetic techniques
Escherichia coli competent cells were transformed with supercoiled plasmid DNA by the calcium chloride procedure (Sambrook et al. 1989). Transformation of B. subtilis was carried out by the method of Dubnau and Davidoff-Abelson (1971). The amy− phenotype was assayed with colonies grown during 48 h in LB starch plates, by flooding the plates with 1% I2-KI solution (Sekiguchi et al. 1975). amy+ colonies produced a clear halo, while amy− colonies gave no halo.
Plasmid and strains construction
In all cases, DNA fragments were obtained by PCR using the oligonucleotides described in the text (restriction sites underlined). Chromosomal DNA from strain JH642 was used as the template. The PCR products of the expected sizes were cloned into pCR-Blunt II-Topo (Promega, Madison, WI) and transformed in E. coli DH5α (Sambrook et al. 1989). Plasmid DNA was prepared using the Wizard DNA purification system (Promega Life Science) and sequenced to corroborate the identity and correct sequence of the cloned fragments.
To generate an integrative vector containing a tightly regulated IPTG-inducible Pspac-oid promoter, but without a transcriptional fusion to lacZ, we decided to replace the Pspac promoter of plasmid pDH88 (Henner 1990) by Pspac-oid of pMUTIN4 (Vagner et al. 1998). A 603-bp DNA fragment, generated by PCR using primers TerPspacOid Up (CGTGAGGAATTCAATAAAACGAAAGGCTCAGTCGAAAGA) and TerPspacOid Lw (CTGGGATCCGCATGCTGTACATCAAGCTTAATTGTGAG), containing Pspac-oid promoter from pMUTIN4, was digested with EcoRI and HindIII and cloned into the integrative plasmid pDH88, previously digested with the same enzymes to release its Pspac, yielding plasmid pLUP30.
The plsC isogenic conditional mutant was constructed as follows: a 489 bp DNA fragment, corresponding to the ribosome binding site and a 5′ portion of plsC gene, was obtained by PCR amplification using the oligonucleotides PyhdOHindIII (AATCAAAGCTTACGACAAAGGAAGTGCGAT) and YhdOSphI (TTTTTTGCATGCTTCTTTTCCGCTTGAA). The fragment was cloned into the HindIII and SphI sites of vector pLUP30, which allows expression of the plsC gene under the control of Pspac-oid. The resulting plasmid was named pLUP32. This construct was then integrated by a single-crossover event at the plsC locus of B. subtilis JH642, yielding strain BLUP34. This approach results in the conditional inactivation of plsC gene, whose expression can be controlled by Pspac-oid. This strain was checked by PCR to ensure that the plasmid is integrated in the correct site.
To allow the introduction of the reporter fusion contained in the plasmid pJM116 (Cmr) (Perego 1993), the chloramphenicol cassette present in the plsC locus of BLUP34 was changed for a spectinomycin resistance cassette through transformation and homologous recombination using the plasmid pCm::Spc (Steinmetz and Richter 1994). The resulting strain was named BLUP102. To introduce the transcriptional fusion of lacZ to the promoter region of the desaturase gene (Pdes-lacZ) into the plsC conditional mutant, the plasmid pAR11 (Aguilar et al. 2001) was linearized with ScaI and introduced by a double crossover event at the amyE locus of BLUP102 chromosome yielding strain BLUP103.
To ectopically express the Pdes-lacZ fusion in a B. subtilis JH642 cerulenin-resistant strain, plasmid pAR11 (Aguilar et al. 2001) was linearized with ScaI and introduced by a double crossover event at the amyE locus of strain GS77 (Schujman et al. 1998) giving rise to strain BLUP87.
To construct plasmid pLUP124, a 1227 bp DNA fragment containing desK was obtained by PCR amplification using the oligonucleotides DesKB33-Up (AGTAACATGGATCCCAGAAAATGAGGTAAGATC) and desKP-Dw (GCTGATCTTCTGCAGTAAATATACTAATC). The fragment was cloned into the BamHI and PstI sites of vector pARD7 (pHPKS replicative vector containing the Pxyl promoter; M. C. Mansilla, pers. comm.).
β-galactosidase assays
Bacillus subtilis cells harboring a Pdes–lacZ chromosomal fusion were grown in MM at 37°C to an OD525 of 0.35, then were split and half of the culture was treated with either 2.5 μg mL−1 of cerulenin (MIC 5 μg mL−1, Schujman et al. 2001), 0.4 μg mL−1 of triclosan (MIC 2 μg mL−1, Heath et al. 2000), or 0.01% xylose as indicated in each experiment. Then cultures were transferred to 25°C or 37°C, as indicated. B. subtillis BLUP103 cells were grown ON in MM at 37°C supplemented with 0.2 mmol/L IPTG to allow the expression of plsC. Cells were washed twice, resuspended in MM to OD525 values of 0.03 in the presence or absence of 1 mmol/L IPTG and incubated at 37°C to an OD525 of 0.35, then were split and incubated at 25 or 37°C. After each treatment, samples were taken at 1-h intervals and assayed for β-galactosidase activity as described previously (Mansilla and de Mendoza 1997). The specific activity was expressed in Miller units (Miller 1972). The results shown are the average of three independent experiments.
FA analyses
For the measurement of UFA biosynthesis, cultures of strains AKP3 (wild-type) and BLUP87 (cerr) were grown in MM at 37°C to an OD525 of 0.25 and then half of this culture was supplemented with 2.5 μg mL−1 of cerulenin for 45 min. When the strains reached an OD525 of 0.35, 2 mL of these cultures were labeled with 0.2 μCi of [14C]palmitate (specific activity, 58 mCi/mmol/L) and further shifted to 25°C for 5 h. Following incubation, cells were collected and lipids were prepared according to the method of Bligh and Dyer (1959). The FA methyl esters were prepared by transesterification of glycerolipids with 0.5 mol/L sodium methoxide in methanol (Christie 1989) and separated into saturated FA and UFA fractions by chromatography on 10% silver nitrate-impregnated Silica Gel G plates (0.5-mm thickness; Analtech Inc., Newark, DE). About 11,000 cpm of radioactivity were loaded into each lane. Chromatographic separation was achieved in a toluene solvent system at −20°C and detected by using a Typhoon 9200 PhosphorImager screen (STORM840; GE Healthcare Argentina S.A., Buenos Aires, Argentina). The radioactivity levels of the spots were quantified by ImageQuant 5.2 (GE Health Care Argentina S.A.).
Analysis of FA by GC-MS
To determine the FA composition, AKP3 cells were grown in MM to an OD525 of 0.35 at 37°C. Cultures were treated or not with 2.5 μg mL−1 cerulenin and then shifted to 25°C for 5 h. Total lipids were extracted and transesterified to yield FA methylesters as described above. The FA methylesters were analyzed in a Perkin–Elmer Turbo Mass gas chromatography–mass spectrometer on a capillary column (30 mm by 0.25 mm in diameter Varian) of 100% dimethylpolysiloxane (PE-1; Perkin-Elmer, Waltham, MA). Helium at 1 mL/min was used as the carrier gas, and the column temperature was programmed to rise by 4°C/min from 100 to 320°C. Branched-chain FA, straight-chain FA, and UFA used as reference compounds were obtained from Sigma Chemical Co (St. Louis, MO).
Bacillus subtilis strain BLUP103 was grown overnight at 37°C in MM supplemented with 0.2 mmol/L IPTG. On the following day, fresh cultures were started by washing twice, resuspended in MM and grown at 37°C in the presence or absence of 1 mmol/L IPTG. After 6 h of growth in the absence of IPTG addition, cells stopped growing because of PlsC depletion. At this point, 50 mL samples cultures were collected and lipids were prepared and analyzed as described above.
Results
Cerulenin inhibits des transcription and UFA synthesis at low temperatures
During previous work aimed to test whether preexistent lipids were able to regulate the expression of Δ5-Des (Cybulski et al. 2002; and S. G. Altabe, pers. comm.), we found that cerulenin, a specific inhibitor of FabF (Fig. 1), the sole condensing enzyme accomplishing acyl chain elongation in B. subtilis (Schujman et al. 2001) repressed the induction of the des gene during cold shock. This was an unexpected observation as previous results of our laboratory showed that inhibition of FA synthesis by cerulenin significantly increases transcription of at least 10 genes, contained in five different operons, encoding key enzymes involved in FA and phospholipid biosynthetic pathways (Schujman et al. 2001, 2003).
As this intriguing preliminary observation could indicate a new level of control in the well-studied Des signal transduction pathway, we decided to examine in detail the mechanism of des promoter regulation by cerulenin. We first assayed the effect of cerulenin on expression of the des gene using strain AKP3 (Aguilar et al. 2001). This strain contains the lacZ reporter gene under the control of the des promoter, integrated ectopically at the nonessential amyE locus. AKP3 was grown until early exponential phase at 37°C and then transferred to 25°C. Half of the culture was treated with sublethal concentration of cerulenin (2.5 μg mL−1). As shown in Figure 2A, the β-galactosidase levels of AKP3 cells growing with cerulenin were five times lower than the untreated cultures. Consistently, after a temperature downshift, AKP3 cells treated with cerulenin synthesized lower levels of UFAs than cells growing in the absence of the antibiotic (Fig. 2B and C). These data showed that cerulenin strongly inhibit des gene induction at low temperature.
Figure 2.
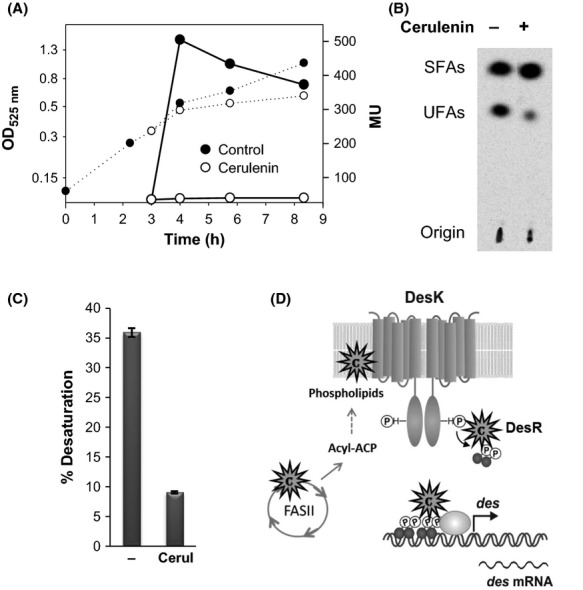
Effect of cerulenin on expression of des promoter and autoradiogram of the products of [14C]palmitate labeling. (A) Bacillus subtillisAKP3 cells (amyE::Pdes-lacZ, cerS) were grown in MM at 37°C to an OD525 of 0.35 and then cultures were split in two. One half was supplemented with cerulenin 2.5 μg mL−1 (white circles), while the other remained untreated (black circles), then cultures were transferred to 25°C. β-galactosidase-specific activities (in Miller units, MU) were determined at the indicated time intervals. Dotted lines: OD525; solid lines: β-galactosidase-specific activities. (B) UFAs synthesized by strain AKP3 after a temperature downshift, in the presence or the absence of cerulenin. Cultures were labeled with 0.2 μCi of [14C]palmitate and incubated at 25°C for 5 h, as described in Material and Methods. Lipids were extracted and transesterified; the resulting methyl esters were separated into saturated (SFAs) and unsaturated (UFAs) fractions by chromatography on silver nitrate-impregnated silica plates. About 11,000 cpm of radioactivity was loaded into all lanes. (C) Percentages of UFA synthesis. The radioactivity levels of the spots of the UFA and SFA shown in panel B were quantified by ImageQuant 5.2. Results are expressed as percentages of the total methyl esters recovered. Values are the average of three independent experiments. (D) Cerulenin could block different steps in the transmission of the cold signal. The putative sites of action of cerulenin are indicated as asterisks.
The TM segments of DesK are essential for cerulenin repression of des transcription
Repression of des transcription by cerulenin could be caused by alternative mechanisms that would block different steps in the transmission of the cold signal (Fig. 2D). It follows that, by a direct action or through inhibition of de novo lipid synthesis, cerulenin could affect sensing properties of DesK, its autophosphorylation and/or the flux of phosphate from DesK-P to the DesR transcription factor. Alternatively, cerulenin could promote dissociation of DesR-P from the des promoter. To distinguish between these possibilities, we used strain CM21, which carries a DesK null mutation, expresses desR from the xylose-inducible Pxyl promoter and contains a Pdes–lacZ fusion integrated at the amyE locus (Albanesi et al. 2004). This strain was transformed either with plasmid pCM9 expressing an N-terminal truncated form of DesK, named DesKC, which lacks the complete TM region, but retains the catalytic core of DesK (Fig. 3A) or with plasmid pLUP124, which express full-length DesK. It has been previously shown that when DesKC is expressed in strain CM21 the Pdes–lacZ fusion is constitutively expressed, even at 37°C, and is not influenced by the addition of exogenous UFAs. This behavior probably takes place because DesKC, which is unable to respond to membrane signals, remains locked in a kinase-dominant state (Albanesi et al. 2004). As shown in Figure 3B, when CM21 is complemented with wild-type DesK cerulenin addition represses des transcription, conversely β-galactosidase activity of CM21/pCM9 was not repressed by cerulenin. These results indicate that the antibiotic does not inhibit the autokinase or the phosphotransferase activities of the truncated DesKC protein, or the activation of the des promoter by phosphorylated DesR. These data support the notion that the TM domain of DesK is essential to sense the inhibitory effect of cerulenin on des transcription. As it is well established that the TM domain of DesK discriminates the surrounding lipid environment to adjust the signaling state of the sensor kinase (Mansilla and de Mendoza 2005), possible changes in membrane lipid composition induced by cerulenin treatment could be responsible for shutting off the cold-induced DesK autokinase activity.
Figure 3.
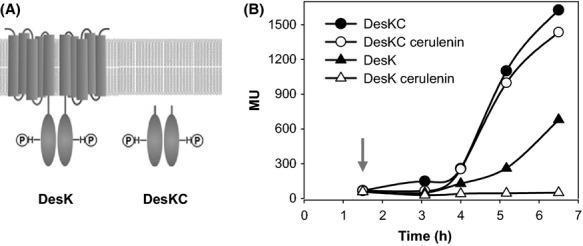
The TM domain of DesK is required for cerulenin repression of des transcription. (A) Scheme of DesK and DesKC proteins. (B) Cells of Bacillus subtillis CM21 (desKR::Km thr::Pxyl-desR, amyE::Pdes-lacZ) transformed with plasmid pCM9 (Pxyl-desKC) or pLUP124 (Pxyl-desK) were grown in LB medium at 37°C to an OD525 of 0.3. Cultures were supplemented with 0.01% of xylose and then were treated with cerulenin 2.5 μg mL−1 (white circles) or untreated (black circles). Cultures were further transferred to 25°C. β-galactosidase specific activities (in Miller units, MU) were determined at the indicated time intervals. Addition of the supplements is indicated by the arrow. Values are representative of three independent experiments.
Downregulation of des expression is linked to inhibition of FA synthesis
It should be noted that at this stage of the work, we were unable to distinguish if inhibition of transcription of the des gene mediated by cerulenin was due to inhibition of its target enzyme, FabF, or by a side effect of the antibiotic, such as its insertion into the membrane. To answer this question, des transcription was evaluated in BLUP87, a cerulenin-resistant mutant of B. subtilis that contains a Pdes-lacZ transcriptional fusion in the amyE locus. Resistance to cerulenin in this strain is given by the I108F substitution of FabF (fabF1 allele), which introduces a residue in the hydrophobic acyl chain-binding pocket that hampers the optimum interaction between the enzyme and the acyl chain of cerulenin (Schujman et al. 2001, 2008). As shown in Figure 4A, after a cold shock, cerulenin produces a slight inhibition of des transcription in this strain (see Fig. 2A). Moreover, the levels of UFAs synthesized by BLUP87 at low temperatures were not diminished by addition of cerulenin (Fig. 4B and C). These results rule out that cerulenin itself could modify the membrane microenvironment of DesK, leading to downregulation of des expression. Thus, this result strongly suggests that repression of des transcription is due to specific inhibition of FA synthesis by cerulenin. To confirm this idea, we tested des expression in the presence of triclosan, an antibiotic that inhibits other enzymes of the fatty acid biosynthetic pathway, FabI and FabL, which catalyze the NADPH reduction of enoyl-ACP to acyl-ACP during the elongation cycle of FA synthesis (Fig. 1, McMurray et al. 1998; Heath et al. 1998, 2000). Addition of sublethal concentration of triclosan (0.4 μg mL−1) to AKP3 (cerS) or BLUP87 (cerR) cells repressed des transcription in both strains (Fig. S2). These results demonstrate that downregulation of des expression is correlated with the specific inhibition of FA synthesis and suggest that this response would be observed when any step in the pathway is blocked.
Figure 4.
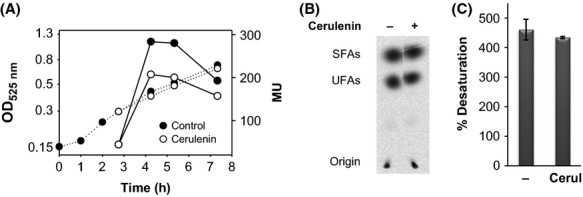
des expression and autoradiogram of the products of [14C]palmitate labeling of a cerulenin-resistant strain. (A) Cells of B. subtillisBLUP87 (fabF1 cerr, amyE::Pdes-lacZ) were grown in MM at 37°C to an OD525 of 0.35 and then were treated with cerulenin 2.5 μg mL−1 (white circles) or untreated (black circles). Cultures were further transferred to 25°C. β-galactosidase specific activities (in Miller units, MU) were determined at the indicated time intervals. Dotted lines: OD525, solid lines: β-galactosidase specific activities. Values are representative of three independent experiments. (B) UFAs synthesized by strain BLUP87 after a temperature downshift, in the presence or the absence of cerulenin. Cultures were challenged with [14C]palmitate as described in Figure 2B. About 11,000 cpm of radioactivity was loaded into all lanes. (C) Percentages of UFA synthesis. The radioactivity levels of the spots of the UFA and SFA shown in panel B were quantified by ImageQuant 5.2. Results are expressed as percentages of the total methyl esters recovered. Values are the average of three independent experiments.
Changes in membrane FA composition in response to cerulenin
The FA composition of strain AKP3, treated or not with cerulenin after a temperature downshift, was determined by gas chromatography–mass spectrometry (GC-MS). In agreement with the experiments shown in Figure 2B, we observed that the proportion of UFAs in cells treated with the antibiotic decreased about threefold (from 10.0 to 3.4%, Table 2). Moreover, and surprisingly, we found that the addition of cerulenin increases the production of shorter FA, as the ratio of short-chain FA (FA of chain length of C13-C15) to long-chain FA (FA of chain length C16 or longer) rose about twofold (1.1–2.2, Table 2). Finally, cerulenin seems not to affect the amount of straight-chain FA nor the iso/anteiso-branched chain FA ratio. These results strongly suggest that inhibition of des expression by cerulenin is due to shortening of the acyl chains of membrane lipids that would be sensed by the TM domain of DesK, acquiring a phosphatase-dominant state.
Table 2.
Fatty acid composition of the phospholipids of strain AKP3.
| Percentage of fatty acid-type1 |
|||
|---|---|---|---|
| Fatty acid | No addition | Cerulenin | ±2 |
| Iso C13:0 | 0.08 | 0.89 | 11.1 |
| Anteiso C13:0 | 0.05 | 1.34 | 26.8 |
| Iso-C14:0 | 3.47 | 4.49 | 1.3 |
| n-C14:0 | 0.43 | 1.27 | 3.0 |
| Iso-C15:0 | 17.90 | 20.36 | 1.2 |
| Anteiso C15:0 | 29.29 | 37.50 | 1.3 |
| n-C15:0 | 1.46 | 1.68 | 1.2 |
| Iso-C16:1 | 2.36 | 0.75 | 0.3 |
| Iso-C16:0 | 9.11 | 6.30 | 0.7 |
| n-C16:1 | 4.40 | 1.76 | 0.4 |
| n-C16:0 | 8.58 | 7.95 | 0.9 |
| Iso-C17:1 | 2.33 | 0.70 | 0.3 |
| Anteiso C17:1 | 0.93 | 0.20 | 0.2 |
| n-C17:0 | 0.66 | 0.44 | 0.7 |
| n-C18:1 | 0.60 | 1.33 | 2.2 |
| UFAs | 10.00 | 3.40 | 0.3 |
| SCFA/LCFA3 | 1.1 | 2.2 | 2.0 |
| Iso/anteiso4 | 1.1 | 0.9 | 0.8 |
| Straight-chain FA | 16.1 | 14.4 | 0.9 |
| Iso + straight/anteiso5 | 1.5 | 1.2 | 0.8 |
Cells were grown in MM at 37°C to an OD525 of 0.35. Cultures were split and cerulenin (2.5 μg mL−1) was added to one half. Cultures were further transferred to 25°C and cells harvested after 6 h of growth. Total lipids were extracted, transesterified to yield FA methylesters and subjected to GC-MS analysis. Values are representative of three experiments.
Ratio of percentages of antibiotic-treated cells compared to untreated cells.
Ratio of short-chain FA (SCFA, C13 to C15) to long-chain FA (LCFA, C16 or longer).
Ratio of iso-branched-chain FA to anteiso-branched-chain FA.
Ratio of (iso-branched-chain FA + straight-chain FA) to anteiso-branched-chain FA.
Effect of long-chain FA on des transcription
As transcriptional repression of des by cerulenin seems to be a consequence of the shortening of the membrane FA chains, it is conceivable that the opposite effect can be obtained in cells whose membranes are enriched in long-chain FA. If this is the case, cells overproducing long-chain FA, could activate des transcription even at 37°C. It has been described that the blockade of phospholipid synthesis in B. subtilis by depletion of PlsC, the enzyme that acylates acyl-glycerol phosphate, leads to a very significant accumulation of free FA (Paoletti et al. 2007). Nevertheless, the accumulation of the 1-acyl-glycerol-3-P intermediate was not observed (Paoletti et al. 2007). Thus, we used strain BLUP103, which contains the plsC gene under the control of the IPTG-inducible Pspac promoter and a Pdes–lacZ fusion integrated at the amyE locus. In this strain, removal of the inducer does not result in the immediate inactivation of the protein or cessation of cell proliferation, but rather, cell growth continues until the preexisting protein is diluted out by subsequent cell divisions, while the synthesis of FA continues at a very significant rate. Thus, during the transition from log phase to growth stasis of BLUP103, abnormally long-chain FA should be incorporated into membrane lipids. To test our hypothesis, we determined the FA composition of phospholipids of BLUP103 cells, grown at 25 or 37°C in the presence or in the absence of the inducer. To this end, the extracted FA was subjected to transesterification with sodium methoxide. This procedure allows analyzing by GC-MS only membrane FA esterified to glycerol. Thus, the FA percentages mostly reflect the composition of complex lipids rather than the content of free FA.
As expected, the mass spectrum of PlsC-depleted cells displayed a significant increase (5.6-fold) in the proportion of long-chain FA (Table 3), including the synthesis of FA of a chain length of 19–22°C. This effect was more pronounced at 37°C. In addition, depletion of PlsC at 37°C led to a threefold increase in the amount of straight-chain FA when compared with cells grown with the inducer (45.9 vs. 13.1%). To determine whether these changes in membrane lipid composition affect des expression, we analyzed the activity of the Pdes-lacZ transcriptional fusion in BLUP103, with or without IPTG addition, on cells grown at 37°C and after a shift to 25°C. As shown in Figure 5, similar levels of des transcription were observed in plsC cells growing at 25°C in the presence or in the absence of the inducer. However, at 37°C the activity of the des promoter in PlsC-depleted cells reached induction levels about eightfold higher than the levels found in presence of IPTG. These data support the notion that the presence of higher amounts of long-chain FA in the membrane of plsC-depleted cells stabilize DesK in a constitutive kinase state.
Table 3.
Fatty acid composition of the phospholipids of a strain deficient in PlsC.
| Fatty acid | Percentage of fatty acid type1 |
|||||
|---|---|---|---|---|---|---|
| 25°C |
37°C |
|||||
| PlsC+ | PlsC− | ±2 | PlsC+ | PlsC− | ±2 | |
| Iso C13:0 | 0.20 | 0.29 | 0.26 | 0.14 | ||
| Anteiso C13:0 | 0.52 | 0.20 | ND | ND | ||
| Iso-C14:0 | 1.67 | 1.77 | 2.27 | 0.83 | ||
| n-C14:0 | 0.43 | 0.50 | 0.70 | 0.44 | ||
| Iso-C15:0 | 14.69 | 10.89 | 23.44 | 7.04 | ||
| Anteiso C15:0 | 38.18 | 27.33 | 30.84 | 11.08 | ||
| n-C15:0 | 1.38 | 1.11 | 1.63 | 0.92 | ||
| Iso-C16:1 | 1.66 | 1.73 | ND | 0.12 | ||
| Iso-C16:0 | 3.12 | 4.34 | 6.54 | 4.99 | ||
| n-C16:1 | 4.25 | 3.99 | 0.28 | 0.32 | ||
| n-C16:0 | 7.09 | 7.20 | 7.43 | 10.76 | ||
| Iso-C17:1 | 4.59 | 5.93 | ND | ND | ||
| Anteiso C17:1 | 1.28 | 2.84 | ND | ND | ||
| n-C17:0 | 0.67 | 1.24 | 0.74 | 12.88 | ||
| Iso-C18:0 | 0.07 | 0.77 | 0.62 | 3.09 | ||
| n-C18:1 | 1.83 | 3.64 | 2.30 | 15.90 | ||
| Iso-C19:0 | ND | 0.26 | 0.48 | 2.13 | ||
| Anteiso C19:0 | ND | 0.48 | 0.13 | 1.86 | ||
| n-C19:0 | ND | 0.16 | ND | 0.79 | ||
| Iso-C20:0 | ND | ND | ND | 1.12 | ||
| n-C20:0 | ND | 0.40 | ND | 3.73 | ||
| Iso-C21:0 | ND | ND | ND | 0.39 | ||
| Anteiso C21:0 | ND | ND | ND | 0.31 | ||
| n-C21:0 | ND | ND | ND | 0.13 | ||
| Iso-C22:1 | ND | ND | ND | 0.19 | ||
| Iso-C22:0 | ND | ND | ND | 0.25 | ||
| UFAs | 11.8 | 14.5 | 1.2 | 0.3 | 0.6 | 2.0 |
| LCFA/SCFA3 | 0.8 | 1.4 | 1.8 | 0.7 | 3.9 | 5.6 |
| Iso/anteiso4 | 0.6 | 0.8 | 1.3 | 1.1 | 1.4 | 1.3 |
| Straight-chain FA | 15.7 | 18.2 | 1.2 | 13.1 | 45.9 | 3.5 |
| Iso + straight/anteiso5 | 0.9 | 1.1 | 1.2 | 1.5 | 3.4 | 2.3 |
ND, not detected.
BLUP103 (Pspac-plsC) cells grown ON at 37°C in MM supplemented with 0.2 mmol/L IPTG were washed and used to inoculate fresh media, in the presence (PlsC+) or absence (PlsC−) of 1 mmol/L IPTG. At DO600 of 0.35 cultures were split and incubated at 25 or 37°C for 6 h. When growth stopped because of PlsC depletion, total lipids were extracted, transesterified to yield FA methylesters and subjected to GC-MS analysis.
Ratio of percentages of not induced cells compared to induced cells.
Ratio of long-chain FA (LCFA, C16 or longer) to short-chain FA (SCFA, C13 to C15).
Ratio of iso-branched-chain FA to anteiso-branched-chain FA.
Ratio of (iso-branched-chain FA + straight-chain FA) to anteiso-branched-chain FA.
Figure 5.
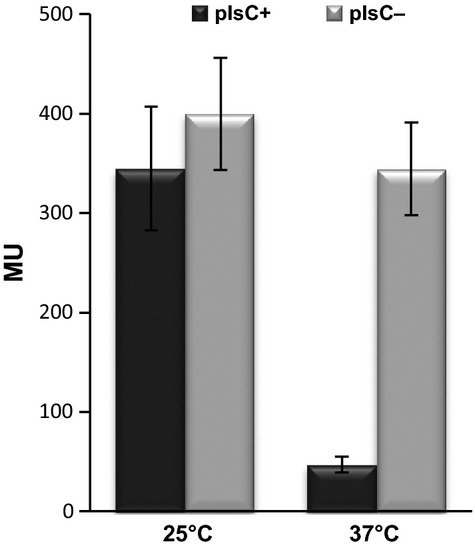
Effect of plsC depletion on des transcription. BLUP103 (Pspac-plsC amyE::Pdes-lacZ) cells grown ON at 37°C in Spizizen minimal medium supplemented with 0.2 mmol/L IPTG were washed and used to inoculate fresh media, in the presence (black bars) or absence (gray bars) of 1 mmol/L IPTG. At OD525 of 0.35 cultures were split and incubated at 25 or 37°C. β-galactosidase specific activities were determined 4 h after the temperature shift. Values expressed in Miller units (MU) are representative of three independent experiments.
Discussion
Bacillus subtilis is a typical mesophile that can be found in the upper layers of the soil, which is subjected to temperature changes both during the course of the day, and over longer time periods, as a consequence of seasonal changes. Rapid and severe temperature downshifts elicit genetic and cellular adaptive reactions that are collectively known as the cold shock stress response (Weber and Marahiel 2002). As cold shock imposes marked changes on the biophysical properties of B. subtilis cytoplasmic membrane (Mansilla et al. 2004), temperature sensing is important to optimize membrane fluidity in this organism. The cold signal induces transcription of the des gene, leading to the introduction of double bonds into the acyl chains of phospholipids. In this paper, we show that the fungal antibiotic cerulenin represses des induction during cold shock. As cerulenin has been extensively used as a tool to understand several aspects of lipid metabolism (Furukawa et al. 1993; Heath and Rock 1995; Loftus et al. 2000; Schujman et al. 2003), the main objective of this work was to uncover the mechanism by which this antibiotic causes inhibition of des transcription.
Cerulenin inhibits lipid synthesis in B. subtilis by the covalent active site-directed inactivation of the FabF condensing enzyme, the enzyme that catalyzes the condensation of malonyl-CoA with acyl-ACP (Fig. 1). Cessation of FA synthesis caused by cerulenin also inhibits the production of phosphatidic acid (PtdOH), the precursor of membrane phospholipids (Fig. 1). However, we have shown here that inhibition of phospholipid synthesis by PlsC depletion does not impair des transcription after cold shock (Fig. 5). So, inhibition of des expression by cerulenin is linked to inhibition of FA synthesis rather than to phospholipid synthesis. Besides, we showed that the addition of sublethal levels of cerulenin, that do not inhibit growth of B. subtilis wild-type strains, alters the length of the acyl chains of membrane phospholipids. In fact, we found that the membrane FA composition of B. subtilis treated with cerulenin is clearly biased toward shorter-chain fatty acyl groups (Table 2).
An important question raised by this work is: how could the FA chain length of B. subtilis phospholipids be decreased by cerulenin? FabF forms part of the fap regulon of B. subtilis (comprising almost all the proteins that catalyzes the later steps of the FASII cycle as well as the earlier steps of phospholipid synthesis, Fig. 1), which is transcriptionally regulated by the FapR repressor. Binding of the repressor to its target sequences is modulated by the levels of malonyl-CoA (Schujman et al. 2006). Antibiotics that specifically inhibit the FASII cycle augment the intracellular levels of malonyl-CoA, which in turn release FapR from its binding sites, increasing the expression of the fap regulon (Schujman et al. 2003). In B. subtilis, two genes involved in the acyl transfer step of phospholipid synthesis, plsX and plsC, are upregulated by inactivation of the FASII cycle (Schujman et al. 2003). Although the experiments shown here suggest that FabF is responsible for the decrease in the acyl chain length of FA of cells exposed to cerulenin, it should be noted that this leftacteristic is dependent upon the competition between the elongation activity of the FASII and the rate of incorporation of FA by the acyltransferase system (Yao and Rock 2013). We envision that in cerulenin-treated cultures, the combined effect of a decrease in the relative rate of the acyl chain elongation by FabF and overproduction of PlsX and PlsC, are responsible for the increased incorporation of FA of shorter chain length. This proposal agrees with the observation that cells with normal rate of FA synthesis, but decreased PlsC activity accumulates long-chain FA (Table 3).
Reconstitution of DesK into bilayers of PC containing acyl chains of different length showed that the longer the FA (the thicker the bilayer) the greater its kinase activity (Martín and de Mendoza 2013). Nevertheless, these data were obtained with vesicles made of phospholipids that are not normally present in B. subtilis membranes. In this paper, we demonstrate that modulation of DesK kinase activity by the thickness of the bilayer indeed take place in vivo under isothermal conditions, using B. subtilis native phospholipids. But, how could the acyl-chain length of membrane phospholipids influence DesK regulation? Changes in membrane thickness can alter the activity of membrane proteins by modifying the orientation or conformation of TM regions (Lee 2003; Cybulski and de Mendoza 2011). Chill stress, among other effects, causes an increase in membrane thickness generated by a decrease in the disorder of the acyl-chains of phospholipids that accompanies cooling (Rafael Oliveira, personal communication).
Periplasmic-sensing histidine kinases comprise the largest group of membrane-bound sensor kinases. They contain a significantly large extracytoplasmic input domain, which generally detects signals by direct interaction with chemically defined small molecules (Mascher et al. 2006). On the other hand, DesK belongs to a group of histidine kinases with the sensing mechanism linked to the TM regions. The molecular basis by which this group of histidine kinases sense environmental signals is largely unknown. We have recently reported that the multimembrane-spanning domain from DesK could be simplified into a chimerical single-membrane-spanning minimal sensor (MS)-DesK that fully retains in vivo and in vitro the cold-sensing properties of the parental system (Cybulski et al. 2010). Mutational and biochemical analysis of this membrane-bound chimera showed that two hydrophilic residues near the N-terminus of DesK's first TM segment are critical for its cold-activation (Cybulski et al. 2010). This region has been named the “buoy,” as its hydrophilicity drives it toward the lipid/water interface, while the hydrophobicity of surrounding residues anchors the buoy to the membrane and can potentially pull it into the membrane interior. The “sunken-buoy” model of thermosensing poses that as the membrane thickens upon cooling, the hydrophilic buoy is pulled into the hydrophobic membrane, an energetically unfavorable situation that elicits conformational changes within the DesK protein that increase the activity of its histidine kinase domain (Cybulski et al. 2010). While the precise structural changes within the TM region remain uncertain, the results described here by manipulating in vivo the chain length of B. subtilis phospholipids, either by inhibiting the elongation activity of FASII or the rate of incorporation by the acyl transfer system, suggest that DesK regulation is indeed linked to changes in membrane thickness that could trigger buoy-dependent conformational changes in this integral membrane cold-sensor.
Acknowledgments
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Agencia Nacional de Promoción Científica y Tecnológica (FONCYT). L. Porrini is a Fellow of CONICET and S. Altabe, L.E. Cybulski, D. de Mendoza and M.C. Mansilla are Career Investigators of the same institution.
Conflict of Interest
None declared.
Funding Information
This work was supported by grants from Consejo Nacional de Investigaciones Cientificas y Técnicas (CONICET) and Agencia de Promoció n Cientίfica y Tecnoló gica(FONCYT).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
The Des signaling pathway for regulation of UFAs synthesis in Bacillus subtilis. DesK could assume different signaling states in response to changes in membrane fluidity. An increase in the order of the acyl chains of membrane lipids (less fluid membrane) promotes a kinase-dominant state of DesK, which autophosphorylates and transfers the phosphate group to DesR. DesK-mediated phosphorylation of DesR enables interaction of DesR-P with des promoter and RNA polymerase, resulting in transcriptional activation of des. Then Δ5-Des is synthesized and desaturates the acyl chains of membrane phospholipids. These newly synthesized UFAs cause a decrease in the order of membrane lipids (more fluid membrane) favoring a phosphatase-dominant state of DesK, leading to dephosphorylation of DesR and thus turning off des transcription.
Effect of triclosan on des transcription in cerulenin-sensitive or cerulenin-resistant strains. Cells of B. subtillis AKP3 (fabF cerS, amyE::Pdes-lacZ) (A) or BLUP87 (fabF1 cerr, amyE::Pdes-lacZ) (B) were grown in LB medium at 37°C to an OD525 of 0.35 and then were treated with triclosan 0.4 µg mL −1 (white circles) or untreated (black circles). Cultures were further transferred to 25°C. β-galactosidase specific activities (in Miller units, MU) were determined at the indicated time intervals. Dotted lines: OD525, solid lines: β-galactosidase specific activities. Values are representative of three independent experiments).
References
- Aguilar PS, Cronan JE, de Mendoza D. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J. Bacteriol. 1998;180:2194–2200. doi: 10.1128/jb.180.8.2194-2200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar PS, Hernandez-Arriaga AM, Cybulski LE, Erazo AC, de Mendoza D. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 2001;20:1681–1691. doi: 10.1093/emboj/20.7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanesi D, Mansilla MC, de Mendoza D. The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator. J. Bacteriol. 2004;186:2655–2663. doi: 10.1128/JB.186.9.2655-2663.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanesi D, Martín M, Trajtenberg F, Mansilla MC, Haouz A, Alzari PM. Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc. Natl. Acad. Sci. USA. 2009;106:16185–16190. doi: 10.1073/pnas.0906699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altabe SG, Aguilar P, Caballero GM, de Mendoza D. The Bacillus subtilis acyl lipid desaturase is a Δ5 desaturase. J. Bacteriol. 2003;185:3228–3231. doi: 10.1128/JB.185.10.3228-3231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Christie W, editor. Gas chromatography and lipids. Ayr, Scotland: The Oily Press; 1989. [Google Scholar]
- Cronan JE, Jr, Gelmann EP. An estimate of the minimum amount of unsaturated fatty acid required for growth of Escherichia coli. J. Biol. Chem. 1973;248:1188–1195. [PubMed] [Google Scholar]
- Cybulski LE, de Mendoza D. Effect of membrane thickness on integral membrane proteins activity. Curr. Protein Pept. Sci. 2011;12:760–766. doi: 10.2174/138920311798841681. [DOI] [PubMed] [Google Scholar]
- Cybulski LE, Albanesi D, Mansilla MC, Altabe S, Aguilar P, de Mendoza D. Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase. Mol. Microbiol. 2002;45:1379–1388. doi: 10.1046/j.1365-2958.2002.03103.x. [DOI] [PubMed] [Google Scholar]
- Cybulski LE, Craig G, del Solar PO, Espinosa M, de Mendoza D. Bacillus subtilis DesR functions as a phosphorylation-activated switch to control membrane lipid fluidity. J. Biol. Chem. 2004;279:39340–39347. doi: 10.1074/jbc.M405150200. [DOI] [PubMed] [Google Scholar]
- Cybulski LE, Martin M, Mansilla MC, Fernandez A, de Mendoza D. Membrane Thickness Cue for Cold Sensing in a Bacterium. Curr. Biology. 2010;20:1539–1544. doi: 10.1016/j.cub.2010.06.074. [DOI] [PubMed] [Google Scholar]
- Dubnau D, Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J. Mol. Biol. 1971;56:209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Tsay JT, Jackowski S, Takamura Y, Rock CO. Thiolactomycin resistance in Escherichia coli is associated with the multidrug resistance efflux pump encoded by emrAB. J. Bacteriol. 1993;175:3723–3729. doi: 10.1128/jb.175.12.3723-3729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood CR, Cuttings SM, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley and Sons; 1990. [Google Scholar]
- Heath RJ, Rock CO. Regulation of malonyl-CoA metabolism by acyl-acyl carrier protein and beta-ketoacyl-acyl carrier protein synthases in Escherichia coli. J. Biol. Chem. 1995;270:1531–15538. doi: 10.1074/jbc.270.26.15531. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Yu YT, Shapiro MA, Olson E, Rock CO. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 1998;273:30316–30321. doi: 10.1074/jbc.273.46.30316. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Su N, Murphy CK, Rock CO. The enoyl-[acyl-carrier-protein] reductases FabI and FabL from Bacillus subtilis. J. Biol. Chem. 2000;275:40128–40133. doi: 10.1074/jbc.M005611200. [DOI] [PubMed] [Google Scholar]
- Henner DJ. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 1990;185:223–228. doi: 10.1016/0076-6879(90)85022-g. [DOI] [PubMed] [Google Scholar]
- Johansson P, Hederstedt L. Organization of genes for tetrapyrrole biosynthesis in gram-positive bacteria. Microbiology. 1999;145:529–538. doi: 10.1099/13500872-145-3-529. [DOI] [PubMed] [Google Scholar]
- Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 2003;1612:1–40. doi: 10.1016/s0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- Loftus TM, Jaworsky DE, Frehywot GL, Townsent CA, Ronjnett GV, Lane DM. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- Mansilla MC, de Mendoza D. L-Cysteine biosynthesis in Bacillus subtilis: identification, sequencing, and functional leftacterization of the gene coding for phosphoadenylylsulfate sulfotransferase. J. Bacteriol. 1997;179:976–981. doi: 10.1128/jb.179.3.976-981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla MC, de Mendoza D. The Bacillus subtilis desaturase: a model to understand phospholipid modification and temperature sensing. Arch. Microbiol. 2005;183:229–235. doi: 10.1007/s00203-005-0759-8. [DOI] [PubMed] [Google Scholar]
- Mansilla MC, Cybulski LE, Albanesi D, de Mendoza D. Control of membrane lipid fluidity by molecular thermo-sensors. J. Bacteriol. 2004;186:6681–6688. doi: 10.1128/JB.186.20.6681-6688.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla MC, Banchio CE, de Mendoza D. Signalling pathways controlling fatty acid desaturation. Subcell. Biochem. 2008;49:71–99. doi: 10.1007/978-1-4020-8831-5_3. [DOI] [PubMed] [Google Scholar]
- Martín M, de Mendoza D. Regulation of Bacillus subtilis DesK thermosensor by lipids. Biochem. J. 2013;451:269–275. doi: 10.1042/BJ20121825. [DOI] [PubMed] [Google Scholar]
- Mascher T, Helmann JD, Unden G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 2006;70:910–938. doi: 10.1128/MMBR.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray LM, Oethinger M, Levy S. Triclosan targets lipid synthesis. Nature. 1998;394:531–532. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- Paoletti L, Lu YJ, Schujman GE, Rock D, de Mendoza CO. Coupling of fatty acid and phospholipid synthesis in Bacillus subtilis. J. Bacteriol. 2007;189:5816–5824. doi: 10.1128/JB.00602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M. Integrational vectors for genetic manipulation in Bacillus subtilis. In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, DC: American Society for Microbiology; 1993. pp. 615–624. [Google Scholar]
- Sambrook JE, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schujman GE, Grau R, Gramajo H, Ornella L, de Mendoza D. De novo fatty acid synthesis is required for establishment of cell type-specific gene transcription during sporulation in Bacillus subtilis. Mol. Microbiol. 1998;29:1215–1224. doi: 10.1046/j.1365-2958.1998.01004.x. [DOI] [PubMed] [Google Scholar]
- Schujman GE, Choi K, Altabe SG, Rock CO, de Mendoza D. Response of Bacillus subtilis to cerulenin and the acquisition of resistance. J. Bacteriol. 2001;183:3032–3040. doi: 10.1128/JB.183.10.3032-3040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schujman GE, Paoletti L, Grossman AD, de Mendoza D. FapR, a bacterial transcription factor involved in global regulation of membrane lipid biosynthesis. Dev. Cell. 2003;4:663–672. doi: 10.1016/s1534-5807(03)00123-0. [DOI] [PubMed] [Google Scholar]
- Schujman GE, Guerin M, Buschiazzo A, Schaeffer F, Llarrull LI, Reh G. Structural basis of lipid biosynthesis regulation in Gram-positive bacteria. EMBO J. 2006;25:4074–4083. doi: 10.1038/sj.emboj.7601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schujman GE, Altabe SG, de Mendoza D. A malonyl-CoA-dependent switch in the bacterial response to a dysfunction of lipid metabolism. Mol. Microbiol. 2008;68:987–996. doi: 10.1111/j.1365-2958.2008.06202.x. [DOI] [PubMed] [Google Scholar]
- Sekiguchi JN, Takada N, Okada H. Genes affecting the productivity of α-amylase in Bacillus subtilis Marburg. J. Bacteriol. 1975;121:688–694. doi: 10.1128/jb.121.2.688-694.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M, Richter R. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene. 1994;142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- Vagner V, Dervyn E, Ehrlich SD. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
- Weber MH, Marahiel MA. Coping with the cold: the cold shock response in the Gram-positive soil bacterium Bacillus subtilis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:895–907. doi: 10.1098/rstb.2002.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MH, Klein W, Muller L, Niess UM, Marahiel M. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol. Microbiol. 2001;39:1321–1329. doi: 10.1111/j.1365-2958.2001.02322.x. [DOI] [PubMed] [Google Scholar]
- Yao J, Rock CO. Phosphatidic acid synthesis in bacteria. Biochim. Biophys. Acta. 2013;1831:495–502. doi: 10.1016/j.bbalip.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Des signaling pathway for regulation of UFAs synthesis in Bacillus subtilis. DesK could assume different signaling states in response to changes in membrane fluidity. An increase in the order of the acyl chains of membrane lipids (less fluid membrane) promotes a kinase-dominant state of DesK, which autophosphorylates and transfers the phosphate group to DesR. DesK-mediated phosphorylation of DesR enables interaction of DesR-P with des promoter and RNA polymerase, resulting in transcriptional activation of des. Then Δ5-Des is synthesized and desaturates the acyl chains of membrane phospholipids. These newly synthesized UFAs cause a decrease in the order of membrane lipids (more fluid membrane) favoring a phosphatase-dominant state of DesK, leading to dephosphorylation of DesR and thus turning off des transcription.
Effect of triclosan on des transcription in cerulenin-sensitive or cerulenin-resistant strains. Cells of B. subtillis AKP3 (fabF cerS, amyE::Pdes-lacZ) (A) or BLUP87 (fabF1 cerr, amyE::Pdes-lacZ) (B) were grown in LB medium at 37°C to an OD525 of 0.35 and then were treated with triclosan 0.4 µg mL −1 (white circles) or untreated (black circles). Cultures were further transferred to 25°C. β-galactosidase specific activities (in Miller units, MU) were determined at the indicated time intervals. Dotted lines: OD525, solid lines: β-galactosidase specific activities. Values are representative of three independent experiments).


