Abstract
Locomotor muscles often perform diverse roles, functioning as motors that produce mechanical energy, struts that produce force and brakes that dissipate mechanical energy. In many vertebrate muscles, these functions are not mutually exclusive and a single muscle often performs a range of mechanically diverse tasks. This functional diversity has obscured the relationship between a muscle's locomotor function and its mechanical properties. I use hopping in toads as a model system for comparing muscles that primarily produce mechanical energy with muscles that primarily dissipate mechanical energy. During hopping, hindlimb muscles undergo active shortening to produce mechanical energy and propel the animal into the air, whereas the forelimb muscles undergo active lengthening to dissipate mechanical energy during landing. Muscles performing distinct mechanical functions operate on different regions of the force–length curve. These findings suggest that a muscle's operating length may be shaped by potential trade-offs between force production and sarcomere stability. In addition, the passive force–length properties of hindlimb and forelimb muscles vary, suggesting that passive stiffness functions to restrict the muscle's operating length in vivo. These results inform our understanding of vertebrate muscle variation by providing a clear link between a muscle's locomotor function and its mechanical properties.
Keywords: length–tension, force–length, muscle stiffness, hopping, landing, passive elasticity
1. Introduction
In most man-made vehicles, the motors that produce mechanical energy and the brakes that dissipate mechanical energy are separate structures, each with their own unique design. However, in many biological systems, muscles are tasked with performing both mechanical functions [1]. To function as a motor, muscles actively shorten while producing force, thereby generating the positive mechanical work that moves an organism. This familiar function is associated with locomotor behaviours such as uphill running, accelerating or jumping during which muscles act to increase the kinetic and/or potential energy of the body. To function as a brake, muscles are lengthened while activated to dissipate mechanical energy and decelerate the body. Active lengthening in muscles is associated with locomotor behaviours such as downhill running, decelerating or landing during which muscles act to decrease the kinetic and/or potential energy of the body.
Within a single muscle, altering the temporal relationship between force production and length change can result in a shift between the production and dissipation of mechanical work. This may be achieved by altering the timing of muscle activation with respect to its length change [2], or as a result of differences in intrinsic muscle properties [3], or in response external factors such as temperature fluctuations [4,5]. However, while the mechanical function of individual muscles can be altered, it is reasonable to predict that some features of muscles are likely to reflect functional specializations as brakes or motors [6].
To function as an effective motor, muscles must maximize their capacity for generating positive mechanical work. As mechanical work is the product of muscle force and shortening, the ability to increase either parameter will result in a more effective motor. A muscle's force–length relationship dictates that maximum muscle force is restricted to intermediate length ranges where myofilament overlap is maximized [7]. In addition, the shape of the force–length curve of vertebrate muscles suggests that the contribution of muscle shortening to work production is limited by the fact that an increase in shortening is counteracted by a decrease in force output at short sarcomere lengths. Given the mechanical constraints associated with the force–length curve, a muscle's operating length range during movement is a critical determinant of work capacity [8].
The effect of muscle's force–length properties on peak force production is determined by the length changes that occur during the period of force development [9]. When a muscle is activated, sarcomeres begin to shorten as cross bridge cycling is initiated and force begins to develop. During tetanic contractions, a muscle may continue to develop force for several hundred milliseconds before reaching peak forces. Shortening of sarcomeres during the period of force development can have a significant effect on peak force as the muscle moves to a shorter position on the force–length curve. When a muscle starts at the plateau of the force–length curve, initial shortening during the period of force development moves the muscle to a more disadvantageous position (ascending limb of the force–length curve), thereby reducing peak forces. By contrast, when a muscle starts at a relatively long length (descending limb of the force–length curve), shortening during the period of force development moves the muscle to a more advantageous position (near the plateau), allowing for higher peak forces [9]. Therefore, operating on the descending limb of the force–length curve appears to improve force production during behaviours requiring significant shortening.
The capacity to produce force is far less constrained during active lengthening. Muscles that are actively stretched produce forces that exceed a muscle's maximum isometric force [10]. As a result, forces are not likely to limit a muscle's capacity to dissipate mechanical work. However, it may be critical to limit the magnitude and rate of the stretch applied to a muscle during energy dissipation because lengthening (eccentric) contractions have been shown to damage muscle fibres [11]. Eccentric contractions can disrupt cytoskeletal structures and damage the Z-discs that define the boundaries of a sarcomere [12]. The disruption and mis-alignment of a subset of sarcomeres reduces a muscle's capacity for force generation and often results in severe soreness and discomfort [13,14].
The exact mechanical factors that cause muscle damage are not broadly agreed upon. Previous studies have shown that muscle damage depends on the magnitude of stretch applied to an active muscle (e.g. [11]). Others have suggested that the amount of mechanical energy dissipated by a muscle is the primary determinant of damage [15]. It is clear, however, that the length over which a muscle is stretched can significantly influence the severity of muscle damage [16–18]. Specifically, stretches applied at a relatively long sarcomere length are more damaging than the same stretch applied at a short sarcomere length. The increased likelihood of muscle damage arises from the fact that operating on the descending limb of the force–length curve (lengths longer than the plateau force–length curve) can compromise sarcomere stability [19]. If sarcomeres acting in series are at slightly different lengths while operating on the descending limb of the force–length curve, local variation in force can cause longer and therefore weaker sarcomeres to ‘pop’ as they are rapidly stretched to lengths beyond myofilament overlap causing permanent damage to the cytoskeletal structures [19]. This instability in sarcomeres particularly on the descending limb of the force–length curve is thought to be the underlying mechanism responsible for muscle damage during active lengthening [20].
The potential trade-off between force production and sarcomere stability may shape variation in the operating lengths of vertebrate muscles functioning as motors versus those functioning as brakes. One mechanism that may alter or restrict where muscles operate on the force–length curve is variation in the passive mechanical properties of muscles specialized for different functions. During movement, muscles may be passively stretched either through contraction of antagonist muscles or by an external load (gravitational, inertial, drag, etc.). The length a passive muscle reaches in response to any loading condition will depend on its passive force–length relationship. In all muscles, the relationship between passive force and length can be described by an exponential function [21]. However, while the general form of this relationship is conserved, the slope of the curve, and the sarcomere length at which passive force is developed can vary significantly between muscles [9,22]. Ultimately, the resistance of a muscle to being stretched will determine its length prior to activation. Therefore, an increase in the passive stiffness of a muscle may function to restrict its maximum operating length during movement.
In this study, I use an ideal model system for revealing the features of muscles performing distinct locomotor functions. The take-off and landing phases of hopping toads provide a unique opportunity to reveal structural and functional features of muscles performing divergent locomotor tasks (figure 1). The take-off phase of a hop involves the generation of mechanical work by the hindlimb muscles, which accelerate the animal into the air [23]. During the landing phase, forelimb muscles act to dissipate mechanical energy and decelerate the body [24,25]. Therefore, muscles of the hindlimb and forelimb are functionally segregated during the primary mode of locomotion in toads. This functional segregation provides an opportunity to determine the muscle properties associated with specialized locomotor functions. A clear benefit of this model system is that the comparison is not confounded by the phylogenetic history of diverse species where the influences of locomotor mechanics and phylogeny can be difficult to tease apart.
Figure 1.

Time-lapse sequence of a hopping bout in Rhinella marinus. This image highlights the role of hindlimbs in accelerating the body into the air and forelimbs in decelerating the body during landing. Images are taken from a high-speed video sequence recorded at 400 frames per second.
I compare the plantaris muscle (an ankle extensor) and anconeus muscle (an elbow extensor) of hopping toads to test two specific hypotheses (figure 2b). First, I hypothesize that the plantaris muscle will operate on the descending limb of the force–length curve, while the anconeus will be restricted to the ascending limb or plateau. Second, I hypothesize that differences in operating lengths are driven by variation in the passive force–length properties of the two muscles. Specifically, I predict that the anconeus muscle will be stiffer than the plantaris, thereby constraining its maximum operating length. To test these hypotheses, I combine in vivo measurements of muscle fascicle lengths in both muscles during hopping with an in vitro characterization of each muscle's force–length curve. I use the same muscle with the same set of transducers to measure lengths during both in vivo and in vitro experiments in order to reliably map muscle fascicle lengths during hopping onto the force–length curve [9,25]. This comparison of the forelimb and hindlimb muscles of hopping toads will elucidate the changes in vertebrate muscle properties associated with diverse locomotor functions.
Figure 2.
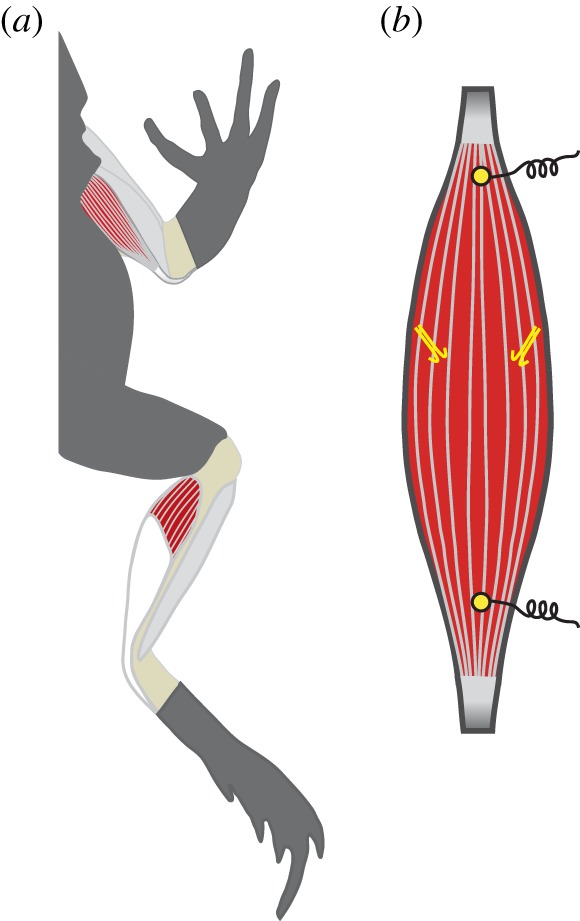
(a) The two muscles used in this study are the anconeus muscle, a primary elbow extensor, and the plantaris muscle, a primary ankle extensor. (b) Each muscle was instrumented with a pair of sonomicrometry transducers, which measured fascicle lengths, and two electromyography electrodes, which measured muscle activity patterns during hopping. The schematic of the muscle is representative of the anconeus muscle. (Online version in colour.)
2. Material and methods
Eight marine toads (Rhinella marinus) were purchased from a herpetological vendor, fed vitamin-enriched crickets ad libitum and housed in glass terraria. All husbandry and experimental procedures were approved by the IACUC at UC Irvine.
In all individuals, sonomicrometry was used to measure fascicle length and electromyography (EMG) was used to measure muscle activation patterns during hopping. Transducers were implanted along a muscle fascicle and care was taken to avoid any intramuscular elastic elements such as aponeuroses. Therefore, the implants allowed us to measure the length changes occurring in the fascicles and not the muscle-tendon unit. In four individuals transducers were implanted in the plantaris muscle, and in four individuals transducers were implanted in the anconeus (figure 2). Surgical procedures were similar to those previously described [9,25].
Sonomicrometry data were collected using an ultrasonic dimension gauge (Sonometrics Inc., London, Ontario, Canada). EMG signals were amplified 1000× (A-M systems, WA, USA). All data were collected at 4000 Hz using a 16-bit A/D converter (National Instruments, TX, USA). Hopping bouts were imaged laterally at 400 frames per second using a Miro 120 high-speed camera (Vision Research, NJ, USA). All data were synchronized using a common external trigger.
Once hopping data were collected, the force–length relationship of the same implanted muscle was quantified using an in vitro preparation. The toads were euthanized with a double-pithing protocol. In four toads, the anconeus muscle along with its nerve (SN 2) was dissected out. In the other four toads, the plantaris muscle along with the sciatic nerve was dissected out. In all muscles, the previously implanted sonomicrometry transducers were left in place and used to measure fascicle lengths in vitro. This protocol allowed us to directly relate in vivo muscle lengths during hopping to the force–length curve characterized in vitro. Muscles were rigidly clamped in place and attached to a dual-mode servomotor (Aurora Scientific Inc., Ontario, Canada) to measure muscle force. A custom-made nerve cuff (with silver electrodes) was placed on the nerve and used to stimulate the muscle. The preparation was placed in an aerated amphibian Ringer's solution at 22°C. The muscle was then stimulated supramaximally at varying lengths to characterize its active and passive force–length properties.
All sonomicrometry and EMG data were recorded and processed according to [25]. Data from high-speed video were analysed using MatLab (Mathworks, Inc., MA, USA) and used to determine the timing of take-off and landing as well as hop distance. As variation in hop distance has been shown to alter muscle operating lengths [25], the analysis in this study was limited to hops ranging from 20 to 30 cm. Ten hops from each individual were included in the analysis.
Following experiments, fascicle lengths, muscle mass and pennation angles were characterized for each muscle (electronic supplementary material, table S1). These data were used to characterize the physiological cross-sectional area and allowed us to compare the maximum specific force (stress) of each muscle. Fits were applied to the active and passive force–length data following Otten [21]. Based on the fit applied to the active force–length data, the peak isometric force (Po) and the fascicle length at peak force (Lo) were determined for each muscle. The passive force–length properties of the anconeus and plantaris were compared in two ways. First, the passive force and length data pooled from all individuals and the forces were log transformed. Log transformation converted the exponential relationship of the passive force–length curve to a linear relationship and allowed for the use of an ANCOVA to compare the two muscles. For this analysis, the muscle was the main effect, log of passive force was the dependent variable and passive fascicle length was the covariate. In addition, using the raw data, the length at which the muscle developed passive force corresponding to 20% of maximum isometric force (L20) was measured and compared between the two muscles [9]. A one-way ANOVA was used to compare the maximum operating lengths of the two muscles (normalized to Lo) during hopping. All statistical analyses were performed using JMP v. 9 (SAS Inc., NC, USA).
3. Results
The plantaris muscle fascicles shortened actively by approximately 20% during the take-off phase of a hop (figure 3a), producing some of the mechanical energy to accelerate the toad into the air. The anconeus muscle shortened actively during aerial phase of a hop as the elbow was extended in anticipation of impact (figure 3b). During the landing phase, the anconeus is stretched actively to dissipate mechanical energy and decelerate the body (figure 3b). During the landing phase, the fascicles of the anconeus stretch by approximately 15% (figure 3b).
Figure 3.
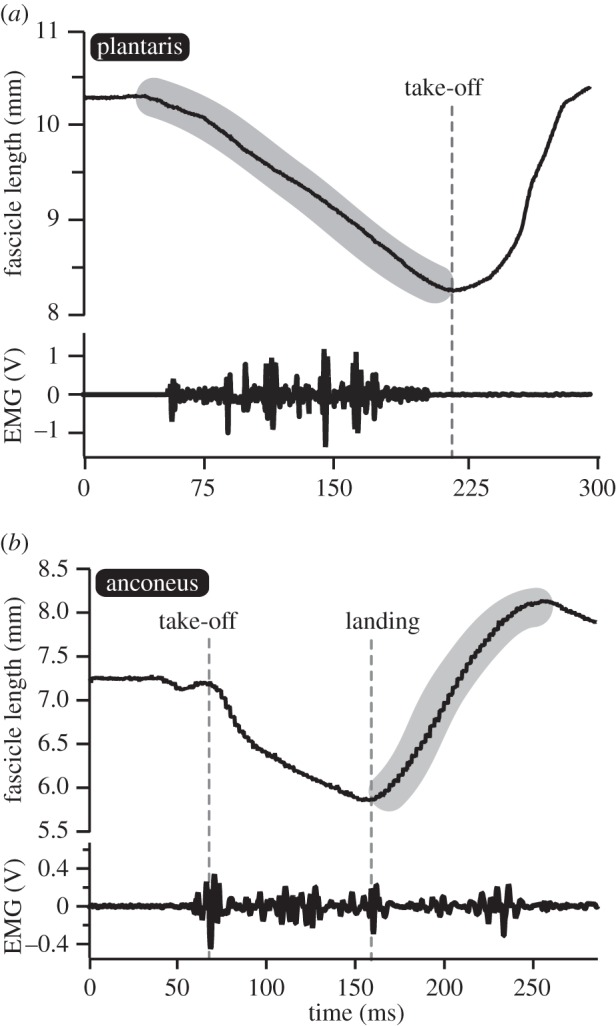
Fascicle lengths and muscle activity patterns during hopping. (a) The plantaris muscle functions as a motor by shortening actively during the take-off phase of a hop. (b) The anconeus muscle shortens actively during the aerial phase in anticipation of landing but functions as a brake by being actively stretched after impact. The shaded areas indicate the regions of muscle fascicle behaviour that are mapped onto the force–length curve.
The active force–length curve of the anconeus and the plantaris did not differ significantly and showed the familiar parabolic shape (figure 4). In addition, the two muscles produced the same maximum isometric force (p = 0.61) when normalized to physiological cross-sectional area (electronic supplementary material, table S1). As a result, all data were normalized to maximum isometric force to facilitate comparisons between muscles of different size. The passive force–length curves of the two muscles were significantly different (figures 4 and 5). An ANCOVA was performed using log-transformed forces and muscle fascicle lengths and used to compare the passive properties of the two muscles. The slope of the log-transformed data differed significantly (p = 0.0001), thereby indicating that the anconeus muscle had significantly higher stiffness than the plantaris muscle (figure 5b). In addition, the length at which a muscle develops passive force corresponding to 20% of maximum isometric force (L20) was compared in the two muscles. The plantaris muscle had to be stretched to significantly (p < 0.0001) longer lengths (1.26 Lo) than the anconeus muscle (1.07 Lo) before developing passive force corresponding to 20% of Po (figure 5c).
Figure 4.
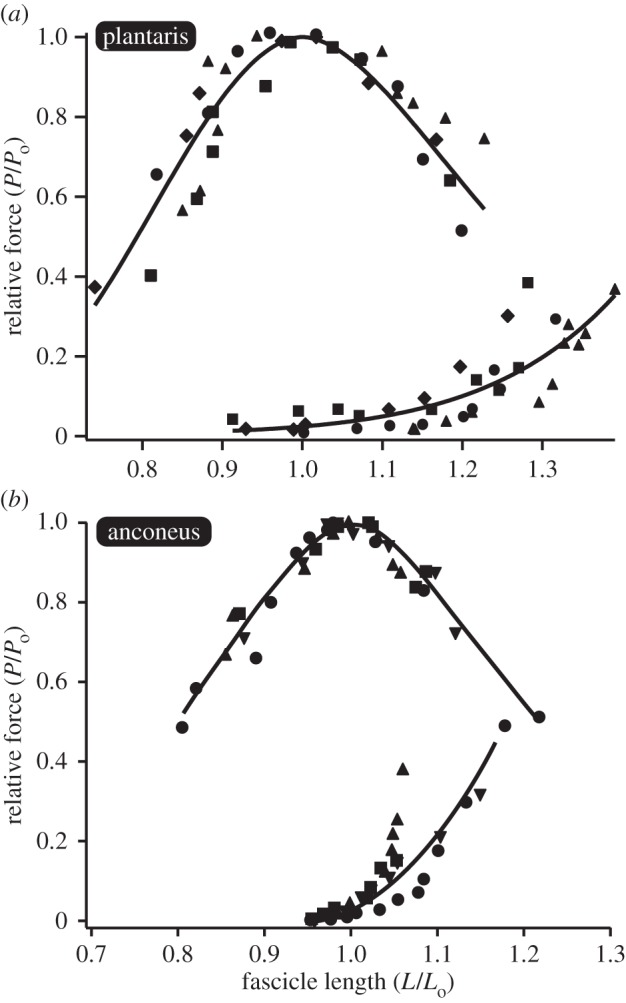
Passive and active force–length curves for the (a) plantaris and (b) anconeus muscles. The same muscles with the same sonomicrometry transducers used for in vivo measurements are used to characterize the force–length curve in vitro. Data are normalized relative to the muscles maximum isometric force (Po) and the muscle's optimal length (Lo) and fit according to [21]. Each individual is shown with a different symbol.
Figure 5.
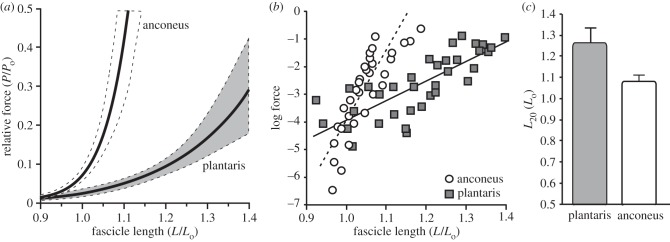
A comparison of the passive properties of the plantaris and anconeus. (a) The passive force–length curves of the two muscles plotted with 95% confidence intervals of the curve (dotted). (b) Log-transformed passive forces. Log transformation converts data from an exponential curve to linear and allows for more direct statistical comparisons using an ANCOVA. Data from the two muscles differ significantly in their slope, suggesting that the two muscles vary in their stiffness (p = 0.0001). (c) A comparison of the lengths at which passive force develops in the two muscles. L20 represents the lengths at which passive force reaches 20% of maximum isomeric force (Po). L20 is significantly longer in the plantaris when compared with the anconeus muscle (p < 0.0001).
The results showed that the operating lengths of the plantaris and anconeus muscle also differed significantly during locomotion. The plantaris operated primarily on the descending limb of the force–length curve (figure 6a). By contrast, the anconeus operated almost entirely on the ascending limb and plateau of the force–length curve (figure 6b). The maximum operating length of the plantaris differed significantly from the anconeus (p = 0.003). On average, the maximum operating length of the plantaris corresponded to 1.18 Lo, while that of the anconeus corresponded to 0.96 Lo (figure 6c).
Figure 6.
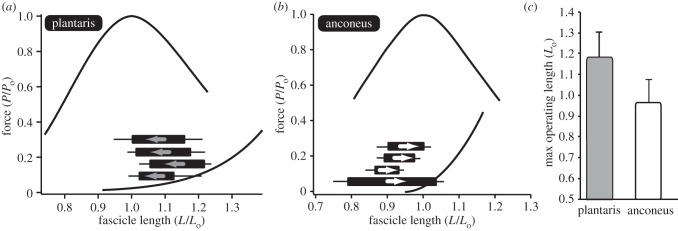
The operating lengths of the (a) plantaris and (b) anconeus muscle. The operating length of each muscle is plotted onto the force–length curve. Each individual is shown as a single bar with the initial and final lengths defining the two ends of the bar. Each bar is shown alongside the standard error of the mean. For the plantaris, the muscle starts at a long length and shortens to the plateau. However, for the anconeus the muscle starts at a short length and lengthens on to the plateau of the force–length curve. Note that the bars only indicate the length range during hopping and do not correspond to the force axis. (c) The maximum operating length of the two muscles differs significantly (p = 0.003). The anconeus muscle is restricted to the ascending limb and plateau of the force–length curve, rarely operating at lengths above Lo.
4. Discussion
These results support the hypothesis that the operating length of muscles functioning to dissipate energy will be restricted to the ascending limb and plateau of the force–length curve, while muscles producing mechanical energy can operate at much longer lengths. The patterns observed in these data are consistent with the idea that the operating length of a muscle is influenced by the potential trade-off between force production during shortening contractions and sarcomere stability during lengthening contractions.
Unlike many muscles that perform diverse mechanical functions and carefully balance the trade-off between force and stability, the hindlimb muscles of anurans are unique in that they are rarely stretched actively during hopping or jumping [23,26]. Therefore, they represent a group of muscles that may safely operate on the descending limb of the force–length curve without bearing the potential risk of eccentric muscle damage. It is therefore not surprising that the plantaris muscle of toads safely operates on the descending limb of the force–length curve during hopping (figure 6). These findings are consistent with two previous studies showing that frog hindlimb muscles operate at relatively long sarcomere lengths during jumping [9,27]. The observed pattern provides some evidence broadly linking the likelihood of eccentric activity with the operating length of muscles during movement.
One mechanism that can restrict operating length during movement is the passive stiffness of the muscle. This study shows a direct link between variation in the passive mechanical property of a muscle and where the muscle operates on the force–length curve (figures 5 and 6). More specifically, the data suggest that the maximum operating length of a muscle is restricted by its resistance to passive stretch. Both the plantaris and the anconeus muscles never operate at lengths where the passive force exceeds 20% of the maximum isometric force (figure 6). This suggests that passive tension may place an upper limit on the operating length of a muscle. Variation in the passive force–length properties of muscles has generally been attributed two distinct mechanisms. First, the passive stiffness of muscle has been shown to arise from collagenous structures within the extracellular matrix (ECM), which surround muscle fibres (endomysium), muscle fascicles (perimysium) or the whole muscle (epimysium). Remodelling of these collagenous structures has been shown to directly alter the passive force–length properties of muscle [28–31]. However, simple measurements of the amount of collagen present in muscle appear to be a relatively poor predictor of muscle stiffness [32,33]. This suggests that subtle changes in the morphology of the ECM may play an important role in defining the passive properties of muscles. Second, the passive mechanical properties of muscles have been attributed to the elastic properties of the intra-sarcomeric protein titin. Titin isoforms vary in size and an increase in titin size corresponds to an increase in its slack length, which is inversely proportional to passive stiffness [34]. The relative contribution of titin has been shown to be significant when assessed at the level of a single muscle fibre where the proportion of passive tension attributed to titin range between 20 and 45% [35]. However, recent studies have shown that the relative contribution of titin to the passive stiffness of a fibre bundle or even a whole muscle is low [33,36]. It is still not known whether the differences observed in the passive properties of the anconeus and plantaris muscle result from variation in intramuscular collagen or titin.
This study shows that muscles performing divergent locomotor functions vary in their passive mechanical properties. Muscles acting as motors are free to operate on the descending limb of the force–length curve, while muscles acting as brakes are restricted to the ascending limb and plateau. Differences in the passive properties of the two muscles are associated with their operating length during locomotion. These results inform our understanding of vertebrate muscle variation by providing a clear link between a muscle's mechanical properties and its locomotor function.
Acknowledgements
The author would like to thank Emily Abbott, Niel Larson, Pooja Rana and Marla Goodfellow for experimental assistance. Nicole Danos and Natalie Holt provided valuable comments on the manuscript.
All husbandry and experimental procedures were approved by the IACUC at UC Irvine.
Funding statement
This work has been supported by the US National Science Foundation grant no. 1051691.
References
- 1.Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S. 2000. How animals move: an integrative view. Science 288, 100–106 (doi:10.1126/science.288.5463.100) [DOI] [PubMed] [Google Scholar]
- 2.Daley MA, Biewener AA. 2003. Muscle force–length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 206, 2941–2958 (doi:10.1242/jeb.00503) [DOI] [PubMed] [Google Scholar]
- 3.Ahn AN, Meijer K, Full RJ. 2006. In situ muscle power differs without varying in vitro mechanical properties in two insect leg muscles innervated by the same motor neuron. J. Exp. Biol. 209, 3370–3382 (doi:10.1242/jeb.02392) [DOI] [PubMed] [Google Scholar]
- 4.Swoap SJ, Johnson TP, Josephson RK, Bennett AF. 1993. Temperature, muscle power output and limitations on burst locomotor performance of the lizard Dipsosaurus dorsalis. J. Exp. Biol. 174, 185–197 [Google Scholar]
- 5.George NT, Sponberg S, Daniel TL. 2012. Temperature gradients drive mechanical energy gradients in the flight muscle of Manduca sexta. J. Exp. Biol. 215, 471–479 (doi:10.1242/jeb.062901) [DOI] [PubMed] [Google Scholar]
- 6.Biewener AA, Roberts TJ. 2000. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc. Sport Sci. Rev. 28, 99–107 [PubMed] [Google Scholar]
- 7.Gordon AM, Huxley AF, Julian FJ. 1966. Variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. Lond. 184, 170–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkholder TJ, Lieber RL. 2001. Sarcomere length operating range of vertebrate muscles during movement. J. Exp. Biol. 204, 1529–1536 [DOI] [PubMed] [Google Scholar]
- 9.Azizi E, Roberts TJ. 2010. Muscle performance during frog jumping: influence of elasticity on muscle operating lengths. Proc. R. Soc. B 277, 1523–1530 (doi:10.1098/rspb.2009.2051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbott BC, Aubert XM. 1952. The force exerted by active striated muscle during and after change of length. J. Physiol. 117, 77–86 [PMC free article] [PubMed] [Google Scholar]
- 11.Lieber RL, Woodburn TM, Friden J. 1991. Muscle damage induced by eccentric contractions of 25-percent strain. J. Appl. Physiol. 70, 2498–2507 [DOI] [PubMed] [Google Scholar]
- 12.Lieber RL, Shah S, Friden J. 2002. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin. Orthop. Relat. Res. 403, S90–S99 (doi:10.1097/00003086-200210001-00011) [DOI] [PubMed] [Google Scholar]
- 13.Proske U, Morgan DL. 2001. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. Lond. 537, 333–345 (doi:10.1111/j.1469-7793.2001.00333.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nosaka K, Newton M, Sacco P. 2002. Muscle damage and soreness after endurance exercise of the elbow flexors. Med. Sci. Sports Exerc. 34, 920–927 (doi:10.1097/00005768-200206000-00003) [DOI] [PubMed] [Google Scholar]
- 15.Brooks SV, Zerba E, Faulkner JA. 1995. Injury to muscle-fibers after single stretches of passive and maximally stimulated muscles in mice. J. Physiol. Lond. 488, 459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbot JA, Morgan DL. 1998. The effects of stretch parameters on eccentric exercise-induced damage to toad skeletal muscle. J. Muscle Res. Cell Motil. 19, 237–245 (doi:10.1023/A:1005325032106) [DOI] [PubMed] [Google Scholar]
- 17.Gosselin LE, Burton H. 2002. Impact of initial muscle length on force deficit following lengthening contractions in mammalian skeletal muscle. Muscle Nerve 25, 822–827 (doi:10.1002/mus.10112) [DOI] [PubMed] [Google Scholar]
- 18.Butterfield TA, Herzog W. 2006. Effect of altering starting length and activation timing of muscle on fiber strain and muscle damage. J. Appl. Physiol. 100, 1489–1498 (doi:10.1152/japplphysiol.00524.2005) [DOI] [PubMed] [Google Scholar]
- 19.Morgan DL. 1990. New insights into the behavior of muscle during active lengthening. Biophys. J. 57, 209–221 (doi:10.1016/S0006-3495(90)82524-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan DL, Proske U. 2004. Popping sarcomere hypothesis explains stretch-induced muscle damage. Clin. Exp. Pharmacol. Physiol. 31, 541–545 (doi:10.1111/j.1440-1681.2004.04029.x) [DOI] [PubMed] [Google Scholar]
- 21.Otten E. 1987. A myocybernetic model of the jaw system of the rat. J. Neurosci. Methods 21, 287–302 (doi:10.1016/0165-0270(87)90123-3) [DOI] [PubMed] [Google Scholar]
- 22.Brown IE, Liinamaa TL, Loeb GE. 1996. Relationships between range of motion, Lo, and passive force in five strap-like muscles of the feline hind limb. J. Morphol. 230, 69–77 (doi:10.1002/(SICI)1097-4687(199610)230:1<69::AID-JMOR6>3.0.CO;2-I) [DOI] [PubMed] [Google Scholar]
- 23.Gillis GB, Biewener AA. 2000. Hindlimb extensor muscle function during jumping and swimming in the toad (Bufo marinus). J. Exp. Biol. 203, 3547–3563 [DOI] [PubMed] [Google Scholar]
- 24.Gillis GB, Akella T, Gunaratne R. 2010. Do toads have a jump on how far they hop? Pre-landing activity timing and intensity in forelimb muscles of hopping Bufo marinus. Biol. Lett. 6, 486–489 (doi:10.1098/rsbl.2009.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azizi E, Abbott EM. 2013. Anticipatory motor patterns limit muscle stretch during landing in toads. Biol. Lett. 9, 20121045 (doi:10.1098/rsbl.2012.1045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson JM, Marsh RL. 1998. Activation patterns and length changes in hindlimb muscles of the bullfrog Rana catesbeiana during jumping. J. Exp. Biol. 201, 2763–2777 [DOI] [PubMed] [Google Scholar]
- 27.Lieber RL, Brown CG. 1992. Sarcomere length-joint angle relationships of 7 frog hindlimb muscles. Acta Anatomica 145, 289–295 (doi:10.1159/000147380) [DOI] [PubMed] [Google Scholar]
- 28.Alnaqeeb MA, Alzaid NS, Goldspink G. 1984. connective-tissue changes and physical-properties of developing and aging skeletal-muscle. J. Anat. 139, 677–689 [PMC free article] [PubMed] [Google Scholar]
- 29.Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. 2004. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin. Orthop. Relat. Res. 426, 258–265 (doi:10.1097/01.blo.0000136831.17696.80) [DOI] [PubMed] [Google Scholar]
- 30.Safran O, Derwin KA, Powell K, Iannotti JP. 2005. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J. Bone Joint Surg. Am. Vol. 87A, 2662–2670 (doi:10.2106/JBJS.D.02421) [DOI] [PubMed] [Google Scholar]
- 31.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. 2011. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J. Physiol. 589, 2625–2639 (doi:10.1113/jphysiol.2010.203364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bensamoun S, Stevens L, Fleury MJ, Bellon G, Goubel F, Tho M. 2006. Macroscopic-microscopic characterization of the passive mechanical properties in rat soleus muscle. J. Biomech. 39, 568–578 (doi:10.1016/j.jbiomech.2004.04.036) [DOI] [PubMed] [Google Scholar]
- 33.Lieber RL, Ward SR. 2013. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am. J. Physiol. Cell Physiol. 305, C241–C252 (doi:10.1152/ajpcell.00173.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granzier HL, Labeit S. 2006. The giant muscle protein titin is an adjustable molecular spring. Exerc. Sport Sci. Rev. 34, 50–53 (doi:10.1249/00003677-200604000-00002) [DOI] [PubMed] [Google Scholar]
- 35.Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA. 2005. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J. Gen. Physiol. 126, 461–480 (doi:10.1085/jgp.200509364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown SHM, Carr JA, Ward SR, Lieber RL. 2012. Passive mechanical properties of rat abdominal wall muscles suggest an important role of the extracellular connective tissue matrix. J. Orthop. Res. 30, 1321–1326 (doi:10.1002/jor.22068) [DOI] [PMC free article] [PubMed] [Google Scholar]


