Abstract
Phenological, biogeographic and community shifts are among the reported responses of marine ecosystems and their species to climate change. However, despite both the profound consequences for ecosystem functioning and services, our understanding of the root causes underlying these biological changes remains rudimentary. Here, we show that a significant proportion of the responses of species and communities to climate change are deterministic at some emergent spatio-temporal scales, enabling testable predictions and more accurate projections of future changes. We propose a theory based on the concept of the ecological niche to connect phenological, biogeographic and long-term community shifts. The theory explains approximately 70% of the phenological and biogeographic shifts of a key zooplankton Calanus finmarchicus in the North Atlantic and approximately 56% of the long-term shifts in copepods observed in the North Sea during the period 1958–2009.
Keywords: shifts, climate change, marine
1. Introduction
Mounting evidence suggests that global warming is altering the biology of the oceans at both the species and the community levels [1,2]. One mechanism by which a species may respond is to track habitat changes, either in time, through a phenological shift, or in space, by a biogeographic shift [1–3]. At the community level, long-term community variations that take place during abrupt ecosystem shifts (AESs) or regime shifts [4] have been documented and sometimes attributed to climate change [5,6]. Although climate-caused environmental changes are often assumed to play a fundamental role in these responses, the underlying pathways by which the environment may trigger phenological, biogeographic and community shifts remain unresolved.
Here, we propose that theoretical frameworks based on the ecological niche sensu Hutchinson [7], frequently applied to investigate the potential spatial distribution of species, can be extended to connect phenological, biogeographic and community shifts. We first establish a theoretical framework to show how the niche can be used to connect phenological and biogeographic shifts at the species level and long-term shifts at the community level. We then test our theory against empirical datasets. Finally, we provide evidence that a significant part of large-scale spatio-temporal changes in both species and communities are predicted from the knowledge of the ecological niche of species.
2. Data
Monthly sea surface temperatures (SSTs) originated from the dataset ERSST_V3 (1958–2009). The dataset is derived from a reanalysis based on the most recently available International Comprehensive Ocean-Atmosphere Data Set. Improved statistical methods have been applied to produce a stable monthly reconstruction, on a 1° × 1° spatial grid, based on sparse data [8].
We used the photosynthetically active radiation (PAR; Einstein m−2 day−1), solar radiation spectrum in the wavelength range of 400–700 nm, as a proxy of the level of energy assimilated by photosynthetic organisms [9]. PAR regulates both the composition and evolution of marine ecosystems, influencing the growth of phytoplankton and in turn the development of zooplankton and fishes. Data were provided by the Giovanni online data system, developed and maintained by the NASA GES DISC (http://gdata1.sci.gsfc.nasa.gov/daac-bin/G3/gui.cgi?instance_id=ocean_month). A monthly climatology of PAR at a spatial resolution of 9 km was carried out by compiling data of the sea-viewing wide field-of-view sensor (SeaWiFS) from September 1997 to December 2010.
Bathymetry data originated from the General Bathymetric Chart of the Oceans database. All environmental data were interpolated on a global grid of 1° longitude × 1° latitude using the inverse squared distance method [10].
Monthly climatology data of upper ocean chlorophyll-a concentration (µg l−1) were retrieved and calculated from the satellite SeaWiFS on a grid of 1° longitude × 1° latitude.
Length of the day (LOD) for any latitude and day of the year was estimated by modelling [11].
Monthly data on the abundance of Calanus finmarchicus at the scale of the North Atlantic and annual data on the abundance of copepods in the North Sea originated from the continuous plankton recorder (CPR) survey (period 1958–2009). This large-scale plankton monitoring programme has sampled the upper layer of the water column (approximately 7 m) on a monthly basis since 1946 [12,13]. Details on methods and contents of this dataset are provided in Reid et al. [13] and Batten et al. [14].
3. Material and methods
(a). Theoretical relationships between the species thermal niche, its spatial distribution and phenology
We first worked on a simple case, where the niche is one-dimensional and represented only by sea surface temperature, to establish at the species level, the theoretical relationships between the (thermal) niche of a species, its spatial distribution and phenology. The response curve of the abundance E of a pseudospecies s in a given site i and time j to change in SSTs was modelled by the following function [15]:
| 3.1 |
With Ei,j,s the expected abundance of a pseudospecies s at location i and time j; cs the maximum value of abundance for species s fixed to one; xi,j the value of temperature at location i and time j; us the thermal optimum and ts the thermal amplitude for species s. The thermal tolerance is an estimation of the breadth (or thermal amplitude) of the species thermal niche (or bioclimatic envelope) [15]. Once the niche was modelled, the expected abundance of such pseudospecies in space (spatial or latitudinal distribution) or time (monthly scale) was determined from the knowledge of SST for a given month and geographical cell. We modelled the niche, spatial distribution and the phenology of an eurytherm psychrophile (us = 15°C and ts = 5°C; figure 1a–c).
Figure 1.
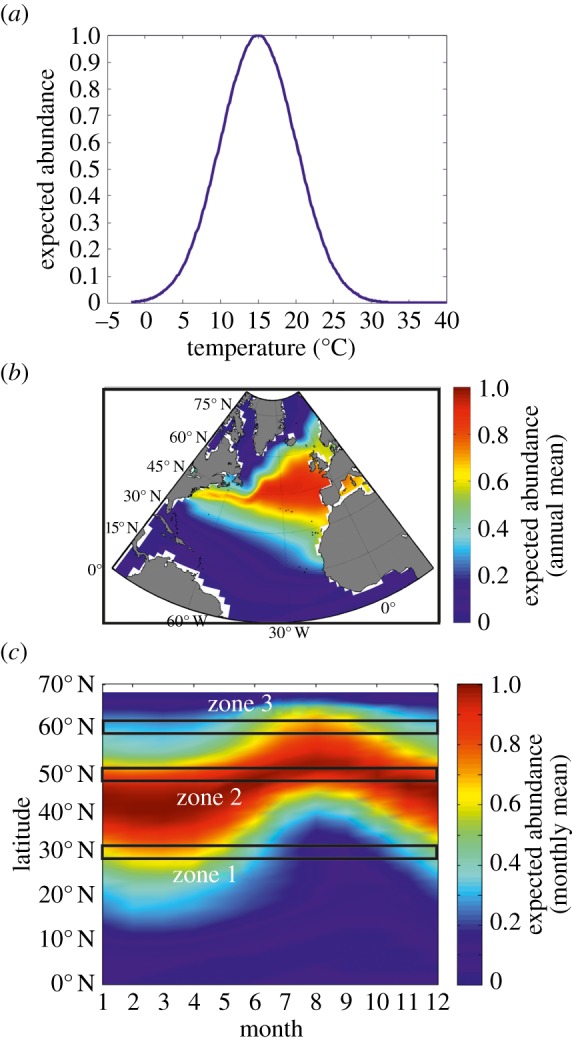
Theoretical relationships between the species distribution, the latitudinal range and the phenology of an eurytherm temperate species calculated from the application of our theory. (a) Theoretical thermal niche. (b) Theoretical mean annual spatial distribution. (c) Theoretical changes in abundance as a function of latitudes and month. Zone 1 is the part of the species distribution where the seasonal maximum occurs in spring or winter. Zone 2 is the part of the species distribution where the seasonal extent is highest. Zone 3 is the part of the species distribution where the seasonal maximum is located at the end of summer.
(b). Relationships between theoretical and observed biogeographic and phenological shifts of Calanus finmarchicus
We tested our theory against actual data using the calanoid copepod C. finmarchicus. In contrast to the idealized example, the niche was four-dimensional. As the species responds not only to SST but also to changes in PAR [16], in chlorophyll-a concentration [17] and bathymetry [18], we also used these three ecological factors.
In contrast to the idealized example that was based on a Gaussian niche, we used the non-parametric probabilistic ecological niche (NPPEN) model [19] to calculate the expected abundance of C. finmarchicus as a function of monthly SST, monthly PAR and monthly chlorophyll-a concentration. The NPPEN model estimates the ecological niche of a species and, because the model is non-parametric, the niche was not constrained to be Gaussian. Once the niche is calculated, the technique projects the expected abundance of the species in space and time. The technique is based on the generalized Mahalanobis distance and a simplified version of the non-parametric test multiple response permutation procedure [19–22]. A high expected abundance corresponds to an environment highly suitable for the species and vice versa. The model NPPEN was applied based on both the macroecological and the macrophysiological knowledge of the species [23–25]. Therefore, the NPPEN model was based on empirical knowledge and not observed data. The reference values were for monthly SST between −1 and 11°C by increments of 1°C. These values were close to those observed empirically [23–25]. The reference thresholds for monthly PAR were between 10 and 50 Einstein m−2 d−1 by increments of 5 and for monthly chlorophyll-a concentration between 0.05 and 1 by increments of 0.05 (unit: log10 µg l−1) [17,23]. The slight changes in these thresholds did not alter substantially model predictions. The NPPEN model estimated the niche of C. finmarchicus based on this theoretical (expert-based knowledge) set of data and we then used the ecological niche to estimate the expected abundance of C. finmarchicus as a function of space and time. The NPPEN model does not need any parametrizing variables [19].
Many findings showed that the abundance of C. finmarchicus declines towards shallow waters (e.g. shallow regions of the North Sea) [18]. To consider this effect, the expected abundance E linearly declines when bathymetry b became inferior to 100 m, as follows:
| 3.2 |
The model NPPEN has never been used on C. finmarchicus with four ecological parameters. More importantly, because the ecological niche is based on expert knowledge, i.e. independent from both the spatial and temporal distribution of sampling, the model can be used at a monthly resolution (instead of a yearly resolution [24,25]), which makes it possible to investigate the relationship between phenology and species distribution.
The expected abundance of C. finmarchicus was compared to the observed abundance at different scales: (i) mean annual spatial distribution (1958–2009); (ii) mean spatial distribution from January to December (1958–2009); and (iii) mean latitudinal monthly changes based on the whole time period based on 1960–1979 (cold decades) and based on 1990–2009 (warm decades) [5]. Data were spatially interpolated for each month and year of the period 1958–2009 using the inverse squared distance method [10]. An estimation was only calculated when the number of samples was above three samples [26,27]. Averages were performed for the time periods mentioned above for both expected and observed abundances. In addition to the control of the number of samples in the spatial interpolations, the use of a large number of years (periods 1958–2009, 1960–1979 and 1990–2009) attenuated the effect of the spatial heterogeneity of the CPR survey. Because the calculation of the niche was based on expert knowledge, there is no circularity between the estimation of the niche and the comparison between expected and observed abundance at both monthly and spatial scales.
(c). Long-term community shifts in the North Sea
We then investigated whether our theory could predict AESs in the North Sea (4° W, 10° E, 51° N, 60° N). We first calculated the annual mean of all North Sea copepods sampled by the CPR survey (see §2) and selected species with an annual relative (i.e. expressed as percentage) abundance of more than 0.001 and a presence of more than 10% for all years of the period 1958–2009, applying the procedure used in Ibanez & Dauvin [28]. The choice of these thresholds was exclusively conditioned by the statistical techniques, which should not be applied on data matrices containing a high proportion of 0 values. This procedure led to the selection of 27 copepods (electronic supplementary material, table S1). Abundance data in the matrix (52 years × 27 species or taxa) were transformed using the function log10(x + 1). A standardized principal component analysis (PCA) was applied to examine long-term changes in copepods.
We then created a large number of pseudospecies, each characterized by a thermal Gaussian niche with the optimum temperature varying between 4 and 25°C (by 2°C increments) and a thermal tolerance ranging from 0.1 to 10°C (by 1°C increments) using equation (3.1). We then eliminated pseudospecies whose ecological niche was too truncated (i.e. expected abundance of more than 0.2 on the cold or hot thermal limit) between an interval of temperature ranging from −1.8 to 40°C. We applied the procedure of Ibanez & Dauvin [28] with the same thresholds we applied to copepods. However, as the number of species were still above observed number of copepods (90 pseudospecies versus 27 species), we randomly selected 27 pseudospecies and performed a standardized PCA to examine long-term changes in these pseudospecies. The first principal component from the PCA performed on pseudospecies and copepods was then compared. We repeated the procedure of selection of the 27 pseudospecies 10 000 times and recalculated each time the standardized PCA on pseudospecies, the comparisons of long-term changes in the first principal components (observed and theoretical) and the calculation of the Spearman correlation.
(d). Spatial and temporal autocorrelation
To account for spatial autocorrelation when geographical patterns were compared, the degrees of freedom n were recalculated to indicate the minimum number of samples (n0.05) needed to maintain a significant relationship at p < 0.05 [5]. The smaller n0.05, the less likely is the effect of spatial autocorrelation on the probability of significance. The reduction of degree of freedom r0.05, expressed as percentage, was then calculated as follows:
| 3.3 |
The higher the reduction in the degree of freedom at probability p < 0.05, the smaller the effect of spatial autocorrelation. Second, when correlations were calculated between time series, the spatial autocorrelation function (ACF) was calculated to allow the adjustment of the actual degree of freedom to assess the probability of significance pACF of correlations more correctly [5].
4. Results and discussion
(a). Theoretical relationships between the species thermal niche, its spatial distribution and phenology
Using only the thermal dimension of the niche as an example, we modelled the expected mean annual distributional range of a hypothetical temperate species with a broad thermal niche (figure 1a; thermal optimum us = 15°C and thermal tolerance ts = 5°C). The abundance of this hypothetical species was then estimated from their (Gaussian) thermal niche and observed monthly SSTs (1958–2009).
The Gaussian model predicts a mean, annual extratropical range with a poleward limit to the south of the Polar Biome and an equatorward limit north of the Atlantic Trade Wind Biome [29] (figure 1b). The calculation of the expected species' abundance as a function of latitude and month leads to three predictions relating latitudinal range and the species phenology (figure 1c). First, in the southern part of its distributional range (zone 1; figure 1c), the species has a seasonal maximum in winter or spring, the latter period is more likely when parameters such as PAR affect the species either directly through its influence on photosynthesis (e.g. phytoplankton) or indirectly through the food web (e.g. herbivorous zooplankton). At its southern range, such a species could not adjust its phenology in response to an increase in sea temperature, resulting in a local reduction of its annual mean and a northward biogeographic shift. Second, at the centre of its range (zone 2; figure 1c), the species will exhibit its maximum seasonal extent, the duration being modulated by the breadth of its thermal niche; here, the species can occur at all months of the year, so long as other niche dimensions such as PAR or LOD do not exert a controlling influence. Consequently, at the centre of the range, an increase in temperature is expected to trigger a shift towards an earlier phenology. All else being equal or held constant, the erosion of the seasonal period of occurrence in late summer should be compensated at an annual scale by higher abundance towards spring or early summer and consequently, no substantial alteration of the species annual mean is expected. Third, at the northern edge of its distributional range (zone 3; figure 1c), the species is likely to peak in summer or late summer. In this case, the temperate species can extend its occurrence in early summer and spring if temperatures are warm, resulting in an increase of its annual mean abundance. If SST warms north of its northern boundary, a northward range shift will occur although the species will be detected in the water first in the late summer when sea temperatures are highest.
(b). Relationships between theoretical and observed biogeographic and phenological shifts of Calanus finmarchicus
We tested our theory against real data for marine species, choosing the key-structural zooplankton species C. finmarchicus. The niche was modelled using the ecological niche model termed NPPEN model [19], which calculated the expected abundance of C. finmarchicus as a function of monthly SST, monthly PAR, monthly chlorophyll-a concentration and bathymetry. These four parameters form the most important niche dimensions for C. finmarchicus [18,24]. Seasonal changes in PAR and LOD are highly correlated positively on average (rmean = 0.97, n = 734 geographical cells). LOD has been assumed to be an important controlling factor of the initiation and termination of the diapause of C. finmarchicus [30]. As NPPEN is non-parametric, the niche was not Gaussian in contrast to the idealized example above.
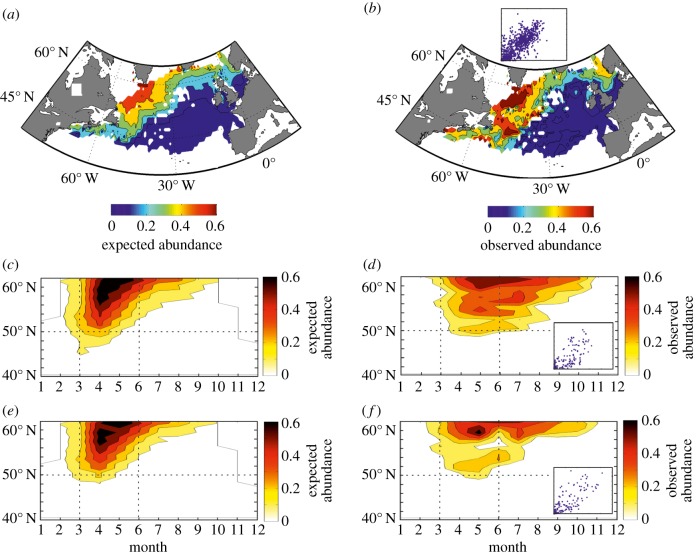
The ecological niche was then used to forecast the spatial distribution of the calanoid (1958–2009; figure 2a). High theoretical abundances occur north of the oceanic polar front [31] and to a lesser extent south of Newfoundland and in the northern part of the North Sea. This corresponds well to the observed spatial distribution (1958–2009) inferred from the CPR survey (figure 2b) [18] and at both monthly (r = 0.73, n = 6713, p < 0.001, n0.05 = 8, r0.05 = 99.88%; electronic supplementary material) and annual scales (r = 0.84, n = 1,046, p < 0.001, n0.05 = 6, r0.05 = 99.43%).
Figure 2.
Relationships between the spatial distribution, the latitudinal ranges and the phenological shifts of both observed and theoretical abundance of C. finmarchicus from the application of our theory. The NPPEN model was used to calculate the expected abundance of C. finmarchicus (electronic supplementary material). (a) Expected and (b) observed spatial distribution of C. finmarchicus in the North Atlantic. Latitudinal and seasonal changes in both the expected and observed abundance of C. finmarchicus based on the periods 1960–1979 (c and d, respectively) and 1990–2009 (e and f, respectively). Both expected and observed abundance of C. finmarchicus were calculated for a meridional band between 30° W and 10° W (northeast Atlantic). Scaled between 0 and 1, scatterplots in (b), (d) and (f) exhibit expected abundance versus observed abundance for (a–b), (c–d) and (e–f), respectively. Both vertical and horizontal dashed lines (c–f) are superimposed to better reveal phenological and biogeographic shifts.
When the expected abundance of C. finmarchicus is represented as a function of latitude and month (1960–1979, two relatively cold decades [5]; northeast Atlantic between 30° W and 10° W), expectations are that the species should have seasonal maxima in spring at the southern edge and between spring and summer towards the centre of its spatial distribution (figure 2c). Observed abundance of C. finmarchicus as a function of latitudes and months provides strong support for both predictions (figure 2d; r = 0.84, n = 259, p < 0.001, n0.05 = 6, r0.05 = 97.68%). The spatio-temporal pattern in expected abundance is however more concentrated than observed abundance (figure 2c,d). At the end of the seasonal occurrence of the species (in summer towards higher latitudes), the level of abundance remains elevated whereas expected abundance drops. This lag may be explained by the fact that when the environment becomes less suitable, the species may remain a certain amount of time before decreasing in abundance (diapause initiation and source/sink dynamics) [17,32]. At the beginning of the seasonal occurrence, the lag between expected and observed abundance is much less pronounced and can be explained by the time needed for the species to increase its level of abundance (reproduction and individual growth) [17].
We next calculated the abundance of C. finmarchicus as a function of both latitude and month for the two warm decades 1990–2009 [33] (figure 2e,f). From our theoretical model, we expect: (i) a reduction in the level of abundance in spring resulting in an erosion of the spatial distribution of the species at its southern margin, and (ii) a reduction in the abundance of the species in late summer to the north. We found good support for both predictions and this explains 70.56% of the total variance of the combined phenological and biogeographic changes (figure 2c–f; r = 0.84, n = 518, p < 0.001, n0.05 = 6, r0.05 = 98.84%). We observed a biogeographic shift of C. finmarchicus northwards in the northeast Atlantic (see the equatorward range limit in figure 2d for 1960–1979 versus figure 2f for 1990–2009). As expected, and because the species overwinters in deep water, and PAR is positively correlated to phytoplankton production in winter in these areas [16], the copepod cannot compensate for the increase in temperature observed between March and September in the southern part of its current distribution during this season. In the central part of its range (approx. 60° N), rising temperature had a negative effect on the abundance of the species in summer. An increase in temperature is expected to generate a poleward shift in the species spatial distribution. Our model predicts that individuals might be first detected in late summer (figure 1), a prediction that is confirmed by observations of the first occurrence of southern zooplankton species (e.g. Centropages typicus, Centropages violaceus and Temora stylifera) along European coasts [34,35].
(c). Long-term community shifts in the North Sea
To test whether our theory might be useful to explain climate-modulated long-term community changes, we first examined long-term modifications among copepods in the North Sea (51° N–60° N, 4° W–10° E) where substantial community changes have occurred already [5]; to do this, we used a standardized PCA performed on a table, years (1958–2009) × annual observed abundance (27 species or taxa; electronic supplementary material, table S1). We generated a total of 90 pseudospecies each characterized by different thermal niches from stenotherms to eurytherms (figure 3a,b) and estimated the expected abundance (as annual mean) of these species in the North Sea as a function of monthly SSTs. The niche was modelled exclusively as a function of monthly SSTs because: (i) bathymetry does not change on a year-to-year basis, and (ii) both PAR and chlorophyll-a concentration were mostly important to reconstruct the seasonal and the distributional range of C. finmarchicus. None of the species had the same thermal niche following the principle of competitive exclusion of Gause [36]. Our objective was to show how, by creating a pool of species with niches differing by their optimum and amplitude, the sum of the temporal changes occurring for each species could create long-term community shifts similar to those observed in the North Sea (figure 3c). We found significant positive relationships (pACF < 0.05) between expected and observed changes in the North Sea copepods (figure 4). The correlations between expected and observed long-term changes were in general (88.90% of the 10 000 simulations) superior to the correlation calculated between annual SSTs and observed changes (r = 0.74, n = 50, pACF < 0.05; figure 3c). Our theory therefore explains 56.25% of the long-term changes in copepods in the North Sea and provides a mechanism to understand how climate-caused changes in temperatures may influence long-term community shifts.
Figure 3.
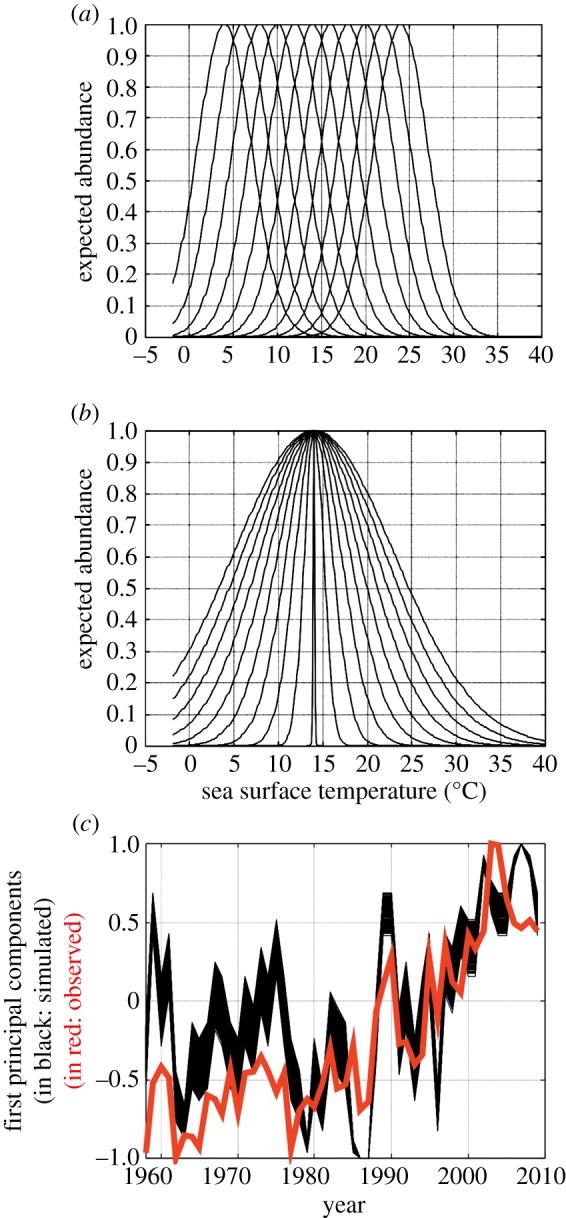
The community shift in the North Sea (4° W–10° E; 51° N–60° N) reconstructed from the application of our theory. Examples of some simulated niches based on (a) different thermal optimums us, and a constant thermal tolerance ts, and (b) a constant average us and different thermal tolerances ts (electronic supplementary material). Only pseudospecies that could establish in the North Sea were used in the analyses. (c) First principal components (10 000 first principal components; in black) from standardized PCAs applied on each simulated table 52 years × 27 pseudospecies and the first principal component (in red) from a standardized PCA performed on the table 52 years × 27 copepods.
Figure 4.
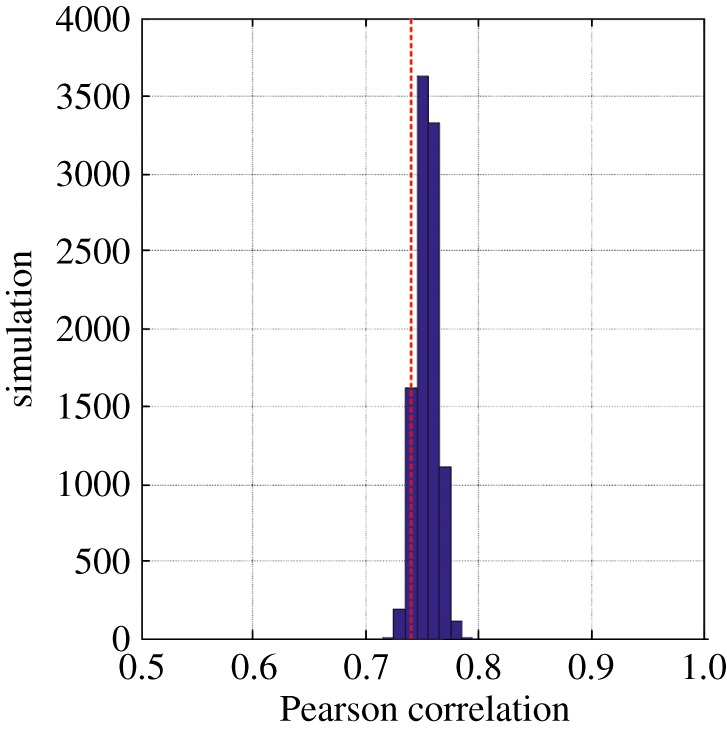
Frequency distribution of the Pearson correlation coefficient calculated between each first principal component calculated for each simulated table 52 years × 27 pseudospecies and the first principal component performed on the table 52 years × 27 copepods (electronic supplementary material). The red dashed vertical line indicates the correlation between observed annual ecosystem changes and changes in annual SST.
(d). Limitations of our theory
Our theory does not resolve species interactions. The climate-induced reorganization of communities is likely to alter species interactions (e.g. predation, competition and facilitation), which may in turn affect both temporal and spatial patterns in species distribution. Modifications in biotic interaction might modulate the ability of a species to inhabit an ecosystem [37]. However, this effect may be negligible at a macroecological scale [38]. Using a macrophysiological model, Helaouët & Beaugrand [18] showed that the fundamental (i.e. niche without the influence of biotic interaction and dispersal) and the realized niches (i.e. niche with effect of biotic interaction and dispersal) of C. finmarchicus did not differ significantly at a global scale.
Our theory also neglects the potential effects of phenotypic plasticity and genetic adaptation. How much can a species alter its niche? Some authors suggest the possibility of rapid genetic responses to natural selection rather than direct reaction of species according to their ecological niche [39]. This might be effective for small and spatially isolated zooplankton or fish populations. However, as already stated, niche conservatism is often observed at palaeoclimatic scales [40]. Our theory works at the scale of the whole spatial distribution of a species, which is likely to integrate all species' capabilities to respond to environmental heterogeneity either by phenotypic plasticity or by genetic adaptation at the time scales covered in this study. In other words, the whole spatial distribution of a species (including de facto population-specific adaptations) reflects its phenotypic plasticity/genetic adaptation at the individual level and small spatial scales.
(e). Concluding remarks
To conclude, our theory enables us to connect phenological, biogeographic and long-term community shifts through a common concept (i.e. the ecological niche that integrates the sum of all environmental constraints on the species physiology). In this way, we provide an explanation for why climate-induced long-term environmental changes—especially changes in temperature—and changes in species and communities, are so often tightly correlated [37]. Even though stochastic effects due to complex abiotic and biotic interactions throughout a species' life cycle, and from demographic effects that control vital processes (e.g. fecundity, survival), make it difficult to forecast the response of species and ecosystems to climate change [41,42], our results demonstrate that a significant proportion of biogeographic, phenological and long-term community shifts is deterministic at some observed emergent spatio-temporal scales and therefore predictable. We propose that a fixed ecological niche offers a way to understand how communities and their species may respond to climatic variability and global climate change. Although the ecological niche is already applied to anticipate the response of a species distributional range to climate change by means of ecological niche models [43], the concept of the ecological niche has never, to our knowledge, been used to link phenological, biogeographic and community shifts, which are the three main documented responses to climate change so far [1–3,5].
Phenological and biogeographic changes can be interpreted as a means by which a species tracks its thermal niche in time and space. At the community scale, a large part of climate-caused long-term community shifts is the result of climate-modulated environmental changes on the ecological niche of each species, which explains why many species remain stable during an AES, and why some may react earlier than others [44]. Our results suggest that where substantial variations in temperature take place over short distances, such as at critical thermal boundaries, long-term biological shifts are likely to be more prominent [5].
Funding statement
This work was supported by the ‘Centre National de la Recherche Scientifique’ (CNRS) and the programme BIODIMAR.
References
- 1.Edwards M, Richardson AJ. 2004. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884 (doi:10.1038/nature02808) [DOI] [PubMed] [Google Scholar]
- 2.Beaugrand G, Reid PC, Ibañez F, Lindley JA, Edwards M. 2002. Reorganisation of North Atlantic marine copepod biodiversity and climate. Science 296, 1692–1694 (doi:10.1126/science.1071329) [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 4.de Young B, Barange M, Beaugrand G, Harris R, Perry RI, Scheffer M. 2008. Regime shifts in marine ecosystems: detection, prediction and management. Trends Ecol. Evol. 23, 402–409 (doi:10.1016/j.tree.2008.03.008) [DOI] [PubMed] [Google Scholar]
- 5.Beaugrand G, Edwards M, Brander K, Luczak C, Ibañez F. 2008. Causes and projections of abrupt climate-driven ecosystem shifts in the North Atlantic. Ecol. Lett. 11, 1157–1168 [DOI] [PubMed] [Google Scholar]
- 6.Weijerman M, Lindeboom H, Zuur AF. 2005. Regime shifts in marine ecosystems of the North Sea and Wadden Sea. Mar. Ecol. Prog. Ser. 298, 21–39 (doi:10.3354/meps298021) [Google Scholar]
- 7.Hutchinson GE. 1957. Concluding remarks. Cold Spring Harbor Symp. Quant. Biol. 22, 415–427 (doi:10.1101/SQB.1957.022.01.039) [Google Scholar]
- 8.Smith TM, Reynolds RW, Peterson TC, Lawrimore J. 2008. Improvements to NOAA's historical merged land-ocean surface temperature analysis (1880–2006). J. Clim. 21, 2283–2296 (doi:10.1175/2007JCLI2100.1) [Google Scholar]
- 9.Asrar G, Myneni R, Kanemasu ET. 1989. Estimation of plant canopy attributes from spectral reflectance measurements. In Theory and application of optical remote sensing (ed. Asrar G.), pp. 252–295 New York, NY: Wiley [Google Scholar]
- 10.Beaugrand G, Reid PC, Ibañez F, Planque P. 2000. Biodiversity of North Atlantic and North Sea calanoid copepods. Mar. Ecol. Prog. Ser. 204, 299–303 (doi:10.3354/meps204299) [Google Scholar]
- 11.Forsythe WC, Rykiel EJ, Jr, Stahl RS, Wu H-I, Schoolfield RM. 1995. A model comparison for daylength as a function of latitude and day of year. Ecol. Model. 80, 87–95 (doi:10.1016/0304-3800(94)00034-F) [Google Scholar]
- 12.Warner AJ, Hays GC. 1994. Sampling by the continuous plankton recorder survey. Prog. Oceanogr. 34, 237–256 (doi:10.1016/0079-6611(94)90011-6) [Google Scholar]
- 13.Reid PC, et al. 2003. The continuous plankton recorder: concepts and history, from plankton indicator to undulating recorders. Prog. Oceanogr. 58, 117–173 (doi:10.1016/j.pocean.2003.08.002) [Google Scholar]
- 14.Batten SD, et al. 2003. CPR sampling: the technical background, materials, and methods, consistency and comparability. Prog. Oceanogr. 58, 193–215 (doi:10.1016/j.pocean.2003.08.004) [Google Scholar]
- 15.Ter Braak CJF. 1996. Unimodal models to relate species to environment. Wageningen, The Netherlands: DLO-Agricultural Mathematics Group [Google Scholar]
- 16.Behrenfeld MJ. 2010. Abandoning Sverdrup's critical depth hypothesis on phytoplankton blooms. Ecology 91, 977–989 (doi:10.1890/09-1207.1) [DOI] [PubMed] [Google Scholar]
- 17.Helaouët P, Beaugrand G, Reid PC. 2011. Macrophysiology of Calanus finmarchicus in the North Atlantic Ocean. Prog. Oceanogr. 91, 217–228 (doi:10.1016/j.pocean.2010.11.003) [Google Scholar]
- 18.Helaouët P, Beaugrand G. 2009. Physiology, ecological niches and species distribution. Ecosystems 12, 1235–1245 (doi:10.1007/s10021-009-9261-5) [Google Scholar]
- 19.Beaugrand G, Lenoir S, Ibanez F, Manté C. 2011. A new model to assess the probability of occurrence of a species based on presence-only data. Mar. Ecol. Prog. Ser. 424, 175–190 (doi:10.3354/MEPS08939) [Google Scholar]
- 20.Lenoir S, Beaugrand G, Lecuyer E. 2011. Modelled spatial distribution of marine fish and projected modifications in the North Atlantic Ocean. Glob. Change Biol. 17, 115–129 (doi:10.1111/j.1365-2486.2010.02229.x) [Google Scholar]
- 21.Rombouts I, Beaugrand G, Dauvin J-C. 2012. Potential changes in benthic macrofaunal distributions from the English Channel simulated under climate change scenarios. Estuar. Coast. Shelf Sci. 99, 153–161 (doi:10.1016/j.ecss.2011.12.026) [Google Scholar]
- 22.Frederiksen M, Anker-Nilssen T, Beaugrand G, Wanless S. 2013. Climate, copepods and seabirds in the boreal Northeast Atlantic: current state and future outlook. Glob. Change Biol. 19, 364–372 (doi:10.1111/gcb.12072) [DOI] [PubMed] [Google Scholar]
- 23.Helaouët P, Beaugrand G. 2007. Macroecology of Calanus finmarchicus and C. helgolandicus in the North Atlantic Ocean and adjacent seas. Mar. Ecol. Prog. Ser. 345, 147–165 (doi:10.3354/meps06775) [Google Scholar]
- 24.Reygondeau G, Beaugrand G. 2011. Future climate-driven shifts in distribution of Calanus finmarchicus. Glob. Change Biol. 17, 756–766 (doi:10.1111/j.1365-2486.2010.02310.x) [Google Scholar]
- 25.Beaugrand G, Mackas D, Goberville E. 2013. Applying the concept of the ecological niche and a macroecological approach to understand how climate influences zooplankton: advantages, assumptions, limitations and requirements. Prog. Oceanogr. 111, 75–90 (doi:10.1016/j.pocean.2012.11.002) [Google Scholar]
- 26.Beaugrand G, Edwards M. 2001. Comparison in performance among four indices used to evaluate diversity in pelagic ecosystems. Oceanol. Acta 24, 467–477 (doi:10.1016/S0399-1784(01)01157-4) [Google Scholar]
- 27.Beaugrand G. 2004. Monitoring marine plankton ecosystems (1): description of an ecosystem approach based on plankton indicators. Mar. Ecol. Prog. Ser. 269, 69–81 (doi:10.3354/meps269069) [Google Scholar]
- 28.Ibanez F, Dauvin J-C. 1998. Shape analysis of temporal ecological processes: long-term changes in English channel macrobenthic communities. Coenoses 13, 115–129 [Google Scholar]
- 29.Longhurst A. 2007. Ecological geography of the sea. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 30.Fiksen O. 2000. The adaptative timing of diapause: a search for evolutionarily robust strategies in Calanus finmarchicus. ICES J. Mar. Sci. 57, 1825–1833 (doi:10.1006/jmsc.2000.0976) [Google Scholar]
- 31.Dietrich G. 1964. Oceanic polar front survey. Res. Geophys. 2, 291–308 [Google Scholar]
- 32.Pulliam HR. 1988. Sources, sinks, and population regulation. Am. Nat. 132, 652–661 (doi:10.1086/284880) [Google Scholar]
- 33.Zhao M, Running SW. 2010. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329, 940–943 (doi:10.1126/science.1192666) [DOI] [PubMed] [Google Scholar]
- 34.Lindley JA, Daykin S. 2005. Variations in the distributions of Centropages chierchiae and Temora stylifera (Copepoda: Calanoida) in the north-eastern Atlantic Ocean and western European shelf waters. ICES J. Mar. Sci. 62, 869–877 (doi:10.1016/j.icesjms.2005.02.009) [Google Scholar]
- 35.Beaugrand G, Lindley JA, Helaouët P, Bonnet D. 2007. Macroecological study of Centropages typicus in the North Atlantic Ocean. Prog. Oceanogr. 72, 259–273 (doi:10.1016/j.pocean.2007.01.002) [Google Scholar]
- 36.Gause GF. 1934. The struggle for coexistence. Baltimore, MD: Williams and Wilkins [Google Scholar]
- 37.Kirby RR, Beaugrand G. 2009. Trophic amplification of climate warming. Proc. R. Soc. B 276, 3053–3062 (doi:10.1098/rspb.2009.1320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson RG, Dawson TP. 2003. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 12, 361–371 (doi:10.1046/j.1466-822X.2003.00042.x) [Google Scholar]
- 39.Lee CE. 2002. Evolutionary genetics of invasive species. Trends Ecol. Evol. 17, 386–391 (doi:10.1016/S0169-5347(02)02554-5) [Google Scholar]
- 40.Crisp MD, et al. 2009. Phylogenetic biome conservatism on a global scale. Nature 458, 754–756 (doi:10.1038/nature07764) [DOI] [PubMed] [Google Scholar]
- 41.Boyce MS, Haridas CV, Lee CT, Group NSDW. 2006. Demography in an increasingly variable world. Trends Ecol. Evol. 21, 141–148 (doi:10.1016/j.tree.2005.11.018) [DOI] [PubMed] [Google Scholar]
- 42.Keith DA, Akçakaya HR, Thuiller W, Midgley GF, Pearson RG, Phillips SJ, Regan HM, Araujo MB, Rebelo TG. 2008. Predicting extinction risks under climate change: coupling stochastic population models with dynamic bioclimatic habitat models. Biol. Lett. 4, 560–563 (doi:10.1098/rsbl.2008.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araujo MB, Guisan A. 2006. Five (or so) challenges for species distribution modelling. J. Biogeogr. 33, 1677–1688 (doi:10.1111/j.1365-2699.2006.01584.x) [Google Scholar]
- 44.Beaugrand G. 2004. The North Sea regime shift: evidence, causes, mechanisms and consequences. Prog. Oceanogr. 60, 245–262 (doi:10.1016/j.pocean.2004.02.018) [Google Scholar]