Abstract
Neocortical neurons mediate the sedative and anticonvulsant properties of benzodiazepines. These agents enhance synaptic inhibition via positive modulation of -aminobutyric acid (GABAA) receptors harbouring 1-, 2-, 3- or 5-protein subunits. Benzodiazepine-sensitive GABAA receptors containing the 5-subunit are abundant in the neocortex, but their impact in controlling neuronal firing patterns is unknown. Here we studied how the discharge rates of cortical neurons are modified by a positive (SH-053-2′F-R-CH3) and a negative (L 655,708) 5-subunit-preferring allosteric modulator in comparison to diazepam, the classical non-selective benzodiazepine. Drug actions were characterized in slice cultures from wild-type and 5(H105R) knock-in mice by performing extracellular multi-unit-recordings. In knock-in mice, receptors containing the 5 subunit are insensitive to benzodiazepines. The non-selective positive allosteric modulator diazepam decreased the discharge rates of neocortical neurons during episodes of ongoing neuronal activity (up states). In contrast to diazepam, the 5-preferring positive modulator SH-053-2′F-R-CH3 accelerated action potential firing during up states. This promoting action was absent in slices from 5(H105R) mice, confirming that it is mediated by the 5-subunit. Consistent with these observations, the negative 5-selective modulator L 655,708 inhibited up state action potential activity in slices from wild-type mice. The opposing actions of diazepam and SH-053-2′F-R-CH3, which both enhance GABAA receptor function but differ in subtype-selectivity, uncovers contrasting roles of GABAA receptor subtypes in controlling the firing rates of cortical neurons. These findings may have important implications for the design of novel anaesthetic and anticonvulsant benzodiazepines displaying an improved efficacy and fewer side effects.
Keywords: GABAA receptor subtypes, alpha-5 containing GABAA receptors, selective benzodiazepines, diazepam, cortex, organotypic slice culture
1. Introduction
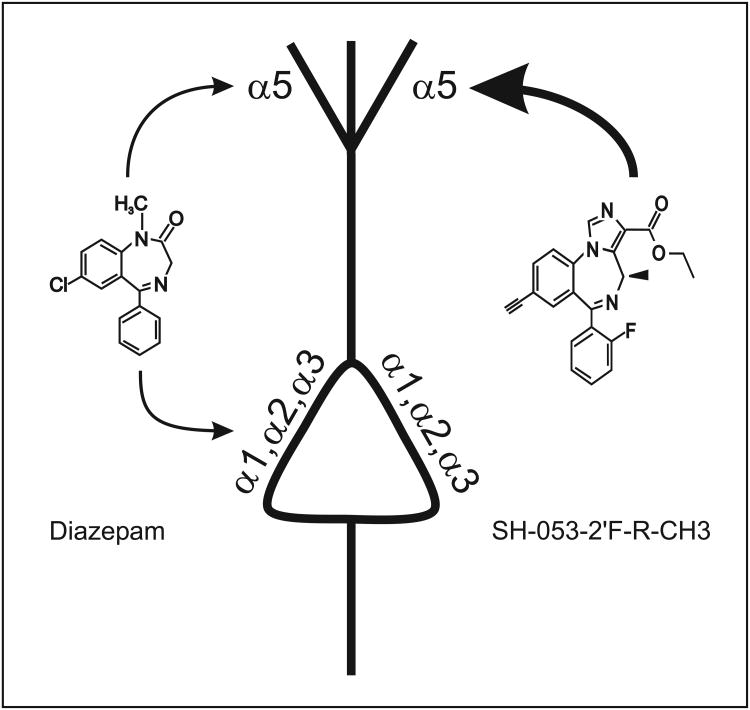
Benzodiazepines enhance -aminobutyric acid (GABAA) receptor mediated inhibition, thereby causing anxiolysis, muscle relaxation, amnesia, sedation and depression of seizure activity (Möhler et al., 2002). These drugs act on a subpopulation of GABAA receptors which is defined by the presence of an 1-, 2-, 3- or 5-, and a -subunit (Puia et al., 1991; Rivas et al., 2009). A recent study provided evidence that glutamatergic cortical pyramidal neurons are a major substrate for mediating the sedative actions of benzodiazepines (Zeller et al., 2008). In accordance with these findings in rodents, functional magnetic resonance imaging studies showed that sedative drugs reduce blood flow predominantly in neocortical circuits of human subjects (Heinke and Koelsch, 2005). On the cellular level, benzodiazepines significantly depress action potential activity of neocortical neurons (Drexler et al., 2010), supporting the idea that this action is causally linked to sedation. Neocortical neurons express a great diversity of GABAA receptor-subtypes (Fritschy and Brünig, 2003). There is ample evidence in the literature that 1-subunit containing receptors largely mediate the motor-sedative properties of diazepam, a non-selective benzodiazepine site agonist (Rudolph et al., 1999; McKernan et al., 2000). However, recent reports suggested that alpha(Greek symbol)5-preferring benzodiazepine site agonists that are structurally related to the newly snthesized compound SH-053-2′F-R-CH3 ((R)-Ethyl-8-ethynyl-6-(2′-fluorophenyl)-4-methyl-4H-benzo[f]imidazo[1,5-a][1,4]diazepine-3-carboxylate) can produce, although this compound was not sedating in primates (Fischer et al., 2010; Savic et al., 2008; Savic et al., 2010). On the other hand, behavioural studies argue against a role of 5 in producing sedation (Crestani et al., 2002; Cheng et al., 2006).
To further elucidate the role of GABAA receptors harbouring 5-subunits in mediating the actions of benzodiazepines in neocortical networks, we compared the effects of SH-053-2′F-R-CH3 and diazepam on the activity patterns of neocortical neurons in organotypic slice cultures. Furthermore, the actions of L 655,708 (ethyl (13aS)-7-methoxy-9-oxo-11,12,13,13a-tetrahydro-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylate) a negative allosteric modulator selective for 5-containing receptors were evaluated.
In previous investigations an excellent correlation between the concentrations of various drugs in causing sedation in behavioural studies and in attenuating spontaneous action potential activity of cultured neocortical neurons has been reported (Hentschke et al., 2005; Drexler et al. 2010). We opted for this in vitro approach because active metabolites are not expected to obscure experimental results. Since benzodiazepines do not only act via the classical high affinity benzodiazepine binding site but also via additional modulatory binding sites on GABAA receptors and further molecular targets in the brain (Baur et al., 2008; Walters et al., 2000), we characterized the effects of SH-053-2′F-R-CH3 in slices derived from wild type mice and in slices prepared from 5 knock-in mice as well. By introducing a histidine-to-arginine point mutation at position 105 of the 5-protein subunit, GABAA receptors containing the mutated subunit are insensitive to allosteric modulation by benzodiazepine-site ligands, whereas regulation by the physiological neurotransmitter GABA is preserved (Benson et al., 1998; Crestani et al., 2002).
2. Material and Methods
2.1. Organotypic slice cultures
Wild type and 5(H105R) mutant mice on the 129X1/SvJ background (Crestani et al., 2002) of both sexes were used for this study. All procedures were approved by the animal care committee (Eberhard-Karls-University, Tuebingen, Germany) and were in accordance with the German law on animal experimentation. Neocortical slice cultures were prepared from two- to five-day old mice as described by Gähwiler (Gähwiler, 1981). Every endeavour has been made to minimize both the suffering and number of animals used. In brief, animals were deeply anaesthetized with isoflurane and decapitated. Cortical hemispheres were aseptically removed and 300μm thick coronal slices were cut. Slices derived from the somatosensory cortex and were fixed on glass coverslips by a plasma clot, transferred into plastic tubes containing 750μl of nutrition medium and incubated in a roller drum at 37°C. After one day in culture, antimitotics were added. The suspension was renewed twice a week. Cultures were used after two weeks in vitro.
Organotypic slice cultures were used for electrophysiological recordings after 15 - 35 days in vitro. As the changes in the reversal potential of GABA-evoked currents occur between postnatal day 5 and 12, all cultures used in the present study had developed into an adult status, which is also indicated by the morphological differentiation of individual cell types (Caeser and Schüz, 1992; Di Cristo et al., 2004).
2.2. Electrophysiology
Extracellular network recordings were performed in a recording chamber mounted on an inverted microscope. Slices were perfused with artificial cerebrospinal fluid (aCSF) consisting of (in mM) NaCl 120, KCl 3.3, NaH2PO4 1.13, NaHCO3 26, CaCl2 1.8 and glucose 11, bubbled with 95% oxygen and 5% carbon dioxide at 34°C. aCSF-filled glass electrodes with a resistance of about 3 to 5 M were advanced into the tissue until extracellular single- or multi-unit spike activity exceeding 100μV in amplitude were visible.
Data were acquired at a sampling frequency of 10 kHz with an AM 1800 amplifier (ZAK, Marktheidenfeld, Germany), the digidata 1200 AD/DA interface, and Axoscope 9 software (Axon Instruments, Foster City, USA). Action potentials were separated from local field potentials by digital bandpass filtering (200-2000Hz). As judged from the size and waveform of single action potentials, about 5-15 different neurons contributed to the signal pick up by a single extracellular electrode.
2.3. Preparation and application of test solutions
Stock solution of SH-053-2′F-R-CH3 (which has previously also been referred to as SH-053-R-CH3-2'F) and L 655,708 (Tocris Bioscience, Bristol, UK) were made by dissolving in DMSO. The final concentration of DMSO did not exceed 0.01% and was found to have no effects on cortical firing patterns. To yield the desired concentration, diazepam (Braun, Melsungen, Germany), SH-053-2′F-R-CH3 and L 655, 708 were diluted in aCSF and filled into syringes. The drug containing aCSF was applied via bath perfusion using syringe pumps (ZAK, Marktheidenfeld, Germany), connected to the experimental chamber via Teflon tubing (Lee, Frankfurt, Germany). The recording chamber consisted of a metal frame with a glass bottom and had a volume of 1.5 ml. The flow rate was approximately 1 ml min-1. When switching from aCSF to drug-containing solutions, the medium in the experimental chamber was replaced by at least 95% within 2 min. To ensure steady state conditions, recordings during diazepam treatment were carried out 10 - 12 min after commencing the change of the perfusate. This time interval has been proven to be sufficient for steady state conditions in organotypic slice cultures (Antkowiak, 1999; Dai et al., 2009), as diffusion times in slice cultures are considerably shorter compared to acute slice preparations (Gredell et al., 2004; Benkwitz et al., 2007).
2.4. Data analysis
Extracellular recorded signals were filtered and counted offline using self-written programs in Matlab R2007b (The Mathworks, Natick, USA). The activity pattern of neocortical slice cultures is characterized by bursts of spontaneous action potential firing separated by periods of neuronal silence. Action potentials were detected using an automated event detection algorithm with a threshold set approximately two times higher than the baseline noise.
Parameters are shown as relative change compared to control condition. We used Student t test for statistical testing, P values <0.05 were considered significant. Results are given as mean ± S.E.M., unless stated otherwise. For comparison of drug effects on episodes of ongoing neuronal activity peri-event time histograms were calculated using self-written routines in Matlab (The Mathworks, Natick, USA). Comparison of neuronal activity was performed using Hedges' g.
3. Results
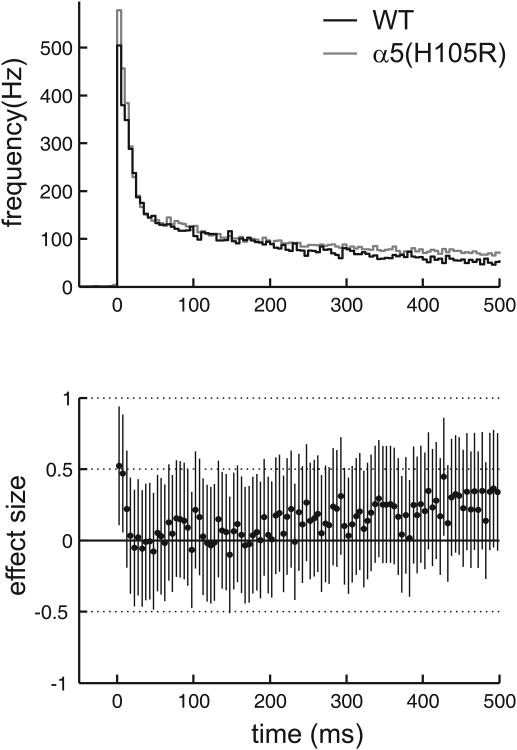
To elucidate the effects of SH-053-2′F-R-CH3 a total of 53 slice cultures from the neocortex of 129X1/SvJ wild type mice and 76 cultures from 5(H105R) knock-in mice on the same genetic background were used. The firing pattern of the neocortical slice cultures was characterized by bursts of action potentials (up states) separated by neuronal silence (down states). The mean up state frequency was slightly higher in wild type slices (0.19 ± 0.02 Hz) compared to slices from the 5(H105R) mutant (0.14 ± 0.01 Hz, P = 0.04). For a more detailed analysis of the neuronal activity the up states of all recordings were collected, divided into bins of 10ms, and averaged. As the up state duration was variable, we restricted our analysis to the first 500ms after the beginning of the up state. The averaged up states derived from extracellular multi-unit recordings were characterized by a high frequency of action potential firing right at the beginning, followed by a step-wise decrease of activity (Fig. 1). Comparison of the activity pattern derived from cultured wild type neurons and from tissue slices derived from the 5(H105R) mutant revealed almost identical neuronal firing under control conditions, as shown in figure 1.
Figure 1.
Neuronal activity of cultured neocortical neurons from wild type (black) and 5(H105R) mutant (grey) mice under control conditions. All activity phases (up states) were collected, divided in bins of 10ms and averaged. The action potential firing frequency of multi-unit recordings is high at the beginning of the up state and declines in a gradual fashion. Comparison between genotypes is given as effect size (Hedges'g including 95% confidence interval). An effect size of 1 would indicate that the two genotypes are one standard deviation apart. A significant difference between genotypes can be assumed, if the 95% confidence interval does not cross the zero-line. Apart from the first 10ms, where slices from the 5(H105R) mutant showed slightly higher activity, neuronal activity between the two genotypes is almost identical.
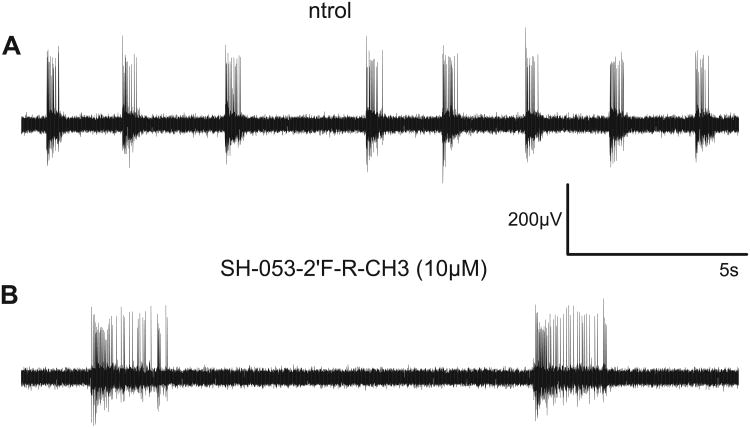
An original recording illustrating the firing pattern of cultured neocortical neurons and the effects of SH-053-2′F-R-CH3 is shown in figure 2. The 5-selective compound SH-053-2′F-R-CH3 modified the cortical network activity in a characteristic way: it reduced the number of up states, thereby depressing the overall activity of the network. However, SH-053-2′F-R-CH3 contemporaneously increased the duration of up states and also the number of action potentials during these up states.
Figure 2. Firing pattern of cultured slices from the neocortex and actions of the 5-selective GABAA receptor modulator SH-053-2′F-R-CH3.
(A) Original extracellular recording from a cultured neocortical slice. Eight bursts of action potentials (up states) are separated by neuronal silence (down states).
(B) SH-053-2′F-R-CH3 in a concentration of 10 μM reduces the number of up states and simultaneously prolongs them. Additionally, the number of action potentials during up states is increased in the presence of SH-053-2′F-R-CH3.
Unlike the 5-selective compound SH-053-2′F-R-CH3 diazepam did not induce an increase of up state duration nor an increase of action potentials within up states (table 1).
Table 1.
Effects of diazepam (DZP) on up state duration and on the number of action potentials (AP) per up state in organotypic slice cultures from the neocortex of wild type mice. Data are normalized to control condition.
| DZP in μM | up state duration | AP per up state |
|---|---|---|
| 6.25 | 0.94 ± 0.12 (n = 19) | 0.80 ± 0.14 (n = 18) |
| 12.5 | 1.30 ± 0.17 (n = 28) | 1.41 ± 0.18 (n = 29) |
| 25 | 1.35 ± 0.15 (n = 33) | 1.29 ± 0.14 (n = 34) |
| 50 | 1.29 ± 0.12 (n = 34) | 0.97 ± 0.10 (n = 35) |
Diazepam induced changes are not significantly different from control condition (t-test, P > 0.05).
Benzodiazepines are used clinically over a wide concentration range to induce desired actions from mild anxiolysis at low doses to abortion of seizure activity or induction of general anaesthesia at high doses. Therefore we were curious to test whether SH-053-2′F-R-CH3 would display 5-selectivity at higher concentrations (Fig. 3).
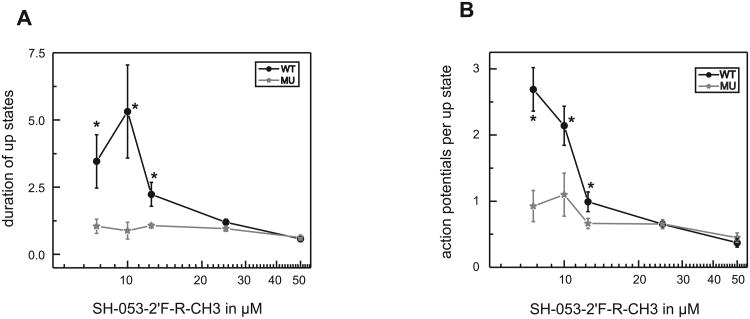
Figure 3.
Concentration-dependent effects of SH-053-2′F-R-CH3 on the duration of up states and on the number of action potentials during up states in cultured cortical neurons from wild type and 5 (H105R) knock-in mice.
(A) The duration of up states is increased by low concentrations of SH-053-2′F-R-CH3 in wild type slices. Values are presented as duration of up states in the presence of SH-053-2′F-R-CH3 divided by duration of up states under control condition. SH-053-2′F-R-CH3 leads to an increase in the wild type (black dots) by 3.5 ± 1.0 at 7.5 μM, by 5.3 ± 1.7 at 10 μM, and by 2.2 ± 0.4 at 12.5 μM, respectively. In cultured slices from the 5(H105R) mutant (grey stars) this effect is absent. * significantly different from 5(H105R) mutant (P < 0.05, t-test).
(B) Low concentrations of SH-053-2′F-R-CH3 lead to an increased number of action potentials during up states in wild type slices. The values are given as number of action potentials during up state in the presence of SH-053-2′F-R-CH3 divided by control condition. SH-053-2′F-R-CH3 increases the number of action potentials during up state in the wild type (black dots) by 2.7 ± 0.3 at 7.5 μM and by 2.1 ± 0.3 at 10 μM, respectively. In slices from the 5(H105R) mutant (grey stars) no such increase is present. * significantly different from 5(H105R) mutant (P < 0.05, t-test). At higher concentrations SH-053-2′F-R-CH3 reduces the number of action potentials during up states in both, wild type and 5(H105R) mutant slices.
We observed that the main effects of SH-053-2′F-R-CH3 were most prominent at low concentrations of the compound. SH-053-2′F-R-CH3 in concentrations up to 15 μM increased the duration of neocortical up states. This effect was most pronounced at 10 μM, where SH-053-2′F-R-CH3 increased the up state duration by 5.3 ± 1.7 fold. The number of action potentials during up states was also enhanced by low concentrations of SH-053-2′F-R-CH3. At 7.5 μM the number of action potentials during up states was 2.7 ± 0.3 fold increased, compared to control condition. However, these characteristic actions were absent in cases where the concentration of SH-053-2′F-R-CH3 was 15 μM or higher.
To test whether the actions of SH-053-2′F-R-CH3 are indeed mediated by 5-subunit containing GABAA receptors, this compound was also tested in cultured slices derived from 5(H105R) knock-in mice, a genetically modified mouse line where 5-containing GABAA receptors are insensitive to benzodiazepines. An effect of SH-053-2′F-R-CH3 should be 5-dependent if present in slices from the wild type, but abolished by the 5(H105R) mutation. As can be seen in figure 3, the typical effects of SH-053-2′F-R-CH3 in the wild type like the prolongation of up states and the increase in number of action potentials during up states are abolished in 5(H105R) mutant slices. To further strengthen the finding that the effects observed with SH-053-2′F-R-CH3 were mediated via 5-containing GABAA receptors, similar experiments were performed with L 655,708, an inverse agonist at 5-containing GABAA receptors. (Quirk et al., 1996) This compound affected the firing characteristics of neocortical wild type neurons in opposite ways compared to SH-053-2′F-R-CH3: the duration of up states was shortened and the number of action potentials per up state was decreased in the presence of L 655,708 (table 2). Based on these experiments we conclude that these characteristic effects are mediated via 5-subunit containing GABAA receptors.
Table 2.
Effects of L 655,708, an inverse agonist specific for the 5 subunit containing GABAA receptor subtype, on up state duration and on the number of action potentials (AP) per up state in organotypic slice cultures from the neocortex of wild type mice.
| L 655,708 in μM | up state duration | AP per up state |
|---|---|---|
| 2.5 | 0.59 ± 0.06 (n = 13) a | 0.61 ± 0.06 (n = 13) a |
| 5.0 | 0.54 ± 0.04 (n = 25) a | 0.57 ± 0.05 (n = 25) a |
Data are normalized to control condition,
= significantly different (P < 0.05) from control condition (t test).
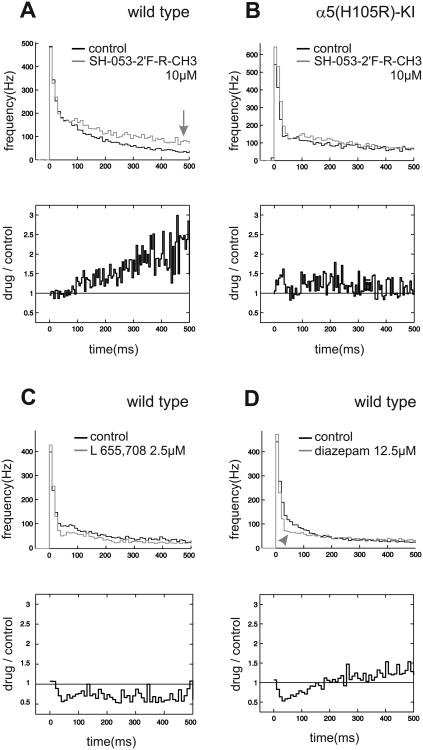
A more detailed analysis of the actions of SH-053-2′F-R-CH3 on the firing pattern of neocortical neurons, performed as outlined above, revealed that in cultured neurons from wild type mice SH-053-2′F-R-CH3 (10 μM) progressively increased the neuronal activity, starting around 100ms after the beginning of the up state. At 500ms after up state onset (the end of our analysis window) action potential activity was roughly twice as high as compared to control (Fig. 4 A). This activity increasing action of SH-053-2′F-R-CH3 was absent in cultured cortical slices from the 5(H105R) mutant (Fig. 4 B), indicating that this SH-053-2′F-R-CH3 action is mediated via 5-containing GABAA receptors. This observation is also supported by the effects of L 655,708 (2.5 μM), showing inverse actions compared to SH-053-2′F-R-CH3, i.e. a depression of neuronal activity (Fig 4 C). Furthermore, we analyzed the actions of the classical benzodiazepine diazepam on cortical up state activity. As expected from a previous study (Drexler et al., 2010), diazepam (12.5 μM) induced a depression of neuronal activity, most pronounced around 50ms after the beginning of the up state (Fig. 4 D).
Figure 4.
The 5-selective compound SH-053-2′F-R-CH3 enhances neuronal activity in cortical slice cultures during up states.
The upper part of the figure displays the mean frequency of action potential firing during up states under control condition (black) and in the presence of the benzodiazepine (grey). All up states were collected, divided into time bins of 10 ms and averaged. The graph shows the mean value of action potential frequency, measures of statistical dispersion have been omitted for clarity reasons. In the lower part of the figure the difference between drug condition divided by control condition is shown to illustrate the effect of the drug on action potential firing during up state.
(A) SH-053-2′F-R-CH3 at a concentration of 10μM (n = 9) leads to an increase in action potential frequency during up states in wild type slices (grey line, marked by the arrow). Although SH-053-2′F-R-CH3 is a positive modulator at GABAA receptors, the compound does not depress, but instead increases neuronal activity during up states. This increase in action potential frequency is the more pronounced the longer the up state lasts (activity approximately twice as high compared to control condition from 300ms to 500ms after the beginning of the up state), as indicated in the lower part of the figure.
(B) In cultured slices from the 5(H105R) mutant the increase in action potential frequency during up states by SH-053-2′F-R-CH3 (10μM, n = 10) is absent. This is consistent with this effect being mediated by 5-containing GABAA receptors.
(C) L 655,708 acts as an inverse agonist at the benzodiazepine site of 5-containing GABAA receptors and can thereby be regarded as an “anti- SH-053-2′F-R-CH3”. In fact, the neuronal activity of cultured cortical neurons shows nearly opposing behaviour in the presence of 2.5 μM L 655,708 compared to SH-053-2′F-R-CH3. The depression of neuronal activity is rather uniform beginning around 50 ms after the onset of the activity phase.
(D) The classical benzodiazepine diazepam (12.5μM, n = 13) leads to a depression of action potential frequency with a maximum around 50 ms after the beginning of the up state (marked by arrowhead in the upper part) in cultured slices from wild type mice. Note the small but steady relative increase in activity, starting at 40ms and reaching control levels at 200ms after up state onset is evident. This upward-sloping course of action potentials points to a similar, activity-enhancing action of diazepam which is superimposed by its overall depression of the cortical network.
4. Discussion
Sedative therapeutics such as benzodiazepines and the anaesthetics act by positive allosteric modulation of GABAA receptors. On the cellular level, enhanced GABAA receptor-mediated inhibition most commonly translates into a decreased action potential activity of neocortical neurons, which have been shown to be a major substrate in mediating sedation and hypnosis induced by the above-mentioned drugs (Hentschke et al., 2005; Zeller et al., 2008). However, it is unknown which of the GABAA receptor subtypes that are expressed in the neocortex are capable of decreasing action potential firing rates of neocortical neurons. There is evidence in the literature that GABAA receptors harboring the 5-subunit are abundant at higher densities in the deep cortical layers (Fritschy and Brünig, 2003). The present study therefore raised the question whether action potential firing of neocortical neurons can be significantly changed by exclusively enhancing the function of the latter GABAA receptor-subtype. To tackle this issue, the effects of 5-selective positive modulator SH-053-2′F-R-CH3 on cortical neurons ex vivo were studied. This agent enhanced cortical action potential firing, an effect that was absent in slices prepared from 5-knock in mice, where SH-053-2′F-R-CH3 cannot bind to 5-containing GABAA receptors. Furthermore, the inverse 5-agonist L 655,708 displayed opposite effects.
In the past years the phenomenon of cortical up- and down states has gained considerable interest in the literature (Johnson and Buonomano, 2007; Lau and Bi, 2005; McCormick et al., 2003; Shu et al., 2003a; Shu et al., 2003b). Neuronal activity in neocortical networks is characterized by phases of persistent activity (termed up states) and phases of relative neuronal quiescence (termed down states). The occurrence of up- and down states is not an artefact produced by culturing neocortical tissue, but is characteristic for neocortical circuits in general, in vivo (Timofeev et al., 2000) and ex vivo (Sanchez-Vives et al., 2010). In the present study we have shown that diazepam decreases action potential activity of cortical neurons during up states. However, SH-053-2′F-R-CH3 did not reduce but increased neuronal activity in slices derived from wild type mice, which is opposite to the effects of diazepam. How to explain these inverse actions of diazepam and SH-053-2′F-R-CH3? Diazepam lacks subtype-selectivity and thus acts via multiple GABAA receptor subtypes whereas SH-053-2′F-R-CH3 only targets receptors containing 5-subunits. It seems likely that action potential enhancing actions are only mediated via 5-containing receptors whereas activation of non-5 receptors reduces neuronal activity. As 5 is only a minor subtype in the neocortex, the overall effect of diazepam is inhibitory. It is interesting to note that the inverse 5-agonist L 655,708 decreased action potential firing during up states. This result is consistent with the inverse action of SH-053-2′F-R-CH3 and might have important implications for the design of novel anaesthetic and anticonvulsive GABAergic drugs. As these agents largely cause their therapeutically desired effect via decreasing the activity of cortical neurons (Hentschke et al. 2005; Zeller et al. 2008; Drexler et al., 2010; Drexler et al., 2011), their efficacy might increase if positive modulation of 5-containing receptors is lacking.
In summary, the dissimilar actions of SH-053-2′F-R-CH3, L 655,708 and diazepam on neuronal activity patterns provide further evidence of highly specific physiological roles of distinct GABAA receptor subtypes in cortical information processing.
On the background of these results it is noteworthy that the sedative properties of SH-053-2′F-R-CH3 reported previously in behavioural studies (Savic et al., 2008; Savic et al., 2010) do not necessarily involve 5-subunit containing GABAA receptors, since interactions with the low affinity binding sites on GABAA receptors may also come into play. Furthermore, benzodiazepines are rapidly cleaved in vivo and the resulting neuroactive metabolites may not maintain subtype selectivity.
4.1. Action potential promoting effects mediated by an 5-prefering benzodiazepine
It was a surprising finding that SH-053-2′F-R-CH3 enhanced action potential firing during cortical up states while L 655,708 depressed neuronal activity. Several different hypotheses can be considered for explaining this finding. For example, it seems possible that 5 is predominantly expressed in inhibitory interneurons. In this scenario, enhancing the function of alpha5-containing GABAA receptors decreases the discharge rates of inhibitory interneurons which in turn reduces synaptic inhibition in postsynaptic cells. As a consequence, action potential firing of postsynaptic neurons is increased by SH-053-2′F-R-CH3. However, so far there is little evidence that 5-subunits reside on inhibitory interneurons in the neocortex (Fritschy and Brünig, 2003; Christie and De Blas, 2002; Ali and Thomson, 2008).
In an alternative scenario, GABA operates as a depolarizing neurotransmitter during up states. Such a mechanism has been elucidated in the literature in some detail (Kaila et al., 1997; Ruusuvuori et al., 2004; Rivera et al., 2005). In brief, long lasting activation of GABAA receptors substantially increases the intracellular concentration of chloride ions in cortical neurons. Chloride mass influx is maintained by an efflux of HCO3- ions, which also flow through activated GABAA receptors. A high intracellular concentration of chloride ions not only shifts the reversal potential of GABAA receptors towards more depolarized potentials, but also enhances the extrusion of chloride ions via the K+-Cl--cotransporter KCC2 (Viitanen et al., 2010). Since removal of chloride ions from the intracellular compartment via KCC2 transporters is linked in a 1:1 manner to an efflux of potassium ions, long lasting activation of GABAA receptors causes a rise in the interstitial potassium concentration, which in turn depolarizes the membrane potential of neurons (Viitanen et al., 2010). Therefore the excitatory action of SH-053-2′F-R-CH3 may involve both a depolarizing shift of the reversal potential of GABAA receptors and a potassium dependent depolarization of neocortical neurons due to an activation of KCC2 transporters.
4.2. Location and function of different GABAA receptor subtypes
Membrane depolarization caused by GABA, as discussed in the last section, does not necessarily facilitate action potential firing. In an elegant study, (Gulledge and Stuart, 2003) demonstrated that, depending on the site of GABA application, GABA-induced membrane depolarization of neocortical pyramidal neurons can affect the mechanism of action potential generation in opposing ways. If GABA was applied at remote dendritic sites and membrane depolarization was observed, the generation of action potentials at the soma was promoted. Interestingly, the opposite was true if membrane depolarization was produced by focal application of GABA in the somatic and perisomatic region. In this case, the generation of action potentials was prevented by an increase in membrane conductance, a mechanism commonly referred to as shunting-inhibition. In summary, these authors showed that the GABA-induced depolarization of the membrane potential can only facilitate action potential activity if there is no shunting inhibition at perisomatic sites.
How do these findings relate to the effects of SH-053-2′F-R-CH3 and diazepam observed in the present study? There is ample evidence that synapse-specific clustering of GABAA receptors is largely determined by the type of the presynaptic GABAergic interneuron (Fritschy and Brünig, 2003; Freund and Katona, 2007; Thomson and Jovanovic, 2010). In the cerebral cortex bitufted interneurons innervate the dendrites of pyramidal cells but not the perisomatic region. It has been shown recently that this branching pattern is well preserved in organotypic tissue cultures of the neocortex (Di Cristo et al., 2004). The 5-subunit is highly enriched at synapses made between bitufted interneurons and pyramidal cells (Ali and Thomson, 2008; Thomson and Jovanovic, 2010). The predominant existence of 5 at remote dendritic sites therefore meets the requirement that GABA-induced membrane depolarization mediated via these receptors is capable of increasing the firing rates of pyramidal cells because activation of 5-subunit containing GABAA receptors does not produce shunting inhibition in the perisomatic region.
As mentioned above, the benzodiazepine-site agonist diazepam largely lacks subtype selectivity (Puia et al., 1991; Rivas et al. 2009). Therefore diazepam not only acts on 5-containing GABAA receptors but also on receptors harboring 1-, 2-, and 3-subunits (Fig. 5). The somata of pyramidal neurons are heavily innervated by parvalbumin positive basket cells and cholecystokin positive basket cells (Freund and Katona, 2007). The GABAA receptors activated by the former interneurons mostly include 1-protein subunits whereas GABAA receptors harboring 2- or 3-subunits are activated predominantly by the latter interneurons. The 2-subunit is also enriched at the axon hillock, a region that is innervated by chandelier cells (Fritschy and Brünig, 2003). We found that unlike SH-053-2′F-R-CH3, diazepam attenuated action potential activity during up-states. The finding that diazepam decreased the discharge rates can be explained by the drug's propensity to modulate synapses located in the perisomatic region, thereby producing shunting inhibition. With regard to the effects of diazepam it is interesting to note that at the onset of episodes of ongoing activity, action potential firing is initially significantly below control values, but then steadily increases, finally reaching drug-free levels (Fig. 4 D). Therefore it is tempting to speculate that similar to SH-053-2′F-R-CH3, diazepam causes a depolarization of the dendritic membrane potential. However, this action is probably overlaid by a prominent shunting inhibition caused by positive modulation of GABAA receptors residing in the perisomatic region.
Figure 5.
Schematic picture of a pyramidal neuron from the cortex. While GABAA receptors containing 1-, 2-, and 3-subunits are enriched at the soma, 5-containing GABAA receptors are mainly found at distal dendritic locations. The unselective benzodiazepine diazepam targets all these GABAA receptor subtypes, resulting in a depression of network activity. The 5-prefering compound SH-053-2′F-R-CH3 acts predominantly at dendritic GABAA receptors. Due to their specific location and properties 5-containing GABAA receptors are capable of inducing excitation during phases of ongoing neuronal activity.
It also seems possible that the opposing drug actions mediated by different subtypes of the GABAA receptor may involve differences in the reversal potential of chloride ions along the somato-dendritic axis or differences in the expression of KCC2 transporters, which are considered as a major mechanism in the mediation of GABA-induced membrane depolarization. However, neither the reversal potential for GABA-induced currents nor the density of KCC2 transporters is significantly different at dendritic and somatic sites (Gulledge and Stuart, 2003; Baldi et al., 2010).
Conclusions
The 5-preferring benzodiazepine SH-053-2′F-R-CH3 enhances the discharge rates of neocortical neurons during up states in vitro. As this action is abolished by the 5(H105R)-mutation and the inverse 5-agonist L 655,708 inversely alters the firing rates of cortical neurons, it is concluded that positive modulation of 5-containing GABAA receptors increases action potential firing of cortical neurons. These results are prompting an unexpected hypothesis, namely that drugs acting as inverse agonists at 5-containing GABAA receptors should enhance the efficacy of anaesthetic and anticonvulsive drugs in current use that act via GABAA receptors and lack subtype-selectivity.
Acknowledgments
We thank Claudia Holt and Ina Pappe for excellent technical assistance. This work has been supported by grant No. AN 321/2-1 from the German Research Foundation (to BA) and by USPHS grant MH046851 (to JMC). UR was supported by Award Number R01GM086448 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Statement of conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali AB, Thomson AM. Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 2008;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- Antkowiak B. Different actions of general anaesthetics on the firing patterns of neocortical neurons mediated by the GABAA receptor. Anesthesiology. 1999;91:500–511. doi: 10.1097/00000542-199908000-00025. [DOI] [PubMed] [Google Scholar]
- Baldi R, Varga C, Tamas G. Differential distribution of KCC2 along the axo-somato-dendritic axis of hippocampal principal cells. Eur J Neurosci. 2010;32:1319–1325. doi: 10.1111/j.1460-9568.2010.07361.x. [DOI] [PubMed] [Google Scholar]
- Baur R, Tan KR, Luscher BP, Gonthier A, Goeldner M, Sigel E. Covalent modification of GABAA receptor isoforms by a diazepam analogue provides evidence for a novel benzodiazepine binding site that prevents modulation by these drugs. J Neurochem. 2008;106:2353–2363. doi: 10.1111/j.1471-4159.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- Benkwitz C, Liao M, Laster MJ, Sonner JM, Eger EI, Pearce RA. Determination of the EC50 amnesic concentration of etomidate and its diffusion profile in brain tissue: implications for in vitro studies. Anesthesiology. 2007;106:114–123. doi: 10.1097/00000542-200701000-00020. [DOI] [PubMed] [Google Scholar]
- Benson JA, Low K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- Caeser M, Schüz A. Maturation of neurons in neocortical slice cultures. A light and electron microscopic study on in situ and in vitro material. J Hirnforsch. 1992;33:429–443. [PubMed] [Google Scholar]
- Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, MacDonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA. Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, De Blas AL. alpha5 Subunit-containing GABAA receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport. 2002;13:2355–2358. doi: 10.1097/00001756-200212030-00037. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABAA receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Perouansky M, Pearce RA. Amnestic Concentrations of Etomidate Modulate GABAA, slow Synaptic Inhibition in Hippocampus. Anesthesiology. 2009;111:766–773. doi: 10.1097/ALN.0b013e3181b4392d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang ZJ. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Drexler B, Zinser S, Hentschke H, Antkowiak B. Diazepam Decreases Action Potential Firing of Neocortical Neurons via Two Distinct Mechanisms. Anesth Analg. 2010;111:1394–1399. doi: 10.1213/ANE.0b013e3181f9c035. [DOI] [PubMed] [Google Scholar]
- Drexler B, Antkowiak B, Engin E, Rudolph U. Identification and characterization of anesthetic targets by mouse molecular genetics approaches. Can J Anaesth. 2011;58:178–190. doi: 10.1007/s12630-010-9414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, Yu J, Zhou H, Johnson EM, Jr, Cook JM, Furtmuller R, Ramerstorfer J, Sieghart W, Roth BL, Majumder S, Rowlett JK. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Brünig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gredell JA, Turnquist PA, MacIver MB, Pearce RA. Determination of diffusion and partition coefficients of propofol in rat brain tissue: implications for studies of drug action in vitro. Br J Anaesth. 2004;93:810–817. doi: 10.1093/bja/aeh272. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- Heinke W, Koelsch S. The effects of anesthetics on brain activity and cognitive function. Curr Opin Anaesthesiol. 2005;18:625–631. doi: 10.1097/01.aco.0000189879.67092.12. [DOI] [PubMed] [Google Scholar]
- Hentschke H, Schwarz C, Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics: strong depression of firing rates and increase of GABA receptor-mediated inhibition. Eur J Neurosci. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- Johnson HA, Buonomano DV. Development and plasticity of spontaneous activity and Up states in cortical organotypic slices. J Neurosci. 2007;27:5915–5925. doi: 10.1523/JNEUROSCI.0447-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci. 1997;17:7662–7672. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau PM, Bi GQ. Synaptic mechanisms of persistent reverberatory activity in neuronal networks. Proc Natl Acad Sci U S A. 2005;102:10333–10338. doi: 10.1073/pnas.0500717102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex. 2003;13:1219–1231. doi: 10.1093/cercor/bhg104. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Rosahl TW, Reynolds DS, Sur K, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor a1 subtype. Nature Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl- currents. Mol Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Rivas FM, Stables JP, Murphree L, Edwankar RV, Edwankar CR, Huang S, Jain HD, Zhou H, Majumder S, Sankar S, Roth BL, Ramerstorfer J, Furtmuller R, Sieghart W, Cook JM. Antiseizure activity of novel gamma-aminobutyric acid (A) receptor subtype-selective benzodiazepine analogues in mice and rat models. J Med Chem. 2009;52:1795–1798. doi: 10.1021/jm801652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific g-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Ruusuvuori E, Li H, Huttu K, Palva JM, Smirnov S, Rivera C, Kaila K, Voipio J. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J Neurosci. 2004;24:2699–2707. doi: 10.1523/JNEUROSCI.5176-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Mattia M, Compte A, Perez-Zabalza M, Winograd M, Descalzo VF, Reig R. Inhibitory modulation of cortical up states. J Neurophysiol. 2010;104:1314–1324. doi: 10.1152/jn.00178.2010. [DOI] [PubMed] [Google Scholar]
- Savic MM, Clayton T, Furtmuller R, Gavrilovic I, Samardzic J, Savic S, Huck S, Sieghart W, Cook JM. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABAA receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Res. 2008;1208:150–159. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic MM, Huang S, Furtmuller R, Clayton T, Huck S, Obradovic DI, Ugresic ND, Sieghart W, Bokonjic DR, Cook JM. Are GABAA receptors containing alpha5 subunits contributing to the sedative properties of benzodiazepine site agonists? Neuropsychopharmacology. 2008;33:332–339. doi: 10.1038/sj.npp.1301403. [DOI] [PubMed] [Google Scholar]
- Savic MM, Majumder S, Huang S, Edwankar RV, Furtmuller R, Joksimovic S, Clayton T, Sr, Ramerstorfer J, Milinkovic MM, Roth BL, Sieghart W, Cook JM. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats? Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J Neurosci. 2003a;23:10388–10401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003b;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Jovanovic JN. Mechanisms underlying synapse-specific clustering of GABAA receptors. Eur J Neurosci. 2010;31:2193–2203. doi: 10.1111/j.1460-9568.2010.07252.x. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol. 2010;588:1527–1540. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RJ, Hadley SH, Morris KD, Amin J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat Neurosci. 2000;3:1274–1281. doi: 10.1038/81800. [DOI] [PubMed] [Google Scholar]
- Zeller A, Crestani F, Camenisch I, Iwasato T, Itohara S, Fritschy JM, Rudolph U. Cortical glutamatergic neurons mediate the motor sedative action of diazepam. Mol Pharmacol. 2008;73:282–291. doi: 10.1124/mol.107.038828. [DOI] [PubMed] [Google Scholar]