Abstract
Since the discovery of the G-protein coupled receptor (kisspeptin receptor) and its ligand, kisspeptin, our understanding of the neurobiological mechanisms that govern the pituitary-gonadal axis has evolved dramatically. In this chapter, we have reviewed progress regarding the relationship between kisspeptin and puberty, and have proposed a novel hypothesis for the role of kisspeptin signaling in the onset of this crucial developmental event. According to this hypothesis, although kisspeptin neurons in the arcuate nucleus (ARC) are critical for puberty, this is simply because these cells are an integral component of the hypothalamic GnRH pulse generating mechanism that drives intermittent release of the decapeptide, as an increase in GnRH is obligatory for the onset of puberty. In our model, ARC kisspeptin neurons play no “regulatory” role in controlling the timing of puberty. Rather, as a component of the neural network responsible for GnRH pulse generation, they subserve upstream regulatory mechanisms that are responsible for the timing of puberty.
Keywords: kisspeptin, kisspeptin receptor, puberty, pulsatile GnRH release, central inhibition
1. Introduction
The discovery nearly a decade ago that the G-protein coupled receptor, KISS1R (aka GPR54) and its ligand, kisspeptin, encoded by the genes KISS1R and KISS1, respectively, play a major role in regulating the hypothalamic-pituitary-gonadal axis has provided a new perspective on the mystery of puberty. As discussed in Chapter 9, Seminara et al. (1) and de Roux et al. (2) first described amino acid mutations of KISS1R in human patients with a delay in puberty onset or an abnormality in pubertal development. Subsequently, several reports also described mutations at different sites of the KISS1R gene in patients with either an absence of or a delay in puberty (3-7) or with precocious puberty (8). Moreover, it has been reported that a genetically targeted deletion of either Kiss1r or Kiss1 in mice results in hypogonadotropic hypogonadism, including delayed pubertal maturation (1, 9-11). Most recently, impairment of pubertal progression in a human family with a mutation of KISS1 was described (12).
Despite a plethora of reports on kisspeptin and its receptor in relation to puberty over the last ten years, a critical evaluation of the role of kisspeptin signaling in the timing of puberty onset is missing. In this review, we will discuss 1) postnatal development of kisspeptin neurons and the kisspeptin receptor in relation to parallel changes in activity of the GnRH neuronal network, as its increase is obligatory for puberty onset, 2) recent findings on development of kisspeptin signaling in the rhesus monkey, and 3) our conceptualization of the role played by kisspeptin signaling in the mechanism that controls the onset and progression of puberty.
2. Developmental changes in GnRH release
An increase in GnRH release from the hypothalamus triggers puberty. Pulsatile infusion of GnRH induces precocious puberty in sexually immature female and male monkeys and female guinea pigs (13-15) and increased pubertal release of GnRH and/or gonadotropin has been described in many mammalian species, including humans (see 16-20). In males, an increase in pulsatile GnRH release at puberty activates tonic gonadotropin secretion, that, in turn, results in the onset of elevated levels of testicular testosterone secretion, which in combination with FSH, initiates spermatogenesis. Tonic LH secretion is composed of intermittent secretory episodes of the hormone, which reflect a corresponding pattern of pulsatile GnRH release by the hypothalamus (21). In females, an increase in pulsatile GnRH release also drives tonic gonadotropin secretion, which is responsible for folliculogenesis and estradiol (E2) secretion. Ovulation in most mammalian species, however, also requires development of the capacity to induce a large surge of GnRH in response to the positive feedback action of the rising circulating E2 levels secreted by the follicle(s) destined to ovulate at mid cycle (22). Currently, the mechanism for these two modes of GnRH release (pulsatile vs. surge) is unclear.
There are two basic developmental patterns of pulsatile GnRH release from birth until the onset of puberty. In highly evolved primates, such as man and macaques, GnRH pulsatility is robust during the infantile period after birth, but is subsequently dampened during juvenile development (and childhood in humans), resulting in a hypogonadotropic state and relative quiescence of the gonad (18, 20). The hiatus in pulsatile GnRH release during the juvenile period may be viewed as a consequence of a neurobiological “brake” that holds GnRH release in check until the initiation of the onset of puberty (23). It is important to note that this is a conceptual brake and may be accounted for by either the imposition of an inhibitory input and/or the loss of a stimulatory input to GnRH neurons (18). Our current viewpoint is that this conceptual brake is an inhibitory neurocircuit in the brain (17). The juvenile phase of primate development is terminated by release from the brake, leading to a REACTIVATION of robust GnRH pulsatility (23). Because this juvenile restraint on pulsatile GnRH release is observed in neonatally castrated monkeys (24, 25) and in agonadal humans (26, 27), and because low levels of LH and GnRH release during the juvenile period in ovariectomized female monkeys are not further suppressed by ovarian steroids (28), the hiatus of pulsatile GnRH release during the juvenile period of primate development is independent of ovarian or testicular steroids.
This control system may be contrasted to that in non-primate species, in which LH release (and presumably GnRH release) immediately after birth is minimal but increases before the onset of puberty, with the prepubertal gonad playing a critical role in restraining GnRH release prior to puberty. For example, in sheep and rodents, gonadotropin secretion (and presumably GnRH release) is suppressed by small amounts of gonadal steroid after birth through the juvenile period, but at a time prior to puberty, low levels of steroid are no longer inhibitory (19, 29). Moreover, neonatal gonadectomy in sheep, rats, and guinea pigs increases LH levels and in sheep and rats administration of gonadal steroids suppresses LH levels (30-33). Therefore, the control system governing reactivation of GnRH release at puberty in primates is different from that regulating the postnatal development of pulsatile GnRH release in non-primates.
3. Developmental changes in the kisspeptin neuronal system
Kisspeptin neurons in the adult hypothalamus are typically found in both the medial basal hypothalamus (MBH) and the preoptic area (POA) (see Chapter 3). In the MBH, kisspeptin neurons are localized in the arcuate nucleus (ARC, synonymous with infundibular nucleus in humans) and in the POA these cells are found in the anteroventral periventricular nucleus (AVPV) in rodents and in similar areas in other species. Kisspeptin neurons in the ARC are considered to be an important component of the hypothalamic control of tonic gonadotropin secretion in all species, while kisspeptin neurons in the AVPV of rodents are critical for surge secretion of GnRH and LH, and therefore for ovulation (34).
Overall expression of Kiss1 mRNA in the hypothalamus (AVPV and ARC combined) is significantly elevated around the time of puberty in both male and female rats (35). In the ARC, Kiss1 mRNA levels in female rats at postnatal day 26 (P26), i.e. 3-4 days before vaginal opening, are over 4-fold higher than those at P21 (36), although changes in the number of Kiss1 expressing neurons from P3 to adulthood are unremarkable (37). In male rats, Kiss1 mRNA levels in the ARC at P45 are significantly higher than those at P15 (38), and the number of Kiss1 neurons increases progressively throughout postnatal development (37). In male mice, however, a developmental increase in Kiss1 mRNA levels in the ARC has not been observed (39-41). Moreover, whereas ovariectomy in female mice at P14 dramatically increases expression of ARC Kiss1 mRNA by P16-P18, i.e. well before puberty onset, castration at P14 in male mice does not result in increased ARC Kiss1 mRNA or LH release at P18 (42). However, expression of both the ARC Kiss1 mRNA and secretion of the gonadotropin were elevated at P45 in males castrated at P14 (42). Interestingly, in both male and female mice Kiss1 expression is detected in ARC on P1, and in females, but not males, kisspeptin receptor signaling appears to be driving gonadotropin release at this early stage of development (43). The absence of a post-castration LH response in prepubertal male mice has been previously reported (44) and differs from the situation in rats and guinea pigs where prepubertal orchidectomy elicits a robust increase in LH secretion (30, 31, 33). In an alternative paradigm to eliminate the confounding effect of testicular steroid feedback on the development of Kiss1 expression in mice, Gill et al. (41) studied the hpg mouse, a GnRH deficient hypogonadal animal, and found that ARC Kiss1 expression increases dramatically between P10 and P30 (as it also did in the hpg female). Clearly, the developmental pattern in Kiss1 expression in the ARC of the male mouse requires further study.
In the rhesus monkey, pubertal increases in KISS1 mRNA in the MBH (presumably in the ARC) in ovarian intact female and agonadal male monkeys have been observed (45). Although a gonadal steroid-independent pubertal increase in KISS1 mRNA expression in female monkeys has not been examined, an ovarian steroid-independent increase in kisspeptin release in the region of the ARC-median eminence (ARC-ME) has been observed (see next section).
The developmental pattern of ARC kisspeptin expression as assessed by immunohistochemistry is less clear. Studies in mice describe an increase in intensity of kisspeptin fibers in the ARC during postnatal development in both males and females but developmental changes in kisspeptin cell number have not been reported (41, 46, 47). It is possible that the pubertal increase in kisspeptin fibers in the ARC may reflect an increased kisspeptin output from kisspeptin cell bodies in the AVPV (see below), as direct innervation of the ARC by AVPV kisspeptin neurons has been reported (48). In the ewe, the number of kisspeptin neurons in the ARC are significantly greater in postpubertal animals compared to prepubertal lambs (49). In the agonadal male monkey, developmental changes in the number of immunopositive kisspeptin neurons in the ARC parallel changes in pulsatile GnRH release with both infant and pubertal animals exhibiting numerous and intensely stained ARC perikarya (50). The importance of ARC kisspeptin neuronal network for generating pulsatile GnRH release in the infant monkey is consistent with the observation that circulating gonadotropin levels were undetectable in a 2-month old infantile boy bearing a loss-of-function mutation of KISS1R (3).
In the case of AVPV kisspeptin neurons, it has been clearly shown that the cell number in female mice progressively increases until the age of puberty (41, 42, 46, 51). Moreover, the developmental increase in the number of kisspeptin neurons in the AVPV in females is dependent on the presence of circulating E2, as ovariectomy of prepubertal mice reduces and/or masks this developmental change (51). Similarly, in the hpg mouse the prepubertal increase in expression of both kisspeptin and Kiss1 is blunted (41), and in aromatase knockout mice there is a complete absence of kisspeptin expression (51). This action of E2 appears to be exerted directly on the AVPV kisspeptin neurons, as conditional knockout of estrogen receptor alpha (ERα) resulted in a marked decrease in the number of kisspeptin immunopositive neurons in this nucleus (52). Kisspeptin or KISS1 expressing neurons have also been described in the POA of women and female monkeys (53-55) but developmental changes in this particular population of neurons have not been studied in primates.
Hypothalamic (POA and ARC combined) levels of Kiss1r mRNA increase at the age of puberty in both male and female rats (35). Specifically, in the female, Kiss1r expression in the AVPV increased at the age of puberty (36). However, neither the neuronal phenotype in the POA/AVPV exhibiting this pubertal increase in Kiss1r expression nor the gonadal steroid dependency of this phenomenon in rodents have been studied. In ovarian intact female rhesus monkeys, KISS1R mRNA in the MBH also increases across puberty onset (45), and functionally, developmental changes in GnRH response to KP-10 depend on the pubertal increase in E2 (56, also see next section). Thus, it is possible that the pubertal increase in Kiss1r/KISS1R mRNA in females is due to the increase in estrogens at this stage of development. However, this view needs further examination, as KISS1R mRNA expression does not change across puberty in agonadal male monkeys (45).
Kiss1r is expressed in approximately 80% of GnRH neurons in cichlid fish, and in adult mice and rats (57-59). During the first few days of postnatal life in mice, only ~40% of GnRH neurons express Kiss1r but this increases to adult levels by P20 (60). Although expression of KISS1R in primate GnRH neurons has not been reported, GnRH neurons in both male and female prepubertal monkeys respond to exogenous kisspeptin (45, 61, 56). Kiss1r may also be present in embryonic mouse GnRH neurons, as they respond to exogenous kisspeptin in vitro (62). Embryonic primate GnRH neurons, however, do not respond to kisspeptin (Keen and Terasawa, unpublished observation), suggesting that GnRH neurons in rhesus monkeys may not acquire KISS1R until later in gestation.
Taking the foregoing considerations together, it seems reasonable to propose that an increase in expression of both kisspeptin mRNA and protein in the ARC occurs in association with the onset of puberty in both sexes of most mammalian species, and this is likely correlated with an increase in kisspeptin release in the ARC-ME region, as demonstrated for the monkey (see next section). In primates, the postnatal pattern in ARC kisspeptin expression is fundamentally dictated by a central inhibition that is independent of gonadal steroids, rather than by ovarian and testicular feedback, as is the case in rats (19, 31). Additionally, it appears that in female rodents an estrogen-dependent developmental increase in kisspeptin peptide and mRNA in the AVPV occurs, leading presumably to an increase in the secretory activity of this rostral population of kisspeptin neurons.
4. Changes in kisspeptin release and KISS1R responsiveness to kisspeptin during the pubertal process
As discussed in Chapter 2, human preprokisspeptin is cleaved to form kisspeptin-54 and further cleaved to kisspeptin-14, -13, or -10, which are all biologically active (63, 64). To determine the role kisspeptin plays in the pubertal increase in GnRH release, it is important to understand 1) the developmental pattern of kisspeptin-54 release and 2) developmental changes in the function of KISS1R expressed by GnRH neurons and/or afferent neurons to the GnRH network. The maturational changes in the responsiveness of the GnRH neurosecretory system can be tested by the kisspeptin agonist, human kisspeptin-10 (hKP-10), and the synthetic kisspeptin antagonist, peptide 234, as described by Roseweir et al. (65). Accordingly, Terasawa and colleagues conducted a series of studies using a microdialysis method, which allows for 1) in vivo measurements of kisspeptin-54 and GnRH release in serially collected dialysate samples from the stalk-median eminence (S-ME) of monkeys and 2) for infusion of hKP-10 and peptide 234 through the microdialysis probe (66).
In an initial series of studies, the developmental pattern of kisspeptin-54 release was examined in both intact and ovariectomized monkeys. Kissspeptin-54 release is pulsatile and mean kisspeptin-54 release increases along with the pubertal increase in mean GnRH release (67). Moreover, kisspeptin-54 pulses during the prepubertal period are of low amplitude with a long inter-pulse interval (IPI), whereas kisspeptin-54 pulses during the pubertal period are of a higher amplitude with a shorter IPI (68). This pubertal modulation of pulsatile kisspeptin-54 release leads to higher mean levels of the peptide in the S-ME of pubertal animals, which is parallel to those seen in GnRH release during the course of puberty in female monkeys (69, 70).
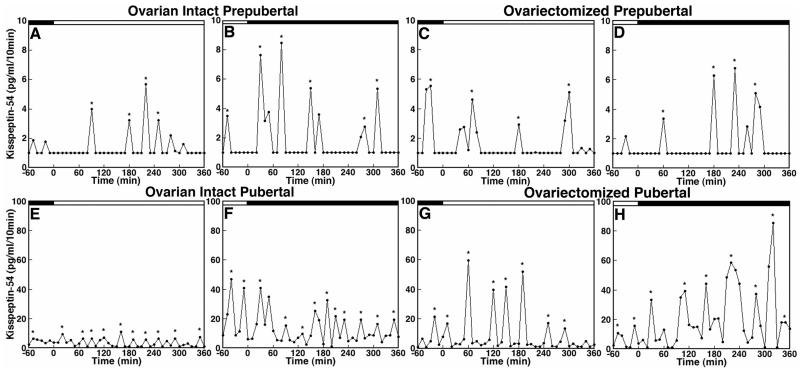
As discussed in the previous section, developmental changes in GnRH release in the rhesus monkey are independent of circulating gonadal steroids. Similar to ovarian intact females (69), developmental increases in the pulse frequency and pulse amplitude of GnRH release do not occur until the age of puberty in ovariectomized monkeys (70). Likewise, the pulse frequency and pulse amplitude of kisspeptin-54 release in ovariectomized monkeys do not increase until the age that puberty would have been anticipated had the animals remained intact (68, Figure 12.1). Importantly, the IPI of kisspeptin-54 release in ovarian intact and ovariectomized females at the prepubertal stage is ~80 min, which is very similar to that of GnRH release (69, 70), whereas the IPI of kisspeptin-54 release at the pubertal stage is ~50 min regardless of the presence or absence of the ovary, which is, again, similar to the IPI of GnRH release in animals at the same developmental stage (69, 70, Figure 12.1). (The role of kisspeptin in GnRH pulse generation will be further discussed in a later section.) An impact of the ovary on kisspeptin-54 release is only observed in the pubertal monkey, where both the pulse amplitude and mean release of kisspeptin-54 is markedly increased by ovariectomy, presumably due to loss of negative feedback from the ovarian steroid E2 (Figure 12.1). In fact, administration of E2 can suppress kisspeptin-54 release in pubertal monkeys, whereas kisspeptin-54 release in prepubertal monkeys is insensitive to E2 (68). This developmental change in ovarian steroid regulation of kisspeptin-54 release is similar to that seen with GnRH release (28). Collectively, these observations indicate that the pubertal increase in kisspeptin-54 release occurs independently from an ovarian steroid hormone feedback mechanism. Rather, the pubertal increase in pulsatile release of kisspeptin-54 in female rhesus monkeys (and presumably male primates) requires a developmental change in an upstream neuronal signal to the kisspeptin neuronal network.
Figure 12.1.
Developmental increases in kisspeptin-54 (KP-54) release are independent of the presence or absence of the ovary in female monkeys. In vivo KP-54 release from the S-ME of ovarian intact prepubertal (A and B) and pubertal (E and F) monkeys as well as ovariectomized prepubertal (C and D) and pubertal (G and H) monkeys are shown. Samples were obtained during the morning period (A, C, E, and G) and during the evening period (B, D, F and H) as indicated by the open and closed bars, respectively, at the top of each graph. Both pulse frequency and amplitude of KP-54 release in ovarian intact pubertal monkeys (E and F) are higher than those in ovarian intact prepubertal monkeys (A and B). Similarly, pulse frequency and amplitude of KP-54 release in ovariectomized pubertal monkeys (G and H) are higher than those in ovariectomzed prepubertal monkeys (A and B). Importantly, ovariectomy does not cause any change in KP-54 release (A and B vs. C and D) in prepubertal monkeys, whereas ovariectomy increases the pulse amplitude of KP-54 release in pubertal monkeys (E and F vs. G and H). Asterisks indicate peaks as determined by PULSAR. Note that the scale of the y-axis in E, F, G, and H (pubertal monkeys) is 10-fold higher than that in A, B, C, and D (prepubertal monkeys). Modified with permission from 68.
Because developmental changes in KISS1R may also contribute to the pubertal increase in GnRH release, in a second series of studies Terasawa and colleagues examined the developmental changes in GnRH release in response to the kisspeptin agonist, hKP-10, and antagonist, peptide 234, administered directly into the S-ME. While the GnRH response to hKP-10 is dose dependent in both ovarian intact prepubertal and pubertal monkeys, a smaller response to a 10 nM dose of hKP-10 is consistently observed in prepubertal monkeys as compared to pubertal monkeys (56). Release of GnRH in both prepubertal and pubertal monkeys is also suppressed by peptide 234. These results suggest that the pubertal increase in pulsatile GnRH release is, in part, due to an increased responsiveness of KISS1R in GnRH neurons during the progression of puberty. This view is consistent with studies in transgenic mice expressing GFP in GnRH neurons, in which electrical firing activity of GnRH neurons stimulated by KP-10 increases across male puberty (40).
To further determine whether the enhanced responses of GnRH neurons to hKP-10 in pubertal monkeys are due to higher levels of circulating E2 at puberty, a similar experiment examining the GnRH responsiveness to hKP-10 in ovariectomized monkeys was conducted. While ovariectomy in prepubertal monkeys did not modify the GnRH response to hKP-10 nor peptide 234, it completely eliminated both the hKP-10-induced stimulation and peptide 234-induced GnRH suppression of GnRH release in pubertal monkeys (56, also Guerriero and Terasawa, unpublished observation). Moreover, replacement of E2 in OVX pubertal monkeys only partially restored the hKP-10-induced GnRH release that was absent in OVX pubertal monkeys (56). These observations suggest that while in prepubertal monkeys the response of KISS1R on GnRH neurons is independent of E2, in pubertal monkeys, functional changes in KISS1R occur as a consequence of the exposure to increased circulating E2 after puberty onset, such that KISS1R responsiveness is enhanced by E2. Collectively, once the pubertal increase in E2 occurs in the female monkey as a consequence of pubertal activation of the GnRH pulse generating mechanism, the presence of E2 appears to enhance the response of GnRH neurons to kisspeptin (56). Although to date, developmental changes in KISS1R mRNA in ovariectomized monkeys have not been examined, it will be important to address this issue further.
5. Kisspeptin signaling and GnRH pulse generation
The hypothesis that kisspeptin neurons are a part of the neurocircuitry underlying the GnRH pulse generating mechanism has been proposed by Goodman and colleagues, Maeda and colleagues, and Steiner and colleagues (71, 72, see also Chapter 14). It is posited that pulsatility originates in ARC kisspeptin neurons containing neurokinin B and dynorphin (called KNDy neurons) by reciprocal interactions of neurokinin B (stimulatory) and dynorphin (inhibitory) and that an intermittent output to the GnRH neuronal network is mediated by kisspeptin. This hypothesis is based on several observations. First, periodic increases in multi-unit activity obtained from electrodes in the MBH are associated with LH pulses in several species (73, 74) and specifically in the ARC, as shown in the goat (75). Second, the neurokinin B receptor agonist, senktide is a potent stimulator of ARC kisspeptin neurons (presumably KNDy neurons) in the mouse (76), and the site of the stimulatory action of neurokinin B on GnRH-dependent LH release in the monkey appears to be upstream of kisspeptin (77). Third, in pubertal monkeys, pulses of kisspeptin-54 released in the ARC-ME correlate to GnRH pulses 75% of the time (67). Fourth, repetitive iv injections of hKP-10 induce trains of GnRH-dependent LH pulses in juvenile male monkeys, in which endogenous GnRH pulsatility is minimal (61), presumably by activating KISS1R on GnRH terminals in the ME, as kisspeptin and GnRH fibers are found in extensive and intimate association in the ME (78, Figure 12.2). Fifth, intra-ARC, not intra-POA, administration of the kisspeptin antagonist, peptide 234, profoundly suppressed LH pulse frequency (79), although again the site of action of the antagonist is likely to be at the ME, as recent electrophysiological studies by Alreja and Steiner indicate that kisspeptin is unable to stimulate KNDy neurons in the mouse (see Chapter 16). The contemporary notion regarding the integral role played by KNDy neurons in GnRH pulse generation is consistent with the classical findings that complete surgical deafferentation of the rat and monkey MBH does not eliminate pulsatile LH release (80, 81), and that selective lesions of the ARC in female monkeys abolishes pulsatile LH release (82). It is also consistent with the recent finding that selective ablation of KNDy neurons in the rat dramatically truncates the ovariectomy-induced increase in LH release (83).
Figure 12.2.
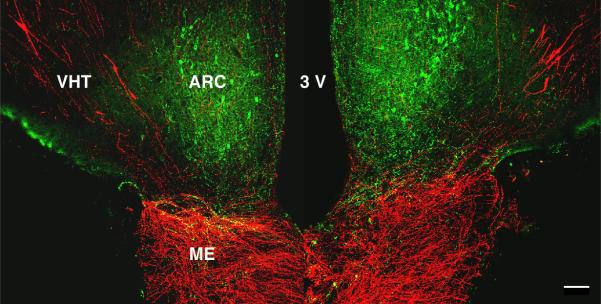
A confocal projection illustrating the relationship between kisspeptin neurons (green) in the arcuate nucleus (ARC) and GnRH cell bodies and projections (red) to the median eminence in a coronal section of the mediobasal hypothalamus of a castrated adult male rhesus monkey. VHT, ventral hypothalamic tract; 3V, third ventricle; ME, median eminence. Scale bar, 100 um. Reprinted with permission from 78.
6. A novel view on the role of kisspeptin in puberty onset
As discussed above, the genetic evidence for the view that kisspeptin neurons are critical for the onset of puberty is overwhelming. Together with results from compelling physiological and pharmacological studies indicating that kisspeptin is the most potent GnRH secretagogue (84), a dogma has emerged that the genes encoding kisspeptin and its receptor regulate puberty, which in turn has led to the perception that kisspeptin signaling represents the key neural substrate that controls the timing of the onset of puberty. Here, we offer an alternative possibility. Namely, while kisspeptin-expressing neurons in ARC are critical for puberty, this is simply because these cells comprise an integral component of the hypothalamic GnRH pulse generating mechanism that generates intermittent release of the decapeptide, an increase of which is obligatory for the onset of puberty. According to this model, kisspeptin neurons in the ARC play no regulatory role in controlling the timing of puberty. Rather, as a component of hypothalamic GnRH pulse generation, they subserve upstream regulatory mechanisms determining the timing of puberty onset. In the case of primates, the upstream control system(s), which are independent of gonadal steroids, first suppress pulsatile GnRH release in infancy and, subsequently, reduction in this suppression reactivates pulsatility of GnRH release at the end of juvenile development (Figure 12.3). In rodents, the early postnatal ontogeny of pulsatile GnRH release is less clear, but later in prepubertal development steroid-dependent mechanisms dictate the timing of puberty by suppressing GnRH pulse generation. This being the case, loss-of-function mutations in KISS1/Kiss1 or KISS1R/Kiss1r, or ablation of neurons expressing either kisspeptin or its receptor, would likely lead to a loss or impairment in GnRH pulsatility that secondarily results in delayed or absent puberty and infertility regardless of species. While this is indeed the case in situations where the genes have been manipulated either spontaneously or experimentally (1, 9-11, 52, 85), interestingly, embryonic ablation of kisspeptin cells in mice did not dramatically influence the timing of puberty or prevent fertility (47). It should be noted that failure to change the timing of puberty in this study may be due to the 5% of kisspeptin neurons in the AVPV that escaped ablation (47). In the context of the results of the study employing kisspeptin neuron ablation, Kiss1 or Kiss1r null mice exhibit some degree of GnRH release as they age (85), and therefore the difference in the phenotypes between these two models may be quantitative, and perhaps be explained by differences in the extent to which the GnRH neuronal network is intrinsically able to generate intermittent GnRH release following a genetic or ablative insult to the GnRH pulse generating mechanism that normally drives gonadotropin secretion in the adult.
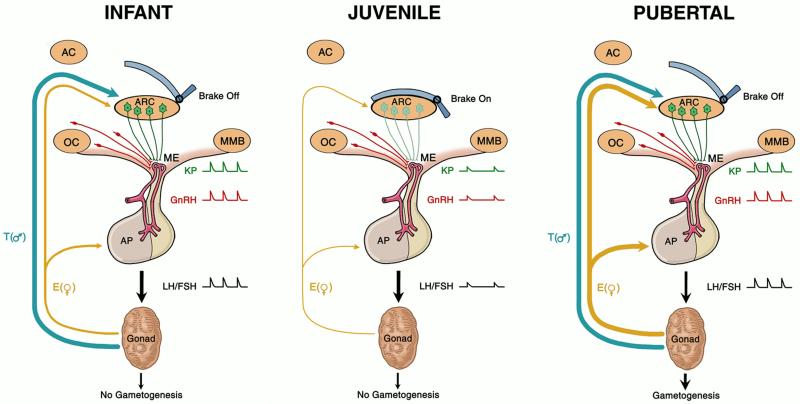
Figure 12.3.
A model for the control of the timing of puberty in primates, in which the role of kisspeptin (KP, green) signaling is posited to be a critical component of the neural machinery essential for generation of pulsatile GnRH (red) release in the hypothalamus. In this model, the GnRH pulse generating mechanism resides in the arcuate nucleus (ARC) and the output of this signaling is relayed to GnRH terminals in the median eminence (ME) by KP projections arising from perikarya in the ARC. During infancy (left panel), ARC GnRH pulse generating activity is robust leading to intermittent release of KP in the ME, resulting in a corresponding pattern of GnRH release into the portal circulation. This, in turn, drives pulsatile gonadotropin (LH and FSH) secretion. In the transition from infancy to the juvenile phase of development (middle panel), a neurobiological brake (central inhibition) holds the ARC GnRH pulse generating mechanism in check and pulsatile release of KP in the ME is markedly suppressed. This leads to reduced GnRH release and to a hypogonadotropic state in the juvenile period. Puberty is triggered when the brake is removed and GnRH pulse generation with robust intermittent release of KP in the ME is reactivated (right panel). According to this model, the mystery of primate puberty lies in the nature of the neurobiological brake, i.e., the mechanism that times its application during infancy and its release at the end of the juvenile phase of development. The thickness of the blue (T, testosterone) and gold (E, estradiol) arrows indicating negative feedback by the testis and ovary, respectively, reflect the degree of gonadal steroid inhibition exerted on LH secretion at these three stages of primate development. AC, anterior commissure; AP, anterior pituitary gland, ARC, arcuate nucleus; OC, optic chiasm; ME, median eminence; MMB, mamillary body.
The notion that kisspeptin signaling is necessary for the onset of puberty only because of its critical role in GnRH pulse generation may be most readily appreciated when the concept is applied to puberty in the male, where initiation of this developmental event requires only robust pulsatile GnRH release to drive tonic LH and FSH secretion. In the case of puberty onset in the human female, the validity of the idea that KISS1 may simply be regarded as a “pulse generating” gene is tenable, because the preovulatory LH surge is triggered by E2 positive feedback action within the MBH-pituitary unit to amplify pulsatile GnRH release and/or the response of the pituitary gonadotrophs to pulsatile GnRH stimulation (22). The situation in the female rodent is more complex because the positive feedback action of E2 is exerted, at least in part, on kisspeptin neurons in the AVPV (34). Nevertheless, as discussed above, the development of kisspeptin neurons in the AVPV in female mice is dependent on ovarian E2 secretion, which, in turn, is dependent on tonic gonadotropin secretion that is driven by pulsatile GnRH release. Thus, it seems reasonable to propose that 1) the primary role of kisspeptin signaling in the control of puberty across species may be restricted to its crucial role in GnRH pulse generation, 2) the time of puberty onset is dictated by kisspeptin-independent mechanisms that control the ontogeny of GnRH pulse generation, and 3) Kiss1 in the rodent may be viewed as a “surge generating gene,” as well as a pulse generating gene (see below for further discussion).
7. Neuronal substrates of central inhibition on GnRH in juvenile primates
According to the model proposed above, the key to the mystery of puberty in primates is to understand 1) the neural substrate that underlies the gonadal steroid independent reduction in GnRH pulse generation from infancy to puberty, and 2) the signals responsible for timing the application and removal of this central neurobiological brake. In this section, we discuss possible neuronal substrates responsible for “central inhibition.”
Two laboratories have each proposed a different neuronal subtype. First, Terasawa and her colleagues have proposed the hypothesis that tonic inhibition by γ-amino butyric acid (GABA) neurotransmission is responsible for this central inhibition in female rhesus monkeys (17). This hypothesis is based on the observations that 1) GABA levels are higher when GnRH release is low in prepubertal monkeys, whereas GABA levels are lower after the onset of puberty when GnRH release is elevated (86), 2) infusion of the GABAA receptor antagonist, bicuculline, into the S-ME stimulates GnRH release to a much greater extent in prepubertal, than in pubertal, monkeys, whereas infusion of GABA is effective in suppressing GnRH release in pubertal, but not prepubertal, monkeys, presumably because of the reduction in tonic GABA inhibition at the onset of puberty (86), and 3) a long-term infusion of bicuculline into the S-ME of juvenile female primates results in precocious puberty and first ovulation (87). Second, Plant and his colleagues have proposed the hypothesis that neuropeptide Y (NPY) neurons are responsible for the central inhibition of pulsatile GnRH release during juvenile development in male monkeys. This hypothesis is based on the finding that mRNA and peptide levels of NPY in the MBH are significantly lower during the neonatal period compared to those during the juvenile period, whereas mRNA and peptide levels of NPY in the MBH decrease, while GnRH mRNA levels increase across puberty in male monkeys (88). Presently whether the sex-differences noted in the “juvenile hiatus” in gonadotropin secretion are attributable to central inhibition mediated by GABA neurons in females vs. NPY neurons in males is unclear. Nonetheless, it is possible that the same population of neurons in the MBH is responsible for gonadal steroid-independent central inhibition, as a large number of GABA neurons in the rat ARC express NPY (89, 90).
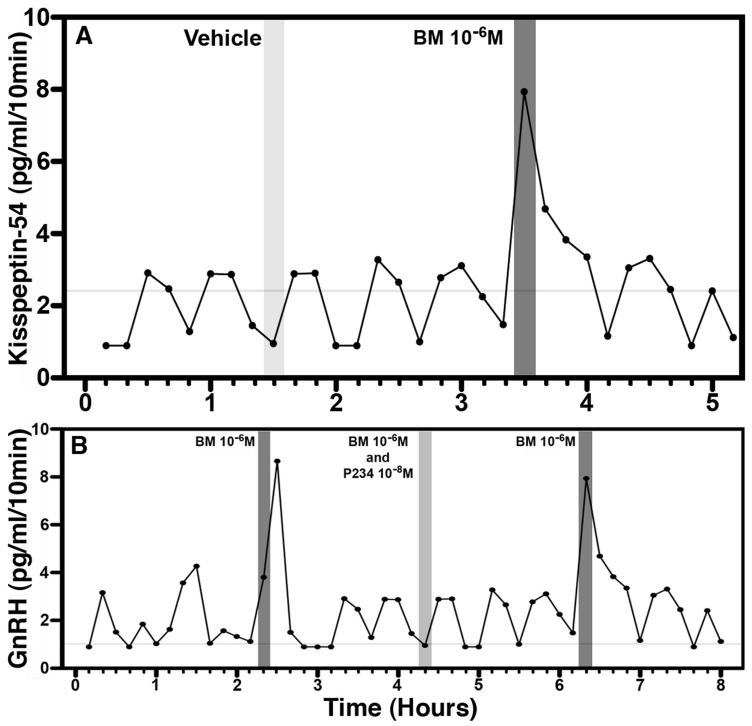
A recent study from Terasawa’s group indicates that bicuculline infusion into the S-ME of prepubertal female monkeys stimulates kisspeptin-54 release (91, Figure 12.4A), similar to the bicuculline-induced stimulation of GnRH release observed in prepubertal monkeys (86). Moreover, the bicuculline-induced GnRH release was blocked by simultaneous infusion of the kisspeptin antagonist, peptide 234 (91, Figure 12.4B). These latter results are consistent with the view that inhibitory GABA neurotransmission is an important component in the upstream suppression of the GnRH pulse generating mechanism during juvenile development in primates. It is, however, unclear what reduces GABA inhibition prior to puberty and whether additional (or alternative) neuronal substrates and somatic cues (92-95) are involved in the upstream control of GnRH pulse generation. Thus, the most important question of exactly what triggers the onset of puberty in primates remains a mystery.
Figure 12.4.
A. GABAA antagonist bicuculline (BM) stimulates KP-54 release in prepubertal female monkeys (but not pubertal monkeys, data not shown). An example showing that bicuculline infusion the S-ME (dark shaded bar) for 10 min induces an increase KP-54 release, whereas vehicle (light shaded bar) infusion does not. B. Blockade of the bicuculline-induced GnRH release by the kisspeptin receptor antagonist, peptide 234 (P234), in a prepubertal monkey. The stimulated GnRH release by bicuculline infusion in the S-ME (dark shaded bars) are not seen in the presence of P234 (light shaded bar). Modified with permission from 91.
8. Neural substrate for steroid inhibition of GnRH release in juvenile rodents
As discussed above, in contrast to primates, the prepubertal restraint on the GnRH pulse generating mechanism in rodents is gonadal steroid dependent. In this regard, studies in sheep and mice indicate that the majority of kisspeptin neurons express ERα (96-98), and it is well established in the adult rodent that ovariectomy increases, and E2 replacement decreases, Kiss1 expression in ARC kisspeptin neurons (34). As might be expected, therefore, transgenic mice with a conditional knockout of ERα in kisspeptin neurons exhibit elevated Kiss1 mRNA levels in ARC at a prepubertal age, and this is associated with high circulating concentrations of LH (and presumably E2) and a dramatic advancement of the age of vaginal opening (52). Interestingly, in contrast to the mRNA data, kisspeptin immunoreactivity in the ARC was greatly reduced in the conditional knockout, suggesting perhaps enhanced release of kisspeptin. Thus, in the case of the female mouse it seems reasonable to conclude that the site of the prepubertal ovarian steroid suppression on pulsatile GnRH release is on the GnRH pulse generating mechanism itself, and specifically on kisspeptin (KNDy) neurons in the ARC. This view is consistent with the long-standing “differential sensitivity to E2” theory, which has been proposed in female rats and sheep (32, 99-101). During the postnatal period through the juvenile period, the hypothalamus (presumably the GnRH neurosecretory system) is inhibited by E2 and sometime prior to puberty the GnRH pulse generating mechanism in the ARC escapes from the GnRH suppression by E2. It has been proposed that this escape is the result of an E2-induced increase in activity of kisspeptin neurons in the AVPV, which in turn amplifies GnRH neuronal activity, leading to puberty onset (39, 51, 52). The precise mechanism by which the initial prepubertal elevation of E2 is triggered in non-primate species, however, is unknown. [Note that, in women and female rhesus monkeys, a similar escape of E2-dependent inhibition of GnRH release occurs well after the initiation of puberty onset, between menarche and first ovulation (102-104)].
9. Summary
In this chapter, we have reviewed progress regarding the relationship between kisspeptin and puberty onset, and have proposed a novel hypothesis for the role of kisspeptin signaling in controlling the timing of this major event in postnatal development. We posit that the profound impact of loss of function mutations in the genes encoding either kisspeptin or its receptor on the onset and progression of puberty in all species can be attributed primarily to the critical role of ARC kisspeptin neurons in the generation of pulsatile GnRH release, which is obligatory for pubertal activation of the pituitary-gonadal axis. According to this hypothesis, kisspeptin neurons do not determine the timing of puberty (see Figure 12.3). Rather, this important developmental event is achieved by upstream neuronal mechanisms that govern the timing of the pubertal activation (rodents) or reactivation (primates) of robust pulsatile GnRH release at the end of the juvenile phase of development. Validation of this hypothesis requires future studies.
Acknowledgements
Supported by grants R01 HD15433 and R01 HD11355 for ET, R01 HD 013254 and U54 HD 08160 for TMP, T32 HD041921 for KAG, and P51 0D011106 for WNPRC.
Abbreviations
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- E2
estradiol
- ERα
estrogen receptor alpha
- GABA
γ-aminobutyric acid
- KISS1
kisspeptin gene (primates)
- Kiss1
kisspeptin gene (non-primates)
- KISS1R
kisspeptin-1 receptor (primates)
- Kiss1r
kisspeptin-1 receptor (non-primates)
- KP
kisspeptin
- MBH
medial basal hypothalamus
- ME
median eminence
- S-ME
stalk-median eminence
- NPY
neuropeptide Y
- POA
preoptic area
References
- 1.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 2.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–76. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O’rahilly S, Aparicio SA. Novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–55. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- 4.Cerrato F, Shagoury J, Kralickova M, Dwyer A, Falardeau J, Ozata M, Van Vliet G, Bouloux P, Hall JE, Hayes FJ, Pitteloud N, Martin KA, Welt C, Seminara SB. Coding sequence analysis of GnRHR and GPR54 in patients with congenital and adult onset forms of hypogonadotropic hypogonadism. Eur J Endocrinol. 2006;155:S3–S10. doi: 10.1530/eje.1.02235. [DOI] [PubMed] [Google Scholar]
- 5.Tenenbaum-Rakover Y, Commenges-Ducos M, Iovane A, Aumas C, Admoni O, de Roux N. Neuroendocrine phenotype analysis in five patients with isolated hypogonadotropic hypogonadism due to a L102P inactivating mutation of GPR54. J Clin Endocrinol Metab. 2007;92:1137–44. doi: 10.1210/jc.2006-2147. [DOI] [PubMed] [Google Scholar]
- 6.Nimri R, Lebenthal Y, Lazar L, Chevrier L, Phillip M, Bar M, Hernandez-Mora E, de Roux N, Gat-Yablonski G. A novel loss-of-function mutation in GPR54/KISS1R leads to hypogonadotropic hypogonadism in a highly consanguineous family. J Clin Endocrinol Metab. 2011;96:E536–45. doi: 10.1210/jc.2010-1676. [DOI] [PubMed] [Google Scholar]
- 7.Lanfranco F, Gromoll J, von Eckardstein S, Herding EM, Nieschlag E, Simoni M. Role of sequence variations of the GnRH receptor and G protein-coupled receptor 54 gene in male idiopathic hypogonadotropic hypogonadism. Eur J Endocrinol. 2005;153:845–52. doi: 10.1530/eje.1.02031. [DOI] [PubMed] [Google Scholar]
- 8.Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358:709–15. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–63. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 10.d’Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MBL, Aparicio SAJR, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–19. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–36. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 12.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–35. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 13.Wildt L, Marshall G, Knobil E. Experimental induction of puberty in the infantile female rhesus monkey. Science. 1980;207:1373–75. doi: 10.1126/science.6986658. [DOI] [PubMed] [Google Scholar]
- 14.Loose MD, Terasawa E. Pulsatile infusion of luteinizing hormone-releasing hormone induces precocious puberty (vaginal opening and first ovulation) in the immature female guinea pig. Biol Reprod. 1985;33:1084–93. doi: 10.1095/biolreprod33.5.1084. [DOI] [PubMed] [Google Scholar]
- 15.Plant TM. Neuroendocrine basis of puberty in the rhesus monkey (Macaca mulatta) Front Neuroendocrinol. 1988;10:215–38. [Google Scholar]
- 16.Grumbach MM, Styne DM. Puberty, Ontogeny, Neuroendocrinology, Physiology, and Disorders. In: Williams RH, Foster DW, Kroenenberg H, Larsen PR, Zorab R, editors. Williams Textbook of Endocrinology. 9th edition W.B. Saunders; Philadelphia, PA: 1998. pp. 1509–1625. [Google Scholar]
- 17.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–51. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- 18.Plant TM, Witchel SF. Puberty in nonhuman primates and primates. In: Neill J, editor. The Physiology of Reproduction. 3rd edition Volume 2. Academic Press; San Diego, CA: 2006. pp. 2177–2230. [Google Scholar]
- 19.Ojeda SR, Skinner MK. Puberty in the rat. In: Neill J, editor. The Physiology of Reproduction. 3rd edition Volume 2. Academic Press/Elsevier; San Diego, CA: 2006. pp. 2061–2126. [Google Scholar]
- 20.Terasawa E, Kurian JR. Neuroendocrine mechanism of puberty. Academic press, Elsevier; Waltham; London: San Diego: 2012. Handbook of Neuroendocrinology; pp. 433–484. [Google Scholar]
- 21.Plant TM. Gonadal regulation of hypothalamic gonadotropin-releasing hormone release in primates. Endocr Rev. 1986;7:75–88. doi: 10.1210/edrv-7-1-75. [DOI] [PubMed] [Google Scholar]
- 22.Plant TM. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Front Neuroendocrinol. 2012;33:160–8. doi: 10.1016/j.yfrne.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Plant TM. Neuroendocrine basis of puberty in the rhesus monkey (Macaca mulatta) Front Neuroendocrinol. 1988;10:215–38. [Google Scholar]
- 24.Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta) Endocrinology. 1985;116:1341–50. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- 25.Pohl CR, de Ridder CM, Plant TM. Gonadal and nongonadal mechanisms contribute to the prepubertal hiatus in gonadotropin secretion in the female rhesus monkey (Macaca mulatta) J Clin Endocrinol Metab. 1995;80:2094–101. doi: 10.1210/jcem.80.7.7608261. [DOI] [PubMed] [Google Scholar]
- 26.Conte FA, Grumbach MM, Kaplan SL. A diphasic pattern of gonadotropin secretion in patients with the syndrome of gonadal dysgenesis. J Clin Endocrinol Metab. 1975;40:670–674. doi: 10.1210/jcem-40-4-670. [DOI] [PubMed] [Google Scholar]
- 27.Ross JL, Loriaux DL, Cutler GB., Jr Developmental changes in neuroendocrine regulation of gonadotropin secretion in gonadal dysgenesis. J Clin Endocrinol Metab. 1983;57:288–293. doi: 10.1210/jcem-57-2-288. [DOI] [PubMed] [Google Scholar]
- 28.Chongthammakun S, Terasawa E. Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology. 1993;132:735–43. doi: 10.1210/endo.132.2.8425492. [DOI] [PubMed] [Google Scholar]
- 29.Foster DL, Jackson LM. Puberty in the sheep. In: Neill J, editor. The Physiology of Reproduction. 3rd edition Volume 2. Academic Press/Elsevier; San Diego, CA: 2006. pp. 1415–1482. [Google Scholar]
- 30.Goldman BD, Gorski RA. Effects of gonadal steroids on the secretion of LH and FSH in neonatal rats. Endocrinology. 1971;89:112–15. doi: 10.1210/endo-89-1-112. [DOI] [PubMed] [Google Scholar]
- 31.Ojeda SR, Ramirez VD. Short-term steroid treatment on plasma LH and FSH in castrated rats from birth to puberty. Neuroendocrinology. 1973-1974;13:100–14. doi: 10.1159/000122201. [DOI] [PubMed] [Google Scholar]
- 32.Foster DL, Ryan KD. Endocrine mechanisms governing transition into adulthood: a marked decrease in inhibitory feedback action of estradiol on tonic secretion of luteinizing hormone in the lamb during puberty. Endocrinology. 1979;105:896–904. doi: 10.1210/endo-105-4-896. [DOI] [PubMed] [Google Scholar]
- 33.Fraser MO, Plant TM. Further studies of the role of the gonads in determining the ontogeny of gonadotropin secretion in the guinea pig (Cavia porcelus) Endocrinology. 1989;125:906–11. doi: 10.1210/endo-125-2-906. [DOI] [PubMed] [Google Scholar]
- 34.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–43. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–74. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 36.Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol. 2009;21:527–37. doi: 10.1111/j.1365-2826.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- 37.Takumi K, Iijima N, Ozawa H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci. 2011;43:138–45. doi: 10.1007/s12031-010-9430-1. [DOI] [PubMed] [Google Scholar]
- 38.Bentsen AH, Ansel L, Simonneaux V, Tena-Sempere M, Juul A, Mikkelsen JD. Maturation of kisspeptinergic neurons coincides with puberty onset in male rats. Peptides. 2010;31:275–83. doi: 10.1016/j.peptides.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Clarkson J, Herbison AE. Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2011;23:293–301. doi: 10.1111/j.1365-2826.2011.02107.x. [DOI] [PubMed] [Google Scholar]
- 40.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive hormone-dependent and –independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One. 2010;5:e11911. doi: 10.1371/journal.pone.0011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297:E1212–21. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology. 2012;153:782–93. doi: 10.1210/en.2011-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jean-Faucher C, el Watik N, Berger M, De Turckheim M, Veyssiere G, Jean C. Regulation of gonadotrophin secretion in male mice from birth to adulthood. Response to LHR injection, castration, and testosterone replacement therapy. Acta Endocrinol (Copenh) 1985;110:193–99. doi: 10.1530/acta.0.1100193. [DOI] [PubMed] [Google Scholar]
- 45.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–34. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2006;147:5817–25. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–10. doi: 10.1038/nn.2818. [DOI] [PubMed] [Google Scholar]
- 48.Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152:2387–99. doi: 10.1210/en.2011-0164. [DOI] [PubMed] [Google Scholar]
- 49.Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153:2756–65. doi: 10.1210/en.2011-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dwarki K, Ramaswamy S, Gibbs R, Plant TM. The arrest of GnRH pulsatility during infancy that guarantees the quiescence of the primate gonad during juvenile development is correlated with a reduction in immunopositive kisspeptin neurons in the arcuate nucleus of the male rhesus monkey (Macaca mulatta). 93rd Annual Meeting of The Endocrine Society; Boston. June 2011; Abstract #P2-262. [Google Scholar]
- 51.Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150:3214–20. doi: 10.1210/en.2008-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107:22693–98. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–50. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 54.Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod. 2010;83:568–77. doi: 10.1095/biolreprod.110.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–98. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- 56.Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female Rhesus monkeys (Macaca mulatta): Implication for the mechanism of puberty. Endocrinology. 2012;153:825–36. doi: 10.1210/en.2011-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (Gpr54) during maturation in cichlid fish. Endocrinology. 2004;145:3613–18. doi: 10.1210/en.2004-0395. [DOI] [PubMed] [Google Scholar]
- 58.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 60.Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:312–21. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- 61.Plant TM, Ramaswamy S, DiPietro MJ. Repetitive activation of hypothalamic G protein coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 62.Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology. 2009;150:1400–12. doi: 10.1210/en.2008-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 64.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffman SN, Vassart G, Parmentier M. The metestasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–36. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 65.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa, Clarke JK, Steiner RA, Miller RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–29. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frost SI, Keen KL, Levine JE, Terasawa E. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the rhesus monkey. J Neurosci Methods. 2008;168:26–34. doi: 10.1016/j.jneumeth.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149:4151–57. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guerriero KA, Keen KL, Terasawa E. Developmental increase in kisspeptin-54 in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta) Endocrinology. 2012;153:1887–97. doi: 10.1210/en.2011-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone (LHRH) increases with puberty in the female rhesus monkey. Endocrinology. 1989;125:92–99. doi: 10.1210/endo-125-1-92. [DOI] [PubMed] [Google Scholar]
- 70.Chongthammakun S, Claypool LE, Terasawa E. Ovariectomy increases in vivo LHRH release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol. 1993;5:41–50. doi: 10.1111/j.1365-2826.1993.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 71.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–60. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 72.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–32. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawakami M, Uemura T, Hayashi R. Electrophysiological correlates of pulsatile gonadotropin release in rats. Neuroendocrinology. 1982;35:63–67. doi: 10.1159/000123356. [DOI] [PubMed] [Google Scholar]
- 74.Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39:256–60. doi: 10.1159/000123988. [DOI] [PubMed] [Google Scholar]
- 75.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21:813–21. doi: 10.1111/j.1365-2826.2009.01909.x. [DOI] [PubMed] [Google Scholar]
- 76.Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–75. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94:237–45. doi: 10.1159/000329045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–95. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O’Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4:e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blake CA, Sawyer CH. Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology. 1974;94:730–36. doi: 10.1210/endo-94-3-730. [DOI] [PubMed] [Google Scholar]
- 81.Krey LC, Hess DL, Butler WR, Espinosa-Campos J, Lu KH, Piva F, Plant TM, Knobil E. Medial basal hypothalamic disconnection and the onset of puberty in the female rhesus monkey. Endocrinology. 1981;108:1944–48. doi: 10.1210/endo-108-5-1944. [DOI] [PubMed] [Google Scholar]
- 82.Plant TM, Moossy J, Hess DL, Nakai Y, McCormack JT, Knobil E. Further studies on the effects of lesions in the rostral hypothalamus on gonadotropin secretion in the female rhesus monkey (Macaca mulatta) Endocrinology. 1979;105:465–73. doi: 10.1210/endo-105-2-465. [DOI] [PubMed] [Google Scholar]
- 83.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153:2800–12. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clarkson J, Han SK, Liu X, Lee K, Herbison AE. Neurobiological mechanisms underlying kisspeptin activation of gonadotropin-releasing hormone (GnRH) neurons at puberty. Mol Cell Endocrinol. 2010;324:45–50. doi: 10.1016/j.mce.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 85.Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21:1015–23. doi: 10.1111/j.1365-2826.2009.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitsushima D, Hei DL, Terasawa E. GABA is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci USA. 1994;91:395–99. doi: 10.1073/pnas.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. Effects of pulsatile infusion of the GABAA receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology. 1999;140:5257–66. doi: 10.1210/endo.140.11.7139. [DOI] [PubMed] [Google Scholar]
- 88.El Majdoubi M, Sahu A, Ramaswamy S, Plant TM, Neuropeptide Y. A hypothalamic brake restraining the onset of puberty in primates. Proc Natl Acad Sci USA. 2000;97:6179–84. doi: 10.1073/pnas.090099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non GABAergic subpopulations. Brain Res. 1997;756:283–86. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- 90.Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–54. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kurian JR, Keen KL, Guerriero KA, Terasawa E. Tonic control of kisspeptin release in prepubertal monkeys: Implications to the mechanism of puberty onset. Endocrinology. 2012;153:3331–6. doi: 10.1210/en.2012-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plant TM, Fraser MO, Medhamurthy R, Gay VL. Somatogenic Control of GnRH Neuronal Synchronization during Development in Primates: A Speculation. In: Delamarre-van de Waal HA, Plant TM, van Rees GP, Schoemaker J, editors. Control of the Onset of Puberty III. Excerpta Medica; Amsterdam: 1989. pp. 111–121. [Google Scholar]
- 93.Wilson ME, Gordon TP, Rudman CG, Tanner JM. Effects of growth hormone on the tempo of sexual maturation in female rhesus monkeys. J Clin Endocrinol Metab. 1989;J68:29–38. doi: 10.1210/jcem-68-1-29. [DOI] [PubMed] [Google Scholar]
- 94.Mann DR, Plant TM. The role and potential sites of action of thyroid hormone in timing the onset of puberty in male primates. Brain Res. 2010;1364:175–85. doi: 10.1016/j.brainres.2010.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Terasawa E, Kurian JR, Keen KL, Shiel NA, Colman RJ, Capuano SV. Body weight impact on puberty: effects of high-calorie diet on puberty onset in female rhesus monkeys. Endocrinology. 2012;153:1696–705. doi: 10.1210/en.2011-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–94. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–30. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 99.Dohrn M, Hohlweg W. Hormonale beziehungen zwischen hypohysenvorderlappen und keimdrusen. Oliver and Boyd; Edinburgh: 1931. Proceedings of the 2nd International Congress on Sex Research; pp. 436–42. [Google Scholar]
- 100.Steele RE, Weisz J. Changes in sensitivity of estradiol-LH feedback system with puberty in the female rat. Endocrinology. 1974;95:513–20. doi: 10.1210/endo-95-2-513. [DOI] [PubMed] [Google Scholar]
- 101.Andrews WW, Advis JP, Ojeda SR. The maturation of estradiol-negative feedback in female rats: evidence that the resetting of the hypothalamic “gonadostat” does not precede the first preovulatory surge of gonadotropins. Endocrinology. 1981;109:2022–31. doi: 10.1210/endo-109-6-2022. [DOI] [PubMed] [Google Scholar]
- 102.Kulin HE, Grumbach MM, Kaplan SL. Changing sensitivity of the pubertal gonadal hypothalamic feedback mechanism in man. Science. 1969;166:1012–13. doi: 10.1126/science.166.3908.1012. [DOI] [PubMed] [Google Scholar]
- 103.Rapisarda JJ, Bergman KS, Steiner RA, Foster DL. Response to estradiol inhibition of tonic luteinizing hormone secretion decreases during the final stage of puberty in the rhesus monkey. Endocrinology. 1983;112:1172–79. doi: 10.1210/endo-112-4-1172. [DOI] [PubMed] [Google Scholar]
- 104.Wilson ME. IGF-1 administration advances the decrease in hypersensitivity to oestradiol negative feedback inhibition of serum LH in adolescent female monkeys. J Endocrinol. 1995;145:121–30. doi: 10.1677/joe.0.1450121. [DOI] [PubMed] [Google Scholar]