Abstract
GABAergic transmission regulates adult neurogenesis by exerting negative feedback on cell proliferation and enabling dendrite formation and outgrowth. Further, GABAergic synapses target differentiating dentate gyrus granule cells prior to formation of glutamatergic connections. GABAA receptors (GABAARs) mediating tonic (extrasynaptic) and phasic (synaptic) transmission are molecularly and functionally distinct, but their specific role in regulating adult neurogenesis is unknown. Using global and single-cell targeted gene deletion of subunits contributing to the assembly of GABAARs mediating tonic (α4, δ) or phasic (α2) GABAergic transmission, we demonstrate here in the dentate gyrus of adult mice that GABAARs containing α4, but not δ, subunits mediate GABAergic effects on cell proliferation, initial migration and early dendritic development. In contrast, α2-GABAARs cell-autonomously signal to control positioning of newborn neurons and regulate late maturation of their dendritic tree. In particular, we observed pruning of distal dendrites in immature granule cells lacking the α2 subunit. This alteration could be prevented by pharmacological inhibition of thrombospondin signaling with chronic gabapentin treatment, shown previously to reduce glutamatergic synaptogenesis. These observations point to homeostatic regulation of inhibitory and excitatory inputs onto newborn granule cells under the control of α2-GABAARs. Taken together, the availability of distinct GABAAR subtypes provides a molecular mechanism endowing spatiotemporal specificity to GABAergic control of neuronal maturation in adult brain.
Keywords: Synaptic homeostasis, neural precursor cell, BrdU, retroviral vector, gabapentin
Introduction
In adult rodent brain, neurogenesis leads to continuous generation of newborn neurons from pools of neural progenitor cells (NPC) in the subventricular zone (SVZ) and subgranular zone (SGZ) of the dentate gyrus (DG). Multiple genetic and epigenetic mechanisms acting in concert with extrinsic signals orchestrate the production and successful functional integration of newborn neurons (Suh et al., 2009; Ma et al., 2010). In particular, activity-dependent regulation by γ-aminobutyric acid (GABA) neurotransmission is known to influence key steps of adult neurogenesis (Abrous et al., 2005; Sernagor et al., 2010).
GABA mediates fast synaptic inhibition by activating ionotropic GABAA receptors (GABAARs), which are assembled from a large family of constituent subunits (Barnard et al., 1998). The composition of GABAAR subtypes determines their pharmacological profile, functional properties and subcellular localization. In particular, phasic and tonic inhibition reflect GABA activation of, respectively, postsynaptic and extrasynaptic GABAARs (Farrant & Nusser, 2005). In the hippocampal formation, distinct GABAAR subtypes mediate the bulk of phasic (α1/βx/γ2, α2/βx/γ2) and tonic (α4/β2/δ, α5/β3/γ2) inhibition (Prenosil et al., 2006; Glykys et al., 2008; Herd et al., 2008). GABAAR gene deletion studies have uncovered the key role of α-subunit variants for receptor assembly and surface expression. Furthermore, they have demonstrated the limited potential of the remaining subunits for compensation, notably the inability to relocate receptors mediating tonic inhibition to synaptic sites (Peng et al., 2002; Kralic et al., 2006).
GABAAR activation typically leads to influx of chloride (Cl−) ions, causing hyperpolarization. In turn, the K+–Cl− exchanger KCC2 maintains low [Cl−]i. However, depending on the Cl− extrusion mechanisms, GABAAR activation can also be depolarizing (Farrant & Kaila, 2007). This effect is relevant especially during early development, when KCC2 expression is low (Ganguly et al., 2001; Ben-Ari, 2002; Owens & Kriegstein, 2002). In adult brain, NPCs and neuroblasts are also tonically activated by GABA, providing a negative feedback on proliferation (Nguyen et al., 2003; Liu et al., 2005). These depolarizing effects of GABA in newborn cells persist for 2–3 weeks during their development and contribute to regulation of their migration, differentiation and functional integration (Overstreet Wadiche et al., 2005; Tozuka et al., 2005; Ge et al., 2006; Karten et al., 2006). However, maturation of newborn granule cells lasts for 2–3 months, and little is known about the subunit composition and temporal expression profile of GABAARs in newborn neurons and the significance of GABAA-mediated tonic and phasic inhibition for completion of neurogenesis.
Here, we addressed these issues by monitoring proliferation, fate decision, survival, migration and dendritic differentiation of newborn neurons in the dentate gyrus of mutant mice lacking subunits contributing to the assemblage of GABAARs mediating tonic (α4, δ) or phasic (α2) GABAergic transmission. Pulse-chase labeling with 5′-bromodeoxyuridine (BrdU) was combined with expression of cell type markers to assess newborn cell proliferation, differentiation and survival in these mutant mice. Next, we used retroviruses to transduce reporter genes for monitoring migration and differentiation of newborn dentate gyrus granule cells and induce conditional, cell-specific gene deletion for assessing cell-autonomous effects of altered GABAergic function. Finally, we investigated the mechanisms underlying homeostatic regulation of excitatory–inhibitory inputs in newborn cells and their consequences for dendritic maturation.
Animals
All animal procedures were performed in accordance with the European Community Council Directives of 24 November 1986 (86/609/EEC) and approved by the cantonal veterinary office of Zurich. The generation of α4-knockout (KO) and δ-KO mice, as well as mice carrying a ‘floxed’ GABA receptor subunit alpha-2 (Gabra2) allele (α2fl/fl), has been described previously (Mihalek et al., 1999; Chandra et al., 2006; Witschi et al., 2011). All lines were bred in the animal facility of the Institute of Pharmacology and Toxicology (University of Zurich) and maintained on a heterozygote background. α2fl/fl and α2-KO mice were backcrossed on the C56BL/6J background for more than nine generations. Experimental wild-type (WT) and mutant mice were either littermates obtained from heterozygote crossings or mutants from homozygous crossings. All genotyping was performed by PCR analysis of tail or ear biopsies.
Retroviral vectors
We used a nonreplicative retroviral vector based on the Moloney murin leukemia virus expressing enhanced green-fluorescent protein (eGFP), monomeric red fluorescent protein (mRFP) or Cre-eGFP under the CAG promoter [containing a cytomegalovirus (CMV) enhancer and chicken β-actin promoter]. Viruses were produced by transfecting HEK 293T cells with three separate plasmids containing the capsid (CMV-vsvg), viral proteins (CMV-gag/pol) and transgene (CAG-eGFP, CAG-mRFP and CAG-Cre-eGFP) using polyethilaminine (Polysciences, Eppelheim, Germany). The supernatant containing the virus was concentrated by two rounds of ultracentrifugation. Typically the retrovirus titer was 108 cfu/mL.
Stereoaxic injection
Adult (8–12 weeks old) male mice anesthetized with 1.5% isoflurane in oxygen were injected stereotaxically with 1 μL of retroviruses encoding either eGFP or mRFP and 1.5 μL of a 1 : 1 mixture of retroviruses encoding Cre-eGFP and mRFP into the dorsal hippocampus (coordinates from Bregma were – anteroposterior, −2 mm; lateral, ± 1.5 mm; dorsoventral, −2.3 mm from the skull). Following surgery, the mice were allowed to recover from anesthesia on a warm pad, injected with 1 mg/kg buprenorphine (Temgesic, Essex Chemie, Luzern, Switzerland) and returned to their home cage with daily monitoring of wellbeing. Mice were killed a 14, 28 or 42 days post-injection (DPI; n = 4 or 5 mice per time-point and genotype) to examine the distribution and maturation of newborn granule cells transduced with the virus.
BrdU treatment
BrdU (Sigma) was dissolved in saline, and mice received eight i.p. injections (50 mg/kg each) in total over 3 days (n = 8–11 mice per group). Animals were killed for histological analysis either 1 or 28 days after the last BrdU injection to investigate NPC proliferation and survival or fate commitment of newborn cells.
Gabapentin treatment
Gabapentin (400 mg/kg; TCI Europe, Zwijndrecht, Belgium) or vehicle (saline) were injected i.p. once daily in WT and α2-KO mice for 14 days (n = 5 mice per group), starting 2 weeks after retrovirus injection. Mice were killed for histological analysis 6 h after the last injection.
Tissue preparation and immunohistochemistry
Mice were deeply anesthetized with sodium pentobarbital and perfused transcardially with phosphate-buffered saline (PBS) followed by fixative containing 4% paraformaldehyde in 0.15 m sodium phosphate buffer and 15% saturated picric acid solution, pH 7.4, as described (Duveau & Fritschy, 2010). After postfixation and cryoprotection, the brain was frozen and 40-μm-thick coronal sections were cut with a sliding microtome.
For BrdU immunohistochemistry, sections were pretreated with a solution of 50% formamide in 2 × saline sodium citrate buffer (SSC; 0.3 m NaCl and 0.03 m sodium citrate, pH 7.4) for 1.5 h at 65 °C. Sections were then washed in 2 × SSC for 10 min and incubated for 30 min at 37 °C in 2N HCl. HCl was thereafter neutralized with 0.1 m boric acid (pH 8.5) for 10 min, followed by three washes in Tris-buffered saline (0.1 m Tris and 0.15 m NaCl, pH 7.4). Brain sections were processed for immunoperoxidase staining with antibodies against BrdU (rat monoclonal, OBT0030, 1 : 400; Oxford Biotechnology, Kidlington, UK), or for triple immunofluorescence staining using a combination of antibodies against BrdU, NeuN (mouse, mAB377, 1 : 1000; Chemicon, Temecula, CA) and GFAP (rabbit, Z334, 1 : 1000; Dako, Glastrup, Denmark). Secondary antibodies coupled to biotin or different fluorochromes were obtained from Jackson Immunoresearch (West Grove, PA, USA).
For Ki67 immunostaining, free-floating sections were incubated in 50 mL citrate buffer (10 mm, pH 6) and heated in a microwave oven for 5 min (800 W). After cooling, sections were washed three times in PBS for 10 min and processed for immunoperoxidase staining with antibodies against Ki67 (mouse, 550609, 1 : 500; BD Pharmingen, Franklin Lakes, NJ).
To investigate the maturation of dendrites and spines in newborn eGFP+ neurons, the signal was intensified by immunofluorescence to eGFP (chicken anti-GFP, GFP-1020, 1 : 1000; Aves, Tigard, OR). When appropriate, Cre expression was verified with a rabbit anti-Cre (PRB-106C, 1 : 1000; Covance, Princeton, NJ) in double labeling experiments. To confirm the absence of α2-GABAARs in Cre-eGFP+/mRFP+ cells, triple immunofluorescence staining was performed as described (Schneider Gasser et al., 2007), using a guinea pig anti-α2 subunit antiserum raised in our laboratory.
Image analysis
Unbiased quantification of cell proliferation
BrdU+ and Ki67+ cells in the SGZ and granule cell layer (GCL) were counted in a one-in-six series of sections (four or five sections per mouse) through the dorsal hippocampus using the optical fractionator method (Mercator software; ExploraNova, France). For each section the DG was outlined and all BrdU+ or Ki67 + cells were counted at high magnification (Axioskop, Zeiss, Jena, Germany; 63 × oil-immersion lens, N.A. 1.3) in the SGZ and GCL. The total number of cells per DG was estimated by multiplying these values by the section sampling factor.
Phenotyping of BrdU+ cells
Triple immunofluorescence staining for BrdU, NeuN and GFAP was used for BrdU+ cell type determination. Sections were analyzed by confocal laser scanning microscopy (LSM 510 Meta; Zeiss). BrdU+ cells were examined following three-dimensional orthogonal reconstruction of stacks of confocal sections spaced by 1 μm using Imaris software (Bitplane, Zurich, Switzerland). At least 20 BrdU+ cells were analyzed per mouse (n = 4 or 5 per genotype).
Topological analysis
The topology of the dendritic tree of newborn neurons was quantified by Sholl analysis in 40-μm-thick sections stained for eGFP. Stacks of confocal images (Zeiss LSM 710 Zen) spaced by 0.5 μm were acquired across the entire thickness of the section with a 40 × objective (N.A. 1.3). Scholl analysis was performed with circles spaced at 10-μm intervals and centered on the cell body in three-dimensional reconstructions of the dendritic tree with the Scholl analysis plugin of Image J (NIH). For statistical comparison, the ‘area under the curve’ of the resulting function (number of intersections as a function of distance from the soma) was calculated. In addition, total dendritic length and number of nodes were determined on 2-D projections of eGFP+ cells obtained with the NeuronJ plugin. For each genotype and time-point, the number of cells analyzed is given in Tables 1 and 2.
Table 1.
Morphological properties of newborn neurons in WT and mutant mice
| Area under the curve | Number of nodes | Total dendritic length | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPI | Genotype | n | (μm2) | F | P | F | P | (μm) | F | P | |
| 14 | WT (control) | 29 | 3531 ± 178 | F2.90 = 2.7 | 0.041 | 5.6 ± 0.3 | F2.90 = 7.7 | 0.0001 | 302.2 ± 16.3 | F2.90 = 2.9 | 0.039 |
| α2-KO | 24 | 3522 ± 187 | N.S | 5.7 ±0.3 | N.S | 289.9 ± 19.9 | NS | ||||
| α4-KO | 21 | 3021 ± 187 | 0.028 | 3.9 ± 0.25 | 0.0002 | 237.4 ± 18.7 | 0.01 | ||||
| δ-KO | 20 | 3715 ± 200 | N.S | 5.85 ± 0.25 | N.S | 314.4 ± 21.3 | NS | ||||
| 28 | WT (control) | 22 | 6368 ± 251 | F2.77 = 4.8 | 0.004 | 5.4 ± 0.21 | F2.77 = 9.68 | 0.001 | 689.2 ± 35.1 | F2.77 = 1.1 | < 0.0001 |
| α2-KO | 26 | 5519 ± 250 | 0.02 | 4.46 ± 0.21 | 0.006 | 581 ± 32.5 | 0.03 | ||||
| α4-KO | 19 | 4964 ± 318 | 0.04 | 4.05 ± 0.3 | 0.0004 | 468.6 ± 41.6 | < 0.0001 | ||||
| δ-KO | 14 | 6814 ± 242 | N.S | 5.9 ± 0.33 | N.S | 778.3 ± 40.2 | N.S | ||||
| 42 | WT (control) | 23 | 5921 ± 216 | F2.52 = 9.79 | 0.002 | 5.9 ± 0.28 | F2.52 = 13.3 | < 0.001 | 639.4 ± 25.9 | F2.52 = 13.1 | < 0.001 |
| α2-KO | 36 | 4709 ± 218 | 0.0002 | 4.2 ± 0.23 | < 0.0001 | 465.5 ± 33.4 | 0.0001 | ||||
| α4-KO | 27 | 4612 ± 191 | 0.0002 | 4.18 ± 0.24 | < 0.0001 | 413.3 ± 28.8 | < 0.0001 | ||||
n, number of GFP+ cells. Values are given as mean ± SEM, with the corresponding P value of post hoc analysis. For each time-point and genotype, the cells analyzed were derived from four or five mice successfully injected with eGFP retroviruses. ‘Area under the curve’ represents the distribution of the number of intersections with concentric circles as a function of distance from the soma (see Fig. 3). Statistical comparisons are between genotypes at defined time-points.
Table 2.
Morphological analysis of mRFP+ (WT) and Cre-eGFP+/mRFP+ (α2-KO) cells at 28 and 42 DPI
| Area under the curve | Number of nodes | Total dendritic length | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPI | Genotype | n | (μm2) | t | P | t | P | (μm) | t | P | |
| 28 | mRFP (control) | 18 | 5929 ± 219 | t25 = –3.45 | 0.002 | 5 ± 0.17 | t25 = –2.63 | 0.01 | 617 ± 30 | t25 = –2.35 | 0.02 |
| eGFP/mRFP | 10 | 4555 ± 351 | 4 ± 0.47 | 460 ± 69 | |||||||
| 42 | mRFP (control) | 27 | 6101 ± 269 | t41 = –2.96 | 0.005 | 5 ± 0.24 | t41 = –4.86 | < 0.0001 | 694 ± 36 | t41 = –4.53 | < 0.0001 |
| eGFP/mRFP | 16 | 4987 ± 197 | 3.937 ± 0.17 | 469 ± 24 | |||||||
Values are given as mean ± SEM, with the corresponding P value. ‘Area under the curve’ represents the distribution of number of intersections with concentric circles as a function of distance from the soma (see Fig. 5). For each time-point, the cells analyzed were derived from five mice successfully injected with retroviruses. Statistical comparisons are between WT and mutant cells at each time point.
Distance of migration
Migration of newborn eGFP+ or BrdU+ cells into the GCL was assessed in photomicrographs taken from epifluorescence microscopy (Zeiss Imager equipped with an Apotome module) with a 40 × oil-immersion objective (N.A. 1.3), using DAPI to counterstain nuclei in the GCL. The distance of migration was measured perpendicularly to a virtual line through the base of the GCL. For each genotype and time-point, 5–10 eGFP+ cells per mouse (n = 4) or 10 BrdU+ cells per mouse (n = 4) were analyzed.
Spine quantification
The density (number per unit length) and shape of spines were determined on third-order dendritic segments of eGFP+ cells (n = 6–8 segments per mouse and four mice per group). Confocal mages were acquired with a 100 × objective (N.A.1.4) with a numerical zoom of 5 (spacing between layers, 0.25 μm). Image deconvolution was performed using a theoretical point-spread function (maximum-likelihood estimate; Huygens, Scientific Volume Imaging, Hilversum, The Netherlands). Deconvolved images were analyzed using the software NeuronStudio (CNIC, Mount Sinai School of Medicine, NY, USA).
Statistics
Statistical analyses were performed using Prism software (GraphPad, La Jolla, CA). For multigroup comparisons, one-way anova followed by Fischer's post hoc test was used. Two-group comparisons were made with unpaired Student's t-tests. Cumulative probability distributions of migration distances were compared using the Kolmogorov–Smirnoff test. Differences were considered significant at P < 0.05.
Electrophysiology
Hippocampal slices were prepared from 2-month-old male C57BL6/J and α4-KO mice ~ 14 days following injection of retroviruses encoding mRFP. Mice were decapitated after isoflurane anesthesia and 250-μm-thick slices through the hippocampus were cut transversally in ice-cold extracellular solution using a HM 650 V vibroslicer (Microm, Switzerland). Slices were incubated at 35 °C for 30 min and subsequently maintained at room temperature. The extracellular solution contained (in mm): NaCl, 125; NaHCO3, 26; NaH2PO4H2O, 1.25; KCl, 2.5; MgSO4, 1.0; CaCl2, 2.5; and glucose, 15; pH 7.4 when bubbled continuously with 95% O2 and 5% CO2.
Slices were transferred to a bath chamber mounted on an upright microscope (Olympus BX51-WI; Olympus, Switzerland). Transduced cells were visualized with epifluorescence combined with IR-DIC for electrode placement. Whole-cell recordings were obtained using a Multiclamp 700A amplifier (Axon Instruments, Sunnyvale, CA). We used electrodes with an open-tip resistance of 4–5 MΩ obtained by pulling borosilicate pipettes with 1.5 mm external diameter and 1.17 mm internal diameter without filament to a tip diameter of ~1 μm on a horizontal Puller (Zeitz GmbH, Germany). The intracellular solution contained (in mm): CsCl, 100; CsGlu, 35; MgATP, 4.5; Tris-phosphocreatine, 11; HEPES, 10; and Tris-GTP, 0.3 (pH 7.4, 303 mOsm). Tonic inhibition was assessed by measuring the holding current before and after the addition of picrotoxin (100 μm) or bicuculline (10 μm). In one series of experiments, the Na+ channel blocker tetrodotoxin (0.5 μm) and the GABA uptake inhibitor NO711 [1-(2-(((diphenylmethylene)amino)oxy)ethyl)-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid; 2 μm] were added to the bath solution. In a second series (WT mice only), the glutamate receptor blockers NBQX (25 μm) and D-APV (50 μm) were added to the bath solution without addition of GABA uptake inhibitors. Both conditions resulted in the same average level of tonic GABAergic currents.
Results
α4-GABAARs regulated cell proliferation in the SGZ
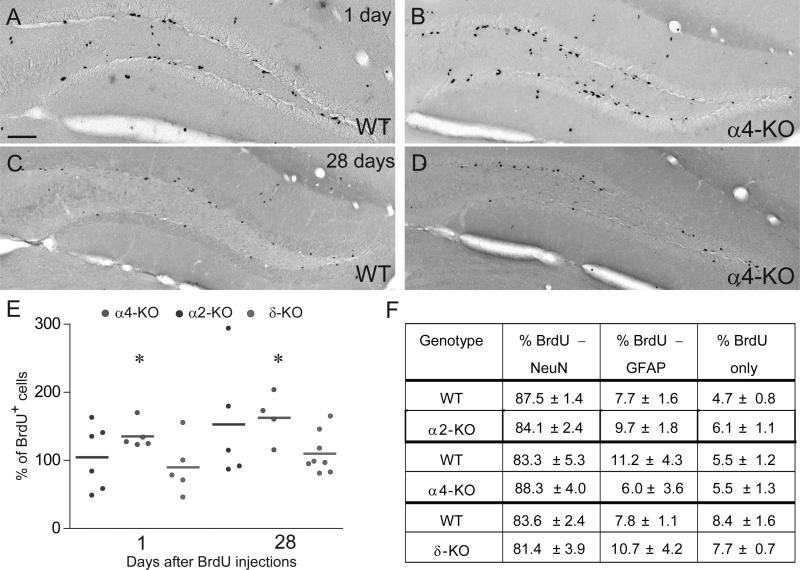
Pulse-chase labeling experiments with BrdU were performed in adult α2-KO, α4-KO and δ-KO mice and their respective WT littermates. These mutants were selected for the contribution of the respective receptors to phasic (α2) and tonic (α4, δ) inhibition in mature DG granule cells (Glykys et al., 2008). One day after completion of BrdU treatment, no difference in the number of BrdU+ cells was observed in the SGZ of α2-KO and δ-KO mice relative to WT. However, a 50% increase was evident in α4-KO mice (Fig. 1A–E). The survival rate of newborn cells, determined as fraction of BrdU+ cells present at 28 days compared to 1 day post-BrdU, was unaltered in all genotypes, reaching 21 and 25% in WT and α4-KO mice, respectively. Consequently, the absolute number of BrdU+ cells was increased by 50% in α4-KO mice without affecting the volume of the DG (data not shown). This observation was further confirmed by counting the number of proliferative cells, using the endogenous marker of dividing cells Ki67 (WT, 345 ± 35; α4-KO, 508 ± 60; unpaired t-test, t6 = 2.36, P = 0.05, n = 4 mice per genotype).
Figure 1.
Role of α2-, α4- and δ-GABAARs in regulating newborn cell proliferation, survival and fate determination. (A–D) Examples of BrdU immunostaining 1 and 28 days after the last of eight BrdU (50 mg/kg) injections delivered over 3 days in WT (A and C) and α4-KO (B and D) mice. One day after the last BrdU injection (A and B), BrdU+ cells were located in the inner part of the GCL and they were either alone or in clusters; note the higher density in α4-KO than in WT. After 28 days (C and D), fewer BrdU+ cells were found and some were located inside the GCL. (E) Unbiased quantification of BrdU+ cells in the six groups investigated. All data are expressed relative to WT littermates; each dot represents the average value of one mouse. A significant increase in BrdU+ cells was evident in α4-KO mice 1 day and 28 days after the last BrdU injection, but not in the other mutants (*P < 0.05; unpaired t-test). (F) Phenotypic analysis of BrdU+ cells by triple immunofluorescence for the neuronal marker NeuN and the astrocytic marker GFAP. None of the mutant mice investigated showed an alteration in the ratio of each class of BrdU+ cells. Scale bar, 75 μm (A–D).
In the same samples, analysis of neuronal versus astrocytic fate commitment of NPC showed no effect of genotype (Fig. 1F), with 81–88% of BrdU+ cells being positive for the neuronal marker NeuN and 6–11% for the astrocytic and stem-cell marker GFAP, corresponding to published figures for C57Bl6/J mice. Although consistent with tonic GABAergic inhibition of NPC proliferation, these results show that none of the GABAARs investigated is critical for modulating newborn cell survival and fate commitment in the adult DG. Furthermore, the lack of effect of GABA receptor subunit delta gene (Gabrd) deletion suggests that the corresponding receptor might be retained (possibly as α4βx-GABAARs) or compensated by upregulation of another GABAAR subunit, such as γ2 (Peng et al., 2002).
Loss of tonic GABAAR-mediated currents in newborn cells of α4-KO mice
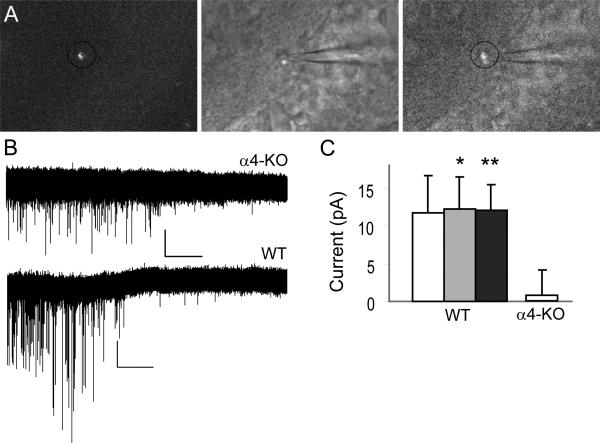
We verified by whole-cell patch clamp recordings that retroviral injection into the hippocampus transduced newborn neurons without altering their basic functional properties. Retroviruses encoding mRFP were used, as the red fluorescence was better seen in combination with IR/DIC microscopy. Confirming published reports, 70% of transduced cells in WT mice displayed tonic GABAergic activity at 14 DPI (n = 16; Fig. 2). The amplitude of tonic currents recorded in whole-cell patch-clamp mode from newborn neurons was similar to that measured in randomly-selected mRFP-negative cells in the same slices.
Figure 2.
Whole-cell patch-clamp recordings from newborn granule cells. (A) Example of a fluorescently labeled cell (left), the corresponding differential interference contrast image (middle) and overlay of the two (right). (B) Representative traces recorded in mRFP+ cells from α4-KO (top) and WT (bottom) mice at 13 and 15 DPI, respectively, depicting the disappearance of phasic events upon application of the GABAAR antagonist bicuculline. A change in holding current, reflecting tonic inhibition, is visible in the trace from WT mouse only. (C) Average amplitude (± SEM) of tonic currents as revealed by application of bicuculline (10 μm; white, n = 4 for WT and n = 5 for α4-KO), picrotoxin (100 μm; gray, n = 12, WT), and the two datasets together (black, n = 16, WT). *P < 0.05, **P < 0.01; paired t-test.
In contrast to these results, no tonic currents were detectable in recordings from mRFP-positive cells in slices from α4-KO mice, although spontaneous bicuculline-sensitive phasic inhibition was detectable in three of five recorded neurons (Fig. 2B). The difference in holding current before and after bicuculline bath application (10 μm) was statistically not significant (I = 73.5 and 74.5 pA, respectively; t4 = 0.346; P = 0.746; unpaired t-test). Therefore, Gabra4 deletion resulted in a marked reduction in tonic GABAergic function in immature neurons.
Differential alteration of dendritic arborization in newborn neurons of α2-KO and α4-KO mice Next, we investigated the role of α2-, α4- and δ-GABAARs in regulating dendritic differentiation of newborn granule cells using a retroviral vector encoding eGFP injected stereotaxically into the dorsal hippocampus. Mice were analyzed 14, 28 and 42 DPI, covering the phases of dendritic growth and spine formation (Toni et al., 2007).
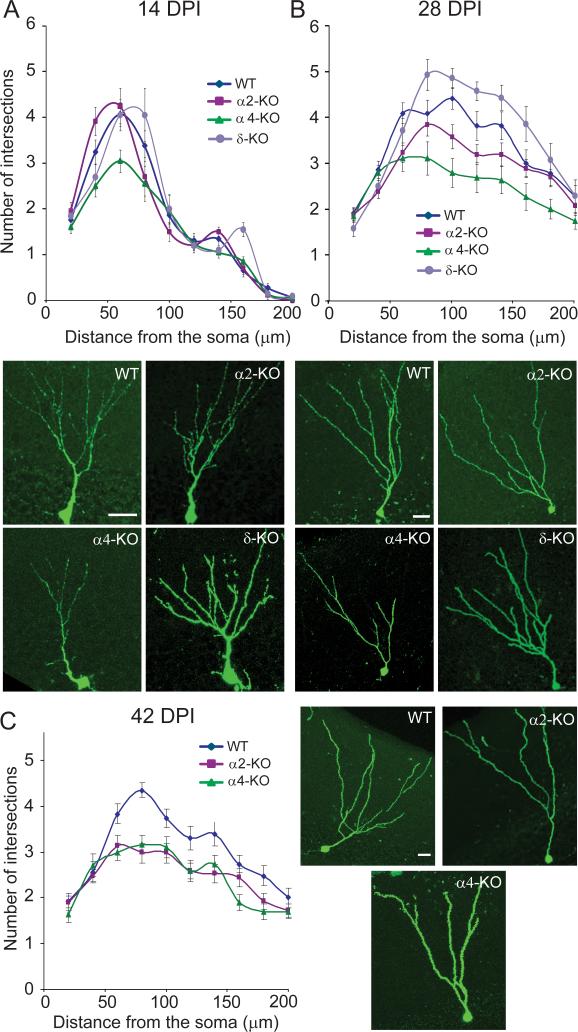
At 14 DPI, eGFP+ cells in WT mice were located inside the GCL and had a well developed dendritic tree extending deep into the molecular layer. Using Sholl analysis to quantify dendritic complexity (number of intersections with concentric circles centered on the soma), branching (number of nodes) and total length (Fig. 3, Table 1), we observed that these parameters were similar in WT, α2-KO and δ-KO mice, whereas in α4-KO mice a significant reduction in dendritic growth and complexity was evident (Fig. 3A, Table 1). Therefore, GABAergic activity mediated by α4-GABAARs probably regulates early dendritic development of newborn granule cells.
Figure 3.
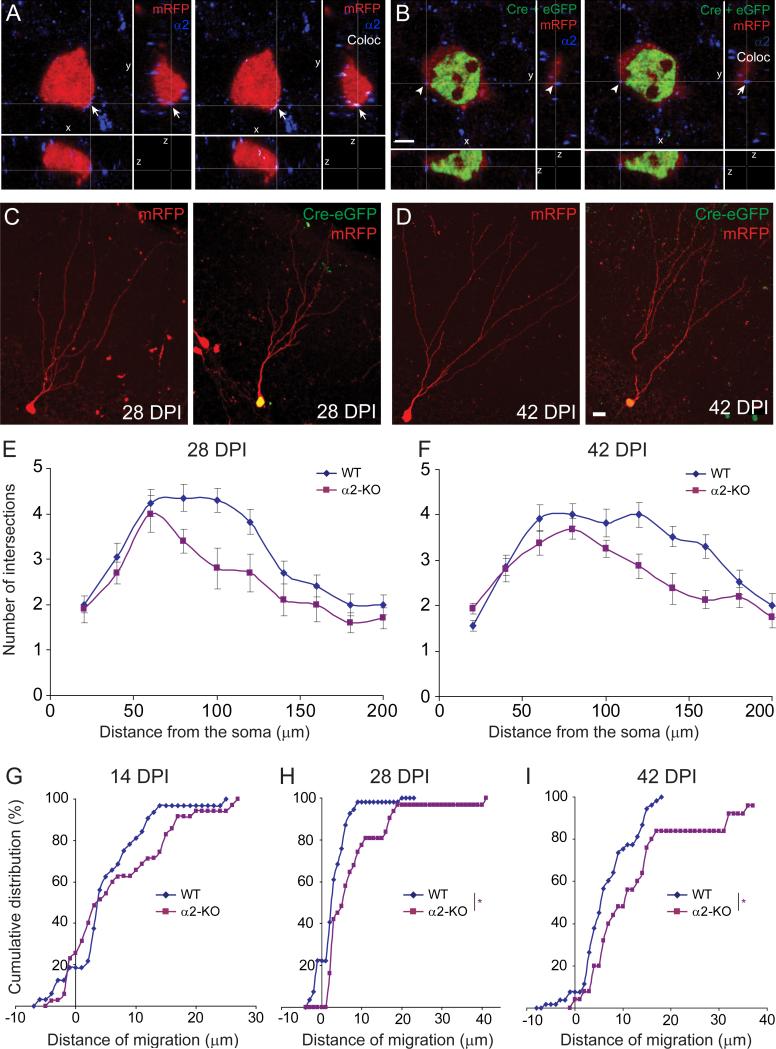
Quantification of dendrite development in eGFP+ newborn neurons by Sholl analysis at 14, 28 and 42 DPI in WT and mutant mice. The number of intersections between eGFP+ dendrite segments and virtual concentric lines centered on the soma and spaced by 10 μm is displayed graphically. For statistical comparison between genotypes, the corresponding AUC was calculated in each graph (see Table 1 for mean values and statistical results). Representative examples of eGFP+ cells in each genotype are illustrated. (A) At 14 DPI, no difference in the number of intersections was observed between WT, α2- and δ-KO mice, whereas a significant reduction was seen in α4-KO mice, mainly proximally. (B) At 28 DPI, a significant reduction in the number of intersections was evident for both α2- and α4-KO mice compared to WT, whereas no difference was observed for δ-KO mice. (C) This alteration was maintained at 42 DPI, mainly due to loss of branches in newborn neurons from α2-KO mice. Scale bars, 20 μm.
At 28 DPI further dendritic growth was evident in all mutant lines, with total length increasing by 97–148% compared to 14 DPI (Table 1). The number of intersections with concentric circles at > 75 μm from the soma was much increased (Fig. 3B), indicating elongation of distal segments. However, the number of nodes was unchanged compared to 14 DPI except in α2-KO mice, where it was reduced (unpaired t-test, t48 = 3.15, P = 0.0028; Table 1). Between-genotype comparisons at 28 DPI revealed a significant reduction in all parameters analyzed in α2-KO and α4-KO mice compared to WT littermates, whereas no difference was observed in δ-KO mice (Table 1). These observations suggest differential alterations in dendritic arborization in mice lacking α2- and α4-GABAARs, with loss of distal branches in α2-KO and overall deficit of dendritic growth in α4-KO mice.
At 42 DPI, we further focused on the latter mutants (Fig. 3C; Table 1). Compared to 28 DPI, no further change in dendritic arborization was observed in newborn cells from WT mice. In contrast, total dendritic length and complexity were significantly reduced in α2-KO mice [unpaired t-test, t60 = −2.42, P = 0.018 for the area under the curve (AUC) and t60 = −2.4, P = 0.019 for the dendritic length], suggesting pruning of dendrites during late maturation. When compared to WT at 42 DPI, dendritic complexity, number of branches and total length were reduced in cells from both α2-KO and α4-KO mice (Table 1), indicating that a deficit in GABAergic function mediated by either receptor subtype precludes the formation of a normal dendritic tree in newborn granule cells.
α4- and α2-GABAARs differentially regulated positioning of newborn neurons
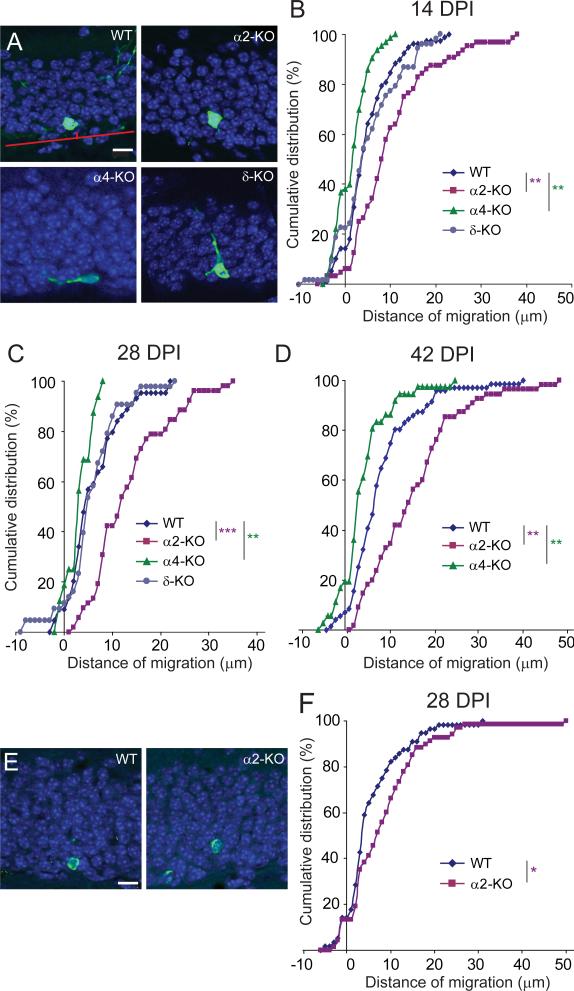
Analysis of the position of eGFP+ cells at different time points showed that newborn cells in WT and in δ-KO mice gradually migrated into the inner third of the GCL (Fig. 4A), as reported (Mathews et al., 2010). In α4-KO mice, however, the distance of migration was significantly shorter and a large fraction of newborn cells remained confined within the SGZ (Fig. 4B). This difference was selective for α4-KO mice and was evident at all developmental stages examined (Fig. 4B–D). In striking contrast, the opposite effect was seen in α2-KO mice, where eGFP+ cells migrated deeper into the GCL than in WT mice (Fig. 4B–D). To verify that these differences in migration behavior were not related to a nonspecific effect of the viral transduction, we analyzed the location of BrdU+ cells in WT and α2-KO mice and observed a similar differential localization in the GCL at 28 DPI (Fig. 4E and F).
Figure 4.
Differential localization of eGFP+ newborn neurons and BrdU+ cells in the GCL of WT and mutant mice. (A) Example of images illustrating the localization of eGFP+ cells at 14 DPI, along with the method used to measure their distance of migration from the SGZ. The red line denotes the SGZ–GCL boundary (d = 0). Note the difference in localization between α2-KO and α4-KO mice. (B–D) Cumulative distribution analysis of the migration distance covered by eGFP+ cells at (B) 14, (C) 28 and (D) 42 DPI. At each time-point investigated, a significant rightward shift was evident in α2-KO mice and a leftward shift in α4-KO mice compared to WT, whereas no distinct phenotype was seen in δ-KO mice (Kolmogorov–Smirnov test, **P < 0.01, ***P < 0.001). (E) Representative images showing that BrdU+ cells (green) in α2-KO mice are located further away from the SGZ than in WT; DAPI staining of nuclei (blue) outlines the GCL. (F) Cumulative distribution analysis revealing the significant rightward shift in the distance of migration of newborn BrdU+ cells in α2-KO mice (Kolmogorov–Smirnov test *P < 0.05). Scale bars in A and E, 10 μm.
As the apparent thickness of the GCL varies from section to section, we also calculated the relative distance of migration from the SGZ as a function of the local GCL thickness, with similar results as in Figure 4 (data not shown).
Cell-autonomous effects of Gabra2 deletion
To determine whether the alterations in newborn granule cell migration and dendritic arborization observed in α2-KO mice are cell-autonomous or induced upon global loss of α2-GABAARs, we investigated the effects of single-cell Gabra2 deletion using retroviruses encoding Cre recombinase in mice carrying Gabra2 alleles flanked by LoxP sites (α2fl/fl). To ensure labeling of the entire cell in addition to Cre immunofluorescence in the nucleus, α2fl/fl mice were injected intrahippocampally with a mixture of retroviruses encoding Cre–eGFP and mRFP, respectively. This approach allowed comparison of newborn neurons carrying a Gabra2 deletion (Cre-eGFP+/mRFP+) with WT neurons (mRFP+ only) in sections from the same animal. Single-cell deletion of Gabra2 was confirmed at 28 DPI by immunofluorescence staining for the α2 subunit, which was readily detected in mRFP+ cells but not in Cre-eGFP+/mRFP+ cells (Fig. 5A and B).
Figure 5.
Single-cell deletion of the Gabra2 gene upon injection of retroviruses encoding Cre-eGFP and mRFP recapitulates the phenotype seen in α2-KO mice. (A and B) Confirmation of Cre activation and α2 subunit deletion, shown by triple staining – left panel, representative image of a newborn mRFP+ (WT) neuron displayed in the three Cartesian planes (x-y, x-z and y-z) demonstrating the presence of aggregates positive for the α2 subunit (blue) on the soma (arrow); right panel, colocalization of the α2 subunit and mRFP is displayed in white. (B) Representative image of a newborn mRFP+/Cre-eGFP+ neuron (α2-KO) demonstrating that no α2-subunit immunoreactivity was detected on these cells. Colocalization analysis (right panel) confirmed that the clusters positive for the α2 subunit were not in contact with the labeled cell (arrowhead). (C and D) Examples taken at 28 and 42 DPI of newborn cells single- and double-transduced with retroviruses encoding Cre-eGFP and mRFP. Only the latter carry the Gabra2 deletion. (E and F) Quantification of dendrite development by Scholl analysis of mRFP+ cells at 28 and 42 DPI (see Table 2 for average values and statistics). Similarly to α2-KO mice, single-cell deletion of Gabra2 caused a significant reduction in the number of intersections compared to WT (mRFP+-only) cells. (G–I) Differential localization of WT and single-cell mutants in the GCL, as depicted by cumulative probability distribution of migration distances. While no difference was evident at 14 DPI, cells lacking Gabra2 migrated deeper into the GCL at later time-points (Kolmogorov–Smirnov test; *P < 0.05). Scale bars, 3 μm (B), 10 μm (D).
Similar to the phenotype observed at 28 and 42 DPI in α2-KO mice, Cre-eGFP+/mRFP+ cells exhibited a significant reduction in dendritic arborization complexity, branching and total length at these time points compared to mRFP+ WT cells (Fig. 5C–F, Table 2). Therefore, the delayed pruning of distal dendritic branches was cell-autonomous, probably occurring as a consequence of reduced GABAergic input. Moreover, cells carrying the Gabra2 deletion were located significantly deeper in the GCL at 28 and 42 DPI, but not at 14 DPI (Fig. 5G–I), compared to WT cells, indicating that migration is controlled, at least in part, by α2-GABAARs in newborn granule cells.
Pharmacological reduction of glutamatergic synaptogenesis in α2-KO mice prevented dendritic pruning in newborn granule cells
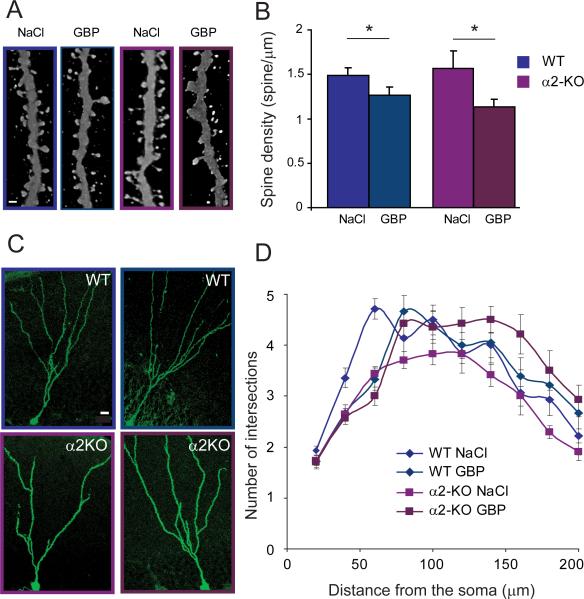
Pruning of dendritic branches in α2-KO newborn neurons might reflect a compensatory mechanism for limiting the amount of glutamatergic input in the absence of α2-GABAARs. To test this hypothesis and address possible underlying mechanisms, we first investigated whether newborn mutant granule cells control the amount of glutamatergic afferents by reducing spine outgrowth on their dendrites. Quantification of spine density in eGFP+ neurons in WT and α2-KO mice at 28 DPI revealed no difference between genotypes (1.45 ± 0.21 vs. 1.23 ± 0.05 spines per μm in WT and α2-KO mice, respectively; unpaired t-test, t5 = 0.88, P = 0.41). Therefore, Gabra2 deletion did not affect spine growth and distribution in newborn granule cells, suggesting similar glutamatergic input per unit of dendrite length in WT and α2-KO mice.
To test the mechanisms underlying pruning of distal dendritic branches in α2-KO mice, we used a pharmacological approach aimed at reducing the formation of glutamatergic synapses on newborn neurons. Based on the report that gabapentin impairs formation of glutamatergic synapses on developing neurons in vitro and in vivo by blocking the thrombospondin receptor α2δ-1 (Eroglu et al., 2009), we investigated the effects of chronic gabapentin administration on spine formation and dendritic arborization of eGFP+ newborn granule cells. To this end, WT and α2-KO mice were injected first with eGFP retroviruses and, starting at 14 DPI, treated daily with either NaCl or gabapentin for 2 weeks.
As glutamatergic synapses are formed on spines, the effect of gabapentin treatment was assessed by quantification of dendritic spines in the four different groups. In both WT and α2-KO mice, a significant reduction in spine density was observed in the gabapentin-treated groups compared to saline controls (Fig. 6A and B), suggesting that blockade of thrombospondin receptor α2δ-1 limits glutamatergic synapse formation on newborn neurons in adult brain. Chronic gabapentin treatment did not affect the maturation of dendrites in WT mice (Fig. 6C and D), indicating that the deficit in spine formation was not due to nonspecific drug effects on cell development. In contrast, chronic gabapentin treatment in α2-KO mice induced a significant increase in dendritic arborization complexity compared to saline controls (one-way anova– AUC, F3,75 = 5.65, P = 0.001; nodes, F3,75 = 15.8, P < 0.0001; total length, F3,75 = 10.8, P < 0.0001). Consequently, gabapentin treatment prevented the pruning of dendrites that normally occurs in α2-KO mice after 14 DPI (one-way anova– AUC, F3,75 = 5.18, P < 0.05; nodes, F3,75 = 23.4, P < 0.0001; total dendritic length, F3,75 = 9.1, P < 0.003; Table 3). Therefore, dendritic pruning appears to be a compensatory mechanism in newborn granule cells to counteract the effects of Gabra2 deletion.
Figure 6.
Effects of gabapentin treatment on spine formation and dendrite development in newborn granule cells in WT and α2-KO mice. (A) Representative images of spine coverage of eGFP+ dendrites at 28 DPI, illustrating the reduction in density upon chronic gabapentin (GBP; 400 mg/kg daily for 14 days) in both WT (blue frame) and α2-KO (red frame) mice. (B) Group quantification of gabapentin effects (one-way anova, F3,15 = 3.9, P = 0.029; WT/NaCl vs. WT/GBP, P = 0.04; α2-KO/NaCl vs. α2-KO/GBP, P = 0.03; *P < 0.05, Bonferroni post-hoc test). (C and D) Scholl analysis of eGFP+ cells in WT and α2-KO mice treated with either NaCl or gabapentin. In WT mice, no difference was observed between the two groups. In α2-KO mice, gabapentin treatment prevented the reduction in number of intersections seen distally in the NaCl-treated group (see Table 3 for mean values and statistics). Scale bars, 1 μm (A), 10 μm (C).
Table 3.
Morphological analysis of eGFP+ cells after chronic gabapentin treatment
| Area under the curve | Number of nodes | Total dendritic length | ||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Treatment | n | (μm2) | P (treat.) | P (treat.) | (μm) | P (treat.) | |
| WT | NaCl | 14 | 6660.7 ± 315.1 | N.S | 6.1 ± 0.3 | N.S | 755.9 ± 29.9 | N.S |
| WT | Gabapentin | 18 | 6669.4 ± 270.1 | 5.7 ± 0.2 | 835.6 ± 38.2 | |||
| α2-KO | NaCl | 33 | 5937.7 ± 164 | < 0.01 | 4.2 ± 0.15 | < 0.0001 | 633.1 ± 22.6 | < 0.0001 |
| α2-KO | Gabapentin | 14 | 6946.4 ± 304.3 | 5.6 ± 0.2 | 825.1 ± 46.2 | |||
Values are given as mean ± SEM. ‘Area under the curve’ represents the distribution of number of intersections with concentric circles as a function of distance from the soma (see Fig. 6). For each group, the cells analyzed (n) were derived from five mice succesfully injected with retrovirus.
Discussion
The results demonstrate the spatiotemporal and functional specificity of α2- and α4-GABAARs in regulating major steps of adult neurogenesis in mouse DG and suggest a homeostatic control of dendritic arborization by the relative contributions of GABAergic and glutamatergic afferents on newborn granule cells. Importantly, newborn cell survival and fate commitment were not affected in any of the mutants analyzed, even upon loss of tonic inhibition by α4-GABAARs, supporting the views that neuronal differentiation is determined at early postmitotic stages (Tozuka et al., 2005) and that survival depends on successful synaptic integration (Tashiro et al., 2006). Single-cell deletion of Gabra2 replicated all major features seen in global α2-KO mice, indicating cell-autonomous function of α2-GABAARs in regulating maturation of newborn granule cells. Our results underscore the relevance of GABAAR heterogeneity as a molecular mechanism ensuring specificity of GABAergic control of neuronal maturation in adult brain.
Immunohistochemistry has failed so far to provide reliable data on GABAAR subunits expressed in adult-born NPC. Most information available is thus derived from mRNA analysis (Overstreet Wadiche et al., 2005; Gascon et al., 2006) and functional studies (Ge et al., 2006; Markwardt et al., 2009). Both approaches unambiguously demonstrate early expression of GABAARs in NPC but provide little insight into their subunit composition and localization. Our results show that deletion of Gabra4, but not Gabra2 or Gabrd, selectively alters NPC proliferation in the SGZ. Therefore, the increased density of BrdU+ and Ki67 + cells in α4-KO mice suggests that α4-GABAARs mediate the negative feedback of tonic GABA action on cell proliferation.
Furthermore, abrogation of tonic GABAergic inhibition in newborn granule cells from α4-KO mice confirmed that this α subunit variant is required for assembly of functional GABAARs. While limited compensation by remaining GABAARs has been reported in α4-KO mice (Liang et al., 2008), based on a moderate region-specific increase in γ2 subunits, this compensation does not appear to rescue tonic currents in immature newborn cells. Therefore, the defective migration and altered dendritic maturation observed in these mutants probably reflect the absence of α4-GABAAR-mediated signaling in immature granule cells and, possibly, in the SGZ. However, the relative contribution of cell-autonomous mechanisms to these effects remains to be determined.
The lack of phenotype in δ-KO mice was unexpected, as these mice display a drastic reduction in tonic inhibition in adult DG (Glykys et al., 2008). Moreover, in mature granule cells the δ subunit is associated mainly, if not exclusively, with the α4 (and β2) subunits (Herd et al., 2008), and the corresponding GABAAR subtype is much reduced in δ-KO mice (Peng et al., 2002). Therefore, the lack of phenotype of δ-KO mice in our study might reflect compensatory changes maintaining tonic inhibition in NPCs (but not in mature granule cells). Alternatively, one might consider that NPCs do not express the δ subunit. No information is available from published studies but data from rodent ontogeny studies point to delayed onset of Gabrd expression during brain development. According to these reports, the δ subunit is undetectable until the second postnatal week (Wisden et al., 1992; Luscher et al., 1997), thus supporting the view that GABAARs incorporating the δ subunit might be operant in mature neurons only.
The effects of Gabra2 gene deletion on GABAergic synaptic transmission have not yet been reported, despite several behavioral and pharmacological studies in α2-KO mice (Dixon et al., 2008, 2010; Vollenweider et al., 2011). In the hippocampal formation, α2-GABAARs contribute mainly to perisomatic synaptic inhibition (Prenosil et al., 2006; Kasugai et al., 2010). Morphologically, the α2 subunit is located postsynaptically (Brunig et al., 2002), possibly bound to the scaffolding protein gephyrin (Tretter et al., 2008). Evidence from our laboratory indicates that Gabra2 deletion does not affect other major GABAAR subunits and causes no loss of GABAergic terminals in the hippocampus (Panzanelli et al., 2010). However, it causes neuron-specific alteration in gephyrin clustering, underscoring the role of α2-GABAARs for synaptic function in the hippocampus. Therefore, dendritic pruning seen in newborn granule cells probably reflect functional deficits in GABAergic synaptic transmission.
The effects of GABAAR agonists on newborn neurons are probably due to depolarization-induced activation of voltage-gated Ca++ channels (Sernagor et al., 2010). However, the relative contribution of ‘tonic’ and ‘phasic’ GABAergic currents has not been determined. Furthermore, the downstream pathways activated by GABA to control cell cycle and postmitotic cell differentiation remain to be identified. A prominent candidate is the CREB pathway, necessary for maturation and long-term survival of newborn neurons (Obrietan et al., 2002; Jagasia et al., 2009). However, it is also conceivable that Ca++-independent mechanisms play a role in regulating cell proliferation. Thus, GABAAR activation negatively regulates stem cell proliferation through activation of S-phase checkpoint kinases and the histone variant H2AX (Andang et al., 2008).
The finding that migration of newborn granule cells is curtailed in α4-KO mice (Fig. 4) reveals a new potential role of tonic GABAergic currents for initiating radial migration, which is necessary for proper localization within the GCL and presumably adequate formation of perisomatic GABAergic inputs. Initiation of radial migration depends on establishment of cell polarity and, notably, the ability to extend a radial dendrite towards the molecular layer (Seki et al., 2007). Several signaling pathways contribute to this key step, including activation of cdk5 (Jessberger et al., 2008). Chronic application of pentobarbital to enhance GABAAR function promotes dendrite outgrowth of newborn neurons (Ge et al., 2006). Furthermore, related to GABA-induced depolarization of immature neurons, the growth-promoting effect of GABA was curtailed by overexpression of NKCC1 (Ge et al., 2006). GABA-mediated depolarization also increases CREB phosphorylation, which in turn promotes dendritic growth (Jagasia et al., 2009). A possible downstream mediator of activated CREB is BDNF, whose expression is necessary for dendritogenesis (Chan et al., 2008). All these effects occur during the first 2 weeks of neuronal development, prior to formation of GABAergic synaptic contacts (Esposito et al., 2005; Markwardt et al., 2009), underscoring the relevance of tonic inhibition. It is noteworthy, however, that α4-KO mice exhibited a mild phenotype with respect to alteration of cell polarity and dendritic arborizations. Compared to the studies cited above, we did not observe ectopic basal dendrites and cell survival was not compromised. Therefore, α4-GABAAR-mediated tonic currents might not signal via these pathways. Furthermore, total dendritic length of newborn cells doubled in α4-KO mice between 14 and 28 DPI, as in WT, reinforcing the notion that the effects of tonic GABA action might be restricted to initial growth of dendrites before the onset of synaptogenesis (Gascon et al., 2006; Sernagor et al., 2010).
In contrast to Gabra4, Gabra2 deletion induced a marked increase in newborn granule cell migration, an effect most pronounced after 14 DPI. Thus, GABAergic currents depending on α2-GABAARs might provide an early signal to stop newborn cells at their correct location in the GCL (Esposito et al., 2005), in line with a similar conclusion concerning embryonic neurons migrating into the cortical plate (Behar et al., 2000). Enhanced newborn granule cell migration also has been reported following targeted deletion of PLCβ1 (which typically is activated by mGLUR signaling; Manning et al., 2010), indicating that multiple extracellular signals regulate this process. Upon inactivation of DISC-1 in newborn neurons, which leads to accelerated maturation, excessive migration into the molecular layer was related to lost ability to receive or interpret positioning signals restricting them to the GCL (Duan et al., 2007), possibly mediated by reelin signaling.
The initial growth of dendrites was normal in α2-KO mice, in which α4-GABAAR function is probably preserved. However, starting during the third postnatal week, absence of α2-GABAARs led to pruning of distal dendrites, suggesting a functional deficit critical for maintenance of dendrites. Therefore, this dendritic pruning might reflect a compensatory mechanism to limit glutamatergic inputs received by newborn neurons. It is important to note that granule cells also receive phasic inhibition by α1-GABAARs (Sun et al., 2004). Therefore, deletion of Gabra2 will not cause a complete loss of GABAergic synaptic inputs onto granule cells. Furthermore, this dendritic pruning is cell-autonomous rather than due to changes in network activity that might occur in α2-KO mice.
The fact that spine density on newborn dendrites was not altered in α2-KO mice confirms reports that spine formation in granule cells is governed by afferent inputs (Frotscher et al., 2000). Furthermore, the effect of chronic gabapentin on spinogenesis in newborn neurons suggests recapitulation of a developmental program, as gabapentin antagonizes glutamatergic synapse formation during ontogeny by blocking the thrombospondin receptor (Eroglu et al., 2009); finally, these data suggest a role for astrocytes, which release thrombospondin, in regulating afferent innervation of newborn granule cells.
Developmental pruning of dendritic branches in granule cells normally occurs under control of NMDA receptor activity (Espinosa et al., 2009). Single-cell deletion of grin2b (NR2B) considerably reduced this pruning, in line with the existence of a homeostatic regulation of inhibitory–excitatory balance. Therefore, demonstration of this mechanism, albeit in response to reduced GABAergic input, again reflects reactivation of a developmental program in adult neurogenesis.
In conclusion, these results underscore the preeminent specificity of GABAergic regulation of adult neurogenesis. Moreover, they show that α4- and α2-GABAARs are not interchangeable and mediate specific roles, most probably related to tonic and phasic inhibition, respectively. Finally, our work suggests that dendritic growth is regulated homeostatically to ensure appropriate balance of excitatory and inhibitory inputs onto newborn granule cells. This form of compensation might represent a novel mechanism to cope with abnormal extrinsic regulation of adult neurogenesis that probably operates also under pathological conditions.
Acknowledgements
This work was supported by the Swiss National foundation (National Center of Competence in Research in Neuroscience, Neural Plasticity and Repair) and NIH grants AA10422 and DE14184. We would like to thank Dr Sebastian Jessberger and Krishna Vadodaria (ETH Zurich) for providing retrovirus constructs and Dr Steven Brown and Ludmila Cuninkova (University of Zurich) for help with retrovirus production.
Footnotes
The authors have no competing interests to declare.
References
- 1.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol. Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 2.Andang M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez S, Nishimaru H, Wilbertz J, Arenas E, Koltzenburg M, Charnay P, El Manira A, Ibanez CF, Ernfors P. Histone H2AX-dependent GABAA receptor regulation of stem cell proliferation. Nature. 2008;451:460–464. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- 3.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acid A receptors: classification on the basis of subunit structure and function. Pharmacol. Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 4.Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb. Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 6.Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- 7.Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol. Cell. Neurosci. 2008;39:372–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl Acad. Sci. USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon CI, Rosahl TW, Stephens DN. Targeted deletion of the GABRA2 gene encoding α2-subunits of GABAA receptors facilitates performance of a conditioned emotional response, and abolishes anxiolytic effects of benzodiazepines and barbiturates. Pharmacol. Biochem. Behav. 2008;90:1–8. doi: 10.1016/j.pbb.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Dixon CI, Morris HV, Breen G, Desrivieres S, Jugurnauth S, Steiner RC, Vallada H, Guindalini C, Laranjeira R, Messas G, Rosahl TW, Atack JR, Peden DR, Belelli D, Lambert JJ, King SL, Schumann G, Stephens DN. Cocaine effects on mouse incentive-learning and human addiction are linked to α2 subunit-containing GABAA receptors. Proc. Natl Acad. Sci. USA. 2010;107:2289–2294. doi: 10.1073/pnas.0910117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duveau V, Fritschy JM. PSA-NCAM-dependent GDNF signaling limits neurodegeneration and epileptogenesis in temporal lobe epilepsy. Eur. J. Neurosci. 2010;32:89–98. doi: 10.1111/j.1460-9568.2010.07272.x. [DOI] [PubMed] [Google Scholar]
- 13.Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62:205–217. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABAA receptor signalling. Prog. Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 17.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 18.Frotscher M, Drakew A, Heimrich B. Role of afferent innervation and neuronal activity in dendritic development and spine maturation of fascia dentata granule cells. Cereb. Cortex. 2000;10:946–951. doi: 10.1093/cercor/10.10.946. [DOI] [PubMed] [Google Scholar]
- 19.Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 20.Gascon E, Dayer AG, Sauvain MO, Potter G, Jenny B, De Roo M, Zgraggen E, Demaurex N, Muller D, Kiss JZ. GABA regulates dendritic growth by stabilizing lamellipodia in newly generated interneurons of the olfactory bulb. J. Neurosci. 2006;26:12956–12966. doi: 10.1523/JNEUROSCI.4508-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herd MB, Haythornthwaite AR, Rosahl TW, Wafford KA, Homanics GE, Lambert JJ, Belelli D. The expression of GABAAβ subunit isoforms in synaptic and extrasynaptic receptor populations of mouse dentate gyrus granule cells. J. Physiol. 2008;586:989–1004. doi: 10.1113/jphysiol.2007.146746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J. Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jessberger S, Aigner S, Clemenson GD, Jr, Toni N, Lie DC, Karalay O, Overall R, Kempermann G, Gage FH. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6:e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karten YJ, Jones MA, Jeurling SI, Cameron HA. GABAergic signaling in young granule cells in the adult rat and mouse dentate gyrus. Hippocampus. 2006;16:312–320. doi: 10.1002/hipo.20165. [DOI] [PubMed] [Google Scholar]
- 27.Kasugai Y, Swinny JD, Roberts JD, Dalezios Y, Fukazawa Y, Sieghart W, Shigemoto R, Somogyi P. Quantitative localisation of synaptic and extrasynaptic GABAA receptor subunits on hippocampal pyramidal cells by freeze-fracture replica immunolabelling. Eur. J. Neurosci. 2010;32:1868–1888. doi: 10.1111/j.1460-9568.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J, Suryanarayanan A, Chandra D, Homanics GE, Olsen RW, Spigelman I. Functional consequences of GABAA receptor alpha 4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol. Clin. Exp. Res. 2008;32:19–26. doi: 10.1111/j.1530-0277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luscher B, Hauselman R, Leitgeb S, Rulicke T, Fritschy JM. Neuronal subtype-specific expression directed by the GABAA-receptor δ-subunit gene promoter in transgenic mice and in cultured cells. Mol. Brain Res. 1997;51:197–211. doi: 10.1016/s0169-328x(97)00242-8. [DOI] [PubMed] [Google Scholar]
- 31.Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat. Neurosci. 2010;13:1338–1344. doi: 10.1038/nn.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning EE, Ransome MI, Burrows EL, Hannan AJ. Increased adult hippocampal neurogenesis and abnormal migration of adult-born granule neurons is associated with hippocampal-specific cognitive deficits in phospholipase C-β1 knockout mice. Hippocampus. 2010 doi: 10.1002/hipo.20900. doi: 10.1002/hipo.20900 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J. Neurosci. 2009;29:15063–15072. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathews EA, Morgenstern NA, Piatti VC, Zhao C, Jessberger S, Schinder AF, Gage FH. A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. J. Comp. Neurol. 2010;518:4479–4490. doi: 10.1002/cne.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor δ subunit knockout mice. Proc. Natl Acad. Sci. USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, Belachew S, Rigo JM. Autocrine/paracrine activation of the GABAA receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSANCAM+) precursor cells from postnatal striatum. J. Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obrietan K, Gao XB, Van Den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism--a positive feedback circuit in developing neurons. J. Neurophysiol. 2002;88:1005–1015. doi: 10.1152/jn.2002.88.2.1005. [DOI] [PubMed] [Google Scholar]
- 38.Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J. Neurophysiol. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- 39.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 40.Panzanelli P, Schlatter M, Sidler C, Crestani F, Scheiffele P, Rudolph U, Fritschy J-M. Neuroscience Meeting Planner. Society for Neuroscience Online; San Diego, CA: 2010. Selective dependence of gephyrin on GABAA receptors containing the a2 subunit for clustering at perisomatic and axo-axonic postsynaptic sites in CA1 pyramidal cells. Program No. 239.10. 2010. [Google Scholar]
- 41.Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J. Comp. Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 42.Prenosil GA, Schneider Gasser EM, Rudolph U, Keist R, Fritschy JM, Vogt KE. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J. Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- 43.Schneider Gasser EM, Duveau V, Prenosil GA, Fritschy JM. Reorganization of GABAergic circuits maintains GABAA receptor-mediated transmission onto CA1 interneurons in α1-subunit-null mice. Eur. J. Neurosci. 2007;25:3287–3304. doi: 10.1111/j.1460-9568.2007.05558.x. [DOI] [PubMed] [Google Scholar]
- 44.Seki T, Namba T, Mochizuki H, Onodera M. Clustering, migration, and neurite formation of neural precursor cells in the adult rat hippocampus. J. Comp. Neurol. 2007;502:275–290. doi: 10.1002/cne.21301. [DOI] [PubMed] [Google Scholar]
- 45.Sernagor E, Chabrol F, Bony G, Cancedda L. GABAergic control of neurite outgrowth and remodeling during development and adult neurogenesis: general rules and differences in diverse systems. Front Cell Neurosci. 2010;4:11. doi: 10.3389/fncel.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 47.Sun C, Sieghart W, Kapur J. Distribution of α1, α4, γ2, and δ subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 49.Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat. Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 50.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 51.Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ. The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor α2 subunits to gephyrin. J. Neurosci. 2008;28:1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vollenweider I, Smith KS, Keist R, Rudolph U. Antidepressant-like properties of α2-containing GABAA receptors. Behav. Brain Res. 2011;217:77–80. doi: 10.1016/j.bbr.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a.Witschi R, Punnakkal P, Paul J, Walczak JS, Cervero F, Fritschy JM, Kuner R, Keist R, Rudolph U, Zeilhofer HU. Presynaptic α2-GABAA receptors in primary afferent depolarization and spinal pain control. J. Neurosci. 2011;31:8134–8142. doi: 10.1523/JNEUROSCI.6328-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]