Abstract
Blood CXCR5+ CD4+ T cells share phenotypic and functional similarities with T follicular helper cells. Studies by He et al. (2013) and Locci et al. (2013) in this issue of Immunity provide insight into their ontogeny and functionally distinct subsets.
T follicular helper (Tfh) cells are CD4+ T cells specialized for providing help to B cells (Crotty, 2011). Tfh cells are identified by a combination of markers, including the chemokine receptor CXCR5, costimulatory molecules ICOS and PD-1, and the transcription repressor BCL6. The main effector site of Tfh cells is germinal centers (GCs) in secondary lymphoid organs, and GC Tfh cells selectively provide help to high-affinity B cells and promote their survival and differentiation into memory cells and plasma cells. Accordingly, Tfh cells are essential for the generation of protective antibody responses, whereas exaggerated or dysregulated Tfh responses cause autoimmunity. A majority of these conclusions were obtained from mouse studies. An important question is whether and how these observations can be translated in human immunology and applied to understand human disease pathogenesis. The challenge here is an extremely limited accessibility to secondary lymphoid organ samples from individuals with diseases. An alternative approach is the analysis of blood samples. Human blood contains memory CXCR5+ CD4+ T cells that share phenotypic and functional properties with Tfh cells and therefore are often called “blood Tfh cells” (herein we also use this term for simplicity). In this issue of Immunity, studies by He et al. (2013) and Locci et al. (2013) substantially extended the knowledge on the ontogeny and subsets of blood Tfh cells.
BCL6 is not expressed by blood Tfh cells, even by “activated” ICOS+ PD-1+ subsets (Bentebibel et al., 2013; Chevalier et al., 2011; He et al., 2013; Locci et al., 2013; Morita et al., 2011). Thus, blood Tfh cells are different from bona fide Tfh cells in secondary lymphoid organs, and direct evidence demonstrating a relationship between blood Tfh cells and GC Tfh cells has been lacking. Here, He et al. showed that similar to GC Tfh cells, the development of blood Tfh cells was totally dependent on ICOS and BCL6. Furthermore, they demonstrated that an increase of “activated” Tfh subset in blood (described later) correlates with the magnitude of newly generated Tfh cells in secondary lymphoid organs. Of note, unlike GC Tfh cells, the development of blood Tfh cells did not require SAP or GC formation (He et al., 2013), suggesting that the major precursors of blood Tfh cells are developing Tfh cells rather than GC-Tfh cells. Nonetheless, these observations suggest that analysis of blood Tfh subsets permits the assessment of ongoing Tfh responses.
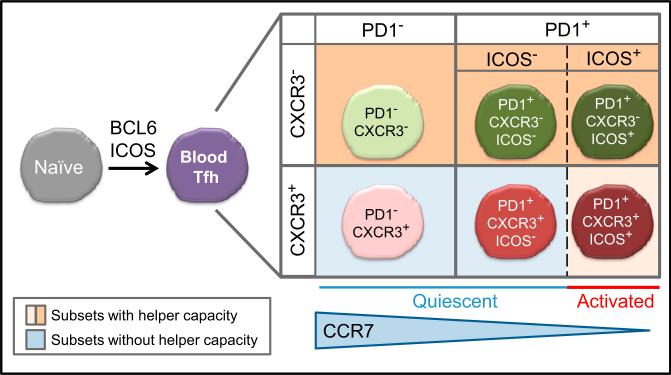
We previously showed that blood Tfh cells are composed of functionally distinct subsets that share properties with Th1, Th2, and Th17 cells, defined by the differential expression of the chemokine receptors CXCR3 and CCR6. Importantly, whereas CXCR3– subsets (containing CCR6– Th2- and CCR6+ Th17-like cells) can help naive B cells to become immunoglobulin-producing plasmablasts, the CXCR3+ Th1-like subset lacks this capacity (Bentebibel et al., 2013; Morita et al., 2011). The two new studies show that that PD-1 expression can define additional aspects of human blood Tfh subsets (He et al., 2013; Locci et al., 2013). Accordingly, human blood Tfh cells can be subdivided into four subsets by the expression of CXCR3 and PD-1 (Figure 1). The PD-1+ subsets can be further subdivided into two subgroups according to the expression of ICOS. The ICOS+PD-1+ subpopulations represent activated blood Tfh cells. These cells are barely present in healthy individuals (He et al., 2013; Locci et al., 2013) but increase after influenza vaccination (Bentebibel et al., 2013). He et al. concluded that the PD-1+ subset expressing the lowest amounts of CCR7 (thus the PD-1+ CCR7lo subset) represents activated blood Tfh cells. This PD-1+CCR7lo subset seems largely overlapping with the ICOS+PD-1+ subsets because the PD-1+ CCR7lo subset contained ICOS+ cells (He et al., 2013), and the kinetics of the increase of PD-1+CCR7lo subset after influenza vaccination (He et al., 2013) was identical to that of the ICOS+PD-1+ subset increase (Bentebibel et al., 2013).
Figure 1. Human Blood Tfh Subsets.
Studies by He et al. (2013) and Locci et al. (2013) provide insights regarding the ontogeny and functionally distinct subsets of blood Tfh cells. Human blood Tfh cell cells can be divided into four subsets according to the expression of CXCR3 and PD-1. CCR7 expression negatively correlates with PD-1 expression. The PD-1+ subsets contain cells expressing ICOS, which represent “activated” blood Tfh cells. Among the “quiescent” blood Tfh subsets, the PD-1+CXCR3– subset is the most efficient at providing help to B cells. The CXCR3+ subsets is able to provide help to B cells only when activated (ICOS+PD-1+ cells), but their ability is limited to memory B cells.
The ICOS–PD-1+ and the PD-1– blood Tfh subsets did not express activation markers and thus represent quiescent blood Tfh subsets (He et al., 2013; Locci et al., 2013). Locci et al. focused on the analysis of the four quiescent ICOS– blood Tfh subsets and found that the PD-1+CXCR3– subset displayed a gene-expression profile the most similar to that of tonsillar GC Tfh cells. Furthermore, the PD-1+CXCR3– subset was the most efficient at inducing memory B cells to differentiate into immunoglobulin G (IgG)-producing plasmablasts. These results show that the PD-1+CXCR3– subset is the most functional among the quiescent blood Tfh subsets.
These new findings will provide immediate implications to the studies on human blood Tfh cells. First, the integration of PD-1, ICOS, and CCR7 together with CXCR3 and CCR6 in the analysis of human blood Tfh cells will facilitate determining the phenotype correlating with disease activity in human autoimmune diseases and generation of protective antibody responses in infections and vaccinations. Indeed, He et al. demonstrated that the frequency of the PD-1+CCR7lo subset positively correlated with disease activity and the titers of several serum autoantibodies in human autoimmune diseases. Locci et al. found that the frequency of PD-1+CXCR3– subset in blood Tfh cells positively correlated with development of broadly neutralizing antibodies against HIV in a large cohort of HIV-infected individuals.
Second, these studies have provided the framework and the directions for the future studies on the biology of human blood Tfh cells. Remaining questions include: What is the function of each blood Tfh subset in vivo? How is the stability of each blood Tfh subset in vivo? Which blood Tfh subsets represent “memory” Tfh cells that immediately differentiate into GC Tfh cells and promote GC responses? What is the cause of the alteration in the blood Tfh subsets in autoimmune diseases? Such studies will provide substantial insights regarding the pathogenesis of autoimmune diseases and for the design of novel vaccines. For example, in our recent study on influenza vaccines, we showed that the vaccination induces the emergence of the ICOS+ PD-1+ CXCR3+ Tfh subset in blood (Figure 1). Despite the findings that the CXCR3+ subsets are the least functional (Locci et al., 2013; Morita et al., 2011), their increase at day 7 after vaccination positively correlated with the generation of protective antibody responses. We found that the ICOS+PD-1+CXCR3+ Tfh cells were able to induce memory B cells to differentiate into plasma cells that produce influenza-specific antibodies ex vivo but lacked the capacity to help naive B cells (Bentebibel et al., 2013). These observations suggest that current influenza vaccine is largely dependent on the CXCR3+ Tfh subsets, which can help only memory B cells, which likely explains its limited efficacy. Together with the observation by Locci et al. in HIV-infected individuals, these studies suggest the importance of the induction of CXCR3– Tfh responses in humans for the generation of optimal protective antibody responses.
REFERENCES
- Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. Science Translational Medicine. 2013;5:176, ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, Sallusto F, Tangye SG, Mackay CR. J. Immunol. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- Crotty S. Annu. Rev. Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- He J, Tsai LM, Leong YA, Hu X. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. this issue. [DOI] [PubMed] [Google Scholar]
- Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, et al. International AIDS Vaccine Initiative Protocol C Principal Investigators Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Schmitt N, Bentebibel SE, Rangana-than R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]